Abstract
Mice that have been subjected to cecal ligation and puncture (CLP) have an impaired ability to clear a subsequent Pseudomonas aeruginosa challenge when compared to sham CLP controls. We hypothesized that that outcome is dependent upon a caspase-1 mechanism and tested that by measuring caspase-1 after CLP and by measuring clearance of a bacterial challenge in caspase-1-deficient mice after CLP. Wild-type mice subjected to CLP had increased caspase-1 activity as well as increased IL-1β and increased IL-18 production in splenocytes stimulated with heat-killed Pseudomonas, and had increased plasma concentrations of IL-1β and IL-18 and impaired clearance of a Pseudomonas aeruginosa challenge when compared to sham controls. Healthy, uninjured caspase-1−\− mice did not differ from wild-types in their ability to clear a Pseudomonas challenge. However, unlike wild-type mice, caspase-1−/− mice subjected to CLP had no impairment of bacterial clearance of the Pseudomonas challenge, suggesting that caspase-1 induction after CLP played a role in impairment of bacterial clearance. This was further substantiated by the use of a specific caspase-1 inhibitor, Ac-YVAD-CMK. Wild-type mice treated with AC-YVAD-CMK (10mg/kg SQ BID, initiated at time of CLP) did not have impaired clearance of a Pseudomonas challenge when compared to sham mice, and had significantly improved bacterial clearance when compared to untreated CLP mice. Increased caspase-1 expression and activity after CLP injury appears to contribute to diminished innate immune function.
Introduction
Sepsis occurs most commonly as an adverse complication in patients who have suffered major trauma or illness, and it is commonly believed that these patients are predisposed to sepsis because they have impaired immune function secondary to their primary lesion. We have tried to mimic this clinical scenario in our investigations of post-injury immunosuppression by assessing clearance of a bacterial challenge in mice 5 days after cecal ligation and puncture. Mice subjected to CLP have impaired ability to clear the subsequent bacterial challenge as efficiently as sham control mice, suggesting that the innate immune response was adversely affected by CLP(1,2). CLP induces activation & mobilization of immune cells to the abdomen and results in local and systemic inflammatory responses. CLP also induces an increase in cellular apoptosis & death, particularly in cells of the lymphocytic lineage(3). Some of these responses have a beneficial role in the immediate response to CLP, but are not inducible to the same degree upon secondary immune challenge. Other host responses do not appear necessary for an effective defense against CLP but, in combination with attenuation of the necessary responses, may play a role in diminished innate immune function against secondary immune challenges.
Caspases (cysteinyl aspartate-specific proteinases) are a group of 15 enzymes which are involved in processing proteins critical to major changes in cell state, including nuclear proteins, transcription factors, cell cycle regulators, kinases, and cytoskeletal proteins(4). Caspases have been recognized to play an important role in inflammation and cell death. Caspase-1, also known as IL-1β converting enzyme (ICE), is a class I cysteine protease that lies in quiescence in a complex of molecules termed the ‘inflammasome’. Cellular activation by microbial ligands of toll-like receptors (TLRs) leads to a chain reaction resulting in release of caspase-1 from the inflammasome complex. Caspase-1 activity results when the released caspase-1 molecules form dimers which cleave the pro-forms of IL-1β and IL-18 (IFNγ-inducing factor) into their mature, active forms(5,6), as well as cleaving other proteins involved in glycolysis and chaperone activities(7). Because of this activity, caspase-1 has been categorized as an inflammatory caspase, although activation of caspase-1 was also reported to induce apoptosis in macrophages and fibroblasts(8,9). Further, although mice deficient in caspase-1 do not have obvious defects in apoptosis, overexpression of caspase-1 can induce cell death(8). Caspase-1 deficient mice are markedly resistant to endotoxic shock(10), presumably due to deficits in secretion of mature IL-1β and IL-18.
The purpose of this study was to determine if caspase-1 was activated in response to CLP and, if so, whether caspase-1 activity played a role in the development of CLP-induced immune suppression.
