Abstract
Objective
Physical activity may confer protective effects in terms of the development of anxiety and its disorders. These effects may be particularly strong among individuals who have elevated levels of anxiety sensitivity (i.e., the fear of somatic arousal; AS), an established cognitive-based risk factor for anxiety and its disorders. The present study performed a laboratory test of the interplay between physical activity and anxiety sensitivity.
Methods
Participants were adults free of Axis I psychopathology (N = 145) who completed measures of physical activity and AS prior to undergoing a recurrent 20% carbon dioxide-enriched air (CO2) challenge.
Results
Consistent with hypothesis, physical activity was significantly related to CO2 challenge reactivity among persons with elevated levels of AS, at high levels of physical activity (p < .001) but not at low levels of physical activity (p = .90). Also consistent with hypothesis, irrespective of the level of physical activity, physical activity did not relate significantly to CO2 challenge reactivity among persons with normative levels of AS (p = .28).
Conclusions
These findings provide novel empirical insight into the role that physical activity may play in terms of resiliency for the development of anxiety disorders. Specifically, the protective effects of physical activity may only be evident at higher doses and among persons who are at increased risk of developing anxiety disorders because they have elevated anxiety sensitivity.
Keywords: physical activity, exercise, anxiety, anxiety sensitivity, biological challenge
INTRODUCTION
Growing evidence suggests that regular physical activity is a possible protective factor in terms of the development of anxiety and its disorders. For example, Goodwin (1) observed that regular physical activity predicted lower prevalence of panic attacks (OR = 0.73), social phobia (OR = 0.65), specific phobia (OR = 0.78), and agoraphobia (OR = 0.64) among a representative adult sample in the United States. These effects were evident after controlling for demographic variables and co-occurring physical and mental health conditions. In this same study, there also was evidence for a dose-response relationship between physical activity and mental health problems, such that the lowest prevalence rates of anxiety disorders were observed among persons who exercised regularly, followed by those who exercised occasionally, rarely and never, respectively (1). A recent prospective study has further found that physical activity is associated with a reduced incidence of anxiety disorders (2). Specifically, using a representative sample (N = 2,548) of adolescent and young adults (ages 14–24) from Munich, Germany, Ströhle and colleagues found that the incidence of any anxiety disorder during a 4-year follow-up was significantly lower among persons who reported engaging in regular physical activity versus those who reported no physical activity (OR = 0.52).
Extant work on the role of physical activity in the development of anxiety and its disorders has primarily focused on establishing the general overall effects of physical activity (1, 2). With any observed main effects, there is evidence of individual variability, which prompts research on identifying moderators. Determining whether the putative protective effects of physical activity with respect to anxiety vulnerability are equally applicable to all persons, or whether these effects are relevant to only a subset of the population has important implications for prevention. Accordingly, there is a need to empirically explore the interplay between physical activity and established risk factors for anxiety disorders.
Perhaps one of the most well-known cognitive-based risk factors for anxiety disorders is anxiety sensitivity (AS) (3). AS is defined as the extent to which individuals believe that anxiety and anxiety-related bodily sensations (i.e., somatic arousal) have harmful consequences (4, 5). AS is distinct from the temperament variables of trait anxiety (6) and negative affectivity (7); it reflects beliefs about internal sensations as opposed to frequency of (negative) mood symptoms. Laboratory (8–11) and prospective (12–16) studies consistently indicate that AS increases the risk for more intense anxiety symptoms and anxiety psychopathology.
Building upon previous work (1, 2), the present study sought to perform a test of the potential interplay between regular physical activity and AS with respect to anxiety vulnerability. We theorized that physical activity would interact with AS to significantly decrease vulnerability in anxiety-based emotional reactivity to bodily perturbation. This hypothesis was guided by extant work suggesting that the apparent protective effects of regular physical activity on the development of anxiety and its disorders may be centered upon reduced reactivity to anxiety-provoking stimuli (17). Indeed, a series of recent studies have shown that an acute bout of exercise reduces fearful responding to panicogenic agents such as carbon dioxide-enriched air (CO2) (18, 19) and cholecystokinin tetrapeptide (20, 21). More importantly, results from both animal and human studies converge to indicate that regular physical activity reduces physiological and psychological reactivity to psychological stressors (17, 22–26). Accordingly, because it is associated with reduced reactivity to stressors, regular physical activity may buffer the effects of bodily perturbation on anxiety for persons who are prone to respond to such stressors with anxiety (i.e., persons with elevated levels of AS) but not for those who do not tend to respond to such stressors with anxiety (i.e., persons with non-elevated levels AS). In addition, there is some work to suggest that the buffering effects of PA on responses to bodily perturbation among individuals with elevated AS may vary across levels of physical activity. Indeed, Dishman and colleagues (27) reported that substantial reductions in trait anxiety become only evident when persons reach a certain threshold of physical activity (i.e., four to five months of exercise training). This observation coupled with the finding that reduced stress reactivity has been linked to regular physical activity may suggest that the PA-buffering effects for individuals with elevated AS may only be evident among individuals who engage in physical activity frequently.
