Abstract
Background
Incidence of pulmonary embolism (PE) for different cancer types in oncology outpatients is unknown. The purpose of the study is to determine the incidence of PE in oncology outpatients, and investigate whether the incidence for PE is higher in certain cancers.
Methods
Cohort of oncology outpatients who had imaging studies at Dana-Farber Cancer Institute, a tertiary outpatient cancer institute, from January 2004 through December 2009 was identified using research patient data registry. Radiology reports were reviewed to identify patients who developed PE. Incidence of PE in the total population and each of 16 predefined cancer groups was calculated. Risk of PE for each cancer was compared using Fisher’s exact test.
Results
A total of 13,783 patients was identified, of which 395 (2.87%, 95%CI: 2.59–3.16) developed PE. The incidence of PE was highest in central nervous system (CNS) (12.90%, 95%CI: 8.45–18.59), hepatobiliary (6.85%, 95%CI: 3.33–12.24), pancreatic (5.81%, 95%CI: 3.59–8.84) and upper gastrointestinal (5.81%, 95% CI: 3.96–8.20) malignancies. The risk of PE was significantly higher for CNS (p<0.0001, OR:5.28), pancreatic (p=0.0027, OR:2.15), upper gastrointestinal (p=0.0002, OR:2.18) and lung/pleural malignancies (p=0.0028, OR:1.45). Significantly lower risk of PE for hematologic (incidence: 1.16%, 95%CI: 0.79–1.64; p<0.0001, OR:0.35) and breast malignancies (incidence: 1.50%, 95%CI: 1.02–2.11; p<0.0001, OR:0.47).
Conclusion
The incidence of PE in oncology outpatients in a tertiary cancer center over a 6-year period was 2.87%. CNS, pancreatic, upper gastrointestinal and lung/pleural malignancies had a significantly higher risk for PE than other malignancies, while hematologic and breast malignancies had a significantly lower risk.
Keywords: cancer, pulmonary embolism, oncology, outpatient, anticoagulation
INTRODUCTION
Over the past century, our knowledge of venous thromboembolism (VTE) has evolved from basic understanding of deep venous thrombosis, to uncovering of genetic links between cancer and thromboembolic complications. It is now well-established that the incidence of deep venous thrombosis and pulmonary embolism (PE) is higher in patients with cancer than in the general population. Recognition of PE as an important complication in cancer patients has led to consideration of primary antithrombotic prophylaxis, even in ambulatory patients with cancer1–3. Since the majority of cancer patients are now treated and followed-up in an outpatient setting, this population consisting of oncologic outpatients is of considerable interest.
Knowing the incidence of PE in individual cancer types in ambulatory oncologic patients is the first step towards defining the high-risk outpatient population that may benefit from primary antithrombotic prophylaxis. However, despite the abundance of literature on PE, the incidence of PE in oncology outpatients, as a whole and in different cancer types, is unknown. There are several studies that report the incidence of VTE and/or PE in cancer patients in general4–8, or in individual cancer types9–20. However, most of them focus on hospitalized patients, and many of them report the incidence of VTE in general and not PE specifically. A few studies that do report the incidence of PE in outpatients do not specifically focus on oncology patients21–22.
Advances in multidetector computed tomography (CT) have allowed better assessment of the pulmonary arterial tree and have led to improved detection of pulmonary embolism, making CT pulmonary angiography the imaging modality of choice for the diagnosis of PE23–26. Although the technique used for a routine chest CT is different from that used for CT pulmonary angiography, clinically unsuspected PE is frequently detected on routine chest CT27–33. Although these episodes of PE are often clinically unsuspected, almost 75% of them are in fact, symptomatic34. While a treatment similar to symptomatic PE has been recommended for patients with unsuspected PE35, the exact incidence and clinical significance of the unsuspected PE is unknown27,32–34,36. Even though literature on the incidence of unsuspected PE is growing21–22,28,30,32,37–38, most of these studies include a relatively small number of patients, the results are not consistent, and there are still gaps in our knowledge. Specifically, the incidence of unsuspected PE in oncologic outpatients remains unknown.
The aim of this study is to determine the incidence of clinically suspected and unsuspected PE in oncologic outpatients at a tertiary cancer center over a period of six years, and to investigate whether the risk for PE is higher in certain cancer types than in others.
MATERIAL AND METHODS
In this institutional review board-approved, Health Insurance Portability and Accountability Act (HIPAA) compliant retrospective study, we identified all the outpatients with cancer who had imaging studies at our institution from January 2004 through December 2009, using the research patient data registry (RPDR). The RPDR maintains records of all the patients who are registered at our institution for any reason. All the oncology inpatients were excluded. If any patient had imaging studies as both outpatient and inpatient at different time-points, all the imaging studies performed while the patient was hospitalized were excluded. The final study cohort consisted of the oncologic outpatients who had imaging studies at our institution.