Materials and Methods
Animal model
All experiments were conducted in accordance with the National Institutes of Health’s Guidelines for the Use of Laboratory Animals (National Institutes of Health Publication 85–23, revised 1996) and with the approval of the Institutional Animal Care and Use Committee at the University of Texas Medical Branch. Male mice (6–8 weeks) were purchased from Jackson Laboratories (Bar Harbour, ME) and included C57BL/6J, Caspase-1 knockout mice (NOD.129S2(B6)-Casp1tm1Sesh/LtJ) and their corresponding wild-type controls (NOD/ShiLtJ). The animals were allowed to acclimatize for at least 7 days after delivery and were maintained on 12 hr light-dark cycles with ad libitum food and water at all times. Cecal ligation was performed as previously described (11) with some modifications. Isoflurane anesthesia (2.5% in 100% O2) was initiated in an induction chamber and maintained by delivery through a face mask. After shaving and cleaning the ventral abdominal wall with alcohol, a midline incision was made and the cecum was exteriorized. Cecal contents were massaged out of the tip and towards the base of the cecum and the distal 0.5 cm of the tip was ligated with 3-0 silk suture. A 25-gauge needle was used to perforate the ligated portion of the cecum once in a through-and-through manner. Some groups of mice were studied for survival after a more severe model of CLP in which cecal contents were massaged into the cecal apex, 1cm of the cecal apex was ligated, and a 23-gauge needle was used to perforate the cecum. The cecum was returned to the abdominal cavity, the abdominal wall was closed with 4-0 Vicryl suture, and the skin was reapposed with cyanoacrylate tissue adhesive. Mice were allowed to recover for 5 days before bacterial challenge. Some groups of mice were treated with the caspase-1 inhibitor Ac-YVAD-CMK (10mg/kg SQ BID; Bachem, Torrance, CA) starting immediately after CLP and continuing until the Pseudomonas challenge. Treatment controls were treated with vehicle (1:1 v/v saline/polyethylene glycol 300) in the same manner.
Bacterial challenge and clearance
Pseudomonas aeruginosa (strain 19660, American Type Culture Collection, Rockville, MD) was inoculated into tryptic soy broth and allowed to replicate overnight in a shaking incubator at 37oC. The resulting bacterial culture was washed with 10ml of sterile 0.9% saline. Viable numbers of colony-forming units (cfu) were determined by plating serial dilutions overnight on tryptic soy agar. Bacteria were suspended in sterile 0.9% saline at a final concentration of 1 × 109 cfu/ml. Mice were challenged with 0.1 ml of this suspension (1 × 108 cfu; i.v.) 5 days after the CLP (or sham) procedure.
The mice were sacrificed under isoflurane 6 hours after intravenous injection of Pseudomonas aeruginosa. Lungs, spleens, and liver tissue samples were aseptically excised, weighed and homogenized in sterile saline (1:10 w/v) using sterile tissue grinders. Serial dilutions of tissue homogenates were plated on tryptic soy agar and incubated overnight at 37°C. Pseudomonas bacterial colony-forming units were identified by colony color and morphology and were counted to assess bacterial clearance. Pseudomonas colony identification was confirmed as necessary by the use of Taxo N Discs (BD, Franklin Lakes, NJ).
Measurement of plasma and cell culture supernatant cytokine concentrations
Commercially available enzyme-linked immunosorbent assay (ELISA) kits were used to measure plasma concentrations of IFNγ and IL-10, as well as plasma and cell culture supernatant concentrations of IL-1β (eBioscience, San Diego, CA) and IL-18 (MBL International, Woburn, MA) in cell culture supernatant following the manufacturers recommended procedures.
Measurement of cell-associated caspase-1 activity by flow cytometry
Caspase-1 activity was measured in splenocytes using the cell-permeable fluorochrome inhibitor of caspase-1 (FLICA) FAM-YVAD-FMK (Immunochemistry Technologies, Bloomington, MN). Spleens were aseptically excised 24, 48, or 120 hours after CLP (or sham) and transferred to 6-well culture plates containing RPMI 1640. The tissue was minced and passed through sterile mesh. Erythrocytes were lysed (erythrocyte lysis kit, R&D Systems). The remaining splenocytes were resuspended to a concentration of 1 × 106 cells/ml in PBS and incubated with the FLICA reagent (1:30 dilution) for 1 hour at 37oC. The cells were washed, resuspended, and incubated for 30 minutes at 4oC with fluorescence-labeled antibodies against cell-surface antigens including anti-F4/80, anti-CD3, anti-CD4, and anti-CD8 (Invitrogen Laboratories, Carlsbad, CA). Isotype-matched antibodies were used as controls. Cells were washed with PBS and resuspended in 1% paraformaldehyde before analysis by a FACScan flow cytometer (BD Biosciences, San Jose, CA). Alternatively, some groups of cells were counterstained with PI after the FLICA incubation and were analyzed on the flow cytometer immediately without fixation. Data were analyzed and graphed by FloJo software (Tree Star Inc, Ashland, OR).
Splenocyte production of IL-1β and IL-18
Spleens were excised from mice 5 days after CLP and transferred to 6-well culture plates containing RPMI 1640. The tissue was minced and passed through sterile mesh. Erythrocytes were lysed (erythrocyte lysis kit, R&D Systems). The remaining splenocytes were resuspended to a concentration of 1 × 106 cells/ml in RPMI and incubated with heat-killed Pseudomonas (1 × 108 cfu/ml) overnight in 5% CO2 at 37°C. Cell supernatants were collected and assayed for IL-1β and IL-18 by ELISA.