We employed a CO2 challenge laboratory paradigm for testing the present research questions. Inhalation of CO2-enrichedair is an ideal paradigm for the current test as it induces the somatic arousal characteristic of panic attacks (27) and CO2 challenge reactivity has been found to significantly prospectively predict the onset of spontaneous panic attacks in the future (28, 29). In the current study, participants were young adults free of both Axis-I psychopathology and history of panic attacks who reported various levels of regular physical activity. Nonclinical healthy persons were sampled in order to rule out the possibility that any observed differences would be attributable to pre-existing psychological or health problems (30). We hypothesized that CO2 challenge reactivity would vary as a function of the interaction between physical activity and AS. Specifically, we expected no relation between physical activity and CO2 challenge reactivity among individuals with low or normative levels of AS. Further, we expected that, among individuals with elevated levels of AS, the relationship between physical activity and CO2 challenge reactivity would be significant but discontinuous, such that greater physical activity would be related to lower CO2 reactivity only for physical activity levels above a certain threshold.
METHODS
Participants
Participants were 145 adults (83 females) between the ages of 18 and 59 (Mage = 21.6 years; SD = 7.43) from the University of Vermont and the greater Burlington, Vermont community. Individuals were recruited through newspaper and other local advertisements posted in university and non-university settings. The racial distribution of the sample generally reflected that of the local population (31): 93.1% (n = 135) white; 2.8% (n = 4) African American; 1.4% (n = 2) Hispanic; 1.4% (n = 2) Asian; 0.7% (n = 1) “other;” and 0.7% (n = 1) did not specify race. The marital status for the vast majority of the sample was single (n = 141; 97.2%), and on average, participants had completed 13.2 years of education (SD = 1.80).
Participants were excluded for a history of Axis I psychiatric disorders, including nonclinical panic attacks, based upon their responses to the Structured Clinical Interview for DSM-IV, non-patient version (SCID-NP) (32). Inter-rater reliability for the SCID-NP for axis I diagnoses and sub-clinical panic attack history has been high in our group (33). Participants also were excluded from participation if they reported (a) current suicidal or homicidal ideation, (b) current use of psychotropic medication, (c) pregnancy, (d) current serious medical conditions (e.g., cancer), (e) serious breathing difficulties or respiratory-based illness (e.g., asthma, emphysema), or (f) limited mental competency or inability to give informed, written consent. As in past work (33), these additional exclusionary criteria were assessed within the context of the structured interview as an additional supplemental set of (standardized) interview-based medical screening questions. Finally, we excluded from our investigation participants who reported no physical activity. This exclusion criterion was used for three reasons. First, the present study was an investigation of the influence of the amount of physical activity and not the occurrence of physical activity. Second, those who never engage in physical activity tend to differ from those who do on numerous psychological and or physical health dimensions (27), which would make the interpretation of effects of physical activity difficult. Third, from a statistical perspective, calculating unbiased estimates of causal effects requires that the distributions of the effects must be completely overlapping (34). It is unlikely that the distributions of the effects of persons who report no physical activity and those who do engage in regular physical activity will be completely overlapping.
Procedures
A detailed description of the procedures has been provided elsewhere (35).1 In short, interested participants responding to community-based advertisements for a laboratory study of anxiety were screened by a trained research assistant using the SCID-NP and scheduled by phone for an individual laboratory appointment. Eligible participants completed a self-report questionnaire packet before beginning the laboratory component of the study. Participants were instructed not to consume caffeine or engage in strenuous physical activity for 3 hours prior to their scheduled laboratory visit. During the laboratory component of the study, participants sat at a desk supporting a computer, which was programmed to administer the CO2 administrations. After completing physiological hookup and listening to experimental instructions, participants were fitted with a positive-pressure C-pap mask. The experimenter observed participants from an adjacent control room containing a computer-operated apparatus designed to automatically provide participants with either room air or a mixture of 20% CO2 - enriched room air. The apparatus (36) assured that all participants received 6 CO2 inhalation trials and 18 room-air inhalation trials each lasting 90 seconds. Furthermore, all participants received the same trial order with CO2 administrations occurring on trials 3, 6, 9, 14, 19, and 22. Lastly, because the original study (35) examined whether CO2 challenge reactivity would vary as a function of the predictability of the biological stressor, each inhalation trial began with an instruction informing the participant of the trial type (room air or 20% CO2).2 Thus, participants received a total of 24 trials, of which 6 were CO2 inhalations (3 predictable and 3 unpredictable) and 18 were room air inhalations (9 predictable and 9 unpredictable). After the laboratory component of the study, participants were debriefed and paid $30 for their participation. All procedures were approved by the University of Vermont Institutional Review Board. Data were collected between September 2003 and August 2008.
Measures
Physical activity
The Exercise Health Survey (EHS) (37) is a self-report measure that includes questions pertaining to the weekly amount of time spent in physical activity and is comparable to that employed in previous work documenting physical activity-anxiety relations (1, 2, 38) as well as measures of physical activity used in large-scale epidemiological studies relating regular physical activity to morbidity and mortality (39–41). Specifically, the measure lists a variety of types of moderate- and vigorous-intensity activities and asks participants how many days per week on average they engage in these or other activities, and the duration of their exercise sessions per occasion. Using this information, we calculated the average minutes per week of physical activity.
