Abstract
Stiff extracellular matrix, elevated interstitial fluid pressure, and the affinity for the tumor cells in the peripheral region of a solid tumor mass have long been recognized as significant barriers to diffusion of small-molecular-weight drugs and antibodies. However, their impacts on nanoparticle-based drug delivery have begun to receive due attention only recently. This article reviews biological features of many solid tumors that influence transport of drugs and nanoparticles and properties of nanoparticles relevant to their intratumoral transport, studied in various tumor models. We also discuss several experimental approaches employed to date for enhancement of intratumoral nanoparticle penetration. The impact of nanoparticle distribution on the effectiveness of chemotherapy remains to be investigated and should be considered in the design of new nanoparticulate drug carriers.
Keywords: extracellular matrix, intratumoral distribution, interstitial fluid pressure, nanoparticles, solid tumors
Introduction
Many solid tumors develop several biological features distinguished from those of normal tissues (1, 2). These features include defective blood vessels and the lack of functional lymphatics (3), as well as abnormal interstitial properties such as increased stiffness of tumor extracellular matrix (ECM) (4) and relatively high interstitial fluid pressure (IFP) (5–11). In particular, the high IFP and abnormal ECM structure are known to be significant barriers to drug diffusion into the tumor mass. Ideally, an anti-cancer therapeutic should be able to reach tumors without systemic loss, penetrate all the way into the core of the tumor mass, enter tumor cells where their target molecules reside, and completely eradicate the tumors. However, the effect of an anti-cancer therapeutic is often limited to the periphery of the tumor mass close to the vasculature (12, 13). More centralized regions of the tumor remain unaffected (14), becoming a potential source for tumor relapse or metastasis. Several review articles discuss the challenges in intratumoral delivery of anti-cancer drugs (1, 11, 12).
In recent years, nanoparticles have been widely explored as a promising vehicle for delivery of anti-cancer therapeutics. The popularity of nanoparticles is attributable to size with a scale pertinent to cellular and subcellular functions and a surface that allows for multiple functionalizations (15). Submicron particles with appropriate surface protection can circulate for a prolonged period and accumulate in solid tumors via leaky vasculature, increasing drug delivery to tumors. On the other hand, due to the size of the system, nanoparticles are likely to face greater difficulties in penetrating tumors (Fig. 1) than free drugs. The effectiveness of nanoparticulate drug carriers may be fundamentally limited if they are designed without considering these physiological barriers (2). Despite the potential impact on clinical success of new delivery approaches, the tumor interstitium has not been actively investigated in the context of the nanoparticle-mediated chemotherapy. To enhance our understanding of tumor interstitium as an active player in nanoparticle transport into solid tumors, thereby facilitating the development of effective nanomedicines, here we review the properties of tumor interstitium and discuss recent approaches to address consequential challenges in nanomedicine delivery.
Fig. 1.
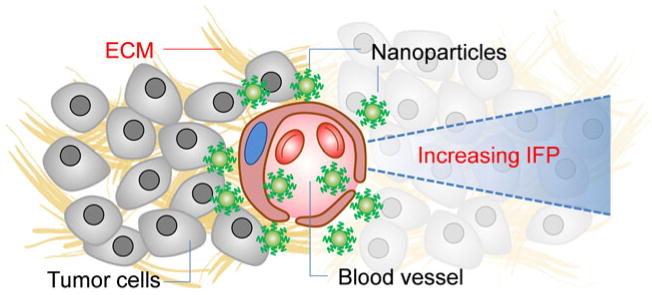
A diagrammatic representation illustrating potential barriers to intratumoral transport of nanoparticles post-extravasation. ECM: extracellular matrix; IFP: interstitial fluid pressure.
Stiff Tumor Extracellular Matrix
Composition and Functions of Extracellular Matrix (ECM)
Tissue stroma consists of fibroblasts, vascular pericytes, endothelial and immune cells, secreted growth factors, and ECM (16, 17). The ECM is composed of collagen networks, microfibril-elastin system (18, 19), glycosaminoglycan (GAG) such as hyaluronan, and proteoglycans (GAGs bound to protein) (18, 20). The ECM acts as structural support for neighboring vasculature and the basal membrane as well as a platform for cell attachment (18). Cell attachment to the ECM promotes intracellular signaling responsible for various cell activities such as cell cycle regulation, migration, and differentiation (21). Components of ECM have the ability to bind growth factors (20, 22) or drugs (6, 12), serving as a reservoir for the molecules.
As a dynamic entity, the ECM is involved in cell migration and remodeling of tissues in both healthy and diseased states (20). For example, proteoglycans in the tumor ECM communicate with growth factors or their receptors (23–27) and influence fibrosis and tumor growth (23, 28–32). Conversely, the ECM is remodeled according to the tumor progression (23, 33–35). Relatively stiff ECM is a common feature of many solid tumors. For example, high density of breast tissues has often been associated with tumorigenesis and metastasis in breast cancer patients (21, 36).
Causes of Stiff Tumor ECM
There are numerous reasons for the stiffness of tumor ECM, such as relatively high collagen levels, an increased presence of lysyl oxidase (LOX), and enhanced integrin signaling in the tumor microenvironment.
High Collagen Levels
Collagen is produced by fibroblasts in the ECM and contributes significantly to the tensile strength of the tissues (37). Many solid tumor types exhibit stiff tumor ECM as a result of high collagen I levels (21). Studies have indicated that elevated levels of collagen I are an indicator of poor prognosis, metastasis and tumor reemergence (21, 38–40). Moreover, the high collagen levels in the tumor ECM are a major barrier in the transport of small-molecular-weight drugs, macromolecules, and nanoparticles (1, 33, 41).
Increased Presence of Lysyl Oxidase (LOX)
Copper-dependent lysyl oxidase (LOX) covalently cross-links collagen and, thus, stiffens the ECM (33, 42). LOX exists in a relatively high level in tumors (33, 43), induced by hypoxia inducible factor (HIF-1) and transforming growth factor beta (TGF-β) (33, 44). Relatively high expression of LOX has been linked to metastasis in squamous carcinomas (21, 45) and poor prognosis. Inhibition of LOX reduces collagen crosslinking as well as LOX-mediated focal adhesions that lead to mammary epithelial cell invasion (33).
Enhanced Integrin Signaling
Integrins are cell receptors that allow communication between cells and the ECM (21). Integrins bound to components of the ECM influence cell survival through signaling, while unbound integrins signal a need for apoptosis (46–48). Different integrins are expressed in various tumor types. For example, the αvβ3 integrin is expressed in melanoma and glioblastoma as well as tumors of the breast, prostate, pancreas, ovary, and cervix (46). It is suggested that ECM stiffness enhances integrin expression, and integrins subsequently promote focal adhesions of cells (4, 33, 49), promoting cytoskeleton remodeling (33, 50). Additionally, integrins can directly influence tumor cell growth (46, 51, 52).
Influence of Stiff Tumor ECM on Drug Transport
Transports of immunoglobulin G (IgG, 150 kDa) and bovine serum albumin (BSA, 68 kDa) in the tumor ECM were compared in a variety of tumor xenograft models, such as human colon adenocarcinoma (LS174T), human glioblastoma (U87), human soft tissue sarcoma (HSTS 26T), and murine mammary carcinoma (MCaIV), which have different elastic moduli (41). BSA diffusion in the tumor ECM was moderately hindered as compared to that in buffered saline, but there was no significant difference according to the tumor models. On the other hand, IgG diffusion was slowed to a greater extent in relatively stiff tumors (41). By comparing the compositions and structures of the tumors, the authors concluded that the resistance to macromolecule penetration was due to the difference in collagen organization rather than the hyaluronan or total GAG content, suggesting that it is the collagen network that should primarily be addressed for efficient intratumoral delivery of macromolecules (41). Here, one should note that the hydrodynamic radii of BSA and IgG are approximately 4.5 nm (53) and 5.3 nm (54), respectively, at least one or two orders of magnitude smaller than those of nanoparticles used for biomedical applications (gold nanoparticles (10–100 nm) (55–57), liposomes (100–1,000 nm) (58–60), or polymeric drug delivery vehicles (50–300 nm) (61–63)), yet their movements in the tumor ECM were significantly hindered by the collagen network.
