Abstract
Chronic allograft vasculopathy (CAV) contributes to heart transplant failure, yet its pathogenesis is incompletely understood. While cellular and humoral alloimmunity are accepted pathogenic mediators, animal models suggest that T cells and antibodies reactive to graft-expressed autoantigens, including cardiac myosin (CM), could participate. To test the relationship between CAV and anti-CM autoimmunity in humans we performed a cross-sectional study of 72 heart transplant recipients; 40 with CAV and 32 without. Sera from 65% of patients with CAV contained anti-CM antibodies, while <10% contained antibodies to other autoantigens (p<0.05), and only 18% contained anti-HLA antibodies (p<0.05 vs. anti-CM). In contrast, 13% of sera from patients without CAV contained anti-CM antibodies (p<0.05, odds ratio, OR, associating CAV with anti-CM antibody=13, 95% CI: 3.79–44.6). Multivariate analysis confirmed the association to be independent of time posttransplant and the presence of anti-HLA antibodies (OR=28, 95% CI: 5.77–133.56). PBMC from patients with CAV responded more frequently to, and to a broader array of, CM-derived peptides than those without CAV (p=0.01). Detection of either CM-peptide-reactive T cells or anti-CM antibodies was highly and independently indicative of CAV (OR=45, 95% CI: 4.04–500.69). Our data suggest detection of anti-CM immunity could be used as a biomarker for outcome in heart transplantation recipients and support the need for further studies to assess whether anti-CM is a pathogenic mediator of CAV.
Introduction
Significant improvements in medical therapy and advances in immunosuppressant management strategies have made heart transplantation the treatment of choice for patients with end stage heart disease. One and 2 year patient and heart graft survival rates are outstanding but long term outcomes are suboptimal, with 5 and 10 year survivals of 72.1% and 53.2% respectively (1). A key pathological manifestation of late cardiac allograft failure is chronic allograft vasculopathy (CAV), an entity which develops in up to 50% of transplant recipients within 5 years. CAV is characterized by intimal thickening, smooth muscle cell proliferation and accumulation of extracellular matrix, which result in arterial narrowing and ultimately graft ischemia and fibrosis (2). Current concepts are that the etiology of CAV is multifactorial but that immune mechanisms dominate (3). Data derived from animal models indicate that alloreactive T cells and antibodies reactive to donor MHC molecules are key participants in the pathogenesis of CAV (4–6). Increasing associative evidence also suggests that cellular and humoral alloimmunity contribute to CAV in human transplant recipients (6–8). Still, the pathogenesis of this disease remains incompletely understood, as CAV can occur in the absence of detectable anti-donor alloimmunity (6–8).
T cells and antibodies reactive to non-HLA molecules, including nonpolymorphic, self-antigens may also contribute to late cardiac allograft failure. Autoreactive T cells and antibodies specific for heart antigens, including cardiac myosin (CM), underlie the pathogenesis of some forms of primary heart failure including autoimmune myocarditis (9–15). Such preexisting memory autoimmunity is expected to be long-lived and resistant to immunosuppression (16–18) and thus could contribute to the development of post-transplant allograft injury. Indeed, reports indicate that acute rejection episodes seem to be more frequent in heart transplant recipients with preexisting serum anti-CM antibodies (19).
In addition to preexisting autoimmunity, autoimmunity could develop de novo posttransplant as a consequence of graft damage initially induced by the alloimmune response (20, 21); immune presentation of self-antigens within an inflammatory environment could break self-tolerance. Animal studies from Fedoseyeva, Benichou and colleagues documented that anti-CM (CM) immunity can be induced following heart transplantation in mice and this recipient MHC-restricted, autoimmunity is an important pathogenic mediator of graft failure (20, 22). A separate research group reported associations among anti-donor alloimmunity, autoimmunity to cardiac antigens and CAV in heart transplant recipients, and provided evidence that the alloimmunity could predate the autoimmune responses (23). Other than these limited reports, evidence supporting a role for organ specific autoimmunity as a pathogenic mediator of CAV in heart transplant recipients is lacking.
To test for a link between autoimmunity and CAV, we obtained peripheral blood samples from heart transplant recipients with and without CAV, measured serum anti-CM antibodies, and quantified T cell reactivity to a panel of CM-derived peptides. We observed a strong and independent association between autoimmunity to CM and the presence of CAV, together identifying a novel biomarker and providing supporting evidence that autoreactivity could contribute to chronic graft injury in human heart transplant recipients.
Methods
Study Patients
We obtained peripheral blood samples from 72 heart transplant patients, at single time points, followed at the heart transplant practice at the Mount Sinai Hospital, NY, NY. 40 patients had CAV and 32 patients had no evidence of CAV (nCAV group) as documented by angiography performed as part of the routine clinical care (24). Immunosuppression for all heart transplant recipients at Mount Sinai, including those in this study is standardized and consists of long term tacrolimus (or rarely cyclosporine A for those who cannot tolerate tacrolimus) and mycophenolate mofetil plus corticosteroids. The steroids are routinely weaned and stopped by 6 mo. posttransplant. Alterations in immunosuppressants and changes in drug dosing are triggered by rejection episodes, the frequency which occurred equally between groups. Patients with angiographically significant CAV are typically treated with sirolimus and a CNI after withdrawing anti-metabolites such as MMF and azothiaprine. Fewer than 5% of patients in the cohort were taking sirolimus for treatment of CAV at the time of sample acquisition.
We did not have access to pretransplant sera. We obtained blood samples at a follow up visit (see Table II for time posttransplant). We collected clinical, pathological and demographic data from the electronic medical record and, for HLA typing data, from the United Network for Organ Sharing (UNOS). Cellular rejection was graded by ISHLT 2004 scoring. Antibody mediated rejection (AMR) was defined by the treating physician based on specific histopathological criteria (macrophage and neutrophil infiltration along capillaries with endothelial cell swelling) with or without hemodynamic compromise. Positive C4d staining and/or serum anti-donor HLA antibodies were considered supportive evidence for the diagnosis of AMR. The studies were approved by the Institutional Review Board of Mount Sinai Hospital.
Table II.
Patient Characteristics
| CAV (n=40) |
no CAV (n=32) |
p value | |
|---|---|---|---|
| Age | 58 +/− 11 y | 60 +/− 14 y | 0.63 |
| Male Gender | 62% | 67% | 1.00 |
| Race/Ethnicity African American |
21% | 20% | 0.65 |
| Caucasian | 42% | 51% | |
| Hispanic | 28% | 17% | |
| Asian | 4% | 0% | |
| Other | 5% | 12% | |
| Time post-transplant to sample acquisition | 10.2 +/− 4.2 y | 7.4 +/− 3.0 y | 0.01 |
| History of Treated Humoral Rejection | 6% | 5% | 0.86 |
| History of Treated Cellular Rejection | 18% | 42% | 0.06 |
Preparation of Peripheral Blood mononuclear cells (PBMC)
Peripheral blood samples were obtained in green top (sodium heparin) and red top tubes. Peripheral blood mononuclear cells (PBMCs) were isolated from the green top tubes by ficoll density-gradient centrifugation. Live cells were counted using acridine orange/ethidium bromide staining and ultraviolet microscopy and were frozen in aliquots. Serum samples were obtained from the red top tubes and stored at −80°C in aliquots.
ELISA assays
We quantified serum anti-CM antibodies using an enzyme-linked immunosorbent assay (ELISA). 96 well Immulon HBX ELISA plates (Thermo Fisher Scientific, Rochester NY) were coated with 1 µg/ml recombinant porcine CM (MW 460,000, Sigma, St Louis MO) or an equal molar concentration (0.14µg/ml) of human serum albumin (MW 67,000, Sigma) as a specificity control in coating buffer (one capsule of carbonate-bicarbonate buffer in 100ml of deionized water, Sigma). As a negative control we used coating buffer alone. The following day, the plate was washed and then blocked with 2% human serum albumin in PBS-0.25% Tween20 at room temperature for 3 h. Patient serum was added at dilutions of 1:50, 1:300, 1:1000, and 1:3000 and incubated for 2 h at 4° C. The plates were washed and biotinylated anti-human IgG (Sigma) was added at a concentration of 1:10,000 and incubated at 4°C for 45 min. Following another wash we added streptavidin-horseradish peroxidase (Dako, Carpenteria, CA) diluted 1:2000 in PBS-0.25% Tween20 for 30 min at 4° C. Following a final wash we added 100µl/ well 3,3,5,5-tetramethylbenzidine liquid substrate for ELISA (Sigma) for 20 min. The reaction was stopped with stop reagent (Sigma) and the plate was read at OD 450 nm using a Biotek ELISA reader (Biotek, Winooski VT).