Anxiety sensitivity
The Anxiety Sensitivity Index (ASI) is a 16-item questionnaire on which respondents indicate on a 5-point Likert-type scale (0 = very little to 4 = very much) the degree to which they fear anxiety symptoms and their negative consequences (42). The ASI is widely used and has demonstrated good psychometric properties (43). A large body of work suggests that AS is an important cognitive-based predictor of emotional response to biological challenge (8, 11, 44) and AS has been shown to affect estimations of cardiovascular fitness obtained by cycle ergometer testing (45). In the present investigation, we utilized the total ASI score, as it represents the global-order AS factor, and therefore takes into consideration different types of fears, including fears of anxiety-related somatic, cognitive, and social cues.
Challenge measures
Participants were asked to provide ratings of their current level of anxiety at baseline and at the end of each inhalation trial using an 11-point Likert-type rating scale, similar to those used in past work (e.g. Subjective Units of Distress Scale; SUDS) (46), ranging from ‘0’ (no anxiety) to ‘10’ (extreme anxiety). Specifically, participants provided a SUDS rating at the end of each of the 24 trials (regardless of whether or not CO2 was delivered on that trial).
DATA ANALYSIS
In order to test whether AS moderated the effect of physical activity on CO2 challenge reactivity, we employed a multi-level, within-subjects, mixed effects regression analysis (MLM) using the program Hierarchical Linear and Nonlinear Modeling version 6.06 (HLM) (47). MLM was selected because of its flexibility in modeling complex relationships, its lack of restrictive assumptions, and its ability to include all of the observations of all subjects regardless of missing data. In addition, HLM 6.06 provides significance tests using “robust” standard errors, which are robust to violations of multivariate normality. The Level 1 portion of the MLM analysis modeled outcomes (SUDS) from each inhalation trial as a function of inhalation type (room air = 0; CO2 = 1). Additionally, since previous findings with this sample (35) indicated that predictability was a significant determinant of SUDS, we included predictability (predictable = 0; unpredictable = 1) as a covariate. As such, the Level 1 portion of the MLM model was:
Given this coding of the Level 1 predictors, β0j represented average SUDS ratings for the predictable room air trials, β1j represented the mean difference between SUDS reported for CO2 versus room air inhalations (herein referred to as “CO2 challenge reactivity”), and β2j is the mean difference between the predictable and unpredictable trials.
The Level 2 portion of the MLM model allowed us to examine whether individual characteristics, such as physical activity and AS, predicted differences in mean SUDS ratings for the room air trials (β0j) and for CO2 challenge reactivity (β1j). As noted above, we included “predictability” in the Level 1 model because predictability was significantly associated with SUDS for room air trials (35). Since we had no hypotheses regarding the effect of predictability on CO2 challenge reactivity, we included it in the model to account for variance related to predictability, but did not include any Level 2 predictors for it.
Level 2 predictors of β0j and β1j included physical activity (PA) and AS. In order to test for discontinuous relations between PA and CO2 challenge reactivity, we followed guidelines put forth by Singer and Willett (48). Specifically, we added an additional term to allow the relation between PA and CO2 challenge reactivity to change at 1 SD above the mean of PA (the “threshold”). We selected this threshold of 1 SD above the mean following common practice when investigating interactions (49). The PA “slope difference” term was coded 0 for PA below threshold, and coded with the amount by which the participant’s PA exceeded the threshold for those with PA levels >1 SD above the mean (i.e., if PA was 1.55, the slope difference term would be coded 1.55–1 = 0.55) (48). Because we expected a “slope difference” only for those with high AS, we included a term for the interaction between AS and the slope difference (i.e., AS × PA Slope Difference). Although we did not expect that AS would moderate the relation between PA and CO2 challenge reactivity among individuals below the PA threshold, we included the AS × PA interaction in our initial model to examine this possibility. Further, we included gender (0 = male; 1 =female) as a covariate because females tend to report both higher AS (43) and lower levels of PA than men (50). Finally, because the age of our participants varied, we included age (log transformed to reduce skewness) as an additional covariate in the model. All predictors were converted to z-scores to enhance interpretation of the findings. Accordingly, the Level 2 equations were:
β0j (mean SUDS, predictable air trials) = γ00+γ01*(PA)+γ02*(AS)+ γ03*(AS × PA)+ γ04*(PA Slope Difference Term)+γ05*(AS × PA Slope Difference Term)+γ06*(gender)+ γ07*(ln(age))+ r0j
β1j (CO2 challenge reactivity) = γ10+γ11*(PA)+γ12*(AS)+γ13*(AS × PA)+ γ14*(PA Slope Difference Term)+γ15*(AS × PA Slope Difference Term)+γ16*(gender)+ γ17*(ln(age))+r1j
β2j (predictability) = γ20+r2j
RESULTS
Preliminary Analysis
On average participants were relatively active (MPA = 148.28, SD = 77.98) and had AS levels similar to those observed in other community samples (MAS = 15.88, SD = 9.44) (3). As expected, women reported higher AS levels than men (17.42 vs. 15.88; F [1, 141] = 5.43, p < .05) and men reported more weekly minutes of PA than women (169.28 vs. 132.60; F [1, 143] = 8.25, p < .01).