High Tumor Interstitial Fluid Pressure
Tumor Interstitial Fluid Pressure (IFP)
Interstitial fluid is similar to plasma in the electrolyte composition and contains approximately 50–60% of the proteins present in the plasma (18). The composition of interstitial fluid depends on the size and charge of substances that diffuse from the blood across capillary walls (18, 64). Traditionally it has been thought that the interstitial fluid is a reservoir for the extra fluids, and its volume is passively controlled in response to the perturbations of capillary filtration and lymph flow (65). However, a recent view suggests that IFP is actively controlled by tension on the collagen network exerted by fibroblasts, a process mediated by various growth factors like platelet-derived growth factor (PDGF) (66, 67) and transforming growth factor-β (TGF-β) (68).
Relatively high IFP is a common feature of many solid tumors (11, 69, 70). Normal IFP is typically between −3 and 3 mmHg, but tumor IFP is significantly higher than normal, ranging from 5 to 40 mmHg and even reaching 100 mmHg (71–75). Hofmann et al. determined that IFP increases with increasing tumor volume (75–78). The degree of elevated IFP in the tumor is linked with poor prognosis (72, 79, 80).
Causes of High Tumor IFP
High tumor IFP is attributable to the relatively high permeability of the vasculature, increased contractility of stroma cells, and lack of functional lymphatic system (1, 71, 73, 75, 81, 82). Rapidly growing tumors recruit new blood vessels via secretion of growth factors like vascular endothelial growth factor (VEGF) (83–85), PDGF (86, 87), and TGF-β as well as other angiogenic factors (11). Due to the lack of elaborated control of angiogenic processes, tumor vasculature is typically irregular and convoluted and lacks regular pericyte coverage, accounting for the leakiness of the blood vessels (11). Fibroblasts of tumor stroma gain contractile function by differentiating towards smooth-muscle cells (88) and exert increasing pressure on the ECM (11). Moreover, many tumors do not have normal lymph vessels, which are responsible for returning macromolecular solutes and interstitial fluids back to the bloodstream (1). The lack of functional lymph flow results in inefficient removal of solutes and fluids from the tumor interstitium, further increasing the IFP (11).
Influence of High Tumor IFP on Tumor Progression
IFP influences tumor metastasis, responses to radiation treatment, and patient survival, although the biological mechanisms remain unclear (89). Rofstad et al. reported that high IFP promotes pulmonary and lymph node metastasis of A-07 tumors (90). This group also observed in a large A-07 melanoma xenograft model that tumors with high IFP had high fractions of acutely hypoxic cells and were resistant to radiation treatment (89). In a subsequent study with small A-07 and R-18 melanoma xenografts without hypoxia, they observed that tumors with high IFP were relatively less sensitive to radiation therapy, indicating that the high IFP negatively influenced the radiocurability in a hypoxia-independent manner as well (71). High IFP also stimulates tumor cell proliferation by exerting the mechanical forces on the cells (91, 92).
Influence of High Tumor IFP on Drug Transport
Tumor IFP is a significant physiological barrier in the delivery of therapeutics to the tumor site, resulting in uneven drug distribution within the tumor mass (1, 93). The difficulty increases with the size of a therapeutic molecule, which is transported by convection rather than a concentration gradient (diffusion) (94, 95). The high IFP induces fluid flow in an undesirable direction—from the high-pressure core to the tumor periphery, preventing effective penetration of macromolecular therapeutics (94).
Distribution of Nanoparticles in Tumor Mass
With the recent advances in imaging techniques, a number of studies have demonstrated biodistribution of nanoparticles in animal models (96–99). Irrespective of the presence of a cell-specific ligand on the surface, nanoparticles tend to accumulate in the solid tumors via the leaky vasculature and the impaired lymphatic drainage, as long as they can circulate for a prolonged period (100–102).
On the other hand, the post-extravasation fate of nanoparticles varies with particle properties, including size, surface charge, and affinity for the cells. For example, Zhang et al. found that penetration of transferrin receptor-targeted lipopolyplexes into three-dimensional cell clusters was relatively limited as compared to a free payload (antisense oligonucleotide) (103). Consequently, the targeted lipopolyplexes were less effective in down-regulating the target gene (Bcl-2) expression than free oligonucleotides in vivo, despite the greater amount deposited in the tumors (103). This result may first be attributed to the large size of lipopolyplexes (140 nm vs. 18-mer oligonucleotide), but the contribution of surface charge (positive charge for lipopolyplexes) and affinity for the cells (due to the transferrin-mediated interaction) cannot be ignored.
Particle Size
An ideal nanoparticle size for tumor accumulation via the leaky vasculature is considered to be in the range of 10–100 nm, above the threshold for the renal filtration, although the upper limit is not well defined (15). In earlier studies, particles even at the upper end of this range were thought to be able to penetrate the tumors. For example, Nomura et al. observed that 85 nm emulsion and 120 nm liposomes appeared immediately in the venous outflow after intratumoral injection (104). In addition, Reddy et al. reported that polymeric nanoparticles with an average size of 178 nm penetrated through the tumor interstitium after peritumoral injection (105). In another example, 65 nm polymeric micelles penetrated a multicellular spheroid (106). The micelles released doxorubicin in the cells over 3–24 h, unlike free doxorubicin that appeared in the cells in <1 h, implying that extracellular drug release was minimal and intact micelles penetrated the spheroid until they reached the cells (106). However, it is uncertain whether it was indeed the intact nanoparticles of those sizes that traveled through the tumor matrix, since these studies were based on observation of particle components or payloads, which may not necessarily represent the nanoparticle assemblies.
Recent studies suggest that the size threshold appropriate for tumor penetration would be much lower than expected. Based on a mathematical model, Goodman et al. predicted that polystyrene particles of 20–40 nm would be able to accumulate in the interior of a multicellular spheroid, but 100 or 200 nm particles would not (107). A similar size limit was experimentally observed in a recent study using a mouse model of human breast cancer (108). This study reported that the intratumoral distribution of 25 nm polymeric micelles was relatively broad. In contrast, 60 nm micelles were localized in proximity to the blood vessel (108).
Surface Charge of Particles
Neither cationic nor anionic particles are desirable for long-term circulation, due to the propensity to recruit opsonins and stimulate phagocytosis among many other effects (109–111). On the other hand, cationic surface charge increases particle binding to the vessels via electrostatic interactions with the vascular glycocalyx (112, 113). Combined with sluggish and irregular tumor blood flow, this feature is expected to contribute to targeted drug delivery to tumoral vasculature (112).
On the other hand, Campbell et al. observed that cationic liposomes (150 nm) did not travel far into the tumor interstitium (112). The effect of surface charge on nanoparticle penetration through the tumor interstitium was more systematically investigated in recent studies. Kim et al. compared the penetration of oppositely charged gold nanoparticles (+30 vs. −36 mV, 6 nm) into cylindroidal cell aggregates (114). Cationic nanoparticles were taken up by the proliferating cells on the periphery of the cylindroids, releasing drug in the cells, whereas anionic nanoparticles were better at penetrating the extracellular matrix and entered hypoxic, necrotic cells in the core of the mass (114). The authors also compared diffusivity of the gold nanoparticles in Matrigel, a tumor extracellular matrix material (114). Cationic nanoparticles had a diffusion coefficient significantly lower than that of anionic particles, due to the attractive electrostatic interactions with negatively charged proteoglycans (114). Stylianopoulos et al. also showed both in modeling and in experimentation that quantum dots with a positive charge (33.1 mV, 5.8 nm) had a lower diffusion coefficient than PEGylated quantum dots (−11.5 mV, 5.6 nm) in a collagen gel (115).
Not only cationic particles but anionic particles are also likely to be limited in intratumoral movement (116). Lieleg et al. observed that the mobility of highly charged particles (160–170 nm), either negative or positive, was significantly reduced compared to PEGylated ones, even though the particles were sufficiently smaller than the mesh size (2–3 μm) of an ECM hydrogel (116). The limited mobility of particles in the ECM was attributed to electrostatic attractive interactions between the particles and the ECM biopolymer network, which had both cationic and anionic patches (116). Addition of agents that could shield the surface charge of particles improved the particle movement (116). Recently, Stylianopoulos et al. discussed that the hindrance in intra-ECM movement of highly charged particles was also due to repulsive interactions with biomacromolecule fibers, especially when the fibers were relatively thin (115). According to these studies, neutral particles would be the most desirable for transport into the tumor matrix (115, 116).