We used commercially available ELISA kits to test sera for reactivity to anti-nuclear antigens, ANA (Calbiotech, Spring Valley, CA), Scl-70 IgG (Calbiotech) and Cardiolipin IgG (Calbiotech).
ELISPOT assays
After pilot optimization and standardization, the interferon gamma (IFNγ) ELISPOT assay was performed under good laboratory practice conditions using antibodies and reagents as previously published (25, 26).
IL-17 ELISPOT assays were performed similarly using anti-human IL17 (clone 64CAP17, 1mg/ml, eBioscience, San Diego, CA) for coating, and FITC anti-human IL-17 (clone 64Dec17, 0.5mg/ml, eBioscience) as the secondary antibody. IL-4 ELISPOT assays were performed using anti-human IL-4 (clone 8D4–8, 0.5mg/ml, eBioscience) for coating and FITC-anti-human IL-4 (clone MP4-25D2, 0.5mg/ml, eBioscience) as the secondary antibody. For the IL-17 and IL-4 assays a monoclonal anti-FITC alkaline phosphatase antibody (Sigma) was added as a tertiary antibody for detection. NBT/BCIP (nitro-blue tetrazolium and 5-bromo-4-chloro-3'-indolyphosphate, Thermo Fisher Scientific, Rochester NY) was used as the developing reagent.
We tested responder PBMCs reactivity against 32 overlapping synthesized peptides derived from the S2 region of the CM heavy chain (Table I) (27). To optimize cell numbers PBMCs from each responder were tested against the peptides in pairs (e.g. peptide 1 and 2 were combined) each at a final concentration of 10µg/ml. PBMCs were also tested for reactivity against whole 10 µM human CM isolated as previously described (27). PBMCs were incubated with the antigens for 48 h, second antibodies and color reagents were added as previously described (26).
Table I.
Overlapping synthetic peptides (25-mer) of HCM S2 region
| Peptide | Sequence | Residue No. |
|---|---|---|
| S2-1 | SAEREKEMASMKEEFTRLKEALEKS | 842–866 |
| S2–2 | FTRLKEALEKSEARRKELEERMVS | 856–880 |
| S2–3 | RKELEEKMVSLLQEKNDLQLQVQAE | 870–894 |
| S2–4 | KNDLQLQVQAEQDNLADAEERCDQL | 884–908 |
| S2–5 | LADAEERCDQLIKNKIQLEAKVKEM | 898–922 |
| S2–6 | KIQLEAKVKEMNERLEDEEEMNAEL | 912–936 |
| S2–7 | LEDEEEMNAELTAKKRKLEDECSEL | 926–950 |
| S2–8 | KRKLEDECSELKRDIDDLELTLAKV | 940–964 |
| S2–9 | IDDLELTLAKVEKEKHATENKVKNL | 954–978 |
| S2–10 | KHATENKVKNLTEEMAGLDEIIAKL | 968–992 |
| S2–11 | MAGLDEHAKLTKEKKALQEAHQQA | 982–1006 |
| S2–12 | KKALQEAHQQALDDLQAEEDKVNTL | 996–1020 |
| S2–13 | LQAEEDKVNTLTKAKVKLEQQVDDL | 1010–1034 |
| S2–14 | KVKLEQQVDDLEGSLEQEKKVRMDL | 1024–1048 |
| S2–15 | LEQEKKVRMDLERAKRKLEGDLKLT | 1038–1062 |
| S2–16 | KRKLEGDLKLTQESIMDLENDKQQL | 1052–1076 |
| S2–17 | IMDLENDKQQLDERLKKKDFELNAL | 1066–1090 |
| S2–18 | LKKKDFELNALNARIEDEQALGSQL | 1080–1104 |
| S2–19 | IEDEQALGSQLQKKLKELQARIEEL | 1094–1118 |
| S2–20 | LKELQARIEELEEELESERTARAKV | 1108–1132 |
| S2–21 | LESERTARAKVEKLRSDLSRELEEI | 1122–1146 |
| S2–22 | RSDLSRELEEISERLEEAGGATSVQ | 1136–1160 |
| S2–23 | LEEAGGATSVQIEMNKKREAEFQKM | 1150–1174 |
| S2–24 | NKKREAEFQKMRRDLEEATLQHEAT | 1164–1188 |
| S2–25 | LEEATLQHEATAAALRKKHADSVAE | 1178–1202 |
| S2–26 | LRKKHADSVAELGEQIDNLQRVKQK | 1192–1216 |
| S2–27 | QIDNLQRVKQKLEKEKSEFKLELDD | 1206–1230 |
| S2–28 | EKSEFKLELDDVTSNMEQUKAKAN | 1220–1244 |
| S2–29 | NMEQIIKAKANLEKMCRTLEDQMNE | 1234–1258 |
| S2–30 | MCRTLEDQMNEHRSKAEETQRSVND | 1248–1272 |
| S2–31 | KAEETQRSVNDLTSQRAKLQTENGE | 1262–1286 |
| S2–32 | ETQRSVNDLTSQRAKLQTENGELSR | 1265–1289 |
The ELISPOTs were quantified using the ImmunospotS4 Core Analyzer (CTL, Shaker Heights, OH). We determined mean numbers of IFNγ ELISPOTs per 500,000 responder PBMCs from single wells. Spots detected in control wells without stimulators were subtracted from the total number of spots. The frequencies of IFNγ producers ranged from 10–292. Patients were assigned a positive reactivity when the frequency of IFNγ ELISPOTs was ≥ 10 after subtracting spontaneous IFNγ production in medium control wells.
Alloantibody Detection
We screened for serum alloantibody using the LABScreen Mixed screening beads (One Lambda Inc., Canoga Park, CA) on a BioPlex Protein Array System (BioRad, Hercules, CA) using BioPlex Manager software (BioRad) as per manufacturer’s instructions. In this assay serum is tested for binding to 12 beads coated with mixtures of polymorphic HLA class I molecules and 5 beads coated with mixtures of polymorphic class II molecules. Results were analyzed using the HLA Fusion software (One Lambda Inc.). We defined a positive screening result for an individual bead as a binding ratio ≥ 3.0 (compared to controls, as per manufacturer’s instructions).
We defined allelic specificity of the alloantibodies in the subgroup of CAV patients with positive screening assays using LABScreen Single Antigen Class I and Single antigen Class II beads (One Lambda Inc.). A positive result was defined as reactivity above background negative control serum. The results were analyzed using the HLA Fusion software (One Lambda Inc.) and compared to donor HLA types to define those with donor reactive anti-HLA antibody.
In vitro Depletion of CD4+CD25+ cells
We depleted CD4+CD25+ cells from PBMC using the Human CD25 MicroBeads II kit (Milenyi Biotech, Auburn, CA). The percentages of CD4, CD8 and CD4+CD25+Foxp3+ cells were determined before and after depletion by flow cytometry. After staining, the cells were acquired using a BD FACS Canto II flow cytometer (BD Biosciences, San Jose, CA) and analyzed with Diva software. FITC anti-human CD4 clone RPA-T4 (eBioscience), APC conjugated anti-human CD25 clone BC96 (eBioscience), and PE mouse anti-human CD8 (BD Biosciences) were used for cell surface staining. Intracellular staining for Foxp3 was performed with Foxp3 intracellular staining kit (eBioscience) followed by the addition of PE conjugated anti-human Foxp3 antibody clone PCH101 (eBioscience).
