Abstract
CD40L is critically important for the initiation and maintenance of adaptive immune responses. It is generally thought that CD40L expression in CD4+ T cells is regulated transcriptionally and made from new mRNA following antigen recognition. However, recent studies with two-photon microscopy revealed that the majority of cognate interactions between effector CD4+ T cells and APCs are too short for de novo synthesis of CD40L. Given that effector and memory CD4+ T cells store preformed CD40L (pCD40L) in lysosomal compartments and that pCD40L comes to the cell surface within minutes of antigenic stimulation, we and others have proposed that pCD40L might mediate T cell-dependent activation of cognate APCs during brief encounters in vivo. However, it has not been shown that this relatively small amount of pCD40L is sufficient to activate APCs, owing to the difficulty of separating the effects of pCD40L from those of de novo CD40L and other cytokines in vitro. Here we show that pCD40L surface mobilization is resistant to cyclosporine or FK506 treatment, while de novo CD40L and cytokine expression are completely inhibited. These drugs thus provide a tool to dissect the role of pCD40L in APC activation. We find that pCD40L mediates selective activation of cognate but not bystander APCs in vitro and that mobilization of pCD40L does not depend on Rab27a, which is required for mobilization of lytic granules. Therefore, effector CD4+ T cells deliver pCD40L specifically to APCs on the same time scale as the lethal hit of CTLs but with distinct molecular machinery.
Introduction
CD40L (CD154), a member of the TNF superfamily, mediates T cell help for APCs during humoral and cellular immune responses (1–4). It is generally thought that CD40L is synthesized from new mRNA (de novo CD40L) and delivered while effector CD4+ T cells are engaged in intimate interactions with cognate APCs in the time frame of a few hours (5). However, we and others have demonstrated that human and mouse effector and resting memory CD4+ T cells retain preformed CD40L (pCD40L) intracellularly, and that pCD40L can come to the cell surface within a few minutes of antigenic stimulation (6, 7).
Given that the interactions between effector CD4+ T cells and APCs are typically brief in vivo (8–12), we propose that pCD40L is rapidly delivered to cognate APCs on a time scale of minutes. Our previous study (7) showed that pCD40L is stored in lysosome-related organelles known as secretory lysosomes, a category of secretory vesicles which includes the lytic granules in CTLs and NK cells (13). Lytic granules are secreted through the center of adhesion ring of the immunological synapse formed between the T cell and target cell (14), indicating that the immunological synapse may serve to ensure efficient delivery of effector functions to specific targets but not to bystanders (15, 16). Although the findings mentioned above suggest that effector CD4+ T cells may selectively activate cognate APCs by directional delivery of pCD40L, it remains to be shown that pCD40L is delivered to the cell surface in sufficient amounts to activate APC, and is not merely in the process of being degraded in lysosomes following CD40 engagement and internalization (17). Also, it has not been addressed whether compartments containing pCD40L use the same trafficking machinery used by lytic granules in CTLs and NK cells, such as the small GTPase, Rab27a (18).
In the present study, taking advantage of selective suppression of de novo CD40L and cytokine expression by the calcineurin inhibitors, cyclosporine A (CsA) and FK506, we show that pCD40L from effector CD4+ T cells is sufficient to activate APCs and provide help selectively to cognate APCs. We also show that the delivery of pCD40L by effector CD4+ T cells depends on distinct molecular machinery from that required for delivery of lytic granules to target cells by CTLs and NK cells.
Materials and Methods
Mice
Mice were housed under specific pathogen–free conditions. These studies were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University. BALB/c, C57BL/6, C.B-17 (Ighb), Cd40lg−/−, CD45.1 congenic, and DO11.10 mice were from the Jackson Laboratory (Bar Harbor, ME). Cd40lg−/− DO11.10 mice were bred in-house. DO11.10 Rag2−/− and BALB/c nu/nu mice were obtained from Taconic Farms (Germantown, NY). ashen mice on a C57BL/6 background were obtained from Dr. Miguel Seabra (Imperial College London, United Kingdom). SMARTA mice, which have a transgenic TCR specific for a lymphocytic choriomeningitis virus (LCMV) epitope (19), were obtained from Dr. J. Lindsay Whitton (The Scripps Research Institute). ashen/+ or ashen/ashen SMARTA mice were bred in-house. Cd40−/− spleens were provided by Dr. David Hinrichs (Veterans Affairs Medical Center, OR).
Antibodies and reagents
PE-Cy7-anti-Fas was purchased from BD Biosciences (San Jose, CA). FITC-PNA was from Vector Lab. Inc. (Burlingame, CA). All other antibodies for flow cytometry were purchased from eBioscience (San Diego, CA). Recombinant cytokines were purchased from Peprotech (Rocky Hill, NJ). Anti-IL-4 was from Bio X Cell (West Lebanon, NH). Easy Sep mouse CD4+ T cell enrichment and B cell enrichment kits were from Stemcell Technologies (Vancouver, Canada). Papain was from Calbiochem (San Diego, CA). Endotoxin-free OVA protein was from Profos AG (Regensburg, Germany). OVA peptide (323–339) was from AnaSpec, Inc. (Fremont, CA). LCMV peptide gp67 was from New England Peptide (Gardner, MA). LPS (L6761), PMA, ionomycin, CsA, and FK506 were from Sigma-Aldrich (St Louis, MO).
In vitro-generated Th1 cells
In vitro-generated Th1 cells were prepared by culturing spleen cells from DO11.10 mice in the presence of 1 µM of antigenic peptide (OVA 323–339) for 4 days in the presence of 1 ng/ml IL-12 and 10 µg/ml anti-IL-4. To prepare ash/+ and ash/ash polyclonal Th1 cells, purified CD4+ T cells were incubated with Mouse T-Activator CD3/CD28 beads (Invitrogen, Carlsbad, CA) at a 1:1 ratio for 4 days in Th1 conditions.
