Abstract
Recent reports demonstrate the anti‐correlated behaviors between the default (DF) and the dorsal attention (DA) networks. We aimed to investigate the roles of the frontal parietal control (FPC) network in regulating the two anti‐correlated networks through three experimental conditions, including resting, continuous self‐paced/attended sequential finger tapping (FT), and natural movie watching (MW), respectively. The two goal‐directed tasks were chosen to engage either one of the two competing networks—FT for DA whereas MW for default. We hypothesized that FPC will selectively augment/suppress either network depending on how the task targets the specific network; FPC will positively correlate with the target network, but negatively correlate with the network anti‐correlated with the target network. We further hypothesized that significant causal links from FPC to both DA and DF are present during all three experimental conditions, supporting the initiative regulating role of FPC over the two opposing systems. Consistent with our hypotheses, FPC exhibited a significantly higher positive correlation with DA (P = 0.0095) whereas significantly more negative correlation with default (P = 0.0025) during FT when compared to resting. Completely opposite to that observed during FT, the FPC was significantly anti‐correlated with DA (P = 2.1e‐6) whereas positively correlated with default (P = 0.0035) during MW. Furthermore, extensive causal links from FPC to both DA and DF were observed across all three experimental states. Together, our results strongly support the notion that the FPC regulates the anti‐correlated default and DA networks. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: functional MRI, functional connectivity, resting BOLD
INTRODUCTION
Resting functional connectivity MRI (rfcMRI) [Biswal et al.,1995], relying on temporal synchronization of blood oxygen level dependent contrast (BOLD) signal among different brain regions in the absence of any goal‐directed task, has recently been exploited to depict different brain functional networks, comprising highly correlated brain regions. With this approach, not only the well‐documented brain functional networks, for example, motor [Biswal et al.,1995], visual [Lowe et al.,1998], language [Hampson et al.,2002], and attention [Fox et al.,2006] but also novel networks such as the default network [Buckner et al.,2008; Greicius et al.,2003; Raichle et al.,2001] have been reported. Apart from the positively correlated regions, regions negatively correlated with each other have also been observed [Fox et al.,2005]. That is, when the BOLD signal is increased in a set of regions, the negatively correlated regions exhibit a reduction in BOLD signal and vice versa. Although the exact functional underpinnings among the “anti‐correlated” brain regions are yet to be established, the general wisdom suggests these anti‐correlated regions representing, potentially, networks that process competing functions [Fox et al.,2005].
Two anti‐correlated networks, the dorsal attention (DA) and the default networks (DF), have recently attracted substantial interests [Corbetta and Shulman,2002; Fox et al.,2005,2006]. To some extent, considering the distinct functional differences and somewhat competing functions between the DA and the default networks, it may not be surprising to learn that the DA and the default network exhibit anti‐correlated behaviors. Specifically, it has been well documented that the DA is associated with externally directed cognitions [Corbetta and Shulman,2002] whereas the default network is more associated with internally directed cognitions [Andrews‐Hanna et al.; Buckner et al.,2008; Buckner and Vincent,2007; Gusnard et al.,2001; Mason et al.,2007]. However, do these two networks exhibit such opposing/competing relation on their own or is there another network(s) that potentially regulates them? Vincent and colleagues [Vincent et al.,2008] have recently revealed that the frontal parietal control (FPC) network is “anatomically positioned to integrate information from these two opposing brain systems.” Furthermore, the cognitive control roles of the brain regions within FPC have been reported in numerous studies [Koechlin et al.,1999; Koechlin and Hyafil,2007; Kompus et al.,2009; Ramnani and Owen,2004]. The most anterior part of the prefrontal cortex (aPFC) has been suggested as the apex of the executive network underlying decision‐making [Koechlin et al.,1999; Koechlin and Hyafil,2007; Kompus et al.,2009; Ramnani and Owen,2004]. Activations of insula (INS) and anterior cingulate cortex (ACC) are commonly observed with a variety of cognitive control processes, particularly those involving conflict monitoring, information integration, and response selection [Cole and Schneider,2007; Posner and Rothbart,2007; Roberts and Hall,2008]. Finally, the anterior inferior parietal lobule (aIPL) region has been reported to increase activity during role transition in stimulus‐response association tasks [Crone et al.,2006] as well as tasks involving control of spatial attention [Sapir et al.,2005]. Therefore, it is not surprising that both Vincent et al. [2008] and Sridaran et al. [2008] have suggested the potential regulation role of FPC to both the default network and the task‐positive network [DA in Vincent et al. and central‐executive networks (CEN) in Sridaran et al.].