Data analysis
Statistical analyses were performed using GraphPad Prism 4 software (La Jolla, CA). All data are presented as mean ± SEM. Multiple group data were analyzed by ANOVA and post-hoc Tukeys test while comparisons between 2 groups were performed by an unpaired t-test. A p value less than 0.05 was considered statistically significant.
Results
CLP was associated with a diminished ability to clear a subsequent bacterial challenge
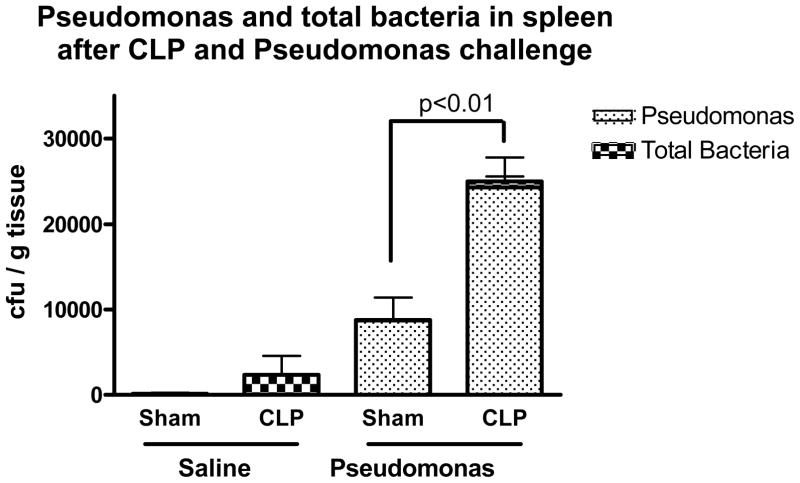
CLP, or sham CLP, was performed and the mice were allowed to recover for 5 days. Mice subjected to CLP had overt signs of injury, including piloerection and lethargy for the first 1–2 days, but continued to eat, drink, and maintain near normal levels of activity afterward. There were no deaths in any of the groups after CLP or sham procedures. On the 5th day after CLP or sham CLP, the mice were anesthetized briefly and subjected to an intravenous challenge of live Pseudomonas aeruginosa (or saline control). The mice were sacrificed 6 hours later for collection of tissues. Five days after CLP, the site of the cecal puncture was typically walled off by abscess formation and there was little to no bacterial growth on culture of the spleens from mice that were not subject to further bacterial challenge. The small amount of other bacteria cultured from the spleens of mice subjected to CLP were mostly gram-negative rods and occasional gram-positive cocci. No Pseudomonas colonies were identified in spleens of mice that were not challenged with Pseudomonas. Mice that had been subjected to CLP and were subsequently challenged with Pseudomonas had increased number of Pseudomonas colony-forming units in spleen tissue samples when compared to sham control mice. (Figure 1)
Figure 1. CLP was associated with a diminished ability to clear bacterial challenge.
Mice challenged with live Pseudomonas aeruginosa (or saline control) 5 days after CLP were sacrificed 6hrs later and spleen homogenates were plated and cultured overnight to measure bacterial growth. CLP was associated with a diminished ability to clear the Pseudomonas challenge when compared to sham controls. Mice subjected to CLP or sham procedures and sacrificed 5 days later with no Pseudomonas challenge had little to no bacterial growth on culture of their spleen. (* = p<0.01; N = 7–9/group).
CLP was associated with increased caspase-1 activity in splenocytes, in both macrophage and granulocyte cell populations
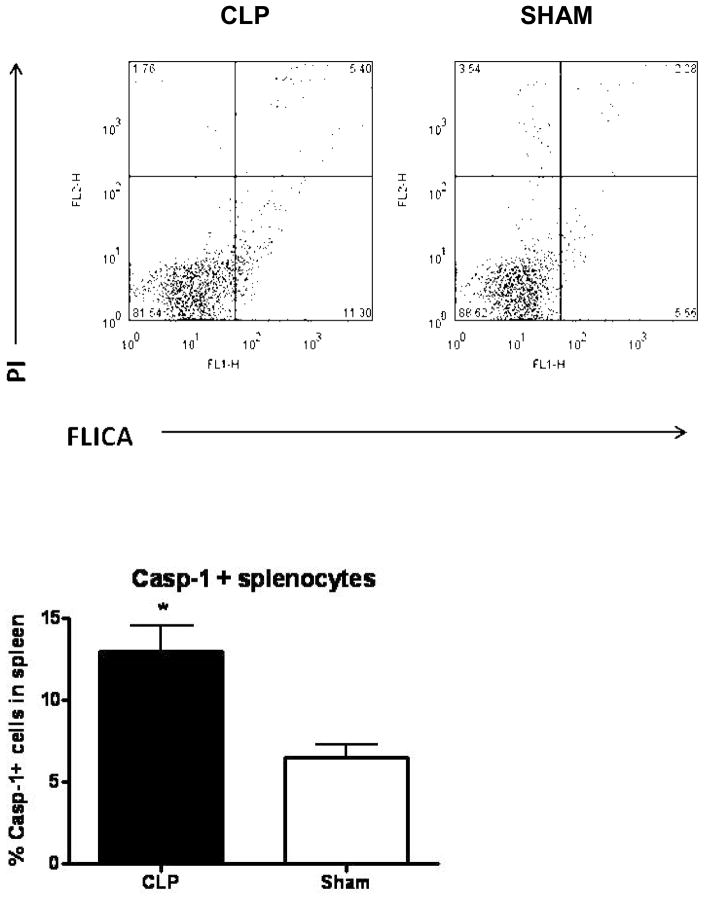