In vivo-generated effector CD4+ T cells
In vivo-generated effector CD4+ T cells were obtained from the draining lymph nodes (dLNs) of BALB/c nu/nu recipients which had received 5 × 105 naïve purified CD4+ T cells from DO11.10 Rag2−/− mice followed by subcutaneous immunization with OVA protein (50 µg) plus papain (50 µg) (20). For Fig.7, antigen-specific in vivo Th1 cells were recovered on day 8 post-infection from LCMV (i.p. infection with 2 × 105 PFU of LCMV Armstrong 53b)-infected recipient C57BL/6 mice given 2 × 104 ash/+ or ash/ash SMARTA cells (7).
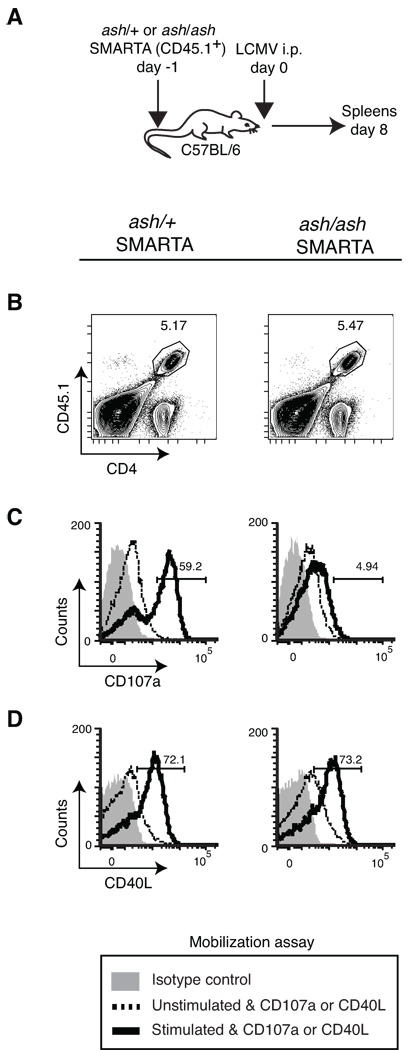
Figure 7. Surface mobilization of pCD40L in ex vivo effector CD4+ T cells upon antigenic stimulation is Rab27a-independent.
A, CD4+ T cells from ash/+ or ash/ash SMARTA TCR transgenic mice were transferred into WT mice. Recipients were intraperitoneally infected with 2 × 105 PFU of LCMV. On day 8 post-infection, spleen cells were collected for the mobilization assay. B, Gating strategy for in vivo-generated ash/+ and ash/ash SMARTA effector CD4+ T cells (CD4+CD45.1+). C and D, In vivo-generated ash/+ and ash/ash SMARTA effector CD4+ T cells were analyzed for mobilization of CD107a (C) and pCD40L (D) upon incubation with antigen-unpulsed APCs (unstimulated) or antigen-pulsed APCs (stimulated) ex vivo for 30 minutes. The numbers in the histograms indicate the percentage of stimulated cells that mobilize CD107a or CD40L. The mobilization assay was conducted in the absence of either CsA or FK506 treatment. Data are representative of two independent experiments (n = 2– 3 per group).
Flow Cytometry
The surface mobilization assay was described previously (7). Briefly, in the surface mobilization assay, fluorochrome-labeled anti-CD40L mAb is included in the culture during the activation of cells at 37°C. Compared to the “snap shot” nature of conventional staining at 4°C after completion of stimulation, the mobilization assay captures CD40L that has been delivered to the cell surface during stimulation while blocking CD40-dependent internalization, thereby providing the “long exposure” view of CD40L expression. By limiting the stimulation period to 30 minutes, we were able to exclude the expression of de novo CD40L as shown previously (7). Data were obtained with an LSR II (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR).
In vitro and ex vivo T helper assay for B cells
In vitro Th1 cells and in vivo effector CD4+ T cells were generated as described above. WT or Cd40−/− spleen cells were differentially labeled with CFSE (21) or the CellTrace Violet dye (Invitrogen) to distinguish cognate (peptide-pulsed) and bystander (unpulsed) populations. Antigenic peptide was pulsed at 1 µM concentration at 37 °C for 2 hours followed by extensive washes. WT or Cd40lg−/− in vitro-generated Th1 or in vivo-generated effector CD4+ T cells were pretreated with 1 µM CsA at 37 °C for one hour and co-incubated with a mixture of cognate and bystander spleen cells from WT or Cd40−/− mice in the presence of CsA throughout the assay. After the indicated hours of incubation, B cell activation was evaluated by staining for CD86, MHC class II, ICAM-1, CD62L, and IL-21 receptor. For analysis of cell division, cognate and bystander B cells were labeled with the same concentration of CFSE and were distinguished by IgM allotype (IgMa versus IgMb), and cell division was measured by CFSE dilution. For analysis of differentiation, cognate and bystander B cells were labeled with the same concentration of CFSE and were distinguished by the CD45 congenic marker (CD45.1 versus CD45.2). The T helper assay was conducted in the presence of IL-4 for 3 days to assess proliferation by CFSE dilution, and in the presence of IL-4 (day 0–4) plus IL-21 (day 2–4) to assess B cell differentiation by staining for Fas, GL7, and CD138. Alternatively, cognate and bystander B cells were distinguished by differentially labeling them with CFSE or CellTrace Violet dye.
In vitro T helper assay for dendritic cells (DCs)
Bone marrow-derived DCs (BMDCs) were generated as previously described (22). Drug pretreated or untreated WT or Cd40lg−/− in vitro-generated Th1 cells were co-cultured with BMDCs in the presence or absence of antigenic peptide, CsA (1 µM) or FK506 (0.1 µM), and isotype control Ab or anti-CD40L for 18 hours. The level of CD70 on DCs was analyzed by flow cytometry. IL-6 production from DCs was analyzed by ELISA (eBioscience).
Statistics
p values were determined by the unpaired Student’s t test. All results are shown as the mean and the standard deviation of the mean. A p value of < 0.05 was considered significant.