In this study, we aimed to further extend the findings of Vincent et al. and Sridharan et al. [Sridharan et al.,2008; Vincent et al.,2008]. Specifically, we focused on how goal‐directed tasks may further shed light on our understanding of the regulatory roles of FPC exerted on the DA and the default networks. Two relatively simple but contrasting tasks were exploited in this study, namely self‐paced/attended (1 Hz) sequential finger tapping (FT) and natural movie watching (MW). The basis of the choice of these two tasks is that FT task will require the engagement of the DA network [Gordon et al.,1998; Rao et al.,2001; Shibasaki et al.,1993] whereas MW will involve the default network [Furman et al.,2007; Golland et al.,2007; Iacoboni et al.,2004]. With these two tasks, we hypothesize that the FPC will selectively augment/suppress either the DA or the default network depending on the nature of the tasks. Specifically, we hypothesize that the FT task (associated with DA) will lead to an increased positive correlation between FPC and DA (increased coordination) and an increased negative correlation (increased disassociation/suppression) between FPC and default when compared to the resting condition. Conversely, the MW task (actively involving the default network) will result in enhanced positive correlation of FPC with default whereas an increased negative correlation with the DA. In addition, we further hypothesize that significant causal links from FPC to both DA and DF should be observed during all three experimental conditions employed to support the initiative regulating role of FPC over the two opposing systems.
METHODS
MR Acquisition
A total of 19 healthy subjects (age 25–33, 7F, all right‐handed) were recruited in this study. Informed consent was obtained from all participants and the experimental protocols were approved by the institutional review board. All images were acquired using a Siemens Allegra 3T MR scanner (Siemens Medical, Erlangen, Germany). Anatomical images were acquired using a 3D MP‐RAGE sequence and these images were subsequently used for spatial normalization. The imaging parameters were: repetition time (TR) = 1,820 ms; echo time (TE) = 4.38 ms; inversion time = 1,100 ms; 144 slices; and voxel size = 1 × 1 × 1 mm3. For the rfcMRI studies, a T2*‐weighted echo‐planar imaging (EPI) sequence was used with the following imaging parameters: TR = 2 s, TE = 32 ms; 33 slices; and voxel size = 4 × 4 × 4 mm3. This sequence was repeated 150 times (∼5 min) for each experimental condition, including resting (RS), self‐paced/attended sequential finger tapping (FT) and natural movie watching (MW). During RS, subjects were instructed to relax and remain still while keeping eyes closed. During FT, subjects were instructed to lie still with eyes closed while continuously touching the thumb to each finger in a sequential manner using only the dominant hand. In addition, subjects were also instructed to actively maintain a consistent pace (∼1 Hz) of finger tapping throughout the entire scan. Each subject was visually monitored during the scan and good compliance was observed. For the natural movie watching task, the movie clip contained shallow sea scenes with a variety of animal activities. Subjects were asked to report what they saw in the movie after the experiment.
Preprocessing
The brain extraction tool of the FSL (FMRIB, Oxford University, UK) was first applied to exclude voxels outside of the brain. Subsequently, rfcMRI data went through several preprocessing steps, including time shifting, motion correction, spatial smoothing (6‐mm full width at half maximum Gaussian kernel), linear trend removal, and low pass filtering (<0.08 Hz). Nuisance sources of variance including white matter, CSF, and global mean signal were removed using regression technique [Fox et al.,2005,2006]. Three subjects were excluded from subsequent analysis because of excessive head motion during scan. For the remaining 16 subjects, images from the first 10 time points were excluded to allow magnetization reaching a steady state.
Functional Network Definition and Spatial Normalization
The anatomical locations reported by Vincent et al. [Vincent et al.,2008] were used to define the DA, default, and FPC networks so as to facilitating a direct comparison between our findings and that reported in the literature. In addition, given the apparent involvement of the motor‐sensory (MS) and visual (V) networks for the FT and MW tasks, respectively, these two networks were similarly constructed. The anatomical locations, abbreviations, and the corresponding Montreal Neurological Institute (MNI) template space [Tzourio‐Mazoyer et al.,2002] coordinates of the brain regions within each network are summarized in Table I whereas their anatomical locations are presented in Figure 1. A sphere with a volume of ∼2 cm3 centered at each of the predefined coordinates was used to construct each ROI. Overall, there were 6, 9, 6, 6, and 5 ROIs within the DA, FPC, default, V, and MS networks (altogether 32 nodes), respectively.
Table I.