Statistical Analysis
Graph Pad Prism 5 (GraphPad Software, La Jolla, CA) and SPSS version 18.0 (SPSS, Chicago, IL) were used for data analysis. We used the Chi square (or Fisher’s exact test, as appropriate) to compare categorical, clinical, and demographic characteristics between groups based on the presence of CAV, and Student's t-test to compare continuous variables between groups. Data are reported as percentages of total or means and standard deviations. We used binary logistic regression analysis to determine factors independently associated with a diagnosis of CAV. Two-sided p<0.05 was considered to be statistically significant.
Results
Characteristics of the study cohort
The clinical characteristics of the 72 patients tested in this cross sectional study are shown in Table II. The mean age of the patients in the CAV group was 58 ± 11 y and the mean age of the patients in the nCAV group was 60± 14 years (p=0.63). As is the case for heart transplant populations within the US as a whole (www.unos.org), our cohort consisted of a predominance of males with the majority being Caucasian (there were no differences between the CAV and nCAV groups). Neither the underlying etiology of heart failure nor the prevalence and severity of treated humoral or cellular rejection posttransplantation differed between the groups (Tables II–III). The time of sample collection posttransplantation was longer in the CAV group (10.2 ± 4.2 in CAV vs.7.4± 3 in nCAV, p=0.01).
Table III.
Etiology of Heart Disease
| CAV n=40 | no CAV n=32 | |
|---|---|---|
| Dilated Cardiomyopathy | 16% | 27% |
| Ischemic Cardiomyopathy | 16% | 10% |
| Idiopathic Cardiomyopathy | 9% | 10% |
| Viral Cardiomyopathy | 9% | 2% |
| Other | 4% | 12% |
| Unknown | 46% | 39% |
No statistically significant difference between groups for any variable
Higher prevalence of anti-CM antibodies in patients with CAV
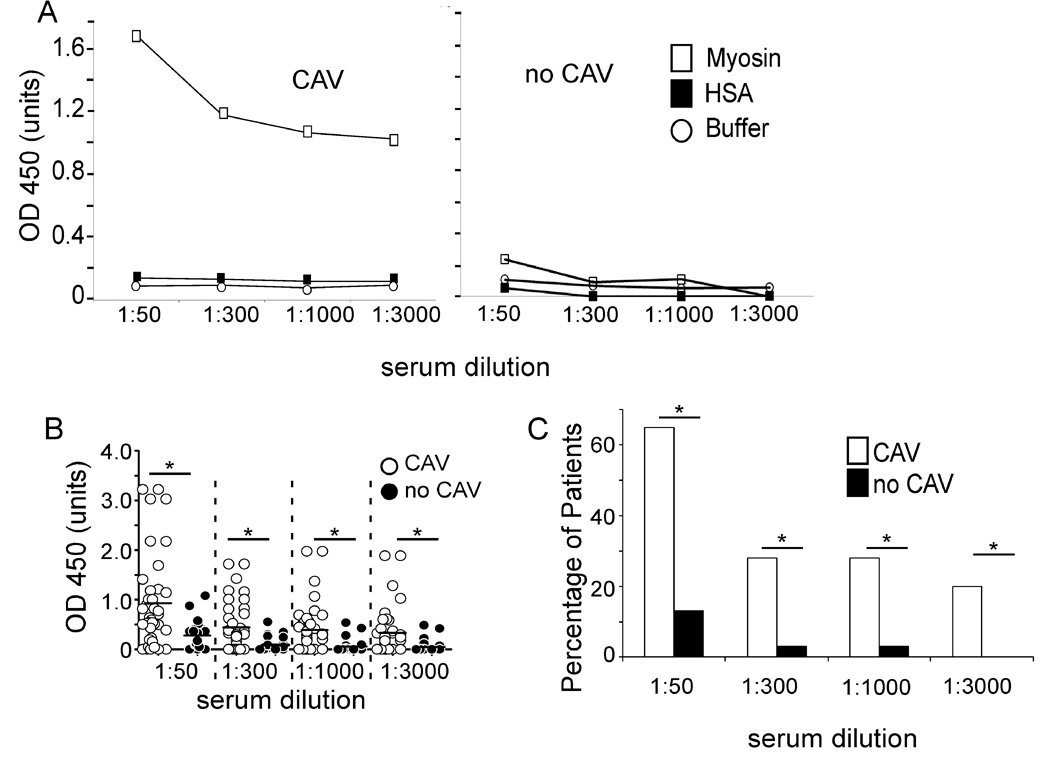
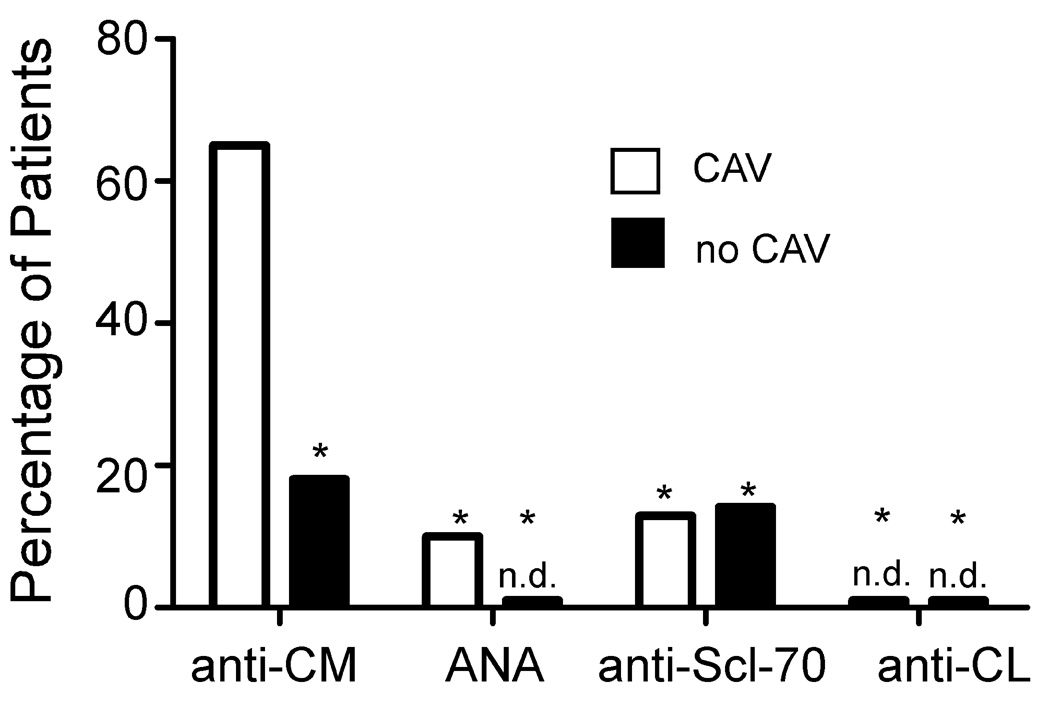
We developed an ELISA to quantify anti-CM IgG antibodies in sera from patients with and without CAV (Fig 1). A representative assay from a single patient with CAV shown in Fig 1A reveals titratable reactivity to CM detectable at a titer of > 1:3000. No response was observed in wells without antigen or in wells containing an equal molar concentration of control human serum albumin (HSA). Fig 1A also shows representative results from a patient without CAV in which we observed essentially no reactivity to CM at any dilution over the control wells. Figures 1B–C depict results for the entire cohort and show a strikingly higher, and statistically significant difference in prevalence, mean reactivity and titer of anti-CM IgG in patients with CAV. As a specificity control we tested each serum sample for reactivity to a panel of autoantigens including anti-nuclear antigens, anti-Scl 70, and anti-cardiolipin (Figure 2). Sera from those with and without CAV reacted at significantly lower frequency to each of these antigens compared to CM.
Figure 1. Anti-CM antibodies are found in serum from patients with CAV.
Sera from 40 patients with CAV and 32 patients without CAV were tested for anti- CM antibodies at serum dilutions of 1:50, 1:300, 1:1000, and 1:3000. A. Representative ELISA results of serum from one patient with CAV (left) and one patient without CAV (right). Open squares represent reactivity to CM, solid squares HSA and open circles buffer alone. B. ELISA results from all CAV and nCAV patients at each serum dilution (1:50, 1:300, 1:1000, 1:3000). C. Summary depicting the percentage of CAV and nCAV patients with anti-CM immunity of OD>0.5 (*p<0.05).