Results
Calcineurin inhibitors block de novo CD40L and cytokine synthesis but do not interfere with pCD40L surface mobilization
We proposed that pCD40L has a physiological role to selectively activate cognate APCs during brief encounters often observed in vivo. Because de novo CD40L and cytokines can cause general activation of APCs regardless of antigen-specificity in vitro, we sought culture conditions in which de novo CD40L and other cytokines made by Th1 cells are blocked, but surface mobilization of pCD40L is preserved. Although we used cycloheximide to block de novo CD40L and cytokines but to preserve pCD40L in the previous study (7), cycloheximide could not be used in the present study because it inhibited the upregulation of activation markers on APCs, as expected (data not shown). We also conducted experiments with the irreversible protein synthesis inhibitor, emetine. However, we found that T cells pretreated with emetine and extensively washed released sufficient emetine to inhibit APC activation (data not shown). We then tested the calcineurin inhibitors, CsA or FK506, known to inhibit de novo CD40L expression (23). CsA does not interfere with CD40-mediated B cell activation by fixed, activated CD4+ T cells (24) or agonistic anti-CD40 (data not shown). These drugs also inhibit T cell production of IFN-γ and IL-4 (25). Upon stimulation with PMA plus ionomycin, Th1 cells showed evident pCD40L mobilization at 30 minutes (Fig. 1A, left) as well as robust induction and surface expression of de novo CD40L at 120 minutes (Fig. 1A, right). CsA or FK506 pre-treatment completely inhibited activation-induced expression of de novo CD40L but had no effect on surface mobilization of pCD40L (Fig. 1B). The efficacy of CsA and FK506 was confirmed by complete suppression of IFN-γ production throughout this study as shown in Fig. 1C.
Figure 1. Calcineurin inhibitors block de novo CD40L expression and cytokine secretion but not surface mobilization of pCD40L.
A, Levels of CD40L mobilization during a 30 minute incubation (left: preformed CD40L) and a 120 minute incubation (right: preformed plus de novo CD40L) upon PMA plus ionomycin stimulation. B, The effect of CsA (left) or FK506 (right) on the levels of CD40L mobilization upon 30 or 120 minute PMA plus ionomycin stimulation. C, WT or Cd40lg−/− Th1 cells were incubated with or without PMA plus ionomycin overnight in the presence or absence of CsA or FK506. IFN-γ production was measured by ELISA with a detection limit of <0.06 ng/mL. N.D., Not detected. Each bar represents the mean ± standard deviation for triplicates. Data are representative of three independent experiments (A,B) or at least five independent experiments (C).
pCD40L in Th1 cells selectively activates cognate B cells and promotes their proliferation and differentiation in vitro
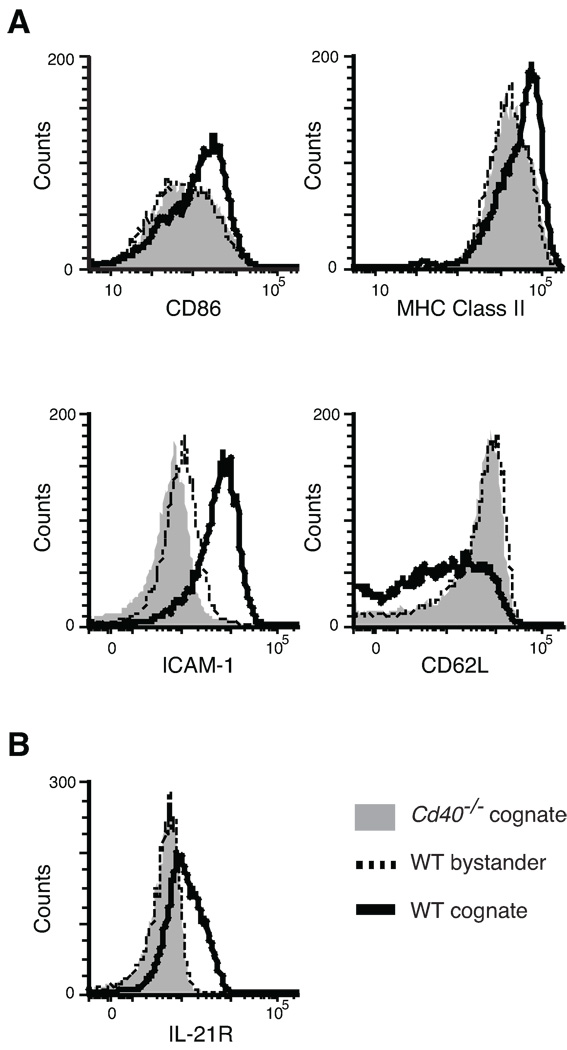
To determine whether surface mobilization of pCD40L is sufficient for antigen-specific T cell help for B cells, we measured B cell activation. Cognate (antigenic peptide-pulsed) and bystander (unpulsed) B cells were differentially labeled with CFSE, and were cultured with Th1 cells in the presence of CsA. Cd40−/− B cells were used as a negative control in separate cultures. CsA was maintained in the culture medium throughout the incubation period. CD40L-dependent up-regulation of CD86, MHC class II, and ICAM-1 (CD54), and down-regulation of CD62L were observed in cognate but not in bystander B cells after overnight culture (Fig. 2A). Increased size (forward scatter) and granularity (side scatter) were also observed in cognate B cells only (data not shown). By day 2, cognate but not bystander B cells up-regulate IL-21 receptor (IL-21R) in a CD40-dependent manner (Fig. 2B). By these measures of B cell activation, the stimulatory capacity of pCD40L (with CsA) was comparable to that of de novo CD40L (no drug) (Supplemental Fig.1). We also observed a weak but noticeable level of bystander activation of B cells by de novo CD40L, but not by pCD40L, when assessed by ICAM-1 and IL-21R levels (Supplemental Fig.1B, C).
Figure 2. pCD40L in Th1 cells is sufficient to mediate selective activation of cognate B cells.
In vitro T helper assay. A–B, CsA pretreated Th1 cells were mixed with cognate (peptide-pulsed) and bystander (unpulsed) spleen cells for 18 hours (A) or 2 days (B). As a control, Cd40−/− spleen cells were used. The figures show the levels of CD86, MHC class II, ICAM-1 (CD54), CD62L (A), and IL-21 receptor (IL-21R) (B) in control, bystander, and cognate B cells. Data are representative of five independent experiments.