MNI coordinates of regions of interest within the five predefined networks
| Dorsal attention | lMT+: (−45, −69, −2) |
| rMT+: (50, −69, −3) | |
| lIPS: (−27, −52, 57) | |
| rIPS: (24, −56, 55) | |
| lFEF: (−25, −8, 50) | |
| rFEF: (27, −8, 50) | |
| Frontal parietal control | laPFC: (−36, 57, 9) |
| raPFC: (34, 52, 10) | |
| ACC: (3, 31, 27) | |
| laIPL: (−52, −49, 47) | |
| raIPL: (52, −46, 46) | |
| ldlPFC: (−50, 20, 34) | |
| rdlPFC: (46, 14, 43) | |
| lINS: (−31, 21, −1) | |
| rINS: (31, 22, −2) | |
| Default | lHF: (−21, −15, −14) |
| rHF: (24, −19, −21) | |
| vmPFC: (0, 51, −7) | |
| PCC: (1, −55, 17) | |
| lpIPL: (−47, −71, 29) | |
| rpIPL: (50, −64, 27) | |
| Visual (V) | lCal: (−8, −72, 4) |
| rCal: (16, −67, 5) | |
| lCS: (−5, −96, 12) | |
| rCS: (18, −96, 12) | |
| lLO: (−23, −89, 12) | |
| rLO: (37, −85, 13) | |
| Motor sensory | lPreC: (−41, −4, 54) |
| rPreC: (42, −13, 53) | |
| lPoC: (−45, −26, 54) | |
| rPoC: (49, −27, 53) | |
| SMA: (6, −5, 54) |
aPFC, anterior prefrontal cortex; dlPFC, dorsal lateral prefrontal cortex; ACC, anterior cingulate cortex; INS, insula; aIPL, anterior inferior parietal lobule; IPS, bilateral intra‐parietal sulcus; FEF, frontal eye field; MT+, middle temporal area; PCC, posterior cingulate cortex; MPFC, medial prefrontal cortex; pIPL, bilateral posterior inferior parietal lobule; HF, hippocampus formation; PreC, precentral gyrus; PoC, postcentral gyrus; SMA, supplementary motor area; Cal, bilateral calcarine; CS, cuneus; LO, lateral occipital.
Figure 1.

The anatomical locations of the five predefined networks are shown, including the dorsal attention (DA), the default, the frontal parietal control (FPC), visual (V), and motor‐sensory (MS) networks, respectively. The MNI coordinates for each region along with the exact anatomical locations for the abbreviations are provided in Table I.
Although the pre‐defined ROIs were used in our study, the corresponding functional connectivity maps of the five networks using the seed‐based approach were also constructed and results are provided in the Supporting Information (Fig. S1). Overall, the anatomical representations of the five networks using the seed‐based approach are highly consistent with that shown in Figure 1 as well as the results reported in the literature [Biswal et al.,1995; Fox et al.,2005; Lowe et al.,1998; Vincent et al.,2008].
Subsequently, these pre‐defined ROIs were warped to each individual subject to minimize perturbation of the intrinsic correlation structures. Specifically, for subject‐template registration, we used a non‐linear HAMMER registration method [Shen and Davatzikos,2002] based on the T1‐weighted MP‐RAGE structural images. For within‐subject registration, we adapted a customized functional‐to‐structural alignment method [Saad et al.,2009] implemented in AFNI to register each individual's T1 images to its EPI rfcMRI images. This method has been shown to improve internal brain structure alignment between these two different modalities. The transformation fields from HAMMER registration and within subject structural‐functional registration were used to warp all defined ROIs to the individual subject's space.
Network Analysis
The defined ROIs within each network were used throughout all subsequent analysis. The mean time course was extracted from each ROI to construct a 32*32 correlation matrix for each subject. After Fisher's z transform and averaging across all subjects, group mean matrices were obtained for each of the three experimental states, which were then used to test the differences among the three experimental conditions. Each individual connection was tested using two‐tailed t test to identify significant positive/negative interactions. The FDR [Benjamini,2001] method was used to correct for multiple comparisons and significant connections were defined at P < 0.05 after FDR correction. For between‐network comparison, group mean interactions among a pair of regions from any two networks (one from each) were concatenated to form a vector and compared across states (RS vs. FT; and RS vs. MW) using nonparametric one‐way ANOVA (kruskwallis test). Similarly, P < 0.05 after FDR correction was considered significance. The same procedures were also done for all within‐network comparisons.
Granger Causality Analysis
Granger causality analysis [Granger,1969] was performed to characterize the effective connectivity among the brain regions in the five predefined networks. Specifically, we aimed to delineate whether or not the FPC initiates the regulating role to the DA and the default networks. The computation was performed using the Causal Connectivity Toolbox (Seth). Granger causality is based on the idea that if the inclusion of the past observations of one time series improves the prediction of the future values of the other, then the second time series is said to be Granger caused by the first one [Granger,1969]. In this study, the 32 time series of all predefined ROIs were simultaneously modeled based on the Granger causality using multivariate regression (MVR) to avoid mediated causality [Ding et al.,2006]. However, since there were only 140 available time points for each subject, this estimation is ill‐posed for individual subjects. To circumvent this difficulty, we concatenated the time series across all subjects for each region. These long‐time series were then entered into conditional Granger causality analysis to obtain results at a group level, essentially assuming a fixed‐effect model. To detect significant Granger causalities between pairs of regions, F test was performed for each individual causality measure and significant causal interactions were defined at P < 0.05 after FDR correcting for multiple comparisons [Benjamini,2001].