Tumor-Targeting Ligands
The presence of cell-specific ligands influences the distribution and retention of nanoparticles in the tumor mass. Kirpotin et al. reported that HER2-targeted immunoliposomes accumulated within tumor cells, whereas non-targeted liposomes were located predominantly in the ECM (101). Epidermal growth factor receptor (EGFR)-targeted immunoliposomes were found inside the tumor cells six times more than non-targeted liposomes (117). In another example, folate receptor (FR)-targeted liposomes were injected intravenously to mice with ascitic lymphoma to examine the distribution of liposomes (118). The overall accumulation of FR-targeted liposomes in ascites was somewhat lower than that of the non-targeted ones, but the fraction of FR-targeted liposomes associated with tumor cells was much higher compared to non-targeted liposomes (118). These studies show that the presence of cell-specific ligands enhances the retention of nanoparticles in the tumor mass via interactions with tumor cells. Farokhzad et al. compared the anti-tumor effects of intratumorally injected nanoparticles and found that non-targeted particles were inferior to targeted ones (119). The difference in the anti-tumor effect between the two particles, given in the same dose directly to the tumors, is attributable to the cell-nanoparticle interaction that would enhance the retention of nanoparticles in the tumors (119).
On the other hand, the cell-specific ligands can interfere with penetration of nanoparticles to the tumor mass. Jang et al. discussed that the spatial distribution of a drug in tumor spheroids depended on the affinity of the drug for the cell membrane (1). While molecules that do not bind to cell membrane readily penetrate the spheroids, those binding to the cellular macromolecules remained at the periphery of the spheroids (1). In earlier studies, Weinstein et al. pointed that intratumoral distribution of cell-specific antibodies is heterogeneous due to the “binding site barrier,” a phenomenon whereby high-affinity antibodies travel a limited depth from the tumor surface (120, 121). The binding site effect was observed with EGFR-targeted micelles in a recent study (108). Lee et al. compared the intratumoral distribution of targeted micelles and non-targeted micelles using confocal microscopy of sectioned tissues and found that the mean diffusion distance of targeted micelles from the blood vessels was significantly shorter than non-targeted ones (108).
Approaches to Enhance Intratumoral Nanoparticle Transport
Enzymes Degrading the ECM
Reducing the stiffness of the ECM and IFP should enhance the permeation of nanoparticulate drug delivery systems through the ECM and enable more homogeneous drug distribution across the tumor mass. Additionally, given that the stiff ECM is associated with tumorigenesis (33, 122), weakening the matrix may interfere with some of the cell-cell and cell-matrix interactions that promote tumor growth. Collagenase (123, 124) and hyaluronidase (123, 125) have been used in conjunction with drug delivery systems to study how these enzymes influence drug distribution in tumors. Moreover, Barkan and others have suggested that hindering the buildup of collagen I as well as LOX-mediated collagen crosslinking may impede tumor reemergence (21).
A few studies reported that hyaluronidase treatment sensitized drug-resistant tumors to chemotherapy, presumably by facilitating drug diffusion into the tumors (126–128). However, Netti et al. suggested that this effect might be due to other mechanisms than enhanced ECM permeability, given the lack of relationship between hyaluronan content and interstitial mobility of macromolecules (41). These authors reported that collagenase treatment improved the penetration of IgG in tumors with rigid ECM and suggested that collagen would be a more reasonable target (41). Additionally, this group showed that chronic treatment of relaxin, a hormone up-regulating collagenase production, induced degradation of collagen and enhanced transport of macromolecules like IgG and 2 MDa dextran into the tumors (129, 130).
The ECM-degrading enzymes have been used to promote intratumoral nanoparticle transport. Hyaluronidase was used to improve the uptake of liposomes in tumors. Liposomal doxorubicin (Caelyx™) was injected intravenously into human osteosarcoma xenografts (subcutaneous or orthotopic) 1 h after intratumoral or intravenous injection of hyaluronidase (125). Intratumoral injection of 1,500 U hyaluronidase reduced IFP by ∼40% in orthotopic xenografts (125). Intravenous injection of hyaluronidase in a subcutaneous xenograft model showed a similar decrease in IFP but required a longer time than intratumoral injection for maximum response (125). Notably, the hyaluronidase treatment enhanced the tumoral distribution of liposomal doxorubicin. Doxorubicin distribution was heterogeneous in tumors of untreated animals. With hyaluonidase, doxorubicin was found in the periphery as well as in the central part of the tumor (125). Hyaluronidase did not influence the microvascular pressure or the intracellular localization of doxorubicin in nuclei, indicating that its main function was to reduce IFP, presumably by degradation of ECM (125).
Goodman et al. used a mathematical model to predict transport of polystyrene nanoparticles (20–200 nm) through spheroids and verified with in vitro experiments. Multicellular spheroids of human cervical carcinoma (SiHa) cells were grown to 400–500 microns for simulation of avascular regions of solid tumors (107). Fluorescent polystyrene nanoparticles were introduced from the periphery of the spheroids and optionally treated with collagenase, and the fluorescence level was observed across the section of the spheroids (107). The authors observed pronounced cell loosening and shedding at the periphery of the spheroids and increasing porosity in the middle quiescent region due to the collagenase treatment (107). Without collagen treatment, the nanoparticle distribution was limited to the periphery of the spheroids, irrespective of particle size. On the other hand, diffusion of 20 or 40-nm nanoparticles was significantly enhanced by the collagen treatment at the periphery of the spheroid as well as toward the necrotic center with a higher porosity (107). However, collagen treatment was ineffective for larger nanoparticles (100 nm or 200 nm) (107).
Kuhn et al. created proteolytic superparamagnetic (SPM) nanoparticles (145 nm in radius), whose surface was modified with collagenase. Penetration of proteolytic SPM nanoparticles was observed in an enhanced ECM matrix, supplemented with additional collagen (131). Under the influence of external magnetic field, the collagenase-conjugated SPM nanoparticles were capable of penetrating the ECM at a rate of ∼90 μm/h (131). The mobility of collagenase-conjugated SPM particles was proportional to the number of functional collagenases on the NPs (131). In contrast, albumin-conjugated SPM nanoparticles were unable to penetrate the enhanced ECM (∼0 μm/h) (131). Similarly, penetration of collagenase-coated polystyrene nanoparticles (100 nm) was four times higher than albumin-coated particles, according to the number of particles delivered to the core of the tumor cell spheroid (132).
Lowering Tumor IFP
Several studies show that reduction of IFP increased transport of therapeutics into tumor masses (11). A representative approach is to normalize the tumor vasculature by inhibiting angiogenic growth factors to an optimal level (133, 134). Jain suggested that inhibitors of the angiogenic growth factors exert an effect on cancer therapy as an adjuvant to chemotherapy rather than an active killer of tumor vasculature as originally envisioned (134). In this context, the role of anti-angiogenic factors was to normalize the abnormal tumor vasculature during the course of therapy, thereby facilitating oxygen and drug delivery (134). One of the desirable effects of anti-angiogenic agents would be to lower tumor IFP. Anti-VEGF monoclonal antibody reduced IFP by more than 70% in human rectal tumors (9) as well as xenograft mouse models of human glioblastoma multiforme (U87) and colon adenocarcinoma (LS174T) (73). Similarly, DC101, a VEGF-receptor-2 antibody, decreased the IFP and induced morphological and functional changes in the vascular network, facilitating BSA penetration in tumors (135).
Alternatively, PDGF antagonists were used to reduce contractility of fibroblasts in the tumor ECM. Imatinib (ST1571), a tyrosine kinase inhibitor of PDGF receptors, was administered concomitantly with epothilone B, a microtubule stabilizer (136, 137), to treat a mouse model of human anaplastic thyroid carcinomas (86). The imatinib treatment decreased tumor IFP, increased the tumor levels of epothilone B by three-fold, and enhanced anti-tumor effects of epothilone B (86). Similar effects were observed with a combination of inhibitory PDGF aptamer or imatinib with Taxol in rodent tumor models (79, 138). However, the effect of imatinib on IFP and drug uptake was transient, and the IFP returned to the pre-treatment level once the imatinib treatment ended (86).