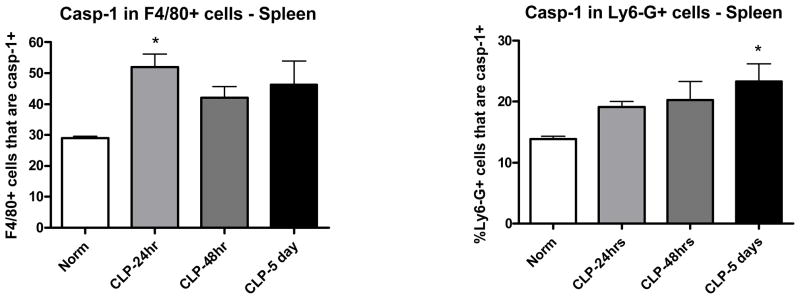
Caspase-1 activity was determined in intact cells from spleen tissue using a cell permeable caspase-1 inhibitor fluorochrome and these results were further extended by examining specific cell populations for higher levels of caspase-1 activity. Caspase-1 activity was found to be higher in splenocytes of mice 24 hours after CLP when compared to splenocytes from sham mice (Figure 2). When concurrently stained with cell surface markers, it was apparent that both macrophages (F4/80+) and granulocytes (Ly6-G+) had a higher level of activity of caspase-1 in mice that had been subjected to CLP (Figure 3).
Figure 2. Caspase-1 activity was increased in splenocytes 24hrs after CLP.
FLICA (fluorochrome inhibitor of caspase-1) was used to detect caspase-1 activity in splenocytes harvested from mice 24hrs after CLP (left) or sham (right) procedures. The top pair of panels shows propidium iodide leakage into dead cells (upper quadrants) and FLICA activation (right-sided quadrants) by caspase-1 in the total splenocyte population. The bottom graph shows caspase-1 activity in splenocytes derived from mice after CLP or sham surgery (* = p<0.05, N = 7/group).
Figure 3. Caspase-1 activity was increased in both macrophages and granulocytes after CLP.
Spleen tissues were collected at 24h, 48h, or 5 days after CLP. Caspase-1 activity was measured by the FLICA assay in both macrophages (F4/80+ cells) and granulocytes (Ly6-G+ cells). (* = p<0.05, N = 4–5/group)
CLP primed for increased production of IL-1β and IL-18 responses to a secondary stimulus
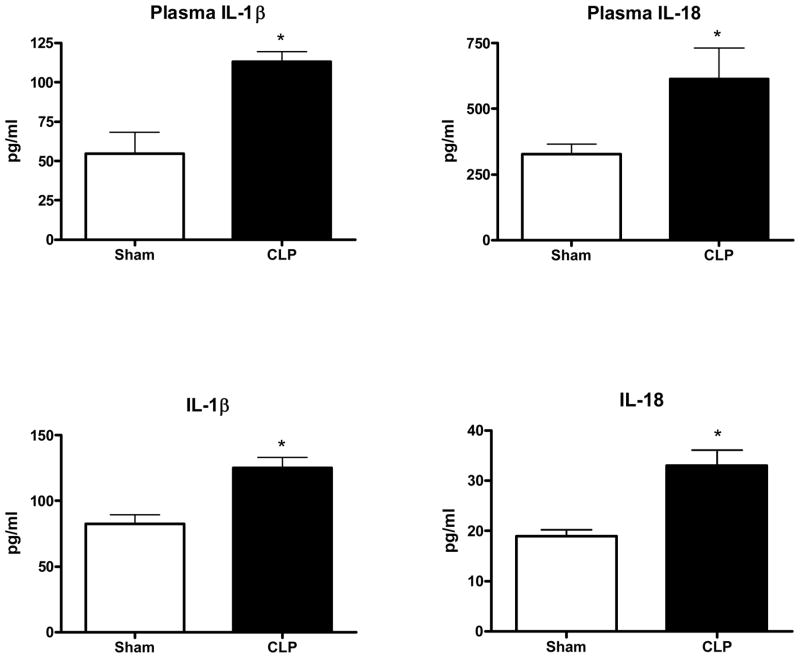
Transcription and translation of pro-forms of IL-1β and IL-18 occur in response to TLR stimulation, but caspase-1 activity is necessary to cleave the pro-forms resulting in the secretion of mature, active IL-1β and IL-18. Therefore, caspase-1 activity may also be indirectly reflected by measurement of IL-1β and IL-18. The top of Fig 4 shows that plasma concentrations of IL-1β and IL-18 were elevated in post-CLP mice 6hrs after Pseudomonas challenge when compared to shams. The bottom of Fig 4 shows that CLP mice had increased induction of splenocyte production of IL-1β and IL- 18 compared to sham mice. Splenocytes were collected 24 hours after CLP or sham and incubated in culture media overnight in the presence of heat-killed Pseudomonas. IL-1β and IL-18 were measured to be significantly higher in cultures of cells collected from post-CLP mice when compared to sham CLP control mice.