Partial Correlation Analysis
Partial correlation analysis was performed to further quantify the regulating role of FPC on the two opposing networks. Specifically, for each subject, partial correlation between pairs of regions within the two networks (i.e., default and DA, one from each) was calculated by regressing out the effect of all signals within the FPC. After Fisher's z transform of both the ordinary correlation and partial correlation values, the differences were then taken as an indicator of the mediating effect of FPC on the two opposing networks. As a result, an N*1 vector for each experimental condition (N: number of subjects) was available to quantify the mediation effect of FPC. Finally, the regulating effect of FPC was compared across different states (using paired t test) to detect the possible task dependence of this role.
RESULTS
To test the hypothesis that the FPC selectively regulates the DA and default networks depending on the nature of the tasks, three regional correlation matrices were constructed for each individual subject using BOLD signal fluctuations obtained during each of the three experimental states (RS, FT, and MW). The resulting correlation matrices with significant connections for the RS and FT states are presented in Figure 2a. As one would have expected [Van Dijk et al.], brain regions within each network (black boxes) are highly synchronized during RS (84.6% significant) whereas significant regional connections between networks are much sparser (35.1%), implying functional disassociation between different networks. For FT, although the regional interaction pattern within each network remains qualitatively similar to that observed during RS, the interactions between networks are dramatically changed (Fig. 2a). Specifically, six pairs of networks are significantly changed between the RS and FT states, including DA‐MS, DA‐default, FPC‐DA, FPC‐default, FPC‐MS, and V‐MS (Fig. 2b). The same comparison was done for all within network connections and no significant changes were detected.
Figure 2.
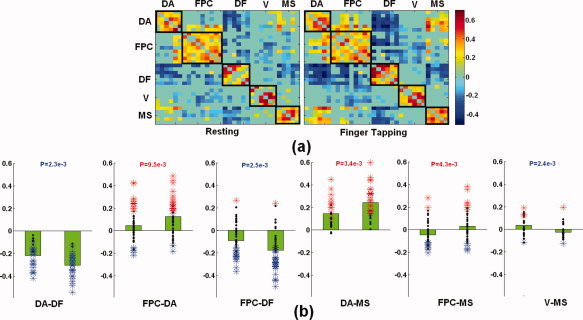
(a) The group mean correlation matrices for resting and finger tapping are shown, respectively. Only the significant correlations are shown here. The hot colors indicate positive correlation whereas the cold colors reflect negative correlation. Each black box highlights the correlation within each network. (b) Six pairs of networks are significantly changed from resting to FT. Each bar chart shown in (b) represents the correlation values during resting (left) and FT (right) for each pair of networks. Black dots represent the individual correlation values overlaid on the bar plots and statistically significant values are represented by colored asterisks. Red: significantly positive correlations; Blue: significantly negative correlations. Finally, the P value associated with the comparison between the pair of networks is provided at the top of each bar chart. DA, dorsal attention; DF, default; FPC, frontal parietal control; MS, motor‐sensory; V, visual networks.
Several interesting and important features are observed in Figure 2b. First, the interaction between DA and MS is significantly increased (more positively correlated) from resting to FT (P = 0.0034), reflecting enhanced attention control over the “task executer (MS)” during this task. Second, consistent with the previously reported results [Kelly et al.,2008], DA and default become significantly more anti‐correlated (P = 0.0023). In addition, during this motor‐oriented task (subjects kept eyes closed during this task), the interaction between MS and V becomes significantly disrupted (P = 0.0024), indicating the potential disassociation between these two functions during a single‐mode task. Finally, the most intriguing results lie on the differences of the interaction patterns of FPC with the two opposing networks (DA and default); FT leads to a significantly higher positive correlation between FPC and DA (P = 0.0095) when compared to RS whereas the interaction between FPC and default becomes significantly more anti‐correlated (P = 0.0025).
In contrast to the active involvement of DA during FT, the MW is designed to predominately engage the default network [Golland et al.,2007; Iacoboni et al.,2004]. Significantly changed network interactions between the two experimental conditions include FPC‐DA, FPC‐default, and V‐MS (Fig. 3). Comparing to the results shown in Figure 2b, several interesting features emerge. First and most importantly, the polarity of the correlation of FPC with DA and default is reversed when compared to that observed during FT (positive with DA and negative with default); the FPC is significantly more anti‐correlated with DA (P = 2.1e‐6) whereas more positively correlated with default (P = 0.0035) when compared to that during RS, strongly supporting our first hypothesis. Second, the interaction between MS and V is similarly disrupted (P = 0.0078) during this vision‐oriented task. Finally, the anti‐correlation between DA and default is comparable to that during RS (P = 0.9193).