Recently, Klosowska-Wardega et al. tested a combination of anti-PDGF and anti-VEGF therapies to augment the response to Taxol-based chemotherapy (83). This combination additively reduced the IFP of KAT-4 tumors in severe combined immunodeficient (SCID) mice, but it did not translate to further enhancement of anti-tumor efficacy of Taxol, likely due to the suboptimal dosing schedule (83).
Additional agents explored to decrease tumor IFP and increase drug uptake are reviewed elsewhere (11). These agents include nicotinamide (139), dexamethasone (140), tumor necrosis factor-alpha (TNF-α) (141–143), Prostaglandin E1 (PGE1) (144, 145), bradykinin agonist (146), TGF-β inhibitor (147), and hyaluronidase (148). However, due to the side effects, systemic application of some agents is cautioned (75, 125, 142).
Hofmann et al. infused 20% human serum albumin (HSA, 66 kDa) in a xenograft model to increase the plasma colloid osmotic pressure so that fluid diffused from the interstitium back into blood vessels (75). The administration of 20% HSA reduced tumor IFP from 8 to 2 mmHg and increased the uptake of dextran (40 kDa) and cetuximab (151.8 kDa) by tumors (75). The tumor growth was better inhibited by the combined administration of 20% HSA and cetuximab than cetuximab alone (75). Dextran was taken up in the tumor mass relatively quickly but flushed from the tumor mass quickly as well (75). On the other hand, cetuximab was taken up by the tumor mass relatively slowly but remained in the tumor tissue due to the affinity for the A431 carcinoma cells (75).
Priming Tumors with an Apoptosis-Inducer
Drug penetration into the tumor mass can be enhanced by expanding the interstitial space of tumors using an apoptosis inducer. Au, Wientjes, and colleagues observed that paclitaxel penetration into three-dimensional tumor histoculture increased abruptly 12–24 h after the treatment (149, 150). They suggested that the enhanced drug penetration was related to the decreasing cell density resulting from drug-induced apoptosis (149). In a subsequent study, they confirmed that paclitaxel accumulation and tumor apoptosis were significantly higher when the tumors were pre-treated with a small dose of paclitaxel (“primed”) prior to the second dose than when the same total dose was administered at once (151). Recently, this group reported a microparticle system that released paclitaxel at different rates so that the first dose of paclitaxel was rapidly released to prime the tumors, followed by the second that provided the sustained drug release (152). This system achieved greater drug level in ovarian tumors, lower toxicity, and longer survival than the commercial Taxol formulation in an ovarian SKOV-3 xenograft model (152). In addition to decreasing tumor cell density, paclitaxel has an effect on tumor vessels (153). Griffon-Etienne et al. observed that paclitaxel increased the diameter of tumor vessels and decreased the microvascular pressure and IFP (153), which may be an additional mechanism of the priming effect of paclitaxel.
Application of Magnetic Force
Kuhn et al. studied magnetic force-induced penetration of SPM nanoparticles through purified ECM (154). The SPM nanoparticles had either a silica coating (135 nm in radius) or a 300 Da polyethylene glycol (PEG) coating (145 nm or 400 nm in radius) (154). The ECM was isolated from Engelbreth-Holm-Swarm murine sarcoma and allowed to polymerize before nanoparticles were applied to the gel surface (154). In the absence of an external magnetic field, none of the nanoparticles penetrated the polymerized ECM (154). When the magnetic field was applied, only 145-nm PEG-coated nanoparticles could travel through the ECM at a velocity of 1.5 mm/h, whereas 400-nm PEG-coated nanoparticles and 135-nm silica-coated particles, which aggregated in the experimental condition, were virtually immobile (154).
Combination with Radiofrequency (RF) Thermal Ablation Therapy
Focal solid tumors can be treated with RF thermal ablation, an induction of coagulation necrosis of tumors using brief heating (<15 min) at >50°C via needle electrodes (155). Goldberg et al. observed a synergistic effect between intratumoral doxorubicin injection and RF ablation in destruction of solid tumors in a rat model (156). In a subsequent study, this group used intravenous application of doxorubicin liposomes (Doxil) in conjunction with RF ablation and achieved higher tumor destruction than Doxil and RF therapy alone (157). The authors attributed this synergistic effect in part to enhanced accumulation of Doxil due to the increased vascular permeability and blood flow (155). Indeed, autoradiographic studies using radiolabelled liposomes demonstrated high liposome distribution in the area peripheral to the RF-ablated region (158). Recently, Zhang et al. predicted, using a mathematical model, that liposome penetration into the avascular central region of tumors would still be limited, but released free drug would diffuse into the central region, which has already been destroyed by RF ablation, and eradicate remaining cells (159). In this context, the authors proposed the use of RF ablation in conjunction with temperature-sensitive liposomes that release drugs by heating (159).
Future Perspectives
Studies have shown in animal models that nanoparticles that circulate for a prolonged period can increase the distribution of the encapsulated drug in solid tumors with leaky vasculature (160). On the other hand, intratumoral distribution of nanoparticles post-extravasation remains relatively unexplored, with a limited number of studies performed in vitro, such as in multicell spheroid models or collagen matrices. Recently, Lee et al. have demonstrated in vivo that the distribution of polymeric micelles in the tumor mass was mainly localized in regions with higher blood vessel densities (108, 161). The heterogeneity of intratumoral nanoparticle distribution would be attributable to biological features of solid tumors that resist intratumoral penetration of nanoparticles, such as the stiff ECM structure, elevated IFP, and affinity for the tumor cells in the peripheral region of a solid tumor, which have long been recognized as diffusion barriers to small-molecular-weight drugs and antibodies (1, 11, 12). Particle size, surface charge, and the presence of cell-specific ligands are found to play critical roles in nanoparticle distribution in tumors. Although it remains to be investigated, particle shape (162) or flexibility (163) may also have an impact on the intratumoral nanoparticle transport.
It is yet unclear whether homogeneous intratumoral distribution of nanoparticles (if it is possible) would be superior to localized distribution of a large amount of nanoparticles to the region proximal to blood vessels. Intratumoral particle penetration may be more desirable as it overcomes the consequences of heterogeneous blood flow in tumors (2) and provides a broad coverage. Alternatively, concentrating nanoparticles in the periphery of tumor may be a more effective way of killing actively proliferating tumor cells in that region. With recent advances in nanotechnology and imaging techniques, we may be able to find answers to these questions: how different attributes of nanoparticles influence their intratumoral distribution and, consequently, the effectiveness of nanoparticle-mediated chemotherapy. It is high time to initiate a systematic exploration of this important yet relatively under-explored area, and it should be an integral part of our effort to build new nanoparticulate drug carriers.
Acknowledgments
This study was supported by a grant from the Lilly Endowment, Inc., to the School of Pharmacy and Pharmaceutical Sciences, Purdue University, and the NIH R21 CA135130.
Contributor Information
Hillary Holback, Department of Industrial and Physical Pharmacy, College of Pharmacy, Purdue University, 575 Stadium Mall Drive, West Lafayette, Indiana 47907, USA.
Yoon Yeo, Email: yyeo@purdue.edu, Department of Industrial and Physical Pharmacy, College of Pharmacy, Purdue University, 575 Stadium Mall Drive, West Lafayette, Indiana 47907, USA; Department of Industrial and Physical Pharmacy, College of Pharmacy, and Weldon School of Biomedical Engineering, Purdue University, West Lafayette, Indiana 47907, USA.