Figure 4. Post-CLP mice had increased IL-1β and IL-18 responses to the subsequent Pseudomonas challenge.
Mice were subjected to CLP and allowed to recover for 5 days. Fig 4-top demonstrates the higher plasma concentrations of IL-1β and IL-18 in post-CLP mice 6hrs after challenge with live Pseudomonas aeruginosa. Fig 4-bottom shows that splenocytes derived from mice 5 days after CLP and cultured in the presence of heat-killed Pseudomonas had higher production of IL-1β and IL-18 than did splenocytes from sham mice. (*p <.05, N = 11/group for IL-1β, N = 4/group for IL-18)
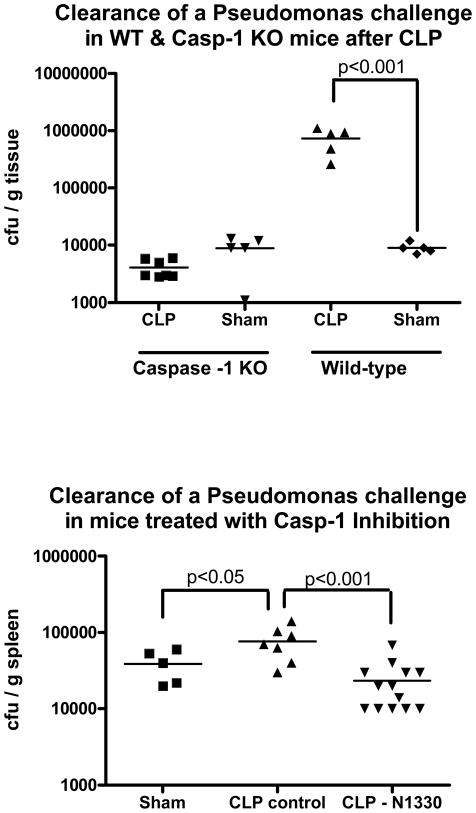
CLP-induced impairment of Pseudomonas clearance did not occur in the absence of caspase-1
To directly test whether induction of caspase-1 activity was involved in the development of post-CLP immunosuppression, caspase-1 knockout mice and their respective wild-type controls were subjected to CLP (or sham CLP) and challenged 5 days later with Pseudomonas aeruginosa. As in the C57BL6 mice used in the aforementioned experiments, the wild-type control mice strain (NOD/ShiLtJ) had diminished ability to clear the bacterial challenge after being subjected to CLP when compared to sham CLP mice. In contrast, there did not appear to be any adverse effect of CLP on subsequent bacterial clearance in the absence of caspase-1 activity as caspase-1 knockout mice cleared the bacterial challenge as readily as mice subjected to sham CLP. (Figure 5, top). To test whether the difference in bacterial clearance may have been due to an attenuation of the CLP-induced injury in the absence of caspase-1, wild-type and caspase-1 knockout mice were subjected to a highly lethal model of CLP and monitored for 5 days without further challenge. There was no significant difference in survival in caspase-1-deficient mice after CLP alone (7/13 vs 5/14 in wild-type mice, p = 0.44). To further confirm the role that caspase-1 induction by CLP had in our investigations, and to test the possibility of targeting capsase-1 therapeutically, mice were subjected to CLP and treated with a specific caspase-1 inhibitor (Ac-YVAD-CMK, N-1330) prior to Pseudomonas challenge. The results, in Figure 5 bottom, show that mice treated with the caspase-1 inhibitor had an attenuation of CLP-induced impairment of bacterial clearance, with treated CLP mice having significantly less Pseudomonas bacterial growth than non-treated CLP mice and equivalent to that seen in sham control mice challenged with Pseudomonas.
Figure 5. Mice deficient of caspase-1 activity did not demonstrate CLP-induced impairment of clearance of a subsequent Pseudomonas challenge.