The demographic data including age and gender, and the type of primary cancer were recorded for this cohort. For patients who had history of more than one malignancy, the more recent malignancy for which the patient underwent imaging was used for categorization. For patients with more than one malignancy being actively treated, the patient was classified under the more advanced stage malignancy. The type of primary cancer was recorded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Cancers were divided into 16 groups based on the ICD-9-CM codes (Table 1).
Table 1.
Incidence of pulmonary embolism (PE) and the risk of PE across tumor types.
| Incidence of PE | Risk of PE across tumor types |
|||||
|---|---|---|---|---|---|---|
| Tumor Type | Total No of patients |
No of patients with PE |
% | 95% CI | p value | OR |
| CNS | 186 | 24 | 12.90 | 8.45–18.59 | *<0.0001 | 5.28 |
| Hepatobiliary | 146 | 10 | 6.85 | 3.33–12.24 | 0.0093 | 2.53 |
| Pancreas | 344 | 20 | 5.81 | 3.59–8.84 | *0.0027 | 2.15 |
| Upper GI | 516 | 30 | 5.81 | 3.96–8.20 | *0.0002 | 2.18 |
| Colorectal | 961 | 40 | 4.16 | 2.99–5.63 | 0.0159 | 1.53 |
| Lung/Pleura | 2350 | 90 | 3.83 | 3.09–4.69 | *0.0028 | 1.45 |
| FGT | 1018 | 35 | 3.44 | 2.41–4.75 | 0.2422 | 1.23 |
| KUB | 473 | 16 | 3.38 | 1.95–5.44 | 0.4815 | 1.19 |
| Melanoma/skin | 519 | 13 | 2.51 | 1.34–4.25 | 0.7877 | 0.87 |
| MSK | 1069 | 25 | 2.34 | 1.52–3.43 | 0.3390 | 0.80 |
| Miscellaneous | 259 | 6 | 2.32 | 0.85–4.97 | 0.8495 | 0.80 |
| MGT | 721 | 16 | 2.22 | 1.27–3.58 | 0.3576 | 0.76 |
| Endocrine | 98 | 2 | 2.04 | 0.25–7.18 | 1.0000 | 0.70 |
| HNF | 373 | 6 | 1.61 | 0.59–3.47 | 0.1574 | 0.55 |
| Breast | 2074 | 31 | 1.50 | 1.02–2.11 | ¶ <0.0001 | 0.47 |
| Hematological | 2676 | 31 | 1.16 | 0.79–1.64 | ¶ <0.0001 | 0.35 |
| Total | 13783 | 395 | 2.87 | 2.59–3.16 | ||
PE, pulmonary embolism; CNS, central nervous system; upper GI, upper gastrointestinal; FGT, female genital tract; KUB; kidney, ureter and bladder; MSK, musculoskeletal; MGT, male genital tract; HNF, head, neck and face.
Significantly higher incidence (p < 0.0031 with Bonferroni correction)
Significantly lower incidence (p < 0.0031 with Bonferroni correction)
The radiology reports of the study cohort were reviewed to identify the patients who had PE detected on contrast-enhanced computed tomography (CT). CT scan of the chest was performed using a 64-row MDCT scanner (Aquilion 64; Toshiba America Medical Systems, CA, USA) or a 4-row MDCT scanner (Volume Zoom; Siemens Medical Solutions, Forchheim, Germany). The standard chest CT protocol at the Dana-Farber Cancer Institute was as follows: (1) 64-row MDCT scanner: 0.5 mm collimation, 120 kVp, maximum tube current of 500 mA using dose modulation with noise index of 12.5 HU, 0.5 s gantry rotation time and a table speed of 26.5 mm per rotation; (2) 4-row MDCT scanner: 2.5 mm collimation, 120 kVp, 165 mAs, 0.5 s gantry rotation time and a table speed of 11.5 mm per rotation. Patients were scanned in the supine position from the cranial to caudal direction from the clavicles to the adrenal glands at end-inspiration. One hundred milliliters of iopromide (300 mg I/mL; Ultravist 300, Bayer HealthCare Pharmaceuticals) was injected intravenously with an automated injector (Stellant, Medrad) at a rate of 2–3 mL/s, with a scan delay of 30 seconds.
Axial images (5 or 7 mm thickness for 4-row MDCT and 5 mm for 64-row MDCT) were reconstructed using standard and lung algorithms and were transferred to the picture archiving communication system (PACS).