References
- 1.Jang SH, Wientjes MG, Lu D, Au JLS. Drug delivery and transport to solid tumors. Pharm Res. 2003;20(9):1337–50. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–64. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92. [PubMed] [Google Scholar]
- 4.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Young JS, Lumsden CE, Stalker AL. The significance of the “tissue pressure” of normal testicular and of neoplastic (Brown-Pearce carcinoma) tissue in the rabbit. J Pathol Bacteriol. 1950;62(3):313–33. doi: 10.1002/path.1700620303. [DOI] [PubMed] [Google Scholar]
- 6.Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001;46(1–3):149–68. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 7.Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvas Res. 1989;37(1):77–104. doi: 10.1016/0026-2862(89)90074-5. [DOI] [PubMed] [Google Scholar]
- 8.Jain RK, Baxter LT. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 1988;48(24 Pt 1):7022–32. [PubMed] [Google Scholar]
- 9.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10(2):145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 1990;50(15):4478–84. [PubMed] [Google Scholar]
- 11.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - An obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–13. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 12.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6(8):583–92. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 13.Kuh HJ, Jang SH, Wientjes MG, Weaver JR, Au JLS. Determinants of paclitaxel penetration and accumulation in human solid tumor. J Pharmacol Exp Ther. 1999;290(2):871–80. [PubMed] [Google Scholar]
- 14.Lankelma J, Dekker H, Luque RF, Luykx S, Hoekman K, van der Valk P, et al. Doxorubicin gradients in human breast cancer. Clin Cancer Res. 1999;5(7):1703–7. [PubMed] [Google Scholar]
- 15.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 16.Fleming JM, Miller TC, Quinones M, Xiao Z, Xu X, Meyer MJ, et al. The normal breast microenvironment of premenopausal women differentially influences the behavior of breast cancer cells in vitro and in vivo. BMC Med. 2010;8:27. doi: 10.1186/1741-7015-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park K. A new ligand for targeted drug delivery to tumor stromal cells. J Control Release. 2010;145(2):75. doi: 10.1016/j.jconrel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Reed RK, Rubin K. Transcapillary exchange: role and importance of the interstitial fluid pressure and the extracellular matrix. Cardiovasc Res. 2010;87(2):211–7. doi: 10.1093/cvr/cvq143. [DOI] [PubMed] [Google Scholar]
- 19.Ushiki T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch Histol Cytol. 2002;65(2):109–26. doi: 10.1679/aohc.65.109. [DOI] [PubMed] [Google Scholar]
- 20.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200(4):448–64. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 21.Barkan D, Green JE, Chambers AF. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer. 2010;46(7):1181–8. doi: 10.1016/j.ejca.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–52. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 23.Koninger J, Giese T, di Mola FF, Wente MN, Esposito I, Bachem MG, et al. Pancreatic tumor cells influence the composition of the extracellular matrix. Biochem Biophys Res Commun. 2004;322(3):943–9. doi: 10.1016/j.bbrc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Comalada M, Cardo M, Xaus J, Valledor AF, Lloberas J, Ventura F, et al. Decorin reverses the repressive effect of autocrine-produced TGF-beta on mouse macrophage activation. J Immunol. 2003;170(9):4450–6. doi: 10.4049/jimmunol.170.9.4450. [DOI] [PubMed] [Google Scholar]
- 25.Csordas G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, et al. Sustained down-regulation of the epidermal growth factor receptor by decorin - A mechanism for controlling tumor growth in vivo. J Biol Chem. 2000;275(42):32879–87. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- 26.Schonherr E, Levkau B, Schaefer L, Kresse H, Walsh K. Decorin-mediated signal transduction in endothelial cells - Involvement of Akt/protein kinase B in up-regulation of p21 (WAF1/CIP1) but not p27(KIP1) J Biol Chem. 2001;276(44):40687–92. doi: 10.1074/jbc.M105426200. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346(6281):281–4. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 28.Theocharis AD, Vynios DH, Papageorgakopoulou N, Skandalisa SS, Theocharis DA. Altered content composition and structure of glycosaminoglycans and proteoglycans in gastric carcinoma. Int J Biochem Cell Biol. 2003;35(3):376–90. doi: 10.1016/s1357-2725(02)00264-9. [DOI] [PubMed] [Google Scholar]
- 29.Reed CC, Gauldie J, Iozzo RV. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 2002;21(23):3688–95. doi: 10.1038/sj.onc.1205470. [DOI] [PubMed] [Google Scholar]
- 30.Santra M, Skorski T, Calabretta B, Lattime EC, Iozzo RV. De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells. Proc Natl Acad Sci USA. 1995;92(15):7016–20. doi: 10.1073/pnas.92.15.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teicher BA, Maehara Y, Kakeji Y, Ara G, Keyes SR, Wong J, et al. Reversal of in vivo drug resistance by the transforming growth factor-beta inhibitor decorin. Int J Cancer. 1997;71(1):49–58. doi: 10.1002/(sici)1097-0215(19970328)71:1<49::aid-ijc10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Tsara ME, Theocharis AD, Theocharis DA. Compositional and structural alterations of proteoglycans in human rectum carcinoma with special reference to versican and decorin. Anticancer Res. 2002;22(5):2893–8. [PubMed] [Google Scholar]
- 33.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9(2):108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kresse H, Schonherr E. Proteoglycans of the extracellular matrix and growth control. J Cell Physiol. 2001;189(3):266–74. doi: 10.1002/jcp.10030. [DOI] [PubMed] [Google Scholar]
- 36.Park CC, Rembert J, Chew K, Moore D, Kerlikowske K. High mammographic breast density is independent predictor of local but not distant recurrence after lumpectomy and radiotherapy for invasive breast cancer. Int J Radiat Oncol Biol Phys. 2009;73(1):75–9. doi: 10.1016/j.ijrobp.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Kolacna L, Bakesova J, Varga F, Kostakova E, Planka L, Necas A, et al. Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix. Physiol Res. 2007;56 1:S51–60. doi: 10.33549/physiolres.931302. [DOI] [PubMed] [Google Scholar]
- 38.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33(1):49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 39.Feng YM, Sun BC, Li XQ, Zhang L, Niu Y, Xiao CH, et al. Differentially expressed genes between primary cancer and paired lymph node metastases predict clinical outcome of node-positive breast cancer patients. Breast Cancer Res Treat. 2007;103(3):319–29. doi: 10.1007/s10549-006-9385-7. [DOI] [PubMed] [Google Scholar]
- 40.Calvo A, Catena R, Noble MS, Carbott D, Gil-Bazo I, Gonzalez-Moreno O, et al. Identification of VEGF-regulated genes associated with increased lung metastatic potential: functional involvement of tenascin-C in tumor growth and lung metastasis. Oncogene. 2008;27(40):5373–84. doi: 10.1038/onc.2008.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60(9):2497–503. [PubMed] [Google Scholar]
- 42.Pfeiffer BJ, Franklin CL, Hsieh FH, Bank RA, Phillips CL. Alpha 2(I) collagen deficient oim mice have altered biomechanical integrity, collagen content, and collagen crosslinking of their thoracic aorta. Matrix Biol. 2005;24(7):451–8. doi: 10.1016/j.matbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Erler JT, Weaver VM. Three-dimensional context regulation of metastasis. Clin Exp Metastasis. 2009;26(1):35–49. doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Postovit LM, Abbott DE, Payne SL, Wheaton WW, Margaryan NV, Sullivan R, et al. Hypoxia/reoxygenation: a dynamic regulator of lysyl oxidase-facilitated breast cancer migration. J Cell Biochem. 2008;103(5):1369–78. doi: 10.1002/jcb.21517. [DOI] [PubMed] [Google Scholar]
- 45.Albinger-Hegyi A, Stoeckli SJ, Schmid S, Storz M, Iotzova G, Probst-Hensch NM, et al. Lysyl oxidase expression is an independent marker of prognosis and a predictor of lymph node metastasis in oral and oropharyngeal squamous cell carcinoma (OSCC) Int J Cancer. 2010;126(11):2653–62. doi: 10.1002/ijc.24948. [DOI] [PubMed] [Google Scholar]