Figure 2. Anti-CM antibodies are more prevalent than antibodies to other common autoantigens in heart transplant recipients.
Results for anti-CM antibodies (CAV, n=40; no CAV, n=32, dilution 1:50) in Figure 1 are re-plotted along with results of assays performed on the same sera testing for reactivity to ANA, anti-Scl-70, and anti-cardiolipin (CL) antibodies by ELISA. Sera from patients with CAV more commonly contained anti-CM antibodies than any of the other antibodies tested (*p <0.05). n.d., none detected.
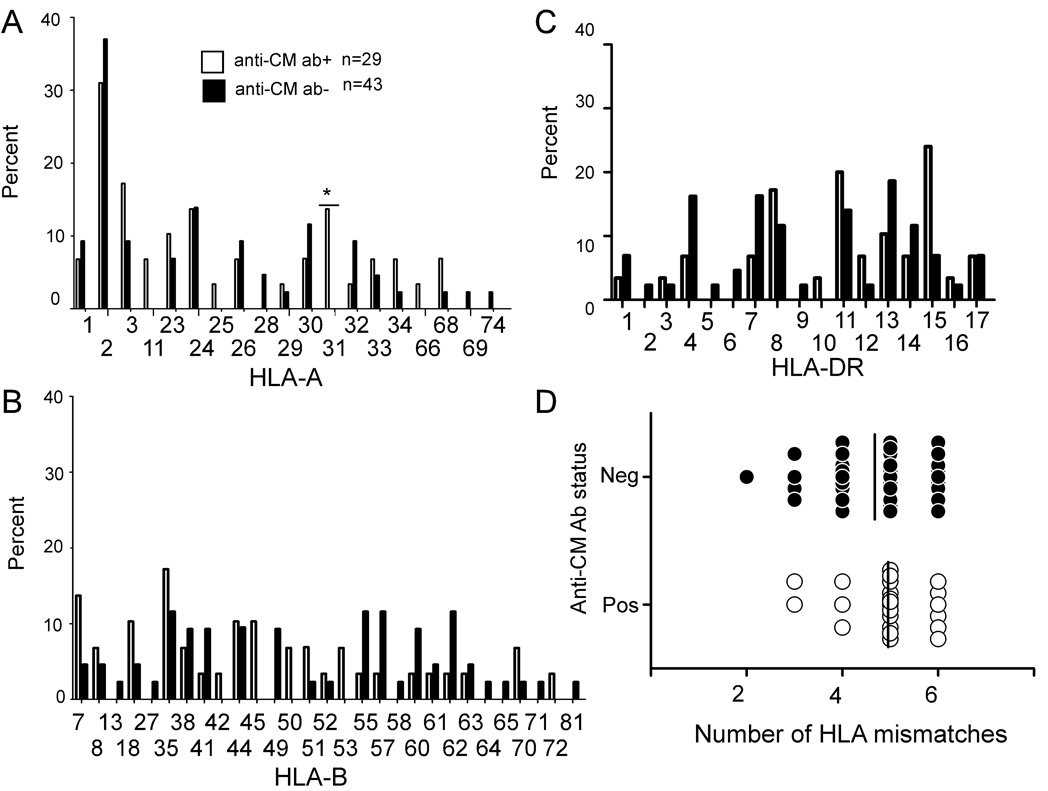
When we examined whether anti-CM antibodies were associated with recipient expression of specific HLA alleles (A, B, DR, Figure 3A–C), we did not observe any relationships. Nor did we observe a relationship between the prevalence of anti-CM antibodies and the number of HLA mismatches between donor and recipient (Fig 3D).
Figure 3. Anti-CM antibodies are not associated with specific recipient HLA alleles or the number of HLA mismatches between donor and recipient.
The percentages of anti-CM antibody positive (+) and anti-CM antibody negative (−) patients expressing each HLA-A (A), HLA-B (B), and HLA-DR (C) allele are shown. Anti-CM antibodies did not associate with any HLA allele in the cohort. Panel D depicts the number of HLA mismatches between donor and recipient at A, B, and DR loci (0–6) plotted against the presence or absence of anti-CM antibody in the serum. The mean number of mismatches is not different between those with and without anti-CM antibodies (vertical line, p=ns).
Independent association of anti-CM antibodies with CAV
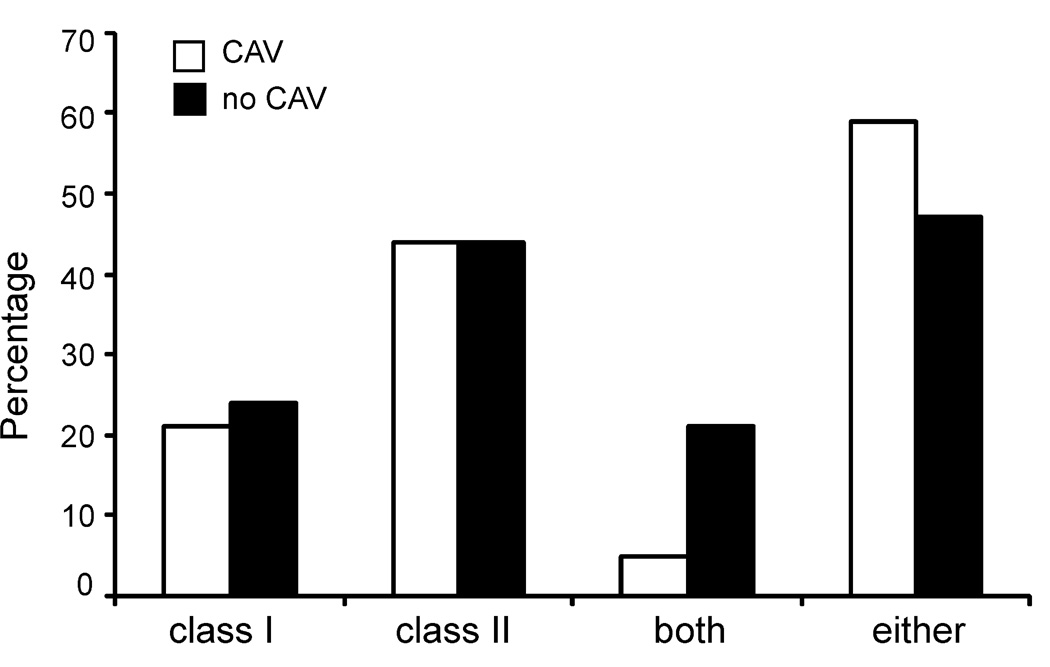
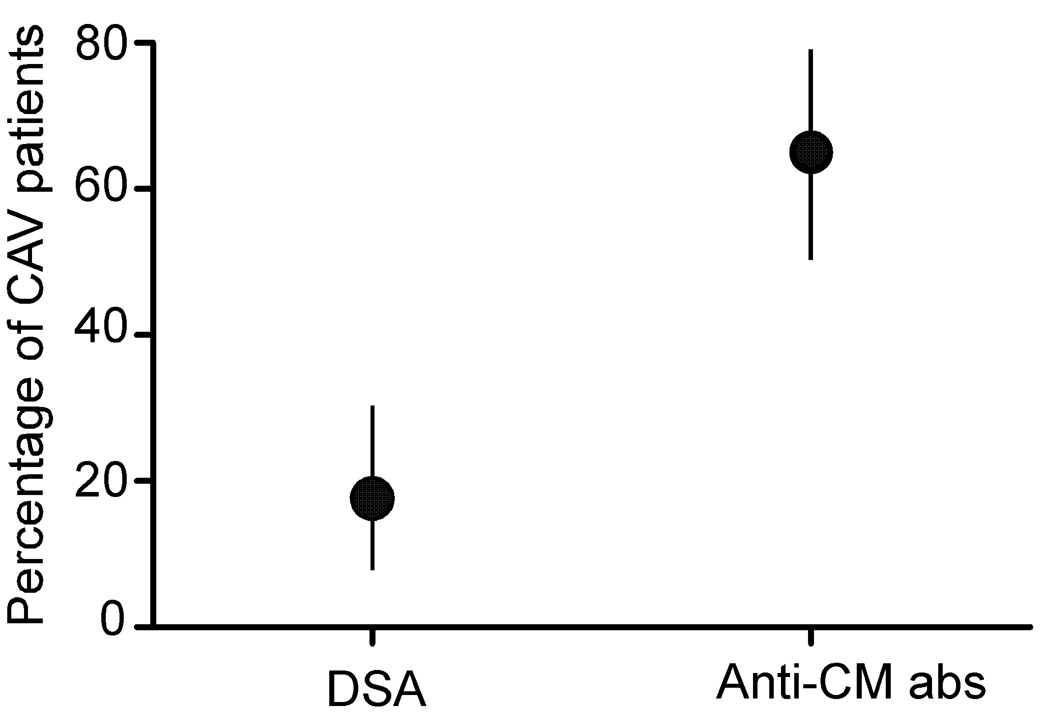
Because anti-HLA antibodies and in particular, anti-class-II-HLA antibodies, have been shown by others to associate with CAV (6, 28), we tested the serum samples from our cohorts for reactivity to class I and class II HLA alleles, initially using Luminex screening beads. We observed a similar prevalence of anti-HLA reactivity in the serum of those with and without CAV reactivity (class I: 21% CAV vs. 24% nCAV, class II: 44%CAV vs. 44% nCAV, both class I and class II 5% CAV vs. 21% nCAV, either class I or II 59% CAV vs. nCAV 47%, p=0.76, 0.96, 0.07, 0.31 respectively, Figure 4). To determine the prevalence of donor-reactive anti-HLA antibodies in those with positive screening assays we examined the allelic specificity of the anti-HLA antibodies using Luminex single antigen beads and compared the results to the HLA alleles expressed by the donor. While serum from 65% of patients with CAV contained anti-CM antibodies (Figure 1) only 18% contained antibodies reactive to donor antigens (p<0.05, Figure 5). We did not observe an association between anti-CM antibodies and donor-reactive, anti-HLA antibodies (DSA) (either anti-class I, anti-class II or both, p=0.65, 1.00, and 0.50 respectively, Table IV).