Hypothesis Testing
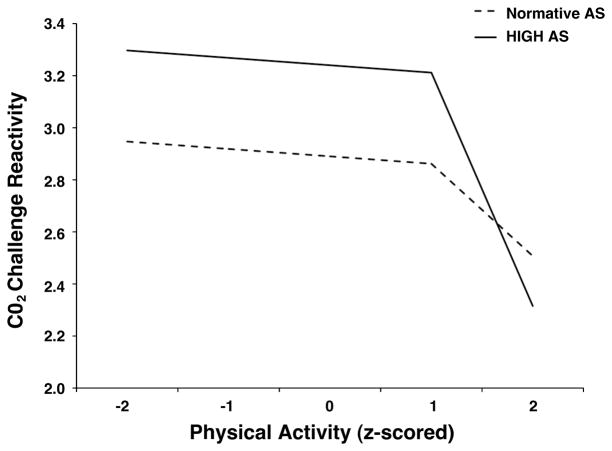
Since our hypotheses centered on CO2 challenge reactivity, we will focus on the results of the second set of Level 2 equations (i.e., predictors of β1j). Initial analyses indicated that neither gender nor the interaction of AS and PA for physical activity below the “threshold” were significant (p = .59 and p = .65, respectively), so they were dropped from the model and the analysis was recomputed. In this final model, age was marginally related to CO2 challenge reactivity (b = −0.38, SEb = .21, t[125] = −1.77, p = .08); we retained this term in the model in order to be conservative. As predicted, CO2 challenge reactivity varied as a function of AS (b = 0.35, SEb = .17, t[125] = 2.05, p < .05) but not as a function of PA (b = −0.03, SEb = .23, t[125] = −0.12, p = .90) among persons whose PA was below the threshold (see Figure 1). Further, consistent with hypothesis, there was a significant interaction between AS and the PA slope difference (b = −0.54, SEb = .24, t[125] = 2.27, p < .05), indicating that the relation between PA and CO2 challenge reactivity changed at 1SD above the mean of PA, and that change depended on the level of AS. Following the guidelines put forth by Aiken and West (49), we examined the change in the relation between PA and CO2 challenge reactivity for participants with high and normative levels of AS. We used a score of 1 SD above the mean AS score (25.32) to represent high levels of AS (51), and used the mean AS score in the sample (15.88) to represent normative levels (51). The results are presented in Figure 1. Consistent with hypothesis, among individuals with high levels of AS, there was a significant discontinuity in the relation between PA and CO2 challenge reactivity when PA exceeded 1SD above the mean (bΔ = −0.87, SEb = .35, t[125] = 2.47, p = .01). Specifically, below the threshold, there was no relation between PA and CO2 challenge reactivity (b = −0.03, SEb = .23, t[125] = −0.12, p = .90), but above the 1 SD threshold, the relation was significant (b = −0.90, SEb = .21, t[125] = 4.25, p < .001; see Figure 1). Also consistent with hypothesis, among individuals with normative levels of AS, there was no discontinuity in the relation between PA and CO2 challenge reactivity (bΔ = −0.34, SEb = .34, t[125] = 1.08, p = .28). Specifically, the relation between PA and CO2 challenge reactivity was not significant either below (b = −0.03, SEb = .23, t[125] = −0.12, p = .90) or above the threshold (b = −0.35, SEb = .21, t[125] = 1.70, p = .09; see Figure 1). The proportion of the total between-subjects variance of CO2 challenge reactivity accounted for by this model was 9.3%.
Figure 1.
CO2 challenge reactivity as a function of the interaction between physical activity and anxiety sensitivity.
In supplementary analyses, we investigated other lower potential “thresholds” (as low as the average level of PA). The results indicated that, as we lowered the threshold, the relation between PA and CO2 challenge reactivity remained significant among individuals with high AS. However, the strength of this relationship generally decreased in magnitude as the threshold decreased. Thus, in this dataset, we could not identify a clear cut-off, over which there was a definite relation between PA and CO2 challenge reactivity and under which such a relation did not exist. Therefore, we can only conclude that PA is related to reduced CO2 challenge reactivity for high AS individuals when physical activity levels are high. Future research should examine whether this relation abruptly changes from non-significant to significant at a certain threshold.
Lastly, we examined the possibility that the moderating effects of AS were accounted for by one of the ASI subscales (ASI-physical, ASI-social, or ASI-mental) (43). Specifically, we reran the analysis three times, each time substituting the one of the ASI subscale scores for the ASI total score. The results of each of these three models mirrored those of the model with the ASI total score (i.e., significant and non-significant terms remained significant and non-significant, respectively). These findings suggest that the relation between AS and PA does not appear to be specific to, or accounted for, by any particular ASI subscale.
DISCUSSION
The present study sought to examine the hypothesis that AS would moderate the relation between physical activity and emotional reactivity to bodily perturbation. We found evidence consistent with this hypothesis. Specifically, we did not observe a significant effect of physical activity on CO2 challenge reactivity for individuals with non-elevated levels of AS. However, consistent with hypothesis, for individuals with elevated levels of AS, higher levels of physical activity were related to significantly lower CO2 challenge reactivity, but only among those who reported high levels of physical activity.