- 46.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001;155(3):459–70. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao HB, Ross FP, Teitelbaum SL. Unoccupied alpha(v)beta(3) integrin regulates osteoclast apoptosis by transmitting a positive death signal. Mol Endocrinol. 2005;19(3):771–80. doi: 10.1210/me.2004-0161. [DOI] [PubMed] [Google Scholar]
- 49.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127(5):1015–26. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4(4):E83–90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 51.Han SW, Khuri FR, Roman J. Fibronectin stimulates non-small cell lung carcinoma cell growth through activation of Akt/mammalian target of rapamycin/S6 kinase and inactivation of LKB1/AMP-activated protein kinase signal pathways. Cancer Res. 2006;66(1):315–23. doi: 10.1158/0008-5472.CAN-05-2367. [DOI] [PubMed] [Google Scholar]
- 52.Vellon L, Menendez JA, Lupu R. alpha(v)beta(3) integrin regulates Heregulin (HRG)-induced cell proliferation and survival in breast cancer. Oncogene. 2005;24(23):3759–73. doi: 10.1038/sj.onc.1208452. [DOI] [PubMed] [Google Scholar]
- 53.Bohme U, Scheler U. Effective charge of bovine serum albumin determined by electrophoresis NMR. Chem Phys Lett. 2007;435:342–5. [Google Scholar]
- 54.Lohle PNM, Verhagen I, Teelken AW, Blaauw EH, Go KG. The pathogenesis of cerebral gliomatous cysts. Neurosurgery. 1992;30(2):180–5. doi: 10.1227/00006123-199202000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Ding Y, Bian X, Yao W, Li R, Ding D, Hu Y, et al. Surface-potential-regulated transmembrane and cytotoxicity of chitosan/gold hybrid nanospheres. ACS Appl Mater Interfaces. 2010;2(5):1456–65. doi: 10.1021/am1001019. [DOI] [PubMed] [Google Scholar]
- 56.Asadishad B, Vosoughi M, Alamzadeh I, Tavakoli A. Synthesis of folate-modified, polyethylene glycol-functionalized gold nanoparticles for targeted drug delivery. J Dispers Sci Technol. 2010;31(4):492–500. [Google Scholar]
- 57.Cho WS, Cho M, Jeong J, Choi M, Han BS, Shin HS, et al. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol Appl Pharmacol. 2010;245(1):116–23. doi: 10.1016/j.taap.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Pornpattananangkul D, Olson S, Aryal S, Sartor M, Huang CM, Vecchio K, et al. Stimuli-responsive liposome fusion mediated by gold nanoparticles. ACS Nano. 2010;4(4):1935–42. doi: 10.1021/nn9018587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang HJ, Zhao PQ, Liang XF, Gong XQ, Song T, Niu RF, et al. Folate-PEG coated cationic modified chitosan - Cholesterol liposomes for tumor-targeted drug delivery. Biomaterials. 2010;31(14):4129–38. doi: 10.1016/j.biomaterials.2010.01.089. [DOI] [PubMed] [Google Scholar]
- 60.Mevel M, Kamaly N, Carmona S, Oliver MH, Jorgensen MR, Crowther C, et al. DODAG; a versatile new cationic lipid that mediates efficient delivery of pDNA and siRNA. J Control Release. 2010;143(2):222–32. doi: 10.1016/j.jconrel.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Barua S, Rege K. The influence of mediators of intracellular trafficking on transgene expression efficacy of polymer-plasmid DNA complexes. Biomaterials. 2010;31(22):5894–902. doi: 10.1016/j.biomaterials.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Contreras J, Xie J, Chen YJ, Pei H, Zhang G, Fraser CL, et al. Intracellular uptake and trafficking of difluoroboron dibenzoylmethane-polylactide nanoparticles in HeLa cells. ACS Nano. 2010;4(5):2735–47. doi: 10.1021/nn901385y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ravindran J, Nair HB, Sung BY, Prasad S, Tekmal RR, Aggarwal BB. Thymoquinone poly (lactide-co-glycolide) nanoparticles exhibit enhanced anti-proliferative, anti-inflammatory, and chemosensitization potential. Biochem Pharmacol. 2010;79(11):1640–7. doi: 10.1016/j.bcp.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79(3):703–61. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- 65.Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73(1):1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- 66.Clark RA, Folkvord JM, Hart CE, Murray MJ, McPherson JM. Platelet isoforms of platelet-derived growth factor stimulate fibroblasts to contract collagen matrices. J Clin Invest. 1989;84(3):1036–40. doi: 10.1172/JCI114227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gullberg D, Tingstrom A, Thuresson AC, Olsson L, Terracio L, Borg TK, et al. Beta 1 integrin-mediated collagen gel contraction is stimulated by PDGF. Exp Cell Res. 1990;186(2):264–72. doi: 10.1016/0014-4827(90)90305-t. [DOI] [PubMed] [Google Scholar]
- 68.Montesano R, Orci L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA. 1988;85(13):4894–7. doi: 10.1073/pnas.85.13.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Milosevic A, Fyles A, Hedley D, Hill R. The human tumor microenvironment: invasive (needle) measurement of oxygen and interstitial fluid pressure. Semin Radiat Oncol. 2004;14(3):249–58. doi: 10.1016/j.semradonc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 70.Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis. 2009;26(1):19–34. doi: 10.1007/s10585-008-9182-2. [DOI] [PubMed] [Google Scholar]
- 71.Rofstad EK, Ruud EBM, Mathiesen B, Galappathi K. Associations between radiocurability and interstitial fluid pressure in human tumor xenografts without hypoxic tissue. Clin Cancer Res. 2010;16(3):936–45. doi: 10.1158/1078-0432.CCR-09-2718. [DOI] [PubMed] [Google Scholar]
- 72.Curti BD, Urba WJ, Alvord WG, Janik JE, Smith JW, Madara K, et al. Interstitial pressure of subcutaneous nodules in melanoma and lymphoma patients: changes during treatment. Cancer Res. 1993;53(10 Suppl):2204–7. [PubMed] [Google Scholar]
- 73.Lee CG, Heijn M, di Tomaso E, Griffon-Etienne G, Ancukiewicz M, Koike C, et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60(19):5565–70. [PubMed] [Google Scholar]
- 74.Aukland K. Interstitial fluid balance in experimental animals and man. In: Staub NC, Hogg JC, Hargens AR, editors. Advances in Microcirculation, Vol 13 Interstitial-Lymphatic Liquid and Solute Movement; Satellite Symposium; Victoria, British Columbia, Canada. July 20–24 1986; Basel, Switzerland; New York, New York, USA Illus: S Karger Ag; 1987. pp. X + 290pp. 110–123. [Google Scholar]
- 75.Hofmann M, McCormack E, Mujic M, Rossberg M, Bernd A, Bereiter-Hahn J, et al. Increased plasma colloid osmotic pressure facilitates the uptake of therapeutic macromolecules in a xenograft tumor model. Neoplasia. 2009;11(8):812–22. doi: 10.1593/neo.09662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boucher Y, Kirkwood JM, Opacic D, Desantis M, Jain RK. Interstitial hypertension in superficial metastatic melanomas in humans. Cancer Res. 1991;51(24):6691–4. [PubMed] [Google Scholar]
- 77.Gutmann R, Leunig M, Feyh J, Goetz AE, Messmer K, Kastenbauer E, et al. Interstitial hypertension in head and neck tumors in patients: correlation with tumor size. Cancer Res. 1992;52(7):1993–5. [PubMed] [Google Scholar]
- 78.Nathanson SD, Nelson L. Interstitial fluid pressure in breast cancer, benign breast conditions, and breast parenchyma. Ann Surg Oncol. 1994;1(4):333–8. doi: 10.1007/BF03187139. [DOI] [PubMed] [Google Scholar]
- 79.Pietras K, Rubin K, Sjoblom T, Buchdunger E, Sjoquist M, Heldin CH, et al. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62(19):5476–84. [PubMed] [Google Scholar]
- 80.Milosevic M, Fyles A, Hedley D, Pintilie M, Levin W, Manchul L, et al. Interstitial fluid pressure predicts survival in patients with cervix cancer independent of clinical prognostic factors and tumor - oxygen measurements. Cancer Res. 2001;61(17):6400–5. [PubMed] [Google Scholar]
- 81.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 82.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60(16):4324–7. [PubMed] [Google Scholar]
- 83.Klosowska-Wardega A, Hasumi Y, Burmakin M, Ahgren A, Stuhr L, Moen I, et al. Combined anti-angiogenic therapy targeting PDGF and VEGF receptors lowers the interstitial fluid pressure in a murine experimental carcinoma. PLoS One. 2009;4(12):e8149. doi: 10.1371/journal.pone.0008149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8(6):464–78. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 85.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146(5):1029–39. [PMC free article] [PubMed] [Google Scholar]