To determine whether pCD40L is sufficient to initiate T cell-dependent B cell proliferation, IL-4 was added to WT or Cd40lg−/− Th1 cells co-cultured with B cells for 3 days in the presence of CsA, and CFSE dilution was analyzed for both cognate and bystander B cells. Cognate and bystander B cells were differentiated by IgM allotype. The data show that Th1 cells promote proliferation of cognate but not bystander B cells in a CD40L-dependent manner in the presence of CsA (Fig. 3A). We obtained the same result when the IgM allotypes of cognate and bystander B cells were switched (data not shown). Both cognate and bystander B cells responded well with anti-CD40 plus IL-4 stimulation (Fig. 3A far right). When antibody was used to neutralize CD40L instead of using Cd40lg−/− Th1 cells, we obtained a similar result (data not shown). The ability of pCD40L to induce B cell proliferation in the presence of IL-4 is slightly inferior to that of de novo CD40L (no drug) (Supplemental Fig. 2A). We also tested whether pCD40L is sufficient to induce B cell differentiation in cultures containing IL-4 (added on day 0) and IL-21 (added on day 2). Cognate B cells but not bystander B cells acquired surface markers of germinal center (GC) B cells (Fas+GL7+) and plasma cells (CD138+) in a CD40-dependent manner (Fig. 3B,C). The ability of pCD40L to induce germinal center and plasma cell markers is comparable to that of de novo CD40L (Supplemental Fig. 2B, C). These results clearly show that pCD40L in Th1 cells is sufficient to mediate antigen-specific B cell activation and promote proliferation and differentiation of cognate B cells in concert with IL-4 and IL-21.
Figure 3. pCD40L in Th1 cells can mediate T cell-induced selective proliferation and differentiation of cognate B cells.
Exogenous helper cytokine(s) were added to assess the potential of pCD40L to support B cell proliferation and differentiation. A, CsA treated WT or Cd40lg−/− Th1 cells were incubated with CFSE-labeled bystander (IgMa) plus cognate (IgMb) B cells in the presence of IL-4. CsA was added daily throughout the culture period. As a positive control, B cells were stimulated with anti-CD40 plus IL-4 in the presence of CsA. On day 3, CFSE dilution was evaluated. B–C, CsA treated Th1 cells were incubated with CFSE-labeled bystander (CD45.2+) and cognate (CD45.1+) B cells in the presence of IL-4 (day 0–4) and IL-21 (day2–4). As a control, Cd40−/− B cells were used. CsA was added daily throughout the culture period. As a positive control, B cells were stimulated with anti-IgM, agonistic anti-CD40, IL-4 and IL-21 in the absence of CsA. On day 4, GC markers (GL7 and Fas, B) and the plasma cell marker (CD138, C) were evaluated. Data are representative of four independent experiments.
In vivo-generated effector CD4+ T cells can selectively activate cognate B cells via pCD40L
Although we observed that pCD40L in Th1 cells generated in vitro can mediate cognate B cell activation, we were concerned that this finding could be an artifact of T cells activated in vitro. Therefore, we conducted a similar experiment using in vivo-generated effector CD4+ T cells. To generate effector CD4+ T cells in vivo, purified naïve CD4+ T cells from DO11.10 Rag2−/− mice were transferred into nude mice, followed by subcutaneous immunization with papain plus OVA. Six days after immunization, cells from dLNs showed a robust expansion of effector CD4+ T cells as well as a vigorous GC reaction in an immunized mouse (Fig. 4A) compared to an unimmunized nude mouse (Fig. 4B), indicating that in vivo-generated effector CD4+ T cells are capable of helping B cells in vivo. The expanded CD4+ T cells are CD44hi effector cells and possess abundant pCD40L (Fig. 4C and data not shown). Highly purified (> 95%) in vivo-generated effector CD4+ T cells were used in an ex vivo T helper assay in the presence of CsA. Like in vitro-generated Th1 cells, in vivo-generated effector CD4+ T cells are capable of activating cognate, but not bystander, B cell activation in a pCD40L-dependent manner as shown by increased ICAM-1 expression (Fig. 4D).
Figure 4. pCD40L in ex vivo effector CD4+ T cells is sufficient to mediate selective activation of cognate B cells.
A–B, Naïve CD4+ T cells from DO11.10 Rag2−/− mice were transferred into BALB/c nu/nu recipient mice followed by subcutaneous immunization with papain plus OVA to obtain in vivo-generated effector CD4+ T cells. The percentages of CD4+ T cells, B cells, and GC (Fas+, PNA+) B cells in dLNs from a BALB/c nu/nu immunized recipient on day 6 after immunization (A) or from a naive BALB/c nu/nu mouse (B) is shown. C, The mobilization assay for pCD40L in donor CD4+ T cells from a BALB/c nu/nu immunized recipient. D, Purified CD4+ T cells from BALB/c nu/nu immunized recipients were incubated overnight in the presence of CsA with cognate and bystander WT B cells, or cognate Cd40−/− B cells, differentially labeled with CFSE. ICAM-1 expression on each B cell population was analyzed. The number in the histogram indicates the percentage of cognate B cells that upregulate ICAM-1. Data are representative of two independent experiments.