The finding that AS moderates the relation between regular physical activity and CO2 challenge reactivity suggests that the apparent protective effects of regular physical activity with respect to anxiety vulnerability (1, 2) may be particularly applicable to persons who have elevated AS. The interplay between AS and physical activity observed in the present study also provides novel empirical insight into the potential effect of regular physical activity on modulating risk conferred by AS in terms of the development of anxiety disorders. Indeed, our results indicate that, for people with high levels of physical activity, those with high levels of AS show no greater CO2 challenge reactivity than those with normal levels of AS. Accordingly, since CO2 challenge reactivity is a predictor of future panic attacks (28, 29), these findings suggest that high levels of physical activity may reduce the risk of panic attacks and other anxiety related problems among those with high levels of AS. Future studies should examine whether regular physical activity indeed reduces the risk for panic attacks among persons high in AS using prospective designs and time sampling approaches (e.g., ecological momentary assessment protocols).
The observation that physical activity interacts with AS to predict CO2 challenge reactivity also may help explain the relative predictive power of AS in any given study. That is, because the effects of AS on fear reactivity to CO2 challenge can vary from small to large (4, 52), it is possible that physical activity may serve as a qualifing, but often unrecognized, factor in understanding such variability. These data therefore highlight the potential importance of documenting physical activity and understanding its contribution to the expression of anxious and fearful responding to bodily sensations.
Although the present data provide evidence that high levels of physical activity were related to significantly lower CO2 challenge reactivity among persons with elevated levels of AS, but not among individuals with normative levels of AS, the exact mechanisms underlying such effects remain unclear. There are a number of non-mutually exclusive possibilities that may warrant further scientific attention. One possibility is that higher levels of physical activity may faciliate corrective fear learning for those high in AS. It also may be possible that high levels of physical activity promotes more adaptive regulation of affect and thereby modulates fear responsivity. A final possibility is that high levels of physical activity for those high in AS may alter brain circuits that mediate fear responsivity. Clearly, elucidating the mechanisms linking risk and protective factors to clinical conditions will facilitate theoretical refinement of models of disorder development and aid in tailoring preventative work on specific conditions.
If replicated and extended, the present laboratory results, in conjunction with earlier field-based work (1, 2), provides guidance for anxiety disorder prevention program development. Initial work in this area has focused on the evaluation of cognitive-behavioral programs designed to target AS (53–55). Numerous scholars have suggested that such prevention programs may benefit from the inclusion of health-oriented tactics to modify AS and related anxiety risk processes (56, 57). Physical activity promotion may represent one such tactic. The present study findings indicate that adding a physical activity component to cognitive (e.g., cognitive restructuring) and behavioral (e.g., interoceptive exposure) strategies may offer a more powerful means to reduce risk for anxiety disorder development. Physical activity programs may also be useful as stand-alone interventions for reducing the deleterious effects of AS. Physical activity programs (either alone or in tandem with other interventions) may be especially efficacious since exercise has also been shown to reduce AS levels (58–60). Thus, incorporating physical activity into prevention programs targeting AS may be particularly potent because it buffers some of the negative consequences of AS (hyper-reactivity), as demonstrated in the present study, and it can reduce overall levels of AS. Lastly, because physical activity also has established physical health benefits, it would likely bolster global-based improvement in multiple domains of life functioning.
The present study has several limitations, some of which provide suggestions for future research. First, the sample was limited in that it was composed primarily of a relatively homogenous group of young adults. To increase the generalizability of these findings, it will be important for researchers to draw from populations other than those used in the present study. Second, although the present investigation examined the interplay between regular physical activity and AS, AS represents only one exemplar risk factor for anxiety psychopathology. Thus, exploration of the role of physical activity in mitigating the effects of other risk factors for anxiety disorders may inform the relative degree of specificity of the observed findings from a vulnerability-resiliance perspective. Finally, the present study was focused on a laboratory model of fear responding. Laboratory findings may not fully generalize to naturally occurring fear behavior (61). Similarly, it is not possible to rule out spuriousness in these types of investigations. Physical activity and symptoms of anxiety and depression may be influenced by common factors (62). Accordingly, scientific attention should be given to the potential influence of other risk factors on the observed relationships, including genetic risk factors (63).
Overall, the present investigation adds uniquely to the extant empirical literature on the role of physical activity in modifying risk for anxious and fearful responding to bodily sensations. Results suggested that those with elevated levels of AS and high levels of physical activity showed significantly reduced CO2 challenge reactivity relative to those with elevated levels of AS and low levels of physical activity. These laboratory findings highlight the potential promise of physical activity as a protective factor for the expression of fear reactivity to somatic peturbation.
Acknowledgments
Dr. Smits is supported by NIH grants R01MH075889 and R01DA027533 and has received royalties from Oxford University Press. Dr. Zvolensky is supported by NIH grants R01DA027533 and R01MH076629. All authors declare that they have no conflicts of interest.
Abbreviations
- OR
Odds Ratio
- AS
Anxiety Sensitivity
- CO2
Carbon Dioxide
- SCID-NP
Structured Clinical Interview for DSM-IV-Non Patient Edition
- EHS
Exercise Health Survey
- ASI
Anxiety Sensitivity Index
- SUDS
Subjective Units of Distress
- MLM
Multi-Level Mixed Effects Regression Analysis
- HLM
Hierarchical Linear and Nonlinear Modeling
- PA
Physical Activity
Footnotes
This study is based on an investigation from which other results have previously been reported (35). None of the predictors of outcome in the present study were investigated in the prior report.