- 86.Pietras K, Stumm M, Hubert M, Buchdunger E, Rubin K, Heldin CH, et al. STI571 enhances the therapeutic index of epothilone B by a tumor-selective increase of drug uptake. Clin Cancer Res. 2003;9(10 Pt 1):3779–87. [PubMed] [Google Scholar]
- 87.Ostman A, Heldin CH. Involvement of platelet-derived growth factor in disease: development of specific antagonists. Adv Cancer Res. 2001;80:1–38. doi: 10.1016/s0065-230x(01)80010-5. [DOI] [PubMed] [Google Scholar]
- 88.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–3. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 89.Rofstad EK, Gaustad JV, Brurberg KG, Mathiesen B, Galappathi K, Simonsen TG. Radiocurability is associated with interstitial fluid pressure in human tumor xenografts. Neoplasia. 2009;11(11):1243–51. doi: 10.1593/neo.91152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rofstad EK, Tunheim SH, Mathiesen B, Graff BA, Halsør EF, Nilsen K, et al. Pulmonary and lymph node metastasis is associated with primary tumor interstitial fluid pressure in human melanoma xenografts. Cancer Res. 2002;62(3):661–4. [PubMed] [Google Scholar]
- 91.Hofmann M, Guschel M, Bernd A, Bereiter-Hahn J, Kaufmann R, Tandi C, et al. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia. 2006;8(2):89–95. doi: 10.1593/neo.05469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hofmann M, Schultz M, Bernd A, Bereiter-Hahn J, Kaufmann R, Kippenberger S. Long-term lowering of tumour interstitial fluid pressure reduces Ki-67 expression. J Biomech. 2007;40(10):2324–9. doi: 10.1016/j.jbiomech.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 93.Boucher Y, Jain RK. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: implications for vascular collapse. Cancer Res. 1992;52(18):5110–4. [PubMed] [Google Scholar]
- 94.Jain RK. Barriers to drug delivery in solid tumors. Sci Am. 1994;271(1):58–65. doi: 10.1038/scientificamerican0794-58. [DOI] [PubMed] [Google Scholar]
- 95.Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47(12):3039–51. [PubMed] [Google Scholar]
- 96.Cho YW, Park SA, Han TH, Son DH, Park JS, Oh SJ, et al. In vivo tumor targeting and radionuclide imaging with self-assembled nanoparticles: mechanisms, key factors, and their implications. Biomaterials. 2007;28(6):1236–47. doi: 10.1016/j.biomaterials.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 97.Choi KY, Min KH, Na JH, Choi K, Kim K, Park JH, et al. Self-assembled hyaluronic acid nanoparticles as a potential drug carrier for cancer therapy: synthesis, characterization, and in vivo biodistribution. J Mater Chem. 2009;19(24):4102–7. [Google Scholar]
- 98.Park K, Kim JH, Nam YS, Lee S, Nam HY, Kim K, et al. Effect of polymer molecular weight on the tumor targeting characteristics of self-assembled glycol chitosan nanoparticles. J Control Release. 2007;122(3):305–14. doi: 10.1016/j.jconrel.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 99.ElBayoumi TA, Torchilin VP. Tumor-targeted nanomedicines: enhanced antitumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibody. Clin Cancer Res. 2009;5(6):1973–80. doi: 10.1158/1078-0432.CCR-08-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gullotti E, Yeo Y. Extracellularly activated nanocarriers: a new paradigm of tumor targeted drug delivery. Mol Pharm. 2009;6(4):1041–51. doi: 10.1021/mp900090z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong KL, Nielsen UB, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66(13):6732–40. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 102.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA. 2007;104(39):15549–54. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang X, Koh CG, Yu B, Liu S, Piao L, Marcucci G, et al. Transferrin receptor targeted lipopolyplexes for delivery of antisense oligonucleotide g3139 in a murine k562 xenograft model. Pharm Res. 2009;26(6):1516–24. doi: 10.1007/s11095-009-9864-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nomura T, Koreeda N, Yamashita F, Takakura Y, Hashida M. Effect of particle size and charge on the disposition of lipid carriers after intratumoral injection into tissue-isolated tumors. Pharm Res. 1998;15(1):128–32. doi: 10.1023/a:1011921324952. [DOI] [PubMed] [Google Scholar]
- 105.Reddy LH, Sharma RK, Murthy RSR. Enhanced tumour uptake of doxorubicin loaded Poly(butyl cyanoacrylate) nanoparticles in mice bearing Dalton's lymphoma tlimour. J Drug Target. 2004;12(7):443–51. doi: 10.1080/10611860400011406. [DOI] [PubMed] [Google Scholar]
- 106.Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug Chem. 2005;16(1):122–30. doi: 10.1021/bc0498166. [DOI] [PubMed] [Google Scholar]
- 107.Goodman TT, Chen JY, Matveev K, Pun SH. Spatio-temporal modeling of nanoparticle delivery to multicellular tumor spheroids. Biotechnol Bioeng. 2008;101(2):388–99. doi: 10.1002/bit.21910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee H, Fonge H, Hoang B, Reilly RM, Allen C. The effects of particle size and molecular targeting on the intratumoral and subcellular distribution of polymeric nanoparticles. Mol Pharm. 2010;7(4):1195–208. doi: 10.1021/mp100038h. [DOI] [PubMed] [Google Scholar]
- 109.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5(4):487–95. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 111.Wang J, Sui M, Fan W. Nanoparticles for tumor targeted therapies and their pharmacokinetics. Curr Drug Metab. 2010;11(2):129–41. doi: 10.2174/138920010791110827. [DOI] [PubMed] [Google Scholar]
- 112.Campbell RB, Fukumura D, Brown EB, Mazzola LM, Izumi Y, Jain RK, et al. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002;62(23):6831–6. [PubMed] [Google Scholar]
- 113.Dellian M, Yuan F, Trubetskoy VS, Torchilin VP, Jain RK. Vascular permeability in a human tumour xenograft: molecular charge dependence. Br J Cancer. 2000;82(9):1513–8. doi: 10.1054/bjoc.1999.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim B, Han G, Toley BJ, Kim CK, Rotello VM, Forbes NS. Tuning payload delivery in tumour cylindroids using gold nanoparticles. Nat Nanotechnol. 2010;5(6):465–72. doi: 10.1038/nnano.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stylianopoulos T, Poh MZ, Insin N, Bawendi MG, Fukumura D, Munn LL, et al. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophys J. 2010;99(5):1342–9. doi: 10.1016/j.bpj.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lieleg O, Baumgärtel RM, Bausch AR. Selective filtering of particles by the extracellular matrix: an electrostatic bandpass. Biophys J. 2009;97(6):1569–77. doi: 10.1016/j.bpj.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mamot C, Drummond DC, Noble CO, Kallab V, Guo ZX, Hong KL, et al. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65(24):11631–8. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 118.Gabizon A, Horowitz AT, Goren D, Tzemach D, Shmeeda H, Zalipsky S. In vivo fate of folate-targeted polyethylene-glycol liposomes in tumor-bearing mice. Clin Cancer Res. 2003;9(17):6551–9. [PubMed] [Google Scholar]
- 119.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci USA. 2006;103(16):6315–20. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Juweid M, Neumann R, Paik C, Perez-Bacete MJ, Sato J, van Osdol W, et al. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 1992;52(19):5144–53. [PubMed] [Google Scholar]
- 121.Graff CP, Wittrup KD. Theoretical analysis of antibody targeting of tumor spheroids. Cancer Res. 2003;63(6):1288–96. [PubMed] [Google Scholar]
- 122.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79(1):144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jiang J, Moore JS, Edelhauser HF, Prausnitz MR. Intrascleral drug delivery to the eye using hollow microneedles. Pharm Res. 2009;26(2):395–403. doi: 10.1007/s11095-008-9756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y, So MK, Rao JH. Protease-modulated cellular uptake of quantum dots. Nano Lett. 2006;6(9):1988–92. doi: 10.1021/nl0611586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eikenes L, Tari M, Tufto I, Bruland OS, Davies CD. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx (TM)) in human osteosarcoma xenografts. Br J Cancer. 2005;93(1):81–8. doi: 10.1038/sj.bjc.6602626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kerbel RS, St Croix B, Florenes VA, Rak J. Induction and reversal of cell adhesion-dependent multicellular drug resistance in solid breast tumors. Hum Cell. 1996;9(4):257–64. [PubMed] [Google Scholar]