pCD40L can trigger DC activation
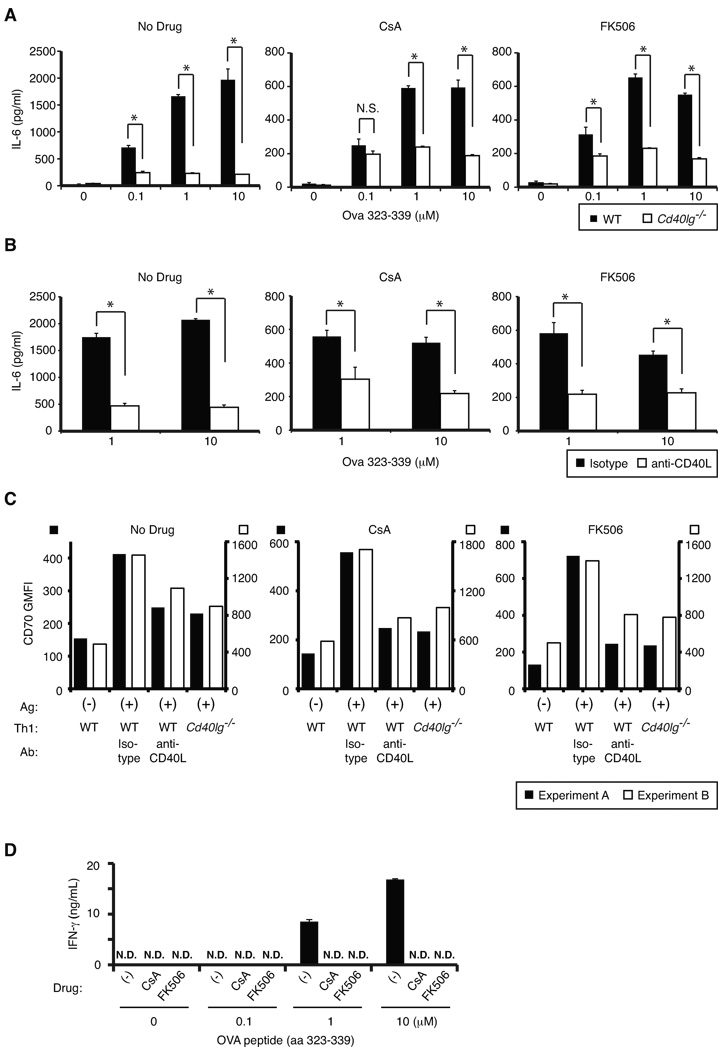
Another important function of CD40L is licensing of DC, which is crucial for generating effective CD4+ and CD8+ T cell responses (4). Taking advantage of the fact that in vitro-generated Th1 cells do not produce detectable IL-6 (data not shown), we measured IL-6 production from bone marrow-derived DCs (BMDCs) to evaluate the ability of pCD40L to activate DCs. WT or Cd40lg−/− DO11.10 Th1 cells were co-cultured with BMDCs in the presence or absence of antigenic peptide and CsA or FK506 for 18 hours. Although the magnitude of IL-6 production is significantly lower in the CsA- or FK506-treated groups than the untreated group, treated Th1 cells still clearly induce IL-6 production from BMDCs in a peptide dose- and CD40L-dependent manner (Fig. 5A). CsA or FK506 treatment did not inhibit IL-6 production from DCs upon LPS stimulation (data not shown). To exclude potential differences between WT and Cd40lg−/− Th1 cells other than CD40L itself, we conducted similar experiments using WT in vitro-generated Th1 cells in the presence of either control Ab or neutralizing anti-CD40L. This experiment yielded a similar result (Fig. 5B). We also evaluated expression of CD70, which is known to be upregulated by a CD40 signaling and facilitates primary and secondary CD8+ T cell responses (26). We observed that Th1 cells induced upregulation of CD70 on DCs in the presence of antigenic peptide in a CD40L-dependent fashion in the presence of CsA or FK506 to levels comparable to the drug untreated group, suggesting that pCD40L is sufficient to efficiently induce CD70 upregulation on DCs (Fig. 5C). The efficacy of CsA and FK506 was confirmed by complete suppression of T cell IFN-γ production induced by DCs in the presence of antigenic peptide (Fig. 5D). Together, these results show that pCD40L can also mediate T cell-dependent DC activation.
Figure 5. pCD40L in Th1 cells is sufficient to activate DCs.
A–B, IL-6 production from BMDCs co-cultured with in vitro-generated WT or Cd40lg−/− Th1 cells for 18 hours in the presence or absence of antigenic peptide, CsA or FK506, and isotype Ab or anti-CD40L was measured by ELISA. Note that different y-axes were used for the groups with drug (CsA or FK506) and without. C, CD70 upregulation was assessed after overnight culture of DCs with Th1 cells in the presence or absence of antigenic peptide, CsA or FK506, and isotype Ab or anti-CD40L. Raw geometric mean fluorescent intensity (GMFI) of CD70 staining from two representative experiments is shown. D, WT Th1 cells were incubated with BMDCs in the presence or absence of OVA peptide overnight. CsA and FK506 were kept in the media throughout the culture. IFN-γ production was measured by ELISA with a detection limit of <0.06 ng/mL. N.D., Not detected. Each bar represents the mean ± standard deviation for triplicates. *p < 0.01. N.S., Not significant. Data are representative of three independent experiments.
The mobilization of pCD40L uses distinct machinery from that of lytic granules
Our previous study indicated that pCD40L exists in secretory lysosomes, a category of secretory vesicles which includes the lytic granules of CTLs and NK cells (7). To investigate the molecular mechanism of pCD40L mobilization from cytoplasm to cell surface, we used ashen mice, which are defective in release of lytic granule contents due to a mutation in the small GTPase Rab27a (18). First, we compared the surface mobilization of pCD40L with CD107a in polyclonal Th1 cells generated with CD4+ T cells from ash/+ or ash/ash mice. The mobilization of CD107a to the cell surface is an indication of the release of lytic granules (27). As expected, ash/ash Th1 cells have defective CD107a mobilization following all three stimuli tested (Fig. 6A). However, the mobilization of pCD40L is unimpaired in ash/ash Th1 cells (Fig. 6B). To confirm this phenotype using in vivo-generated effector CD4+ T cells, ashen mice were bred with SMARTA TCR transgenic mice. WT recipient mice of ash/+ or ash/ash SMARTA CD4+ T cells were infected with LCMV. On day 8 post-infection, splenocytes from infected animals were used ex vivo for evaluation of pCD40L mobilization (Fig. 7A, B). While the mobilization of CD107a is clearly impaired in ash/ash SMARTA effector CD4+ T cells following antigenic stimulation (Fig. 7C), pCD40L mobilization is maintained (Fig. 7D). We conclude that effector CD4+ T cells use distinct machinery for the mobilization of pCD40L from that of lytic granules.
Figure 6. Surface mobilization of pCD40L in Th1 cells is Rab27a-independent.
In vitro-generated polyclonal Th1 cells from ash/+ and ash/ash CD4+ T cells were analyzed for mobilization of CD107a (A) and pCD40L (B) upon stimulation with PMA plus ionomycin, anti-CD3 plus anti-LFA-1, or anti-CD3 plus anti-ICOS for 30 minutes. The numbers in the histograms indicate the percentage of stimulated cells that mobilize CD107a or CD40L. The mobilization assay was conducted in the absence of either CsA or FK506 treatment. Data are representative of three independent experiments (n = 2– 3 per group).