Figure 4. Anti-HLA antibodies are equally prevalent in those with and without CAV.
Sera from 40 patients with CAV and 32 patients without CAV were screened for the presence of anti-HLA antibodies using luminex. There was no statistically significant difference in percentage of patients who screened positive for class I alone, class II alone, class I and class II, or either class I or II between the two groups. p=ns for all groups.
Figure 5. Higher prevalence of anti-CM antibodies than Donor Specific Antibodies (DSA) in patients with CAV.
Graphical depiction of the prevalence of donor-HLA reactive antibodies and anti-CM antibodies, with 95% confidence intervals (vertical lines), in the 20 CAV patients that screened positive for anti-HLA antibodies by screening tests. Because the confidence intervals do not overlap the differences are statistically significant.
Table IV.
Association between DSA and anti-CM antibodies
| DSA +* | DSA − | Total | |
|---|---|---|---|
| Anti-CM IgG + | 6 | 19 | 25 |
| Anti-CM IgG − | 1 | 14 | 15 |
| Total | 7 | 33 | 40 |
DSA=donor specific anti-HLA antibody, p=0.5 (χ2)
In a univariable analysis we calculated the odds ratio (OR) for the presence of CAV using serum anti-CM antibodies as a biomarker (Table V). We found that anti-CM IgG was associated with CAV with an OR of 12–13 (depending on the titer detected, p < 0.001 to 0.022). In stark contrast, the OR for only other variable statistically associated with CAV, time posttransplant, was 1.2 (95% CI: 1.1–1.4). We did not observe a significant association between a history of either AMR or cellular rejection and CAV. We also tested for an association between a history of rejection and anti-CM antibodies. We found no significant associations between the presence of anti-CM antibodies and either AMR (3/5 with AMR vs. 26/67 without AMR were anti-CM Ab+, p=0.64) or cellular rejection (10/24 with cellular rejection vs. 20/48 without cellular rejection were anti-CM Ab+, p= 1.00).
Table V.
Univariable Analysis of Associations with CAV
| 95% CI for OR | ||||
|---|---|---|---|---|
| OR | p value |
lower | upper | |
| Gender | 1.00 | 1.00 | 0.40 | 2.52 |
| Caucasian Race | 0.64 | 0.32 | 0.27 | 1.55 |
| History of treated Humoral Rejection | 1.42 | 0.71 | 0.23 | 9.00 |
| History of treated T cell Rejection | 0.40 | 0.06 | 0.16 | 1.05 |
| Anti-CM Abs1:50 dilution n = 72 | 13.0 | 0.00 | 3.79 | 44.60 |
| Anti-CM Abs1:300 dilution = 72 | 11.8 | 0.02 | 1.43 | 96.90 |
| Anti-CM Abs1:1000 dilution=72 | 11.8 | 0.02 | 1.43 | 96.90 |
| Time post-transplant to sample acquisition | 1.20 | 0.02 | 1.03 | 1.39 |
| HLA Abs: Either Class I or Class II | 1.50 | 0.80 | 0.07 | 31.58 |
We next constructed a logistic regression model to test the independence of the relationship between anti-CM antibodies and CAV (Table VI). We included time posttransplant and the presence of anti-HLA class I or II screening results in the model. We observed a strong and independent relationship between CAV and anti-CM immunity with an OR of > 27 (95% CI: 5.77–133.56).
Table VI.
Multivariable Logistic Regression of associations with CAV
| 95% CI for OR | ||||
|---|---|---|---|---|
| OR | p value | lower | upper | |
| Anti-CM Abs1:50 dilution n=72 | 27.76 | 0.00 | 5.77 | 133.56 |
| Gender | 1.11 | 0.88 | 0.28 | 4.39 |
| Either Anti-Class I or Anti-Class II Ab | 2.05 | 0.31 | 0.52 | 8.01 |
| Race | 0.78 | 0.43 | 0.43 | 1.44 |
| Time post-transplant to sample acquisition | 1.21 | 0.02 | 1.03 | 1.44 |
T cells reactive to CM-derived peptides and CAV
Because T cell help is generally required for isotype switching and because T cell autoimmunity itself could impact transplant outcome, we tested PBMCs obtained from a subset of 31 patients (from whom we were able to obtain sufficient numbers of PBMCs), with and without CAV, for reactivity to human CM by ELISPOT. Because the large MW of CM (480,000 kilodaltons) precludes testing for reactivity to the intact antigen at micromolar quantities, and because previous work by Cunningham and colleagues suggested immune dominance of S2 fragment of human CM heavy chain animal models and in humans with autoimmune myocarditis(9, 27), we tested for reactivity to a panel of 32 synthetic peptides derived from this immune dominant region (Table I).