For unpredictable trials, the instruction “Unpredictable Trial: You will NOT be told whether or not you will receive CO2 on this trial” appeared on the computer screen. For predictable trials, either the instruction “Predictable Trial: You will receive CO2 on this trial” or “Predictable Trial: You will not receive CO2 on this trial” appeared on the screen, depending on whether or not it would be a CO2 trial. Participants were never misinformed. As reported by Yartz and colleagues (35), results indicated that equivalent levels of anxiety were experienced during predictable and unpredictable administrations of 20% carbon dioxide-enriched air. However, since predictability was significantly associated with anxiety for room air trials, this variable was included as a covariate in the present analysis.
References
- 1.Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med. 2003;36:698–703. doi: 10.1016/s0091-7435(03)00042-2. [DOI] [PubMed] [Google Scholar]
- 2.Strohle A, Hofler M, Pfister H, Muller AG, Hoyer J, Wittchen HU, Lieb R. Physical activity and prevalence and incidence of mental disorders in adolescents and young adults. Psychol Med. 2007;37:1657–66. doi: 10.1017/S003329170700089X. [DOI] [PubMed] [Google Scholar]
- 3.Taylor S, editor. Anxiety Sensitivity: Theory, Research, and Treatment of the Fear of Anxiety. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. [Google Scholar]
- 4.McNally RJ. Anxiety sensitivity and panic disorder. Biol Psychiatry. 2002;52:938–46. doi: 10.1016/s0006-3223(02)01475-0. [DOI] [PubMed] [Google Scholar]
- 5.Reiss S, McNally RJ. Expectancy model of fear. In: Reiss S, Bootzin RR, editors. Theoretical Issues in Behavior Therapy. Orlando, FL: Academic Press, Inc; 1985. [Google Scholar]
- 6.McNally RJ. Anxiety sensitivity is distinguishable from trait anxiety. In: Rapee RM, editor. Current controversies in the anxiety disorders. New York, NY: Guilford Press; 1996. pp. 214–27. [Google Scholar]
- 7.Zvolensky MJ, Kotov R, Antipova AV, Schmidt NB. Diathesis stress model for panic-related distress: a test in a Russian epidemiological sample. Behav Res Ther. 2005;43:521–32. doi: 10.1016/j.brat.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Brown M, Smits JA, Powers MB, Telch MJ. Differential sensitivity of the three ASI factors in predicting panic disorder patients’ subjective and behavioral response to hyperventilation challenge. J Anxiety Disord. 2003;17:583–91. doi: 10.1016/s0887-6185(02)00231-1. [DOI] [PubMed] [Google Scholar]
- 9.Carter MM, Suchday S, Gore KL. The utility of the ASI factors in predicting response to voluntary hyperventilation among nonclinical participants. J Anxiety Disord. 2001;15:217–30. doi: 10.1016/s0887-6185(01)00061-5. [DOI] [PubMed] [Google Scholar]
- 10.Rapee RM, Medoro L. Fear of physical sensations and trait anxiety as mediators of the response to hyperventilation in nonclinical subjects. J Abnorm Psychol. 1994;103:693–9. doi: 10.1037//0021-843x.103.4.693. [DOI] [PubMed] [Google Scholar]
- 11.Zvolensky MJ, Feldner MT, Eifert GH, Stewart SH. Evaluating differential predictions of emotional reactivity during repeated 20% carbon dioxide-enriched air challenge. Cogn Emot. 2001;15:767–86. [Google Scholar]
- 12.Li W, Zinbarg RE. Anxiety sensitivity and panic attacks: a 1-year longitudinal study. Behav Modif. 2007;31:145–61. doi: 10.1177/0145445506296969. [DOI] [PubMed] [Google Scholar]
- 13.Mailer RG, Reiss S. Anxiety sensitivity in 1984 and panic attacks in 1987. J Anxiety Disord. 1992;6:214–47. [Google Scholar]
- 14.Schmidt NB, Lerew DR, Jackson RJ. The role of anxiety sensitivity in the pathogenesis of panic: prospective evaluation of spontaneous panic attacks during acute stress. J Abnorm Psychol. 1997;106:355–64. doi: 10.1037//0021-843x.106.3.355. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt NB, Cook JH. Effects of anxiety sensitivity on anxiety and pain during a cold pressor challenge in patients with panic disorder. Behav Res Ther. 1999;37:313–23. doi: 10.1016/s0005-7967(98)00139-9. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt NB, Zvolensky MJ, Maner JK. Anxiety sensitivity: prospective prediction of panic attacks and Axis I pathology. J Psychiatr Res. 2006;40:691–9. doi: 10.1016/j.jpsychires.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev. 2001;21:33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- 18.Esquivel G, Schruers K, Kuipers H, Griez E. The effects of acute exercise and high lactate levels on 35% CO2 challenge in healthy volunteers. Acta Psychiatr Scand. 2002;106:394–7. doi: 10.1034/j.1600-0447.2002.01333.x. [DOI] [PubMed] [Google Scholar]