Only the first episode of PE was included. Each patient was counted only once, even if the PE was detected on several successive scans or there were multiple episodes of PE in the same patient. If patients had PE initially detected on an inpatient CT, any further outpatient CT scans performed in these patients were excluded. The incidence of PE and 95% confidence interval (CI) were calculated for the total population and in the 16 cancer groups. The risk of PE for patients in each cancer group was compared with that of all other patients, using Fisher’s exact test. Using the Bonferroni correction to adjust for multiplicity, p<0.0031 was considered to indicate a significant difference in the incidence of PE. Odds Ratios (ORs) were also computed.
Patients with PE were divided into two subgroups, depending on whether the PE was clinically suspected or unsuspected. The PE was considered as suspected if a dedicated CT pulmonary angiography (CTA) study was ordered by the referring physician, and considered unsuspected if the PE was incidentally detected on routine staging or follow-up CT. The proportion of suspected and unsuspected PE was calculated for the entire population as well as for each cancer group. The percentage and 95% confidence interval (CI) of suspected PE was calculated for each group to determine whether certain cancers are more likely associated with suspected or unsuspected PE.
The most proximal location of PE was recorded based on the radiology reports. The location of PE was categorized as main, lobar, segmental or subsegmental PE. The main PE included PE involving the main pulmonary arterial trunk or the right and left main pulmonary arteries. The lobar PE included the lobar and interlobar arteries. When PE involved multiple locations, the most proximal location was recorded. The percentage of the patients in whom the main pulmonary arteries were involved was separately calculated for the suspected and unsuspected PE groups. These percentage values were compared using the Fisher’s exact test.
Whether the patients received any treatment for PE, and if treated, the type of treatment was recorded from the medical records. The difference in the proportion of treated patients with suspected and unsuspected PE was studied.
The study design, including the methods used for data collection, the type of data collected and the statistical methods, was finalized prior to the beginning of data collection.
RESULTS
A total of 13,783 oncologic outpatients were included in the study, consisting of 6,103 males (44.3%) and 7,680 females (55.7%) with a mean age of 61 years (range 19–101 years). A total of 395 patients developed PE, with an incidence of 2.87% (95% confidence interval, 2.59–3.16). The population who developed PE consisted of 186 males (47.1%) and 209 females (52.9%) with a mean age of 61 years (range 20–91 years).