Wild-type mice subjected to CLP had higher numbers of Pseudomonas colony-forming units in spleen tissue 6 hours after a Pseudomonas challenge than did mice subjected to sham procedures. However, caspase-1 knockout mice subjected to CLP did not differ from sham mice in the amount of Pseudomonas isolated from spleen tissue after a Pseudomonas challenge (Figure 5, top). Figure 5, bottom, shows that wild-type mice treated with a specific caspase-1 inhibitor after CLP had less Pseudomonas bacteria growth from spleen tissue compared to non-treated CLP mice and did not differ significantly from the sham control mice (*p <.05, N = 5–13/group)
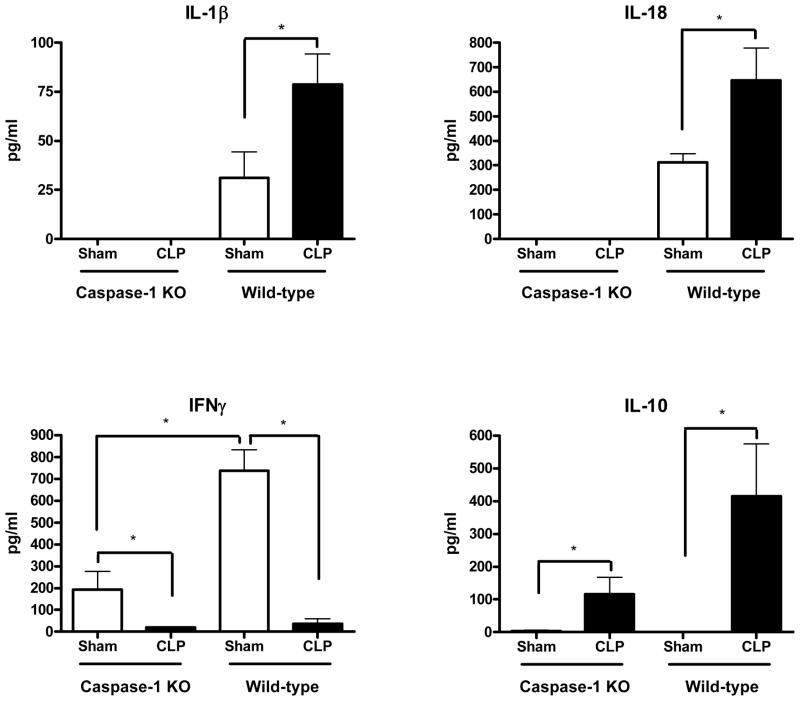
Depletion of caspase-1 activity was associated with smaller, but similar, changes in plasma IFNγ and IL-10 as seen in wild-type mice after CLP
To determine whether induction of caspase-1 activity had any effect on the inflammatory cytokine profile in post-CLP mice challenged with Pseudomonas, blood samples were collected 6hrs after the Pseudomonas challenge for measurement of plasma cytokine concentrations. Wild-type mice subjected to CLP had an attenuation of IFNγ and an exaggeration of IL-10 responses to the Pseudomonas challenge when compared to those of the sham control mice. Caspase-1 KO mice subjected to CLP had a similar change in the pattern of cytokine response (ie, lower IFNγ and higher IL-10) when compared to sham control caspase-1 KO mice, although the concentrations of those cytokines were approximately 4 times less than seen in the wild-type mice. IL-1β and IL-18, which were higher in post-CLP WT mice compared to sham WT mice, were not detectable in mice deficient in caspase-1. (Figure 6)
Figure 6. Mice deficient in caspase-1 activity had no detectable IL-1β or IL-18 but had similar changes in the serum IFNγ and IL-10 cytokine response to a Pseudomonas challenge as seen in wild-type mice after CLP.
Caspase-1 knockout mice subjected to CLP had no detectable circulating concentrations of caspase-1- dependent IL-1β or IL-18 in response to a subsequent Pseudomonas challenge. Both caspase-1 knockout mice and wild-types that were subjected to CLP had similar suppression of the IFNγ and exacerbation of the IL-10 responses to a subsequent Pseudomonas challenge when compared to those responses in sham mice. (*p <.05, N =5–7/group)
Discussion
Sepsis occurs most commonly as an adverse complication in patients who have suffered major trauma or major illness, and it is commonly believed that these patients are predisposed to sepsis because they have impaired immune function secondary to their primary lesion. That belief is supported by the fact that many of the bacteria isolated from septic patients are not typically virulent in people with competent immune function. Sepsis is thought to develop when an initial systemic pro-inflammatory response is followed by a sustained anti-inflammatory state concurrent with immunoparalysis(12-19). Mortality can occur during the proinflammatory phase, such as during infections with meningococcemia or toxic-shock syndrome(16,20); however, most septic patients survive the initial inflammatory response and enter a prolonged immunosuppressive state in which they are unable to resolve their primary infection or continue to develop new secondary infections.