Figure 3.

(a) The group mean correlation matrices for resting and natural movie watching are shown, respectively. Only the significant correlations are shown here. The hot colors indicate positive correlation whereas the cold colors reflect negative correlation. Each black box highlights the correlation within each network. (b) Three pairs of networks are significantly changed from resting to MV whereas no significant changes are observed between DA and default. Each bar chart shown in (b) represents the correlation values during resting (left) and MW (right) for each pair of networks. Black dots represent the individual correlation values overlaid on the bar plots and statistically significant values are represented by colored asterisks. Red: significantly positive correlations; Blue: significantly negative correlations. Finally, the P value associated with the comparison between the pair of networks is provided at the top of each bar chart. DA, dorsal attention; DF, default; FPC, frontal parietal control; V, visual networks.
One potential factor that may affect our findings is the use of global signal regression since it has been suggested that this procedure may alter MR signal [Chang and Glover,2009; Fox et al.,2009; Murphy et al.,2009]. To address this potential concern, identical procedures with the exception of global signal regression were used for data analysis and results are provided in Figure S3 (Supporting Information, “Global Signal Regression”). Overall, while the absolute network correlation differs with and without global mean signal regression, the relative connectivity patterns (both within and between networks) are quite similar and the above findings hold with (Figs. 2 and 3) and without (Fig. S3) global signal regression (refer Supporting Information, “Global Signal Regression” for more details).
Our second hypothesis concerns the initiative regulating role of FPC to the two opposing networks. Results of the Granger causality analysis are provided in Figure 4. Extensive causal flow originated from FPC to both DA and default (pink lines) is apparent (Fig. 4); there are 7/8 forward causal interactions from FPC to DA/default during resting, 8/13 during finger tapping, and 8/9 during movie watching, strongly suggesting that FPC plays a critical role in initiating regulation to the default and the DA networks across different brain states. In addition to the forward interactions originated from the FPC to the DA and default networks, there are also considerable feedback interactions from both DA and default to FPC during all three experimental conditions. Specifically, there are 3/5 feedback interactions from DA/DF to FPC during resting, 5/5 during finger tapping, and 11/8 during movie watching. Overall, there are considerably more causal links from FPC to either DA/DF than feedbacks during both resting and finger tapping (7 (FPC‐>DA)/3(DA‐>FPC), 8 (FPC‐>DF)/5(DF‐>FPC) during resting; 8 (FPC‐>DA)/5(DA‐>FPC), 13 (FPC‐>DF)/5(DF‐>FPC) during finger tapping. However, during movie watching, the forward and feedback interactions are more balanced (8 (FPC‐>DA)/11(DA‐>FPC), 9 (FPC‐>DF)/8(DF‐>FPC)).
Figure 4.

Significant causal interactions between pairs of regions among the three networks: DA, default and FPC. Pink lines: from FPC to DA/default; cyan lines: from DA/default to FPC; gray lines: between DA and default.
To further quantify the regulating effect of FPC over the two opposing systems during different states, partial correlation by regressing out the effects of FPC on each pair of regions in the DA and default (one from each) was computed and the results are presented in Figure 5a–c. Comparing with the results obtained using ordinary correlation, it is immediately apparent that the interaction between DA and default becomes significantly less anti‐correlated after the effects of FPC are removed across all three experimental conditions (RS: P = 7.6e‐6; FT: P = 1.5e‐9; MW: P = 0.0053). Moreover, the relative mediation effects of the FPC across the three experimental conditions also exhibit state‐dependent variation (Fig. 5d); it shows that FPC exerts the strongest mediation effect during FT while the mediation effect during resting and movie watching is comparable.
Figure 5.

The mediation effects of FPC on the interaction between DA and default for (a) resting, (b) finger tapping, and (c) movie watching, respectively. (d) Comparison of FPC's mediating role across the three experimental conditions. RS, resting; FT, finger tapping; MW, movie watching.
DISCUSSION
In this study, we hypothesize that FPC will selectively augment/suppress either the DA or the default network depending on if the task is more engaged with the DA or the default network, respectively. Our results show that FT (Fig. 2), exploited to engage the DA network, leads to a significantly higher positive correlation between FPC and DA (P = 0.0095) when compared to RS whereas the interaction between FPC and default becomes significantly more anti‐correlated (P = 0.0025). Conversely, MW used to engage the default network shows that the FPC is significantly more anti‐correlated with DA (P = 2.1e‐6) whereas more positively correlated with default (P = 0.0035) when compared to that during RS (Fig. 3). This change of interaction polarity between FPC to DA and default during the two experimental tasks strongly supports our hypothesis and demonstrates the important regulating roles of FPC exerting on the default and DA networks. We further hypothesize that FPC possesses the initiative regulating role over the DA and default networks. Indeed, Granger causality analysis reveals extensive causal links originated from FPC to both DA and default, strongly supporting the notation that FPC plays a critical role in initiating regulation to the default and the DA networks. Collectively, our results shed great light on the regulating role of FPC to the two opposing/competing systems, default and DA.