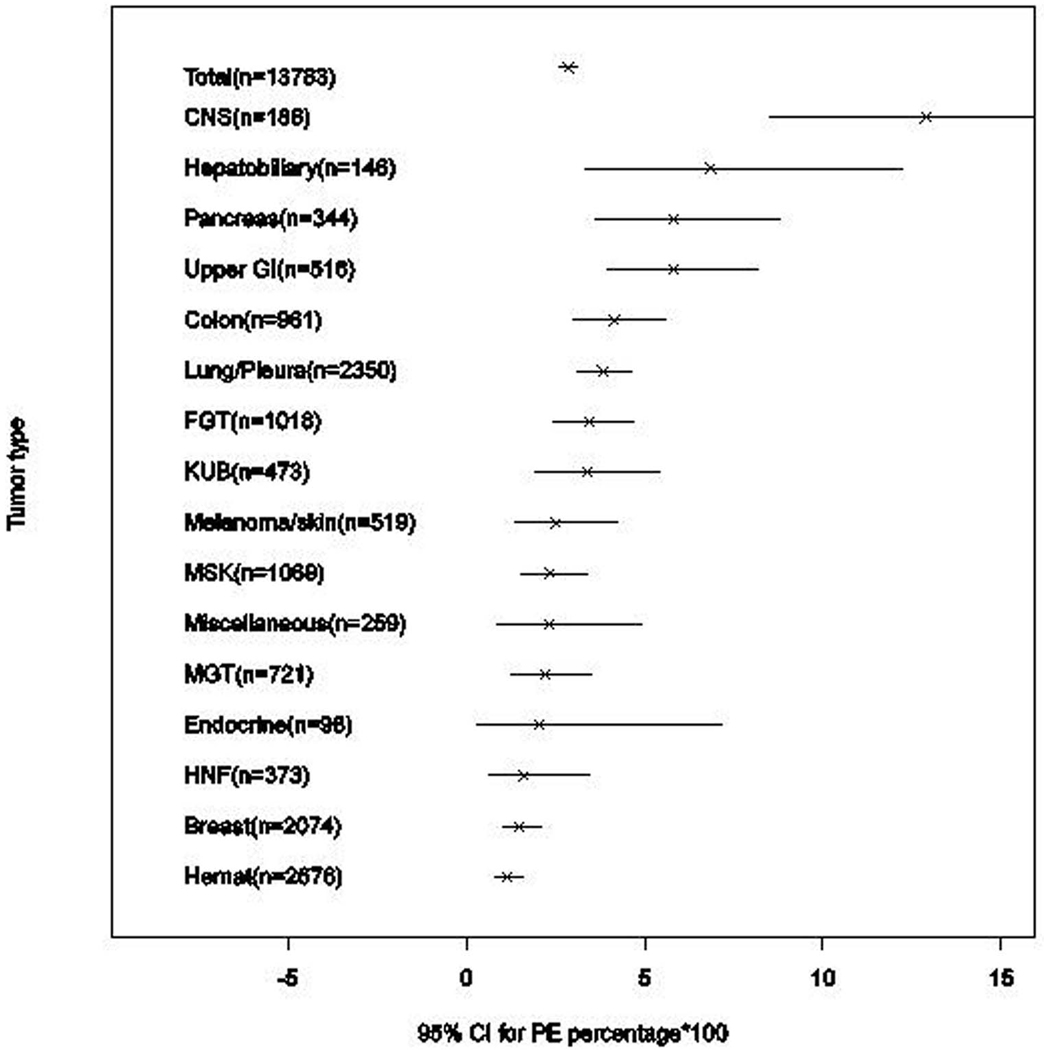
The incidence of PE was highest in malignancies of the central nervous system (CNS) (12.90%, 95%CI: 8.45–18.59), followed by hepatobiliary (6.85%, 95%CI: 3.33–12.24), pancreatic (5.81%, 95%CI: 3.59–8.84) and upper gastrointestinal (GI) (5.81%, 95% CI: 3.96–8.20) malignancies. The lowest incidence of PE was found in hematologic malignancies (1.16%, 95%CI: 0.79–1.64) and in breast cancer (1.50%, 95%CI: 1.02–2.11). Table 1 lists the incidence of PE for the different cancer types (Figure 1).
Figure 1.
Incidence of pulmonary embolism (PE) with 95% CI for different cancer types. The figure indicates the incidence of PE in each type of cancer (x) with the 95% confidence interval (-).
PE, pulmonary embolism; CNS, central nervous system; upper GI, upper gastrointestinal; FGT, female genital tract; KUB; kidney, ureter and bladder; MSK, musculoskeletal; MGT, male genital tract; HNF, head, neck and face; Hemat, hematological.
After applying the Bonferroni correction, the risk for PE was found to be significantly higher for CNS (p<0.0001, OR:5.28), pancreatic (p=0.0027, OR:2.15), upper gastrointestinal (p=0.0002, OR:2.18) and lung/pleural (p=0.0028, OR:1.45) malignancies than other cancers. The hepatobiliary and colorectal cancers have high incidence of PE; however, it did not meet statistical significance after applying the Bonferroni correction (Table 1). A significantly lower risk of PE was noted for hematologic malignancies (p<0.0001, OR: 0.35) and breast cancer (p<0.0001, OR:0.47).
Out of the total 395 patients with PE, 193 patients (48.9%) had suspected PE (overall incidence of suspected PE, 1.40%); and 202 patients (51.1%) had unsuspected PE, detected incidentally on the routine staging/restaging study (overall incidence of unsuspected PE, 1.47%). In certain cancer groups such as CNS, lung/pleural and hematologic malignancies, suspected PE was more common than unsuspected PE; while in certain other groups including pancreatic, hepatobiliary and colorectal cancer, unsuspected PE was more frequent (Table 2). However, these differences in the proportion of suspected and unsuspected PE were not statistically significant, except for pancreatic and CNS cancers. Patients with pancreatic cancer are significantly more likely to develop unsuspected PE than suspected PE (unsuspected PE 85.0%, 95%CI: 62.1–96.8). The PE was clinically suspected in almost all the cases of CNS tumors (23/24, 95.8%; 95%CI: 78.9–99.9).
Table 2.
Proportion of suspected pulmonary embolism (PE).