- 127.St Croix B, Man S, Kerbel RS. Reversal of intrinsic and acquired forms of drug resistance by hyaluronidase treatment of solid tumors. Cancer Lett. 1998;131(1):35–44. doi: 10.1016/s0304-3835(98)00199-2. [DOI] [PubMed] [Google Scholar]
- 128.Baumgartner G, Gomar-Höss C, Sakr L, Ulsperger E, Wogritsch C. The impact of extracellular matrix on the chemoresistance of solid tumors - experimental and clinical results of hyaluronidase as additive to cytostatic chemotherapy. Cancer Lett. 1998;131(1):85–99. [PubMed] [Google Scholar]
- 129.Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9(6):796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 130.Novak K. Measuring the matrix. Nat Rev Cancer. 2003;3:394. [Google Scholar]
- 131.Kuhn SJ, Finch SK, Hallahan DE, Giorgio TD. Proteolytic surface functionalization enhances in vitro magnetic nanoparticle mobility through extracellular matrix. Nano Lett. 2006;6(2):306–12. doi: 10.1021/nl052241g. [DOI] [PubMed] [Google Scholar]
- 132.Goodman TT, Olive PL, Pun SH. Increased nanoparticle penetration in collagenase-treated multicellular spheroids. Int J Nanomedicine. 2007;2(2):265–74. [PMC free article] [PubMed] [Google Scholar]
- 133.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8(4):309–16. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 134.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 135.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–6. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 136.Altmann KH, Wartmann M, O'Reilly T. Epothilones and related structures - a new class of microtubule inhibitors with potent in vivo antitumor activity. Biochim Biophys Acta. 2000;1470(3):M79–91. doi: 10.1016/s0304-419x(00)00009-3. [DOI] [PubMed] [Google Scholar]
- 137.Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55(11):2325–33. [PubMed] [Google Scholar]
- 138.Pietras K, Ostman A, Sjoquist M, Buchdunger E, Reed RK, Heldin CH, et al. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001;61(7):2929–34. [PubMed] [Google Scholar]
- 139.Lee I, Boucher Y, Jain RK. Nicotinamide can lower tumor interstitial fluid pressure: mechanistic and therapeutic implications. Cancer Res. 1992;52(11):3237–40. [PubMed] [Google Scholar]
- 140.Kristjansen PEG, Boucher Y, Jain RK. Dexamethasone reduces the interstitial fluid pressure in a human colon adenocarcinoma xenograft. Cancer Res. 1993;53(20):4764–6. [PubMed] [Google Scholar]
- 141.Kristensen CA, Nozue M, Boucher Y, Jain RK. Reduction of interstitial fluid pressure after TNF-alpha treatment of three human melanoma xenografts. Br J Cancer. 1996;74(4):533–6. doi: 10.1038/bjc.1996.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Curnis F, Sacchi A, Corti A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J Clin Invest. 2002;110(4):475–82. doi: 10.1172/JCI15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Curnis F, Sacchi A, Borgna L, Magni F, Gasparri A, Corti A. Enhancement of tumor necrosis factor a antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13) Nat Biotechnol. 2000;18(11):1185–90. doi: 10.1038/81183. [DOI] [PubMed] [Google Scholar]
- 144.Rubin K, Sjoquist M, Gustafsson AM, Isaksson B, Salvessen G, Reed RK. Lowering of tumoral interstitial fluid pressure by prostaglandin E-1 is paralleled by an increased uptake of Cr-51-EDTA. Int J Cancer. 2000;86(5):636–43. doi: 10.1002/(sici)1097-0215(20000601)86:5<636::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 145.Salnikov AV, Iversen VV, Koisti M, Sundberg C, Johansson L, Stuhr LB, et al. Lowering of tumor interstitial fluid pressure specifically augments efficacy of chemotherapy. FASEB J. 2003;17(12):1756–8. doi: 10.1096/fj.02-1201fje. [DOI] [PubMed] [Google Scholar]
- 146.Emerich DF, Dean RL, Snodgrass P, Lafreniere D, Agostino M, Wiens T, et al. Bradykinin modulation of tumor vasculature: II. Activation of nitric oxide and phospholipase A(2)/prostaglandin signaling pathways synergistically modifies vascular physiology and morphology to enhance delivery of chemotherapeutic agents to tumors. J Pharmacol Exp Ther. 2001;296(2):632–41. [PubMed] [Google Scholar]
- 147.Lammerts E, Roswall P, Sundberg C, Gotwals PJ, Koteliansky VE, Reed RK, et al. Interference with TGF-beta 1 and -beta 3 in tumor stroma lowers tumor interstitial fluid pressure independently of growth in experimental carcinoma. Int J Cancer. 2002;102(5):453–62. doi: 10.1002/ijc.10722. [DOI] [PubMed] [Google Scholar]
- 148.Brekken C, Davies CD. Hyaluronidase reduces the interstitial fluid pressure in solid tumours in a non-linear concentration-dependent manner. Cancer Lett. 1998;131(1):65–70. doi: 10.1016/s0304-3835(98)00202-x. [DOI] [PubMed] [Google Scholar]
- 149.Jang SH, Wientjes MG, Au JLS. Determinants of paclitaxel uptake, accumulation and retention in solid tumors. Invest New Drugs. 2001;19(2):113–23. doi: 10.1023/a:1010662413174. [DOI] [PubMed] [Google Scholar]
- 150.Zheng JH, Chen CT, Au JLS, Wientjes MG. Time- and concentration-dependent penetration of doxorubicin in prostate tumors. AAPS PharmSci. 2001;3(2):E15. doi: 10.1208/ps030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jang SH, Wientjes MG, Au JLS. Enhancement of paclitaxel delivery to solid tumors by apoptosis-inducing pretreatment: effect of treatment schedule. J Pharmacol Exp Ther. 2001;296(3):1035–42. [PubMed] [Google Scholar]
- 152.Lu Z, Tsai M, Lu D, Wang J, Wientjes MG, Au JLS. Tumor-penetrating microparticles for intraperitoneal therapy of ovarian cancer. J Pharmacol Exp Ther. 2008;327(3):673–82. doi: 10.1124/jpet.108.140095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 1999;59(15):3776–82. [PubMed] [Google Scholar]
- 154.Kuhn SJ, Hallahan DE, Giorgio TD. Characterization of superparamagnetic nanoparticle interactions with extracellular matrix in an in vitro system. Ann Biomed Eng. 2006;34(1):51–8. doi: 10.1007/s10439-005-9004-5. [DOI] [PubMed] [Google Scholar]
- 155.Ahmed M, Goldberg SN. Combination radiofrequency thermal ablation and adjuvant IV liposomal doxorubicin increases tissue coagulation and intratumoural drug accumulation. Int J Hyperthermia. 2004;20(7):781–802. doi: 10.1080/02656730410001711655. [DOI] [PubMed] [Google Scholar]
- 156.Goldberg SN, Saldinger PF, Gazelle GS, Huertas JC, Stuart KE, Jacobs T, et al. Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intratumoral doxorubicin injection in a rat breast tumor model. Radiology. 2001;220(2):420–7. doi: 10.1148/radiology.220.2.r01au44420. [DOI] [PubMed] [Google Scholar]
- 157.Goldberg SN, Girnan GD, Lukyanov AN, Ahmed M, Monsky WL, Gazelle GS, et al. Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intravenous liposomal doxorubicin in a rat breast tumor model. Radiology. 2002;222(3):797–804. doi: 10.1148/radiol.2223010861. [DOI] [PubMed] [Google Scholar]
- 158.Monsky WL, Kruskal JB, Lukyanov AN, Girnun GD, Ahmed M, Gazelle GS, et al. Radio-frequency ablation increases intratumoral liposomal doxorubicin accumulation in a rat breast tumor model. Radiology. 2002;224(3):823–9. doi: 10.1148/radiol.2243011421. [DOI] [PubMed] [Google Scholar]
- 159.Zhang A, Mi X, Yang G, Xu LX. Numerical study of thermally targeted liposomal drug delivery in tumor. J Heat Transfer. 2009;131:043209. [Google Scholar]
- 160.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51(4):691–744. [PubMed] [Google Scholar]
- 161.Lee H, Hoang B, Fonge H, Reilly R, Allen C. In vitro distribution of polymeric nanoparticles at the whole-body, tumor, and cellular levels. Pharm Res. 2010;27(11):2343–55. doi: 10.1007/s11095-010-0068-z. [DOI] [PubMed] [Google Scholar]
- 162.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2(4):249–55. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fox ME, Szoka FC, Fréchet JMJ. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. Acc Chem Res. 2009;42(8):1141–51. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]