We have modeled that paradigm in mice by using CLP to induce a major injury and initial systemic proinflammatory response. When the mice are challenged with Pseudomonas 5 days later, compromise of innate immune function is revealed by diminished ability of the mice to clear the bacterial challenge when compared to uninjured (sham) controls. Caspase-1 propagates inflammation in that it does not appear to be part of an apoptotic mechanism but contributes to the inflammatory cytokine response by cleaving pro-forms of IL-1β and IL-18 to their mature, active forms. In this study, we showed that caspase-1 activity was higher in wild-type mice that had been subjected to CLP when compared to tissues from sham control mice. We also demonstrated that CLP primed for increased production of IL-1β and IL-18 in response to subsequent challenges, both of which require caspase-1 activity for cleavage and release from precursor forms. These findings are consistent with a recent paper reporting that circulating concentrations of caspase-1 and IL-18 were higher in septic patients(21).
We further demonstrated that bacterial clearance was adversely affected in wild-type mice subjected to CLP but not in caspase-1-deficient mice subjected to CLP, suggesting that caspase-1 activation in response to injury plays a mechanistic role in the impairment of subsequent innate immune function. This was not due to a baseline difference in innate immune function as the absence of caspase-1 activity did not affect the ability of healthy, uninjured caspase-1 knockout mice to clear the Pseudomonas challenge. Nor does it seem likely to be due to a more severe injury after CLP as there was no difference in mortality of capase-1-deficient mice compared to wild-type mice after each group was subjected to a more severe CLP model. The use of a caspase-1 specific inhibitor further confirmed those results and also suggests the therapeutic potential that caspase-1 inhibition might have for the prevention of post-injury immunosuppression. Other recent studies have confirmed the role of caspase-1 in the proinflammatory process and hinted at the adverse effect caspase-1 activity might have in the immune process. Caspase-1 deficient mice are markedly resistant to endotoxic shock(10), presumably due to deficits in maturation of IL-1β and IL-18. When a caspase-1 inhibitor was nebulized into the lungs of rats subjected to i.v. infusion of LPS, the rats had a decreased IL-1β and IL-18 cytokine response both in the lungs and systemically as well as decreased expression of iNOS and COX-2 in the lung tissue(22). Caspase-1 deficient mice were resistant to E. coli-induced peritonitis in another study when compared to both wild-type and IL-1β knockout mice(23).
Recent investigations have reported an improvement in survival after CLP in animals treated with broad caspase inhibitors, and associated those results with diminished inflammation and apoptosis (24,25). It is not clear how the specific depletion of caspase-1 activity prevented the decline in innate immune function in mice subjected to CLP. We have not been able to demonstrate any difference in cell death in post-CLP mice compared to sham controls, most likely because our model of CLP is less severe than that used by other investigators (24,25). Therefore, this seems to rule out attenuation of cell death in the absence of caspase-1 as a mechanism for our findings. Nor did it appear that the altered ability to clear the bacterial challenge could be explained by an attenuation of the severity of the CLP-induced injury in caspase-1-deficient mice as casp-1−/− mice had a similar level of mortality as wild-type mice when subjected to a more severe model of CLP. Caspase-1 KO mice did have a subdued IFNγ response to the Pseudomonas challenge which is likely due to the impaired release of IL-18, a cytokine which contributes to induction of IFNγ (26). Although circulating concentrations of IL-10 were elevated in caspase-1 KO mice subjected to CLP when compared to sham caspase-1 KO’s, the serum concentrations were lower than measured in wild-type mice subjected to CLP. Thus the anti-inflammatory cytokine balance was not as pronounced in the absence of caspase-1 and perhaps this contributed to the attenuation of impaired immune function in these mice. We also showed that CLP was followed by increased levels of caspase-1 activity. Caspase-1 levels were elevated in macrophages and neutrophils, two cell populations that would be expected to play a primary role in the innate immune response to the Pseudomonas challenge.
Although administration of several TLR agonists such as LPS can induce caspase-1 activation(28,29), TLR signaling does not seem to be required for the activation of caspase-1. Neither TLR2 nor TLR4 were necessary for caspase-1 activation in response to heat-killed Gram negative or Gram positive bacteria(30). Pseudomonas aeruginosa seems to induce caspase-1 independently of TLR5 by introduction of flagellin into the cytosol through a type III secretion system(31,32). However, there are reports that Pseudomonas may also activate caspase-1 by a flagellin-independent pathway(33). Consistent with that, infection of macrophages with flagellin-deficient Pseudomonas induced activation of caspase-1, suggesting an alternate pathway of Pseudomonas-induced caspase-1 activation(31).
The present findings suggest that caspase-1 may play a role in the development of sepsis secondary to a major injury. Selective depletion of caspase-1 did not seem to adversely affect immune competence in uninjured mice and attenuated a decline in immune competence in injured mice. Although caspase-1 may provide a potential therapeutic target in patients at high risk for development of sepsis, further work is necessary to determine if treatments against caspase-1 would be effective in patients in which sepsis is already established.