- 19.Smits JA, Meuret AE, Zvolensky MJ, Rosenfield D, Seidel A. The effects of acute exercise on CO(2) challenge reactivity. J Psychiatr Res. 2009;43:446–54. doi: 10.1016/j.jpsychires.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Strohle A, Feller C, Onken M, Godemann F, Heinz A, Dimeo F. The acute antipanic activity of aerobic exercise. Am J Psychiatry. 2005;162:2376–8. doi: 10.1176/appi.ajp.162.12.2376. [DOI] [PubMed] [Google Scholar]
- 21.Strohle A, Graetz B, Scheel M, Wittmann A, Feller C, Heinz A, Dimeo F. The acute antipanic and anxiolytic activity of aerobic exercise in patients with panic disorder and healthy control subjects. J Psychiatr Res. 2009;43:1013–7. doi: 10.1016/j.jpsychires.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Binder E, Droste SK, Ohl F, Reul JM. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behav Brain Res. 2004;155:197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Forcier K, Stroud LR, Papandonatos GD, Hitsman B, Reiches M, Krishnamoorthy J, Niaura R. Links between physical fitness and cardiovascular reactivity and recovery to psychological stressors: A meta-analysis. Health Psychol. 2006;25:723–39. doi: 10.1037/0278-6133.25.6.723. [DOI] [PubMed] [Google Scholar]
- 24.Rimmele U, Zellweger BC, Marti B, Seiler R, Mohiyeddini C, Ehlert U, Heinrichs M. Trained men show lower cortisol, heart rate and psychological responses to psychosocial stress compared with untrained men. Psychoneuroendocrinology. 2007;32:627–35. doi: 10.1016/j.psyneuen.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Sinyor D, Schwartz SG, Peronnet F, Brisson G, Seraganian P. Aerobic fitness level and reactivity to psychosocial stress: physiological, biochemical, and subjective measures. Psychosom Med. 1983;45:205–17. doi: 10.1097/00006842-198306000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Throne LC, Bartholomew JB, Craig J, Farrar RP. Stress reactivity in fire fighters: An exercise intervention. Int J Stress Manag. 2000;7:235–46. [Google Scholar]
- 27.Dishman RK, Washburn RA, Heath GW. Physical Activity Epidemiology. Champaign, IL: Human Kinetics; 2004. [Google Scholar]
- 28.Schmidt NB, Maner JK, Zvolensky MJ. Reactivity to challenge with carbon dioxide as a prospective predictor of panic attacks. Psychiatry Res. 2007;151:173–6. doi: 10.1016/j.psychres.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt NB, Zvolensky MJ. Anxiety sensitivity and CO2 challenge reactivity as unique and interactive prospective predictors of anxiety pathology. Depress Anxiety. 2007;24:527–36. doi: 10.1002/da.20267. [DOI] [PubMed] [Google Scholar]
- 30.Forsyth JP, Zvolensky MJ. Anxiety sensitivity dimensions in the prediction of body vigilance and experiential avoidance. Cog Ther Res. 2002;26:449–60. [Google Scholar]
- 31.State of Vermont Department of Health. Vermont population estimates: Overview of race and ethnicity data. 2007 Available from: http://healthvermont.gov/research/2007pop/documents/RACENOTE07.PDF.
- 32.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders, Non-patient Edition (SCID-I/NP, Version 2.0) New York, NY: Biometrics Research, New York State Psychiatric Institute; 1995. [Google Scholar]
- 33.Zvolensky MJ, Leen-Feldner EW, Feldner MT, Bonn-Miller WO, Lejuez CW, Kahler C, Stewart G. Emotional responding to biological challenge as a function of panic disorder and smoking. J Anxiety Disord. 2004;18:19–32. doi: 10.1016/j.janxdis.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. New York, NY: Cambridge University Press; 2007. [Google Scholar]
- 35.Yartz AR, Zvolensky MJ, Bernstein A, Bonn-Miller MO, Lejuez CW. Panic-relevant predictability preferences: a laboratory test. J Abnorm Psychol. 2008;117:242–6. doi: 10.1037/0021-843X.117.1.242. [DOI] [PubMed] [Google Scholar]
- 36.Lejuez CW, Forsyth JP, Eifert GH. Devices and methods for administering carbon dioxide-enriched air in experimental and clinical settings. J Behav Ther Exp Psychiatry. 1998;29:239–48. doi: 10.1016/s0005-7916(98)00018-4. [DOI] [PubMed] [Google Scholar]
- 37.Zvolensky MJ. Anxiety and Health Research Laboratory Health Assessment Questionnaire. 2002. unpublished manuscript. [Google Scholar]
- 38.Smits JA, Zvolensky MJ. Emotional vulnerability as a function of physical activity among individuals with panic disorder. Depress Anxiety. 2006;23:102–6. doi: 10.1002/da.20146. [DOI] [PubMed] [Google Scholar]
- 39.Lee IM, Sesso HD, Paffenbarger RS., Jr Physical activity and coronary heart disease risk in men: does the duration of exercise episodes predict risk? Circulation. 2000;102:981–6. doi: 10.1161/01.cir.102.9.981. [DOI] [PubMed] [Google Scholar]