Discussion
In vivo studies using CD40L knockout mice and neutralizing anti-CD40L antibody have firmly established that CD40L mediates T cell help (2). In the classic model, effector CD4+ T cells deliver de novo CD40L, along with cytokines, to cognate APCs during prolonged interactions lasting hours (5). However, recent imaging studies with two-photon microscopy show clearly that effector CD4+ T cells do not usually have enough time to synthesize de novo CD40L. Given that effector CD4+ T cells also store intracellular pCD40L and mobilize it to the cell surface immediately after TCR stimulation, we proposed that pCD40L can mediate cognate APC activation during the brief antigen-specific interactions that dominate in vivo. In the current study, we blocked the synthesis of de novo CD40L and soluble cytokines while preserving the surface mobilization of pCD40L in effector CD4+ T cells, and demonstrated that pCD40L can selectively deliver T cell help to cognate B cells and trigger DC activation.
We speculate that effector CD4+ T cells deliver help to antigen-specific B cells using pCD40L at the T zone-B zone boundary, at extrafollicular sites, and in the GC (3). In situ staining studies have detected CD40L staining in all of locations listed above (6, 28). Studies with neutralizing anti-CD40L clearly show that CD40L is indispensable not only for the initiation but also for the maintenance of GCs, as well as the differentiation and affinity maturation of GC B cells (29, 30). However, we think that pCD40L plays a minor role during stable interactions between effector CD4+ T cells and cognate B cells owing to the opportunity for abundant delivery of de novo CD40L and cytokines. B cells undergoing stable interaction with T cells seem to be poised to differentiate into plasma cells. Several studies have shown that high affinity B cells preferentially become plasma cells (31–33). It is believed that high affinity B cells gather, process, and present more antigen (34, 35). The immunological synapse formed by T cells is a versatile structure which can be re-oriented from APCs bearing fewer peptide/MHC to those bearing more peptide/MHC (36). A study with two-photon microscopy estimated that each cognate GC B cell encounters as many as 50 T cells per hour (9), allowing T cells to form stable interactions with rare high affinity GC B cells (37). During stable interaction, GC B cells would receive a prolonged CD40 signaling as well as cytokines including IL-21 (38). Since IL-21 induces both the transcriptional factors necessary for maintaining GC B cell phenotype (Bcl-6) and plasma cell differentiation (Blimp-1) (39–41), and these two transcriptional factors counter-regulate each other (42), it is likely that a prolonged CD40 signaling owing primarily to de novo CD40L would promote plasma cell differentiation of GC B cells by repressing Bcl-6 via upregulation of IRF-4 expression (43).
We propose that pCD40L plays a major role in facilitating the affinity maturation process during brief interactions between low affinity GC B cells and T cells in the light zone of GCs, as suggested previously (6). Compared to high affinity B cells, low affinity B cells preferentially seed GC reactions (31, 32). Increased peptide/MHC expression on GC B cells regardless of BCR affinity via DEC205-mediated Ag delivery enhanced plasma cell differentiation and compromised affinity maturation (44). This result may be explained partly by prolonged CD40 signaling owing to forced stable interactions between low affinity GC B cells and T cells. Similarly, heightened CD40 signaling caused by agonistic anti-CD40 or a CD40L transgene in B cells also resulted in skewed short-lived plasma cell differentiation of GC B cells accompanied by premature termination of GC reactions, compromised affinity maturation, and diminished generation of long-lived plasma cells (45–47). A recent study suggested a model that explains how transient CD40 signaling facilitates affinity maturation of GC B cells by deletion of cells with damaged DNA (48). Brief CD40 signaling can induce temporary disruption of Bcl-6 repressor functions, promoting rapid, transient expression of DNA checkpoint genes and subsequent apoptosis of GC B cells with unrepaired DNA lesions (48). In fact, one quarter of T cells in GCs are associated with blebs from dead GC B cells (9). In this scenario, surviving GC B cells may undergo further affinity maturation by rapidly recovering Bcl-6 function (49). Whether a low avidity T cell-B cell interaction is sufficient to induce the mobilization of pCD40L, as has been reported for preformed Fas ligand (pFasL) in CTLs (50), needs to be addressed experimentally. A recent study showed that a transient BCR signaling increases B cell sensitivity to CD40L (51). This mechanism may also play an important role for optimizing a pCD40L-mediated selection process. Enrichment of both BCR and CD40 signaling signatures in the light zone compared to the dark zone of GC by microarray analysis further supports this notion (44). Although our data showed that pCD40L could promote acquisition of plasma cell markers by cognate B cells, this may simply reflect favorable conditions for T cell help in vitro owing to the lack of the germinal center environment that enforces intermittent CD40 signaling. Nevertheless, our data reveal the competence of pCD40L for B cell activation.
Another important function of pCD40L may be DC licensing. Two-photon microscopy shows predominantly brief interactions of effector memory CD4+ T cells with APCs in target tissues (11), suggesting a role for pCD40L during the effector phase of inflammation, probably through promoting cytokine secretion and upregulation of costimulatory molecules in DCs. Importantly, pCD40L has been implicated in the pathogenesis of rheumatoid arthritis (52). In this study, peripheral blood CD4+ T cells and synovial fluid CD4+ T cells from patients with rheumatoid arthritis, but not healthy donor CD4+ T cells, stored and mobilized pCD40L. Why healthy donor CD4+ T cells lacked pCD40L in this study is unknown, although it is possible that the sensitivity of the assay and/or human-mouse differences are contributing factors. The authors further showed, using stimulated and fixed patient CD4+ T cells, that the amount of pCD40L that came to the cell surface upon stimulation with PMA plus ionomycin was sufficient to trigger IL-12 production from DCs. CsA-insensitive CD40L, presumably pCD40L, has been detected on CD4+ T cells from systemic lupus erythematosus patients (53, 54). These findings suggest a role for dysregulation of pCD40L in autoimmune settings. Possible regulatory functions of pCD40L (for example, induction of IL-10 production from macrophages (55) or dampening innate immune responses by inhibiting inflammasomes (56)) should also be included in considering pCD40L as a therapeutic target for autoimmune or inflammatory diseases (57).