Although our results are in strong support of the proposed hypotheses, the basis of choosing the two experimental paradigms to test these hypotheses deserves further discussion. The active involvement of attention in timed behavior has been documented in several previous studies [Gordon et al.,1998; Rao et al.,2001]. Specifically, regions within DA, including both middle temporal area (MT) and intra‐parietal sulcus (IPS), have been reported to increase activity during temporal processing tasks [Rao et al.,2001] and bilateral IPS has been documented to be activated during a sequential finger typing task, similar to the FT task in this study [Gordon et al.,1998]. In contrast, given the internal‐directed nature of the default network, its activity should be suppressed during such exogenous tasks. Indeed, we find that FPC exhibits an increased positive connectivity with the DA while becoming more anti‐correlated with default (Fig. 2b) during FT when compared to RS, strongly supporting our hypothesis. Using exactly the same FT task as that in our study, Kawashima et al. conducted a PET study [Kawashima et al.,1993] and reported increased blood flow in the superior prefrontal areas. On the basis of this finding, they suggested that the superior prefrontal areas may possess a regulatory role during such hand movement, which echo nicely with the findings in our study. More generally, the active involvement of brain regions within the FPC network, including aIPL, dorsal lateral prefrontal cortex (dlPFC), and aPFC during timed behaviors have also been well reported [Harrington et al.,1998; Rao et al.,2001].
The active engagement of the default network in various tasks including affective decision making and mental scene construction based on memory [Andrews‐Hanna et al.] makes it a perfect candidate to subserve the movie‐elicited internal thinking and/or mental scene construction. Indeed, several studies [Golland et al.,2007; Iacoboni et al.,2004] have reported the active involvement of the default network during natural movie watching and proposed that this network might possess an equally important role as the stimulus‐driven sensory processing functions during MW [Golland et al.,2007]. Besides, movie‐elicited memory process might also partly account for the involvement of the default network during MW [Vincent et al.,2006,2008]. As a result, we would expect an opposite temporal correlation pattern between FPC and DA/default during this internally driven process in contrast to that observed during FT. Indeed, completely opposite to that observed during FT, FPC was significantly more positively correlated with default while more anti‐correlated with DA during MW, further supporting the proposed hypothesis. Although direct evidence on the activation of FPC during movie watching are scarce (presumably because of the intrinsic cognitive complexity of the task), Iacoboni et al. [2004] did observe activation of superior frontal regions along with several brain regions in the default network during movie watching, which are consistent with our findings of the active regulatory role of FPC during this state.
The observed selective augmentation or suppression of the FPC to the default and DA networks not only suggests that FPC is capable of co‐activating with the DA network to accomplish attention‐demanding tasks [Koechlin et al.,1999; Kompus et al.,2009; Roberts and Hall,2008], but also shows its flexible role in coping with the default network and suppressing DA for primarily internally directed processes. Our results further imply that the default network, similarly to the task positive networks, is under extensive regulation by the FPC network to switch between activation and deactivation to facilitate the performance of certain tasks. Moreover, the observed selective regulating behavior of FPC appears independent of the absence or presence of global signal regression (Fig. S3) as well as is comparable using a different normalization procedure from the employed Fisher z‐transform (Figs. S4 and S5, details in Supporting Information) further underscoring the consistency of our findings.
Although the changes of the temporal correlation patterns of FPC with the default and DA networks during FT and MW strongly suggest that the FPC regulates the two opposing networks, temporal correlation, however, does not provide information on the causality of the observed regulation. In other words, an alternative explanation to our findings could be that the DA/default selectively recruits the FPC depending on the nature of the tasks. To resolve this potential uncertainty, Granger causality analysis [Granger,1969] was conducted. Results reveal extensive forward influences initiated from FPC to both the default and DA across all three experimental states (Fig. 4), supporting the regulatory role of the FPC over these two networks. In addition, the forward influences from FPC to DA/DF are considerably more than the feedbacks from these two networks during resting and finger tapping, suggesting the dominant regulating role of FPC over these two networks during these two rather simple states. In contrast, the forward and feedbacks influences are more balanced between FPC and DA/DF during the more “internally complex” movie watching task, suggesting more interactive information changing among these three networks (both forward and feedback).