| Tumor Type | Total PE |
Suspected PE |
Percentage of Suspected PE |
95% CI | p value | Odds Ratio |
|---|---|---|---|---|---|---|
| Endocrine | 2 | 2 | 100.0 | 15.8–100.0 | 0.2381 | Inf |
| CNS | 24 | 23 | 95.8 | 78.9–99.9 | *<0.0001 | 27.05 |
| HNF | 6 | 4 | 66.7 | 22.3–95.7 | 0.4399 | 2.11 |
| MGT | 16 | 10 | 62.5 | 35.4–84.8 | 0.3133 | 1.78 |
| Hematological | 31 | 19 | 61.3 | 42.2–78.2 | 0.1901 | 1.73 |
| Lung/Pleura | 90 | 51 | 56.7 | 45.8–67.1 | 0.0948 | 1.50 |
| MSK | 25 | 13 | 52.0 | 31.3–72.2 | 0.8373 | 1.14 |
| Miscellaneous | 6 | 3 | 50.0 | 11.8–88.2 | 1.0000 | 1.05 |
| Breast | 31 | 15 | 48.4 | 30.2–66.9 | 1.0000 | 0.98 |
| Colorectal | 40 | 15 | 37.5 | 22.7–54.2 | 0.1370 | 0.60 |
| FGT | 35 | 13 | 37.1 | 21.5–55.1 | 0.1599 | 0.59 |
| Upper GI | 30 | 11 | 36.7 | 19.9–56.1 | 0.1866 | 0.58 |
| KUB | 16 | 5 | 31.3 | 11.0–58.7 | 0.2027 | 0.46 |
| Hepatobiliary | 10 | 3 | 30.0 | 6.7–65.3 | 0.3387 | 0.44 |
| Melanoma/skin | 13 | 3 | 23.1 | 5.0–53.8 | 0.0881 | 0.30 |
| Pancreas | 20 | 3 | 15.0 | 3.2–37.9 | ¶ <0.0001 | 0.17 |
| Total | 395 | 193 | 48.9 | 43.8–53.9 |
PE, pulmonary embolism; CNS, central nervous system; HNF, head, neck and face; MGT, male genital tract; MSK, musculoskeletal; FGT, female genital tract; upper GI, upper gastrointestinal; KUB; kidney, ureter and bladder; inf, infinity.
Significantly higher proportion of suspected PE (p < 0.0031 with Bonferroni correction)
Significantly lower proportion of suspected PE (p < 0.0031 with Bonferroni correction)
The most proximal location of the embolus was in the main pulmonary arteries in 107 patients (27.1%), in lobar arteries in 100 patients (25.3%), segmental arteries in 144 patients (36.5%) and in subsegmental arteries in 44 patients (11.1%). Table 3 depicts the distribution of the location of suspected and unsuspected PE. The proportion of the main pulmonary artery involvement for suspected and unsuspected PE was 23.3% (45/193) and 30.7% (62/202) respectively, which was not significantly different (Fisher’s exact test, p = 0.11).
Table 3.
Location of suspected and unsuspected PE.
| Most proximal site of embolus |
Main | Lobar | Segmental | Subsegmental | Total |
|---|---|---|---|---|---|
| Unsuspected | 62 | 62 | 65 | 13 | 202 |
| Suspected | 45 | 38 | 79 | 31 | 193 |
| Total | 107 | 100 | 144 | 44 | 395 |
Out of the patients with PE, 96.7% (382/395) was treated (anticoagulants in 341 patients, inferior vena cava filter in 15 patients, thrombolysis using tissue plasminogen activator (tPA) in three patients, and a combination of the above in 23 patients) and 2.8% (11/395) received no treatment. Information regarding the management was not available in 0.5% patients (2/395). Out of the 382 treated patients, 34 patients were already on anticoagulation and/or had an IVC filter for prior deep venous thrombosis. Following the diagnosis of PE, the drug and/or dose of anticoagulant was modified in 18 patients, anticoagulation was started in 9 patients who previously had an IVC filter and IVC filter was placed in 7 patients who were previously on anticoagulation. All 11 patients who received no treatment had contraindications to therapy. Of these 11 untreated patients, 4 had suspected PE and 7 had unsuspected PE.
Out of the 202 patients with unsuspected PE, 195 patients were treated (96.5%) and 7 patients were not treated (3.5%). Out of the 193 patients with suspected PE, 187 patients were treated (96.9%), 4 patients (2.1%) were untreated, and the information about treatment was not available in 2 patients (1%). There was no difference in the proportion of treated patients between the suspected and unsuspected PE groups (p = 0.84).
DISCUSSION
To our knowledge, this is the first large study on the incidence of suspected and unsuspected PE in oncologic outpatients. The incidence of PE among different cancer types has also not been previously reported in this patient population. Over a period of six years, we found a 2.87% incidence of PE in a total of 13,783 outpatients with cancer who had imaging studies at our institution. In a meta-analysis, Reynolds et al found an incidence of PE ranging from 0.13% to 8.65% in cancer patients in general39. The incidence of PE in the general population has been reported to be 0.11% 40. The mean age of patients with PE was 61 years (range 20–91 years) in our study, compared with the mean age of 63 years (range, 28–74 years) previously reported39.
The higher risk of PE in CNS, pancreatic, upper GI and lung/pleural malignancies compared to other cancers found in our study is in agreement with the prior studies which have reported a higher incidence of thromboembolic complications in CNS, pancreas, upper GI and lung malignancies, as well as in renal and uterine cancers8,41–42. The possible reasons for the higher risk of PE in CNS malignancy include patients’ factors such as immobility. In our study, the incidence of PE in urinary tract and female genital tract malignancies was above the overall incidence; however it did not reach statistical significance. The risk of PE in hematologic and breast cancer was significantly low in our study. It is possible that there is a "protective effect" against thromboembolsm in patients with hematologic malignancies, which may be related to low platelet counts and low hemoglobin levels. Chew et al reported a lower incidence of VTE in breast and prostate cancer8. The incidence of PE in male genital tract cancers was relatively low in our study as well. Blom et al have reported a higher incidence of VTE in hematologic malignancies42, however, this difference could be due to a small number of patients with hematological malignancies in their study, all of whom were not necessarily outpatients.