Acknowledgments
This work was supported by the NIH-NIGMS (K08 GM072857)
The author would like to thank his K08 sponsor, Dr. Edward Sherwood, for providing additional support for this work
References
- 1.Murphey ED, Sherwood ER. Bacterial clearance and mortality are not improved by a combination of IL-10 neutralization and IFN-gamma administration in a murine model of post-CLP immunosuppression. Shock. 2006;26:417–424. doi: 10.1097/01.shk.0000226343.70904.4f. [DOI] [PubMed] [Google Scholar]
- 2.Murphey ED, Lin CY, McGuire RW, Toliver-Kinsky T, Herndon DN, Sherwood ER. Diminished bacterial clearance is associated with decreased IL-12 and interferon-gamma production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock. 2004;21:415–425. doi: 10.1097/00024382-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Muenzer JT, Davis CG, Chang K, Schmidt RE, Dunne WM, Coopersmith CM, Hotchkiss RS. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun. 2010;78:1582–1592. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nhan TQ, Liles WC, Schwartz SM. Physiological functions of caspases beyond cell death. Am J Pathol. 2006;169:729–732. doi: 10.2353/ajpath.2006.060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 6.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282:36321–36329. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 8.Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 9.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 11.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 12.Lowry SF, Awad S, Ford H, Cheadle W, Williams MD, Qualy RL, McCollam JS, Bates BM, Fry DE. Static and dynamic assessment of biomarkers in surgical patients with severe sepsis. Surg Infect (Larchmt) 2004;5:261–268. doi: 10.1089/sur.2004.5.261. [DOI] [PubMed] [Google Scholar]
- 13.Monneret G, Finck ME, Venet F, Debard AL, Bohe J, Bienvenu J, Lepape A. The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett. 2004;95:193–198. doi: 10.1016/j.imlet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Murphy T, Paterson H, Rogers S, Mannick JA, Lederer JA. Use of intracellular cytokine staining and bacterial superantigen to document suppression of the adaptive immune system in injured patients. Ann Surg. 2003;238:401. doi: 10.1097/01.sla.0000086661.45300.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy TJ, Paterson HM, Mannick JA, Lederer JA. Injury, sepsis, and the regulation of Toll-like receptor responses. J Leukoc Biol. 2004;75:400–407. doi: 10.1189/jlb.0503233. [DOI] [PubMed] [Google Scholar]
- 16.Natanson C, Hoffman WD, Suffredini AF, Eichacker PQ, Danner RL. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med. 1994;120:771–783. doi: 10.7326/0003-4819-120-9-199405010-00009. [DOI] [PubMed] [Google Scholar]
- 17.Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med. 1997;25:1095–1100. doi: 10.1097/00003246-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Osuchowski MF, Welch K, Yang H, Siddiqui J, Remick DG. Chronic sepsis mortality characterized by an individualized inflammatory response. J Immunol. 2007;179:623–630. doi: 10.4049/jimmunol.179.1.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect Immun. 2006;74:5227–5235. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberholzer A, Oberholzer C, Minter RM, Moldawer LL. Considering immunomodulatory therapies in the septic patient: should apoptosis be a potential therapeutic target? Immunol Lett. 2001;75:221–224. doi: 10.1016/s0165-2478(00)00307-2. [DOI] [PubMed] [Google Scholar]
- 21.Delogu G, Famularo G, Tellan G, Marandola M, Antonucci A, Signore M, Marcellini S, Moretti S. Lymphocyte Apoptosis, Caspase Activation and Inflammatory Response in Septic Shock. Infection. 2008;36:485–487. doi: 10.1007/s15010-008-7070-y. [DOI] [PubMed] [Google Scholar]
- 22.Boost KA, Hoegl S, Hofstetter C, Flondor M, Stegewerth K, Platacis I, Pfeilschifter J, Muhl H, Zwissler B. Targeting caspase-1 by inhalation-therapy: effects of Ac-YVAD-CHO on IL-1 beta, IL-18 and downstream proinflammatory parameters as detected in rat endotoxaemia. Intensive Care Med. 2007;33:863–871. doi: 10.1007/s00134-007-0588-0. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson NT, Wewers MD. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1beta and interleukin-18. Am J Respir Crit Care Med. 2006;174:1003–1010. doi: 10.1164/rccm.200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 25.Weber P, Wang P, Maddens S, Wang PS, Wu R, Miksa M, Dong W, Mortimore M, Golec JM, Charlton P. VX-166: a novel potent small molecule caspase inhibitor as a potential therapy for sepsis. Crit Care. 2009;13:R146. doi: 10.1186/cc8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 27.Sherwood ER, V, Enoh T, Murphey ED, Lin CY. Mice depleted of CD8+ T and NK cells are resistant to injury caused by cecal ligation and puncture. Lab Invest. 2004;84:1655–1665. doi: 10.1038/labinvest.3700184. [DOI] [PubMed] [Google Scholar]
- 28.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 29.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Miao EA, puche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 32.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 33.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]