The mobilization of pCD40L is Rab27a-independent, indicating that pCD40L is stored in compartments distinct from lytic granules. Since the acquisition of lytic granules is limited to effector T cells (13), our recent finding of pCD40L expression in CD4 single positive thymocytes, naïve CD4+ T cells, and resting memory CD4+ T cells provides further evidence that the pCD40L-containing compartment is distinct from lytic granules (7) (Koguchi et al., manuscript in preparation). Although pFasL has been observed in lytic granules (58), recent reports indicate that pFasL also resides in compartments distinct from lytic granules because surface expression of pFasL is resistant to a microtubule inhibitor that blocks release of lytic granules (59) and occurs at a lower stimulation threshold than that of lytic granules (50). Our previous study showed that pCD40L is colocalized strongly with FasL in Th1 cells (7). Together, these results suggest the existence of a distinct trafficking mechanism for rapid delivery of pCD40L and other TNF family members to the cell surface in CD4+ T cells. Evidence is accumulating that other TNF superfamily members are stored in secretory compartments in hematopoietic cells, including CD70 and CD40L in DCs (60, 61) and pCD40L and LIGHT in platelets (62, 63). Investigation of this trafficking machinery will provide methods to dissect the functions of preformed versus newly synthesized CD40L in vivo through the generation of knockout and transgenic mice lacking one or the other. It might also offer insight into the regulation of the many biological functions mediated by other TNF family members. Such efforts will provide new therapeutic targets for immunosuppression, including methods to further increase the efficacy of calcineurin inhibitors for established inflammatory conditions by blocking residual T cell-dependent APC activation triggered by pCD40L.
Supplementary Material
Acknowledgements
We thank Dr. Susan Murray for reviewing this manuscript. We thank Dr. Mark Slifka for providing LCMV stock, Dr. J. Lindsay Whitton, The Scripps Research Institute, for providing SMARTA mice, Dr. Miguel Seabra, Imperial College London, for providing ashen mice on a C57BL/6 background, and Dr. David Hinrichs, Veterans Affairs Medical Center in Oregon, for providing Cd40−/− spleens. We also thank Katelynne Gardner Toren and Fanny Polesso for excellent technical assistance.
This study was supported by National Institutes of Health grants R01 AI050823, R01 AI070934, and R21 AI077032 to D.C.P. J. L. G. and T.J.T. have been supported as trainees on an institutional training grant from the National Institutes of Health, T32 AI078903.
Abbreviations
- BMDC
bone marrow-derived dendritic cells
- CsA
cyclosporine A
- dLN
draining lymph node
- pFasL
preformed Fas ligand
- GC
germinal center
- LCMV
lymphocytic choriomeningitis virus
- MP
memory phenotype
- pCD40L
preformed CD40L.
Footnotes
Disclosures
The authors declare no competing financial interests.
References
- 1.Parker DC. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 2.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 3.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 4.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 5.Murphy KM, Travers P, Walport M. Janeway’s Immunobiology. New York, NY: Garland Science, Taylor & Francis Group, LLC; 2008. [Google Scholar]
- 6.Casamayor-Palleja M, Khan M, MacLennan IC. A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J Exp Med. 1995;181:1293–1301. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koguchi Y, Thauland TJ, Slifka MK, Parker DC. Preformed CD40 ligand exists in secretory lysosomes in effector and memory CD4+ T cells and is quickly expressed on the cell surface in an antigen-specific manner. Blood. 2007;110:2520–2527. doi: 10.1182/blood-2007-03-081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O'Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 10.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, de la Maza LM, Monuki E, Flugel A, Pennington MW, Parker I, Chandy KG, Cahalan MD. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity. 2008;29:602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- 14.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins MR, Griffiths GM. The synapse and cytolytic machinery of cytotoxic T cells. Curr Opin Immunol. 2010;22:308–313. doi: 10.1016/j.coi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thauland TJ, Parker DC. Diversity in immunological synapse structure. Immunology. 2010;131:466–472. doi: 10.1111/j.1365-2567.2010.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yellin MJ, Sippel K, Inghirami G, Covey LR, Lee JJ, Sinning J, Clark EA, Chess L, Lederman S. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell-B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J Immunol. 1994;152:598–608. [PubMed] [Google Scholar]
- 18.Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152:825–834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 20.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huddleston CA, Weinberg AD, Parker DC. OX40 (CD134) engagement drives differentiation of CD4+ T cells to effector cells. Eur J Immunol. 2006;36:1093–1103. doi: 10.1002/eji.200535637. [DOI] [PubMed] [Google Scholar]
- 22.Son YI, Egawa S, Tatsumi T, Redlinger RE, Jr, Kalinski P, Kanto T. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J Immunol Methods. 2002;262:145–157. doi: 10.1016/s0022-1759(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 23.Fuleihan R, Ramesh N, Horner A, Ahern D, Belshaw PJ, Alberg DG, Stamenkovic I, Harmon W, Geha RS. Cyclosporin A inhibits CD40 ligand expression in T lymphocytes. J Clin Invest. 1994;93:1315–1320. doi: 10.1172/JCI117089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawakami K, Parker DC. Antigen and helper T lymphocytes activate B lymphocytes by distinct signaling pathways. Eur J Immunol. 1993;23:77–84. doi: 10.1002/eji.1830230113. [DOI] [PubMed] [Google Scholar]
- 25.Hermann-Kleiter N, Baier G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood. 2010;115:2989–2997. doi: 10.1182/blood-2009-10-233585. [DOI] [PubMed] [Google Scholar]