Our study revealed that PE was clinically unsuspected in approximately half of the positive PE cases (51.1%, 202/395), which is consistent with prior clinical and autopsy studies31,43. The incidence of unsuspected PE of 1.47% in this study is in keeping with the estimated incidence of 1.2% in cancer outpatients in a meta-analysis by Dentali et al44. There was a seemingly higher incidence of suspected or unsuspected PE in certain cancers. This difference did not meet statistical significance in most cancer groups, possibly due to the relatively small number of patients in each category. However, incidence of unsuspected PE was significantly higher than suspected PE in pancreatic cancer (85.0% unsuspected PE, 95%CI: 62.1–96.8). The reason for this higher rate of unsuspected PE in pancreatic cancer is unknown and to the best of our knowledge, this has not been previously reported or investigated. The majority of the patients with CNS malignancy had suspected PE. However, this is conceivably because patients with CNS cancers typically do not have chest CT as a part of their routine follow-up evaluation, unless they develop chest symptoms, thereby negating the opportunity to detect a PE incidentally.
To our surprise, there was no significant difference in the proportion of main pulmonary artery involvement between the suspected and unsuspected PE. In this cohort of oncology outpatients, the majority (96.5%) of the patients who developed unsuspected PE were treated, and they received a similar treatment as suspected PE. The suspected or unsuspected nature of PE had no bearing on treatment-related decisions. Although no clear guidelines currently exist for the treatment of unsuspected PE, our results indicate that patients with unsuspected PE usually receive the same treatment as those with suspected PE. Until further data emerges, there is certainly a trend towards treating each detected episode of PE27,33,35,45.
There are two major limitations of the study. One is that only the radiology reports were used to identify the patients with PE without reviewing the images. The other is that standard chest CT without thin-section dedicated CT pulmonary angiography technique was used to detect PE in patients with unsuspected PE. Therefore, the incidence of PE, especially of unsuspected PE involving the smaller branches, may have been underestimated. The difference in the involvement of smaller pulmonary arteries between the suspected and unsuspected PE groups was not calculated, because smaller emboli are better detected with thin-section dedicated CT pulmonary angiography which is performed in patients with suspected PE, than with thicker section routine chest CT on which unsuspected PE is incidentally detected. Some oncologists may routinely place patients with certain malignancies on VTE prophylaxis. However, the patients on VTE prophylaxis were not excluded from the present study, which is another limitation of the study since these patients have decreased likelihood of developing PE.
In conclusion, the incidence of PE in oncology outpatients in a tertiary cancer center over a six-year period was 2.87% (clinically suspected PE, 1.40%; unsuspected PE, 1.47%). CNS, pancreatic, upper gastrointestinal and lung/pleural malignancies had a significantly higher risk for PE than other malignancies, while hematologic and breast malignancies had a significantly lower risk. The evidence reported here on the incidence of PE in the different cancer groups may contribute to the clinical management of the oncology outpatients.
Acknowledgments
The investigators were supported by 2009-11 Agfa HealthCare/RSNA Research Scholar Grant (M.N.), and 5R21 CA11627-02 (H.H.) from the National Institutes of Health
Footnotes
There are no financial disclosures from any authors.