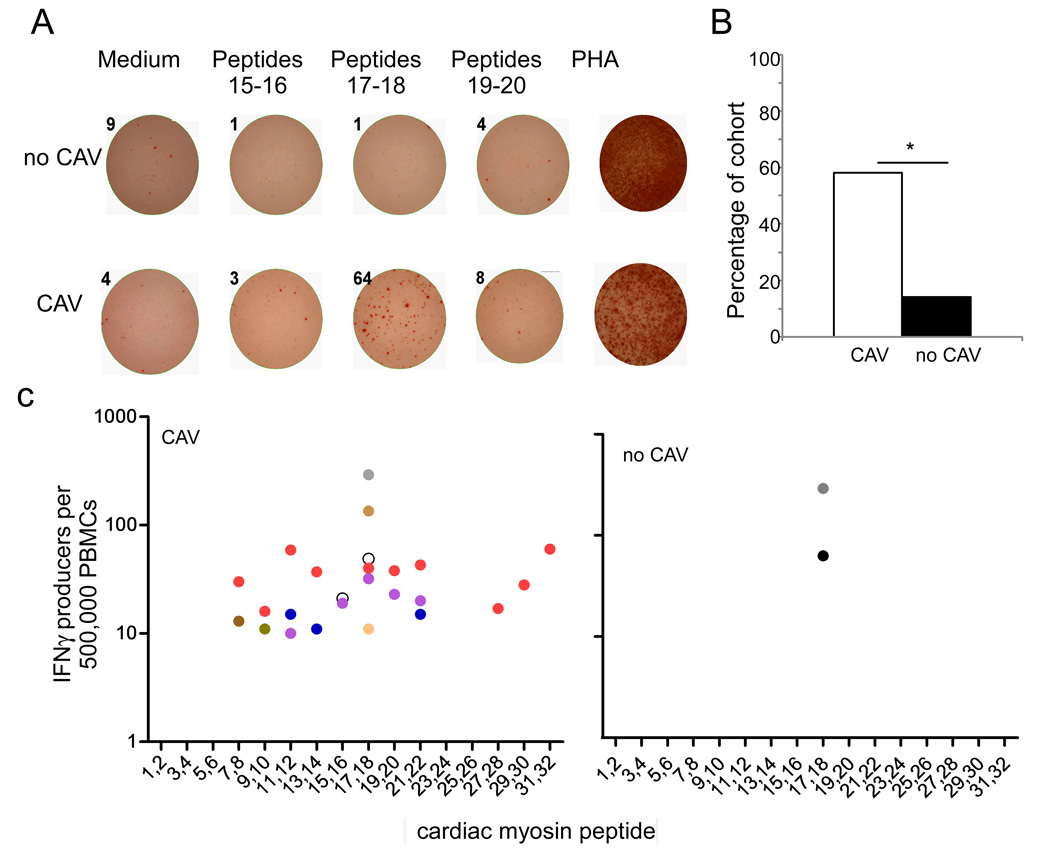
Representative results for one patient with CAV and another without CAV are shown in Fig 6A with a summary of the results for the 31 patients depicted in Fig 6B–D. Ten of 17 patients (58.8%) with CAV responded to at least one CM-derived peptide compared to only 2 of the 14 patients without CAV (15.4%, p=0.01). Reactivity to peptides 17–18 predominated as 5 of 10 patients responded to these epitopes. Interestingly, the peptide antigen reactivity was significantly broader in the patients with CAV; 4 of 10 patients with CAV responded to multiple pairs of peptides while the PBMCs from only 2 patients without CAV responded to two peptides S2 17–18. We tested for peptide-induced IL-17 and IL-4 production by ELISPOT and none was detected (data not shown).
Figure 6. Stronger T cell reactivity to CM in patients with CAV.
A. Representative IFNγ ELISPOT wells in which PBMCs obtained from one patient without CAV (no CAV, top row) and one with CAV (bottom row) were stimulated with medium alone or 3 different pairs of CM-derived peptides. PHA served as a positive control. Numbers above each well are the spots detected. B. The prevalence of T cell reactivity to CM as defined by >10 IFNγ producers per 500,000 PBMCs in response to at least one peptide is shown for 18 patients with CAV and 13 patients without CAV (*p<0.05). C. Peptide mapping of PBMCs from CAV patients (left) and nCAV patients (right). Each color represents the responses of a different patient.
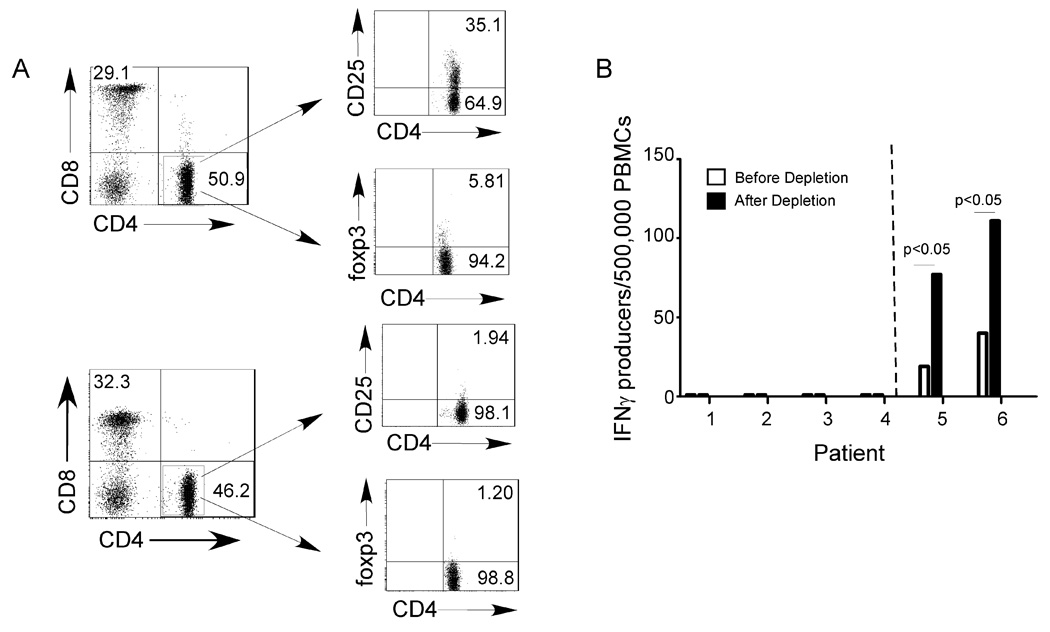
Previous work by others indicated better kidney allograft survival associated with T cell mediated regulation (29). To test whether Tregs suppressed autoreactive anti-CM immunity in our stable heart transplant recipients (without CAV) we repeated the peptide-stimulated IFNγ ELISPOT assays before and after depleting CD4+CD25+Foxp3+ Tregs with magnetic beads (Fig 7A). These experiments showed that for PBMC that did not respond to CM-derived peptides prior to the depletion, Treg depletion had no effect (the depletion did not uncover CM-reactive T cells). Control experiments with PBMCs from patients with detectable CM-peptide-reactive IFNγ producers prior to depletion showed that Treg depletion increased the responses 2–3 fold, thus indicating that the assay can uncover a regulatory response if it is present.
Figure 7. Treg mediated suppression does explain the absence of CM-peptide reactive T cell immunity.
A. Representative flow cytometry plots of PBMC before (above) and after (below) removal of CD25+ cells using magnetic beads, verifying depletion of the CD4+ CD25+ Foxp3+ subset (right panels). B. IFNγ ELISPOT assays for reactivity to CM peptides 17–18 using pre-depletion and post-depletion PBMC from six different patients, 4 without detectable responses pre-depletion (left of dotted line) and 2 with detectable responses pre-depletion (right of dotted line). PBMC from an additional 6 patients without detectable responses pre-depletion showed identical results (no new responses after depletion, not shown).
We used a logistic regression model to determine if a relationship existed between T cell reactivity and CAV independent of time post-transplantation. We observed a strong and independent relationship between CAV and T cell reactivity with an OR of 9.76 (95% CI: 1.4 to 68.2). The difference in T cell reactivity between the groups remained significant even after controlling for time post-transplantation to sample acquisition. When we analyzed for the association between CAV and having either anti-CM IgG or T cell reactivity to CM we found a higher OR of 45 (95%, CI 4.04–500.7). We did not have access to cells from any of the donors so could not quantify anti-donor T cell immunity
Discussion
A better understanding of the pathogenesis of CAV and the development of biomarkers capable of reliably detecting and predicting this prevalent and highly morbid disease are required to improve long term outcomes following heart transplantation. The data reported herein provide new insight to both of these issues by demonstrating a strong and independent relationship between anti-CM immunity and the presence of CAV in human heart transplant recipients. Anti-CM antibodies (Fig 1), but not other autoantibodies (Fig 2), were more prevalent in patients with CAV. Logistic regression analysis revealed a strong association between anti-CM antibodies and CAV independent of time posttransplant and anti-HLA antibodies (OR= 27, Table VI) both of which have been previously associated with CAV (6, 30).
We also observed that CM-peptide-reactive, IFNγ-producing, T cells were more prevalent in patients with CAV (Fig 6), independent of time post-transplantation (OR=9). With the caveat that we did not test for responses to all possible peptides within the CM molecule, the results of the Treg depletion experiments (Fig 7) suggest that absence of CM-reactive T cells in the those without CAV could not be attributed to regulation (which suppressed CM-reactive effector T cells) but rather suggests an absence of T cell priming to this autoantigen.