- 40.Lee IM, Paffenbarger RS., Jr Associations of light, moderate, and vigorous intensity physical activity with longevity. The Harvard Alumni Health Study. Am J Epidemiol. 2000;151:293–9. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- 41.Leon AS, Connett J. Physical activity and 10. 5 year mortality in the Multiple Risk Factor Intervention Trial (MRFIT) Int J Epidemiol. 1991;20:690–7. doi: 10.1093/ije/20.3.690. [DOI] [PubMed] [Google Scholar]
- 42.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 43.Peterson RA, Reiss S. Anxiety Sensitivity Index Manual (Revised Edition) Worthington, OH: International Diagnostic Systems; 1992. [Google Scholar]
- 44.Zinbarg RE, Brown TA, Barlow DH, Rapee RM. Anxiety sensitivity, panic, and depressed mood: a reanalysis teasing apart the contributions of the two levels in the hierarchial structure of the Anxiety Sensitivity Index. J Abnorm Psychol. 2001;110:372–7. doi: 10.1037//0021-843x.110.3.372. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt NB, Lerew DR, Santiago H, Trakowski JH, Staab JP. Effects of heart-rate feedback on estimated cardiovascular fitness in patients with panic disorder. Depress Anxiety. 2000;12:59–66. doi: 10.1002/1520-6394(2000)12:2<59::AID-DA1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 46.Wolpe J. Psychotherapy by Reciprocal Inhibition. Stanford, CA: Stanford University Press; 1958. [Google Scholar]
- 47.Raudenbush SW, Bryk AS, Cheong YF, Congdon RT. HLM 6: Hierarchical Linear and Nonlinear Modeling. Lincolnwood, IL: Scientific Software International; 2004. [Google Scholar]
- 48.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 49.Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 50.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2005. [Google Scholar]
- 51.Peterson RA, Plehn K. Measuring anxiety sensitivity. In: Taylor S, editor. Anxiety sensitivity: Theory research and treatment of the fear of anxiety. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 1999. pp. 61–81. [Google Scholar]
- 52.Bernstein A, Zvolensky MJ. Anxiety sensitivity: selective review of promising research and future directions. Expert Rev Neurother. 2007;7:97–101. doi: 10.1586/14737175.7.2.97. [DOI] [PubMed] [Google Scholar]
- 53.Feldner MT, Zvolensky MJ, Schmidt NB, Smith RC. A prospective test of anxiety sensitivity as a moderator of the relation between gender and posttraumatic symptom maintenance among high anxiety sensitive young adults. Depress Anxiety. 2008;25:190–9. doi: 10.1002/da.20281. [DOI] [PubMed] [Google Scholar]
- 54.Gardenswartz C, Craske M. Prevention of panic disorder. Behav Ther. 2001;32:725–37. [Google Scholar]
- 55.Schmidt NB, Eggleston AM, Woolaway-Bickel K, Fitzpatrick KK, Vasey MW, Richey JA. Anxiety Sensitivity Amelioration Training (ASAT): a longitudinal primary prevention program targeting cognitive vulnerability. J Anxiety Disord. 2007;21:302–19. doi: 10.1016/j.janxdis.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Watt MC, Stewart SH, Conrod PJ, Schmidt NB. Personality-based approaches to treatment of co-morbid anxiety and substance use disorder. In: Stewart SH, Conrod PH, editors. Anxiety and substance use disorders: The vicious cycle of comorbidity. New York, NY: Springer Publishing; 2008. pp. 201–19. [Google Scholar]
- 57.Zvolensky MJ, Smits JAJ, editors. Anxiety in health behaviors and physical illness. New York, NY: Springer; 2007. [Google Scholar]
- 58.Broman-Fulks JJ, Berman ME, Rabian BA, Webster MJ. Effects of aerobic exercise on anxiety sensitivity. Behav Res Ther. 2004;42:125–36. doi: 10.1016/S0005-7967(03)00103-7. [DOI] [PubMed] [Google Scholar]
- 59.Broman-Fulks JJ, Storey KM. Evaluation of a brief aerobic exercise intervention for high anxiety sensitivity. Anxiety Stress Coping. 2008;21:117–28. doi: 10.1080/10615800701762675. [DOI] [PubMed] [Google Scholar]
- 60.Smits JA, Berry AC, Rosenfield D, Powers MB, Behar E, Otto MW. Reducing anxiety sensitivity with exercise. Depress Anxiety. 2008;25:689–99. doi: 10.1002/da.20411. [DOI] [PubMed] [Google Scholar]
- 61.McNally RJ. Panic induction: A critical appraisal. Behav Ther. 1999;30:333–41. [Google Scholar]
- 62.De Moor MH, Boomsma DI, Stubbe JH, Willemsen G, de Geus EJ. Testing causality in the association between regular exercise and symptoms of anxiety and depression. Arch Gen Psychiatry. 2008;65:897–905. doi: 10.1001/archpsyc.65.8.897. [DOI] [PubMed] [Google Scholar]
- 63.Mata J, Thompson RJ, Gotlib IH. BDNF genotype moderates the relation between physical activity and depressive symptoms. Health Psychol. 2010;29:130–3. doi: 10.1037/a0017261. [DOI] [PMC free article] [PubMed] [Google Scholar]