REFERENCES
- 1.Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet. 1994;343(8902):886–889. doi: 10.1016/s0140-6736(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 2.Agnelli G, Gussoni G, Bianchini C, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10(10):943–949. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- 3.Perry JR, Julian JA, Laperriere NJ, et al. PRODIGE: a randomized placebo-controlled trial of dalteparin low molecular weight heparin (LMWH) thromboprophylaxis in patients with newly diagnosed malignant glioma. [Accessed September 4, 2010];J Thromb Haemost. 2010 doi: 10.1111/j.1538-7836.2010.03973.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20598077. [DOI] [PubMed]
- 4.Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann. Surg. 2006;243(1):89–95. doi: 10.1097/01.sla.0000193959.44677.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb. Haemost. 2002;87(4):575–579. [PubMed] [Google Scholar]
- 6.Stein PD, Beemath A, Meyers FA, et al. Incidence of venous thromboembolism in patients hospitalized with cancer. Am. J. Med. 2006;119(1):60–68. doi: 10.1016/j.amjmed.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 7.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110(10):2339–2346. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 8.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch. Intern. Med. 2006;166(4):458–464. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 9.Blom JW, Osanto S, Rosendaal FR. High risk of venous thrombosis in patients with pancreatic cancer: a cohort study of 202 patients. Eur. J. Cancer. 2006;42(3):410–414. doi: 10.1016/j.ejca.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Oh SY, Kim JH, Lee K, et al. Venous thromboembolism in patients with pancreatic adenocarcinoma: lower incidence in Asian ethnicity. Thromb. Res. 2008;122(4):485–490. doi: 10.1016/j.thromres.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Tesselaar ME, Osanto S. Risk of venous thromboembolism in lung cancer. Curr Opin Pulm Med. 2007;13(5):362–367. doi: 10.1097/MCP.0b013e328209413c. [DOI] [PubMed] [Google Scholar]
- 12.Tetzlaff ED, Correa AM, Baker J, Ensor J, Ajani JA. The impact on survival of thromboembolic phenomena occurring before and during protocol chemotherapy in patients with advanced gastroesophageal adenocarcinoma. Cancer. 2007;109(10):1989–1995. doi: 10.1002/cncr.22626. [DOI] [PubMed] [Google Scholar]
- 13.Levi AD, Wallace MC, Bernstein M, Walters BC. Venous thromboembolism after brain tumor surgery: a retrospective review. Neurosurgery. 1991;28(6):859–863. doi: 10.1097/00006123-199106000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Brandes AA, Scelzi E, Salmistraro G, et al. Incidence of risk of thromboembolism during treatment high-grade gliomas: a prospective study. Eur. J. Cancer. 1997;33(10):1592–1596. doi: 10.1016/s0959-8049(97)00167-6. [DOI] [PubMed] [Google Scholar]
- 15.Saphner T, Tormey DC, Gray R. Venous and arterial thrombosis in patients who received adjuvant therapy for breast cancer. J. Clin. Oncol. 1991;9(2):286–294. doi: 10.1200/JCO.1991.9.2.286. [DOI] [PubMed] [Google Scholar]
- 16.Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J. Clin. Oncol. 2007;25(1):70–76. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 17.Satoh T, Matsumoto K, Uno K, et al. Silent venous thromboembolism before treatment in endometrial cancer and the risk factors. Br. J. Cancer. 2008;99(7):1034–1039. doi: 10.1038/sj.bjc.6604658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tagalakis V, Levi D, Agulnik JS, et al. High risk of deep vein thrombosis in patients with non-small cell lung cancer: a cohort study of 493 patients. J Thorac Oncol. 2007;2(8):729–734. doi: 10.1097/JTO.0b013e31811ea275. [DOI] [PubMed] [Google Scholar]
- 19.Chew HK, Davies AM, Wun T, et al. The incidence of venous thromboembolism among patients with primary lung cancer. J. Thromb. Haemost. 2008;6(4):601–608. doi: 10.1111/j.1538-7836.2008.02908.x. [DOI] [PubMed] [Google Scholar]
- 20.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winston CB, Wechsler RJ, Salazar AM, Kurtz AB, Spirn PW. Incidental pulmonary emboli detected at helical CT: effect on patient care. Radiology. 1996;201(1):23–27. doi: 10.1148/radiology.201.1.8816515. [DOI] [PubMed] [Google Scholar]
- 22.Farrell C, Jones M, Girvin F, Ritchie G, Murchison JT. Unsuspected pulmonary embolism identified using multidetector computed tomography in hospital outpatients. Clin Radiol. 2010;65(1):1–5. doi: 10.1016/j.crad.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Raptopoulos V, Boiselle PM. Multi-detector row spiral CT pulmonary angiography: comparison with single-detector row spiral CT. Radiology. 2001;221(3):606–613. doi: 10.1148/radiol.2213010473. [DOI] [PubMed] [Google Scholar]