- 26.Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol. 2005;17:275–281. doi: 10.1016/j.coi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 28.Van den Eertwegh AJ, Noelle RJ, Roy M, Shepherd DM, Aruffo A, Ledbetter JA, Boersma WJ, Claassen E. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T-B cell interactions. J Exp Med. 1993;178:1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- 30.Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J Exp Med. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connor BP, Vogel LA, Zhang W, Loo W, Shnider D, Lind EF, Ratliff M, Noelle RJ, Erickson LD. Imprinting the fate of antigen-reactive B cells through the affinity of the B cell receptor. J Immunol. 2006;177:7723–7732. doi: 10.4049/jimmunol.177.11.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol. 2009;183:3139–3149. doi: 10.4049/jimmunol.0901690. [DOI] [PubMed] [Google Scholar]
- 34.Harwood NE, Batista FD. New insights into the early molecular events underlying B cell activation. Immunity. 2008;28:609–619. doi: 10.1016/j.immuni.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Cyster JG. Shining a light on germinal center B cells. Cell. 2010;143:503–505. doi: 10.1016/j.cell.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 36.Depoil D, Zaru R, Guiraud M, Chauveau A, Harriague J, Bismuth G, Utzny C, Muller S, Valitutti S. Immunological synapses are versatile structures enabling selective T cell polarization. Immunity. 2005;22:185–194. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, Morse HC, 3rd, Lipsky PE, Leonard WJ. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 40.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol. 2003;21:205–230. doi: 10.1146/annurev.immunol.21.120601.141138. [DOI] [PubMed] [Google Scholar]
- 43.Saito M, Gao J, Basso K, Kitagawa Y, Smith PM, Bhagat G, Pernis A, Pasqualucci L, Dalla-Favera R. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell. 2007;12:280–292. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erickson LD, Durell BG, Vogel LA, O'Connor BP, Cascalho M, Yasui T, Kikutani H, Noelle RJ. Short-circuiting long-lived humoral immunity by the heightened engagement of CD40. J Clin Invest. 2002;109:613–620. doi: 10.1172/JCI14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolduc A, Long E, Stapler D, Cascalho M, Tsubata T, Koni PA, Shimoda M. Constitutive CD40L expression on B cells prematurely terminates germinal center response and leads to augmented plasma cell production in T cell areas. J Immunol. 2010;185:220–230. doi: 10.4049/jimmunol.0901689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kishi Y, Aiba Y, Higuchi T, Furukawa K, Tokuhisa T, Takemori T, Tsubata T. Augmented antibody response with premature germinal center regression in CD40L transgenic mice. J Immunol. 2010;185:211–219. doi: 10.4049/jimmunol.0901694. [DOI] [PubMed] [Google Scholar]
- 48.Polo JM, Ci W, Licht JD, Melnick A. Reversible disruption of BCL6 repression complexes by CD40 signaling in normal and malignant B cells. Blood. 2008;112:644–651. doi: 10.1182/blood-2008-01-131813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ci W, Polo JM, Melnick A. B-cell lymphoma 6 and the molecular pathogenesis of diffuse large B-cell lymphoma. Curr Opin Hematol. 2008;15:381–390. doi: 10.1097/MOH.0b013e328302c7df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He JS, Gong DE, Ostergaard HL. Stored Fas ligand, a mediator of rapid CTL-mediated killing, has a lower threshold for response than degranulation or newly synthesized Fas ligand. J Immunol. 2010;184:555–563. doi: 10.4049/jimmunol.0902465. [DOI] [PubMed] [Google Scholar]
- 51.Damdinsuren B, Zhang Y, Khalil A, Wood WH, 3rd, Becker KG, Shlomchik MJ, Sen R. Single round of antigen receptor signaling programs naive B cells to receive T cell help. Immunity. 2010;32:355–366. doi: 10.1016/j.immuni.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacDonald KP, Nishioka Y, Lipsky PE, Thomas R. Functional CD40 ligand is expressed by T cells in rheumatoid arthritis. J Clin Invest. 1997;100:2404–2414. doi: 10.1172/JCI119781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi Y, McNerney M, Datta SK. Regulatory defects in Cbl and mitogen-activated protein kinase (extracellular signal-related kinase) pathways cause persistent hyperexpression of CD40 ligand in human lupus T cells. J Immunol. 2000;165:6627–6634. doi: 10.4049/jimmunol.165.11.6627. [DOI] [PubMed] [Google Scholar]
- 54.Katsiari CG, Liossis SN, Dimopoulos AM, Charalambopoulo DV, Mavrikakis M, Sfikakis PP. CD40L overexpression on T cells and monocytes from patients with systemic lupus erythematosus is resistant to calcineurin inhibition. Lupus. 2002;11:370–378. doi: 10.1191/0961203302lu211oa. [DOI] [PubMed] [Google Scholar]
- 55.Mathur RK, Awasthi A, Wadhone P, Ramanamurthy B, Saha B. Reciprocal CD40 signals through p38MAPK and ERK-1/2 induce counteracting immune responses. Nat Med. 2004;10:540–544. doi: 10.1038/nm1045. [DOI] [PubMed] [Google Scholar]
- 56.Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, Schneider P, Tschopp J. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- 57.Kornbluth RS. An adaptive route to innate immunity? Blood. 2007;110:2221–2222. [Google Scholar]
- 58.Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med. 1999;5:90–96. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- 59.He JS, Ostergaard HL. CTLs contain and use intracellular stores of FasL distinct from cytolytic granules. J Immunol. 2007;179:2339–2348. doi: 10.4049/jimmunol.179.4.2339. [DOI] [PubMed] [Google Scholar]
- 60.Keller AM, Groothuis TA, Veraar EA, Marsman M, Maillette de Buy Wenniger L, Janssen H, Neefjes J, Borst J. Costimulatory ligand CD70 is delivered to the immunological synapse by shared intracellular trafficking with MHC class II molecules. Proc Natl Acad Sci U S A. 2007;104:5989–5994. doi: 10.1073/pnas.0700946104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson S, Zhan Y, Sutherland RM, Mount AM, Bedoui S, Brady JL, Carrington EM, Brown LE, Belz GT, Heath WR, Lew AM. Selected Toll-like receptor ligands and viruses promote helper-independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity. 2009;30:218–227. doi: 10.1016/j.immuni.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 63.Otterdal K, Smith C, Oie E, Pedersen TM, Yndestad A, Stang E, Endresen K, Solum NO, Aukrust P, Damas JK. Platelet-derived LIGHT induces inflammatory responses in endothelial cells and monocytes. Blood. 2006;108:928–935. doi: 10.1182/blood-2005-09-010629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.