The peptide mapping analysis (Fig 6) demonstrated for the first time that peptides 17–18 within the S2 region of the human CM heavy chain are immune dominant. Moreover, we observed that PBMCs from 2/14 patients without CAV responded to a single CM-derived determinant, while PBMCs from the majority of those with CAV responded to multiple epitopes (Fig 6), indicating a broader and more potent autoimmune response. When we combined the presence of anti-CM T cell and/or antibody reactivity into a single variable and repeated the logistic regression analysis, we observed an even stronger and independent association with CAV (OR=45).
We did not observe significant correlations between anti-CM immunity and recipient expression of specific HLA alleles or the number of HLA mismatches between donor and recipient (Fig 3) supporting the prevalence data (Fig 1) that anti-CM immunity occurs commonly posttransplant. Because of the cross-sectional nature of our study design we cannot make definitive conclusions regarding the temporal relationships between anti-CM immunity, anti-HLA immunity and the development of CAV. Anti-CM immunity is often detectable in patients with end stage heart failure and has been reported de novo post-transplant as a putative consequence of damage induced by anti-HLA immunity (28, 31). While our data do not specifically address either of these issues, the detected striking relationship between anti-CM immunity and CAV in our study cohort underscores the need for prospective studies to further assess the utility of anti-CM immunity as a diagnostic and prognostic, pre- and posttransplant biomarker for CAV.
Our findings are associative, but they add to the emerging literature derived from animal models and humans indicating that autoimmunity contributes to the pathogenesis of chronic allograft injury (20, 32–38). Studies in murine models have shown that autoimmunity accelerates allograft injury (20, 32) and that inducing tolerance to autoantigens can prevent alloimmunity and prolong graft survival (39). The observation that anti-type V collagen-reactive antibodies emerge in lung transplant recipients prior to their developing bronchiolitis obliterans supports this hypothesis (40, 41).
There are 22 known coding non-synonymous polymorphisms present in the alpha chain subunit of the cardiac myosin protein (www.ncbi.nlm.nih.gov). While it is conceivable that anti-CM immunity could be directed toward polymorphic regions of donor CM not present in the recipient, the fact that we detected stronger T and B cell reactivity in patients with CAV using one form of CM and one set of CM-derived peptides makes it unlikely that the differences we observe are driven by the polymorphisms.
CM is an intracellular protein (protecting it from exposure to serum autoantibodies), but it is theoretically possible for anti-CM antibodies to participate in graft injury by binding to CM released by injured cells initiating extracellular, complement- and FcR-mediated inflammation (13, 36, 42). This phenomenon was illustrated by Zhang and colleagues who described the ability of CM to act as a pathogen associated molecular pattern, initiating toll like receptor-mediated inflammation, and causing upregulation of inflammatory cytokines. It is also possible that anti-CM antibodies cross react with extracellular matrix proteins, including laminin which contains a highly homologous amino acid sequence (43) and/or β-adrenergic receptors (27) and thus trigger endothelial injury and/or proliferation which could contribute to vasculopathy. Autoantibodies could enter cells via cell surface receptors and once they are internalized, bind to intracellular antigens that trigger cell death, complement activation and finally tissue fibrosis.(43) Alternatively and perhaps more likely, CM-reactive T cells could mediate intragraft injury through cytokine-initiated inflammation and direct cytotoxicity.(44) As a consequence the injury would expose released intracellular CM to the immune system, amplifying the autoimmune response and resulting in antibody isotype switching. The resultant autoantibodies could further amplify the autoimmune injury (13) or act as non-participatory bystanders.
In summary, we demonstrate a strong and independent association between anti-CM immunity and chronic allograft vasculopathy. These findings support the concept that autoimmunity may drive the pathogenesis of CAV and identify CM as a potential therapeutic target for future studies of tolerance induction to prevent graft injury. Risk assessment based on the presence or absence of anti-CM immunity in the periphery could be used for prognostic and/or therapeutic purposes. Together the work supports the need for large-scale prospective studies to address these issues.
Acknowledgements
The authors thank Tina Yao, Praeophayam Wauhop, Anne Tretin, Katya Tawil Ostrow, Paulina Trzcinka, Jennifer Smar, Marvin Lin and Denise Peace for their technical assistance.
Abbreviations
- CM
cardiac myosin
- CAV
chronic allograft vasculopathy
- CI
confidence interval
- OR
odds ration
- nCAV
no CAV
Footnotes
Assay development was supported by NIH AI63594 awarded to PSH. AM is a recipient of a Roche Clinical Science fellowship award from the American Society of Transplantation. SK is supported by NIH T32 AI078892. MWC is supported by NIH R01HL56267
References
- 1. http://optn.transplant.hrsa.gov.
- 2.Johnston DR, Sayegh MH, Madsen JC. Overcoming cardiac allograft vasculopathy (CAV) by inducing tolerance. Front Biosci. 2002;7:e116–e118. doi: 10.2741/johnston. [DOI] [PubMed] [Google Scholar]
- 3.Mehra MR. Contemporary concepts in prevention and treatment of cardiac allograft vasculopathy. Am J Transplant. 2006;6:1248–1256. doi: 10.1111/j.1600-6143.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Demir Y, Valujskikh A, Heeger PS. The male minor transplantation antigen preferentially activates recipient CD4+ T cells through the indirect presentation pathway in vivo. J Immunol. 2003;171:6510–6518. doi: 10.4049/jimmunol.171.12.6510. [DOI] [PubMed] [Google Scholar]
- 5.Sahara H, Shoji T, Ng CY, Weiss MJ, Muniappan A, Guenther DA, Houser SL, Pujara AC, Sayre JK, Wain JC, Sachs DH, Madsen JC, Allan JS. The role of indirect recognition of MHC class I and II allopeptides in a fully mismatched miniature swine model of lung transplantation. Transplant Proc. 2006;38:3256–3258. doi: 10.1016/j.transproceed.2006.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terasaki PI, Cai J. Human leukocyte antigen antibodies and chronic rejection: from association to causation. Transplantation. 2008;86:377–383. doi: 10.1097/TP.0b013e31817c4cb8. [DOI] [PubMed] [Google Scholar]
- 7.Poggio ED, Roddy M, Riley J, Clemente M, Hricik DE, Starling R, Young JB, Gus B, Yamani MH, Heeger PS. Analysis of immune markers in human cardiac allograft recipients and association with coronary artery vasculopathy. J Heart Lung Transplant. 2005;24:1606–1613. doi: 10.1016/j.healun.2004.12.110. [DOI] [PubMed] [Google Scholar]
- 8.Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 9.Galvin JE, Hemric ME, Kosanke SD, Factor SM, Quinn A, Cunningham MW. Induction of myocarditis and valvulitis in lewis rats by different epitopes of cardiac myosin and its implications in rheumatic carditis. Am J Pathol. 2002;160:297–306. doi: 10.1016/S0002-9440(10)64373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Heuser JS, Kosanke SD, Hemric M, Cunningham MW. Cryptic epitope identified in rat and human cardiac myosin S2 region induces myocarditis in the Lewis rat. J Immunol. 2004;172:3225–3234. doi: 10.4049/jimmunol.172.5.3225. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Heuser JS, Kosanke SD, Hemric M, Cunningham MW. Protection against experimental autoimmune myocarditis is mediated by interleukin-10-producing T cells that are controlled by dendritic cells. Am J Pathol. 2005;167:5–15. doi: 10.1016/S0002-9440(10)62948-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Heuser JS, Cunningham LC, Kosanke SD, Cunningham MW. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol. 2006;177:8234–8240. doi: 10.4049/jimmunol.177.11.8234. [DOI] [PubMed] [Google Scholar]