One potential limitation of the Granger causality analysis lies in the relatively poor temporal resolution (TR = 2 s), which might impede the detection of potential causal links occurring at a shorter time scale. Although it is possible to improve the temporal resolution, the spatial coverage will be compromised, making it difficult to encompass all five networks of interest in our study. However, one recent study by Deshpande et al. [Deshpande et al.] has systematically evaluated the effects of TR [among other factors including the neuronal delays, the hemodynamic delays, signal to noise ratio (SNR), etc.] on the delectability of causal influence using fMRI data. They found that even with TR = 2 s Granger Causality analysis can detect neuronal delays ranging from tens to hundreds of milliseconds depending on different degrees of hemodynamic response function (HRF) confounds and SNR. Their findings greatly support the reliability of the detected causal patterns in this study. In fact, several recently published studies focusing on Granger causality also employed a similar TR and physiologically sensible results have been reported, including Deshpande et al. (TR = 2,000 ms) [Deshpande et al.,2008]; Sridharan et al. (TR = 2,000 ms) [Sridharan et al.,2008]; Stevens et al. (TR = 1,500 ms) [Stevens et al.,2009]; and Krueger et al. (TR = 2,000 ms) [Krueger et al.].
Although our results thus far have strongly support the notion that the FPC regulates both the default and DA, partial correlation analysis further quantifies this regulating effect as shown in Figure 5. Although the disrupted anti‐correlation between DA‐DF is somewhat expected after regressing out of the effect of FPC, the differences of the relative regulating strength across the three states are interesting. The FPC exerts a stronger regulating role during finger tapping than movie watching. One possible explanation is that FPC up‐regulates DA during finger tapping more than it up‐regulates DF during movie watching, which can be qualitatively validated from Figures 2 and 3. If this is indeed the main reason, there might be other tasks in which FPC up‐regulates DF more than it up‐regulates DA during finger tapping. However, this is beyond the scope of this article and deserves further investigation.
Finally, at the system level, the intriguing selective regulation pattern reported in this study seems to suggest that the three networks (FPC, DA, and default) form a functional “triad” with FPC at the apex regulating the two branches—DA and default. In addition, the increased DA‐MS and DA‐V (not significant, P = 0.0570) interaction during FT and MW tasks, respectively, may suggest that the motor control and sensory processing networks are hierarchically underneath the DA. This structure is essentially consistent with the top‐down control theory [Buschman and Miller,2007] during volitional shifts of attention contingent on current task requirements. For the other branch, the default network seems to work alone based on our study. Nevertheless, one potential network that may be hierarchically underneath the default network is the emotional network. Evidence to support this hypothesis can be found from several previous studies showing an emotional processing component within the major nodes of the default network [Andrews‐Hanna et al.; Gusnard et al.,2001; Pallesen et al.,2009]. Furthermore, Andrews‐Hanna et al. [Andrews‐Hanna et al.] have recently explicitly showed that the “middle core” of the default network is active during affective decision making. Further studies using specially designed paradigms are needed to directly investigate this issue. Finally, in contrast to the observed alterations of network interaction patterns during different brain states, no significant within‐network changes are observed across different experimental paradigms employed in our study. This finding raises an important fact. That is, in addition to identify regional brain activation, special attention should also be devoted to specifically determine between network interactions.
In conclusion, by applying two simple but contrasting tasks, self‐paced/attended (1 Hz) sequential finger tapping (FT) and natural movie watching (MW), we have demonstrated the selective regulating role of FPC to the two opposing systems, dorsal attention (DA) and default (DF). Specifically, through both ordinary and effective (Granger Causality) connectivity analysis, we have shown that FPC up‐regulates DA/DF and down‐regulates DF/DA during FT/MW, respectively. Moreover, partial correlation analysis detects state‐dependence of this regulation effect. Overall, our findings strongly support the notion that the FPC tightly regulates the opposing DA and default networks for successful fulfillment of specific tasks and/or maintenance of certain behavioral states.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
REFERENCES
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL: Functional anatomic fractionation of the Brain's default network. Neuron 65: 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y ( 2001): The control of the false discovery rate in multilpe testing under dependency. Ann Stat 29: 1165–1188. [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS ( 1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL ( 2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Vincent JL ( 2007): Unrest at rest: Default activity and spontaneous network correlations. Neuroimage 37: 1091–1096; discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK ( 2007): Top‐down versus bottom‐up control of attention in the prefrontal and posterior parietal cortices. Science 315: 1860–1862. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH ( 2009): Effects of model‐based physiological noise correction on default mode network anti‐correlations and correlations. Neuroimage 47: 1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W ( 2007): The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage 37: 343–360. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA ( 2006): Neural evidence for dissociable components of task‐switching. Cereb Cortex 16: 475–486. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Laconte S, James GA, Peltier S, Hu X ( 2008): Multivariate Granger causality analysis of fMRI data. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Sathian K, Hu X: Effect of hemodynamic variability on Granger causality analysis of fMRI. Neuroimage 52: 884–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Chen Y, Bressler SL, editors ( 2006): Granger Causality: Basic Theory and Application to Neuroscience. Wienheim: Wiley. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME ( 2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME ( 2006): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103: 10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME ( 2009): The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101: 3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman O, Dorfman N, Hasson U, Davachi L, Dudai Y ( 2007): They saw a movie: Long‐term memory for an extended audiovisual narrative. Learn Mem 14: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golland Y, Bentin S, Gelbard H, Benjamini Y, Heller R, Nir Y, Hasson U, Malach R ( 2007): Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex 17: 766–777. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Lee JH, Flament D, Ugurbil K, Ebner TJ ( 1998): Functional magnetic resonance imaging of motor, sensory, and posterior parietal cortical areas during performance of sequential typing movements. Exp Brain Res 121: 153–166. [DOI] [PubMed] [Google Scholar]
- Granger C ( 1969): Investigating causal relations by econometric models and cross‐spectral methods. Econometrica 37: 424–438. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V ( 2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME ( 2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC ( 2002): Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp 15: 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY, Knight RT ( 1998): Cortical networks underlying mechanisms of time perception. J Neurosci 18: 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, Knowlton BJ, Molnar‐Szakacs I, Moritz M, Throop CJ, Fiske AP ( 2004): Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage 21: 1167–1173. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Yamada K, Kinomura S, Yamaguchi T, Matsui H, Yoshioka S, Fukuda H ( 1993): Regional cerebral blood flow changes of cortical motor areas and prefrontal areas in humans related to ipsilateral and contralateral hand movement. Brain Res 623: 33–40. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP ( 2008): Competition between functional brain networks mediates behavioral variability. Neuroimage 39: 527–537. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J ( 1999): The role of the anterior prefrontal cortex in human cognition. Nature 399: 148–151. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A ( 2007): Anterior prefrontal function and the limits of human decision‐making. Science 318: 594–598. [DOI] [PubMed] [Google Scholar]
- Kompus K, Hugdahl K, Ohman A, Marklund P, Nyberg L ( 2009): Distinct control networks for cognition and emotion in the prefrontal cortex. Neurosci Lett 467: 76–80. [DOI] [PubMed] [Google Scholar]
- Krueger F, Landgraf S, van der Meer E, Deshpande G, Hu X: Effective connectivity of the multiplication network: A functional MRI and multivariate granger causality mapping study. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA ( 1998): Functional connectivity in single and multislice echoplanar imaging using resting‐state fluctuations. Neuroimage 7: 119–132. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN ( 2007): Wandering minds: The default network and stimulus‐independent thought. Science 315: 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA ( 2009): The impact of global signal regression on resting state correlations: Are anti‐correlated networks introduced? Neuroimage 44: 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallesen KJ, Brattico E, Bailey CJ, Korvenoja A, Gjedde A ( 2009): Cognitive and emotional modulation of brain default operation. J Cogn Neurosci 21: 1065–1080. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK ( 2007): Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol 58: 1–23. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM ( 2004): Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nat Rev Neurosci 5: 184–194. [DOI] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL ( 2001): The evolution of brain activation during temporal processing. Nat Neurosci 4: 317–323. [DOI] [PubMed] [Google Scholar]
- Roberts KL, Hall DA ( 2008): Examining a supramodal network for conflict processing: A systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. J Cogn Neurosci 20: 1063–1078. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW ( 2009): A new method for improving functional‐to‐structural MRI alignment using local Pearson correlation. Neuroimage 44: 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir A, d'Avossa G, McAvoy M, Shulman GL, Corbetta M ( 2005): Brain signals for spatial attention predict performance in a motion discrimination task. Proc Natl Acad Sci USA 102: 17810–17815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK: A MATLAB toolbox for Granger causal connectivity analysis. J Neurosci Methods 186: 262–273. [DOI] [PubMed] [Google Scholar]
- Shen D, Davatzikos C ( 2002): HAMMER: Hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging 21: 1421–1439. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Sadato N, Lyshkow H, Yonekura Y, Honda M, Nagamine T, Suwazono S, Magata Y, Ikeda A, Miyazaki M ( 1993): Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain 116 ( Part 6): 1387–1398. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V ( 2008): A critical role for the right fronto‐insular cortex in switching between central‐executive and default‐mode networks. Proc Natl Acad Sci USA 105: 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD ( 2009): Changes in the interaction of resting‐state neural networks from adolescence to adulthood. Hum Brain Mapp 30: 2356–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M ( 2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL: Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol 103: 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL ( 2006): Coherent spontaneous activity identifies a hippocampal‐parietal memory network. J Neurophysiol 96: 3517–3531. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL ( 2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100: 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