- 24.Ghaye B, Szapiro D, Mastora I, et al. Peripheral pulmonary arteries: how far in the lung does multi-detector row spiral CT allow analysis? Radiology. 2001;219(3):629–636. doi: 10.1148/radiology.219.3.r01jn32629. [DOI] [PubMed] [Google Scholar]
- 25.DeMonaco NA, Dang Q, Kapoor WN, Ragni MV. Pulmonary embolism incidence is increasing with use of spiral computed tomography. Am. J. Med. 2008;121(7):611–617. doi: 10.1016/j.amjmed.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghaye B, Ghuysen A, Bruyere P, D'Orio V, Dondelinger RF. Can CT pulmonary angiography allow assessment of severity and prognosis in patients presenting with pulmonary embolism? What the radiologist needs to know. Radiographics. 2006;26(1):23–39. doi: 10.1148/rg.261055062. discussion 39-40. [DOI] [PubMed] [Google Scholar]
- 27.Goodman LR. Small pulmonary emboli: what do we know? Radiology. 2005;234(3):654–658. doi: 10.1148/radiol.2343041326. [DOI] [PubMed] [Google Scholar]
- 28.Paddon AJ. Incidental pulmonary embolism detected by routine CT in patients with cancer. Cancer Imaging. 2005;5(1):25–26. doi: 10.1102/1470-7330.2005.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storto ML, Di Credico A, Guido F, Larici AR, Bonomo L. Incidental detection of pulmonary emboli on routine MDCT of the chest. AJR Am J Roentgenol. 2005;184(1):264–267. doi: 10.2214/ajr.184.1.01840264. [DOI] [PubMed] [Google Scholar]
- 30.Gladish GW, Choe DH, Marom EM, et al. Incidental pulmonary emboli in oncology patients: prevalence, CT evaluation, and natural history. Radiology. 2006;240(1):246–255. doi: 10.1148/radiol.2401051129. [DOI] [PubMed] [Google Scholar]
- 31.Engelke C, Manstein P, Rummeny EJ, Marten K. Suspected and incidental pulmonary embolism on multidetector-row CT: analysis of technical and morphological factors influencing the diagnosis in a cross-sectional cancer centre patient cohort. Clin Radiol. 2006;61(1):71–80. doi: 10.1016/j.crad.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Cronin CG, Lohan DG, Keane M, Roche C, Murphy JM. Prevalence and significance of asymptomatic venous thromboembolic disease found on oncologic staging CT. AJR Am J Roentgenol. 2007;189(1):162–170. doi: 10.2214/AJR.07.2067. [DOI] [PubMed] [Google Scholar]
- 33.Desai SR. Unsuspected pulmonary embolism on CT scanning: yet another headache for clinicians? Thorax. 2007;62(6):470–472. doi: 10.1136/thx.2006.067884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connell CL, Boswell WD, Duddalwar V, et al. Unsuspected pulmonary emboli in cancer patients: clinical correlates and relevance. J. Clin. Oncol. 2006;24(30):4928–4932. doi: 10.1200/JCO.2006.06.5870. [DOI] [PubMed] [Google Scholar]
- 35.Streiff MB. Diagnosis and initial treatment of venous thromboembolism in patients with cancer. J. Clin. Oncol. 2009;27(29):4889–4894. doi: 10.1200/JCO.2009.23.5788. [DOI] [PubMed] [Google Scholar]
- 36.Sun J, Kim TS, Lee J, et al. Unsuspected pulmonary emboli in lung cancer patients: the impact on survival and the significance of anticoagulation therapy. Lung Cancer. 2010;69(3):330–336. doi: 10.1016/j.lungcan.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Gosselin MV, Rubin GD, Leung AN, Huang J, Rizk NW. Unsuspected pulmonary embolism: prospective detection on routine helical CT scans. Radiology. 1998;208(1):209–215. doi: 10.1148/radiology.208.1.9646815. [DOI] [PubMed] [Google Scholar]
- 38.Sebastian AJ, Paddon AJ. Clinically unsuspected pulmonary embolism--an important secondary finding in oncology CT. Clin Radiol. 2006;61(1):81–85. doi: 10.1016/j.crad.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds MW, Shibata A, Zhao S, et al. Impact of clinical trial design and execution-related factors on incidence of thromboembolic events in cancer patients: a systematic review and meta-analysis. Curr Med Res Opin. 2008;24(2):497–505. doi: 10.1185/030079908x261050. [DOI] [PubMed] [Google Scholar]
- 40.Stein PD, Matta F. Epidemiology and incidence: the scope of the problem and risk factors for development of venous thromboembolism. Clin. Chest Med. 2010;31(4):611–628. doi: 10.1016/j.ccm.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J. Clin. Oncol. 2009;27(29):4839–4847. doi: 10.1200/JCO.2009.22.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blom JW, Doggen CJM, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 43.Pineda LA, Hathwar VS, Grant BJ. Clinical suspicion of fatal pulmonary embolism. Chest. 2001;120(3):791–795. doi: 10.1378/chest.120.3.791. [DOI] [PubMed] [Google Scholar]
- 44.Dentali F, Ageno W, Becattini C, et al. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: a meta-analysis. Thromb. Res. 2010;125(6):518–522. doi: 10.1016/j.thromres.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 45.Ritchie G, McGurk S, McCreath C, Graham C, Murchison JT. Prospective evaluation of unsuspected pulmonary embolism on contrast enhanced multidetector CT (MDCT) scanning. Thorax. 2007;62(6):536–540. doi: 10.1136/thx.2006.062299. [DOI] [PMC free article] [PubMed] [Google Scholar]