- 13.Zhang P, Cox CJ, Alvarez KM, Cunningham MW. Cutting edge: cardiac myosin activates innate immune responses through TLRs. J Immunol. 2009;183:27–31. doi: 10.4049/jimmunol.0800861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis NM, Kurahara DK, Vohra H, Mascaro-Blanco A, Erdem G, Adderson EE, Veasy LG, Stoner JA, Tam E, Hill HR, Yamaga K, Cunningham MW. Priming the immune system for heart disease: a perspective on group A streptococci. J Infect Dis. 2010;202:1059–1067. doi: 10.1086/656214. [DOI] [PubMed] [Google Scholar]
- 15.Ellis NM, Li Y, Hildebrand W, Fischetti VA, Cunningham MW. T cell mimicry and epitope specificity of cross-reactive T cell clones from rheumatic heart disease. J Immunol. 2005;175:5448–5456. doi: 10.4049/jimmunol.175.8.5448. [DOI] [PubMed] [Google Scholar]
- 16.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, Swanson SJ, Mannon RB, Roederer M, Kirk AD. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 17.Valujskikh A. The challenge of inhibiting alloreactive T-cell memory. Am J Transplant. 2006;6:647–651. doi: 10.1111/j.1600-6143.2005.01215.x. [DOI] [PubMed] [Google Scholar]
- 18.Valujskikh A, Lakkis FG. In remembrance of things past: memory T cells and transplant rejection. Immunol Rev. 2003;196:65–74. doi: 10.1046/j.1600-065x.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 19.Morgun A, Shulzhenko N, Unterkircher CS, Diniz RV, Pereira AB, Silva MS, Nishida SK, Almeida DR, Carvalho AC, Franco M, Souza MM, Gerbase-DeLima M. Pre- and post-transplant anti-myosin and anti-heat shock protein antibodies and cardiac transplant outcome. J Heart Lung Transplant. 2004;23:204–209. doi: 10.1016/S1053-2498(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 20.Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162:6836–6842. [PubMed] [Google Scholar]
- 21.Ciubotariu R, Liu Z, Colovai AI, Ho E, Itescu S, Ravalli S, Hardy MA, Cortesini R, Rose EA, Suciu-Foca N. Persistent allopeptide reactivity and epitope spreading in chronic rejection of organ allografts. J Clin Invest. 1998;101:398–405. doi: 10.1172/JCI1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolls HK, Kishimoto K, Illigens BM, Dong V, Sayegh MH, Benichou G, Fedoseyeva EV. Detection of cardiac myosin-specific autoimmunity in a model of chronic heart allograft rejection. Transplant Proc. 2001;33:3821–3822. doi: 10.1016/s0041-1345(01)02617-3. [DOI] [PubMed] [Google Scholar]
- 23.Nath DS, Ilias Basha H, Tiriveedhi V, Alur C, Phelan D, Ewald GA, Moazami N, Mohanakumar T. Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. J Heart Lung Transplant. 2010;29:1277–1285. doi: 10.1016/j.healun.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, Madsen J, Parameshwar J, Starling RC, Uber PA. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29:717–727. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Sawinski D, Uribarri J, Peace D, Yao T, Wauhop P, Trzcinka P, Ostrow K, Poggio ED, Heeger PS. 25-OH-vitamin D deficiency and cellular alloimmunity as measured by panel of reactive T cell testing in dialysis patients. Am J Transplant. 2010;10:2287–2295. doi: 10.1111/j.1600-6143.2010.03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poggio ED, Clemente M, Hricik DE, Heeger PS. Panel of reactive T cells as a measurement of primed cellular alloimmunity in kidney transplant candidates. J Am Soc Nephrol. 2006;17:564–572. doi: 10.1681/ASN.2005030293. [DOI] [PubMed] [Google Scholar]
- 27.Mascaro-Blanco A, Alvarez K, Yu X, Lindenfeld J, Olansky L, Lyons T, Duvall D, Heuser JS, Gosmanova A, Rubenstein CJ, Cooper LT, Kem DC, Cunningham MW. Consequences of unlocking the cardiac myosin molecule in human myocarditis and cardiomyopathies. Autoimmunity. 2008;41:442–453. doi: 10.1080/08916930802031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soleimani B, Lechler RI, Hornick PI, George AJ. Role of alloantibodies in the pathogenesis of graft arteriosclerosis in cardiac transplantation. Am J Transplant. 2006;6:1781–1785. doi: 10.1111/j.1600-6143.2006.01401.x. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez DS, Jankowska-Gan E, Haynes LD, Leverson G, Munoz A, Heisey D, Sollinger HW, Burlingham WJ. Immune regulation and graft survival in kidney transplant recipients are both enhanced by human leukocyte antigen matching. American journal of transplantation. 2004;4:537–543. doi: 10.1111/j.1600-6143.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 30.Valantine H. Cardiac allograft vasculopathy after heart transplantation: risk factors and management. J Heart Lung Transplant. 2004;23:S187–193. doi: 10.1016/j.healun.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Warraich RS, Pomerance A, Stanley A, Banner NR, Dunn MJ, Yacoub MH. Cardiac myosin autoantibodies and acute rejection after heart transplantation in patients with dilated cardiomyopathy. Transplantation. 2000;69:1609–1617. doi: 10.1097/00007890-200004270-00015. [DOI] [PubMed] [Google Scholar]
- 32.Mahesh B, Leong HS, Nair KS, McCormack A, Sarathchandra P, Rose ML. Autoimmunity to vimentin potentiates graft vasculopathy in murine cardiac allografts. Transplantation. 2010;90:4–13. doi: 10.1097/TP.0b013e3181dfa694. [DOI] [PubMed] [Google Scholar]
- 33.Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schonemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 34.Dubel L, Farges O, Johanet C, Sebagh M, Bismuth H. High incidence of antitissue antibodies in patients experiencing chronic liver allograft rejection. Transplantation. 1998;65:1072–1075. doi: 10.1097/00007890-199804270-00011. [DOI] [PubMed] [Google Scholar]
- 35.Braghi S, Bonifacio E, Secchi A, Di Carlo V, Pozza G, Bosi E. Modulation of humoral islet autoimmunity by pancreas allotransplantation influences allograft outcome in patients with type 1 diabetes. Diabetes. 2000;49:218–224. doi: 10.2337/diabetes.49.2.218. [DOI] [PubMed] [Google Scholar]
- 36.Win TS, Pettigrew GJ. Humoral autoimmunity and transplant vasculopathy: when allo is not enough. Transplantation. 2010;90:113–120. doi: 10.1097/TP.0b013e3181e25a59. [DOI] [PubMed] [Google Scholar]
- 37.Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Jr, Chiyo M, Lee J, Mickler E, Smith GN, Petrache I, Brand DB, Burlingham WJ, Gopalakrishnan B, Greenspan DS, Christie JD, Wilkes DS. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181:5738–5747. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jurcevic S, Ainsworth ME, Pomerance A, Smith JD, Robinson DR, Dunn MJ, Yacoub MH, Rose ML. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71:886–892. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 39.Fedoseyeva EV, Kishimoto K, Rolls HK, Illigens BM, Dong VM, Valujskikh A, Heeger PS, Sayegh MH, Benichou G. Modulation of tissue-specific immune response to cardiac myosin can prolong survival of allogeneic heart transplants. J Immunol. 2002;169:1168–1174. doi: 10.4049/jimmunol.169.3.1168. [DOI] [PubMed] [Google Scholar]
- 40.Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, Mohanakumar T. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, Woods K, Smith GN, Cummings OW, Heidler KM, Blum JS, Wilkes DS. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169:1542–1549. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 42.Raedler H, Yang M, Lalli PN, Medof ME, Heeger PS. Primed CD8(+) T-cell responses to allogeneic endothelial cells are controlled by local complement activation. Am J Transplant. 2009;9:1784–1795. doi: 10.1111/j.1600-6143.2009.02723.x. [DOI] [PubMed] [Google Scholar]
- 43.Yung S, Chan TM. Anti-DNA antibodies in the pathogenesis of lupus nephritis--the emerging mechanisms. Autoimmun Rev. 2008;7:317–321. doi: 10.1016/j.autrev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Heeger PS. T-cell allorecognition and transplant rejection: a summary and update. Am J Transplant. 2003;3:525–533. doi: 10.1034/j.1600-6143.2003.00123.x. [DOI] [PubMed] [Google Scholar]