Abstract
Purpose
Prostate apoptosis response protein-4 (Par-4) sensitizes cells to chemotherapy; however, Akt1 inactivates Par-4. Previously we showed that Par-4 overexpressing colon cancer cells responded more readily to 5-FU than did wild type counterparts. In this study we investigated: 1) the effects of the Akt inhibitor, phenylbutyl isoselenocyanate (ISC-4), on tumor growth in nude mice and 2) bystander effect of Par-4 overexpressing cells on wild type tumor growth.
Experimental design
Mice (80) were injected with wild type HT29 human colon cancer cells in the right flank. Forty of the mice were also injected in the left flank with HT29 cells engineered to overexpress Par-4. Mice were treated with 5-FU, ISC-4, a combination, or vehicle.
Results
ISC-4 reduced tumor growth, with or without 5-FU. When Par-4 overexpressing tumors were present, wild type tumors grew more slowly than when no Par-4 overexpressing tumors were present. The level of Par-4 protein as well as the Par-4 binding protein, GRP78, was increased in wild type cells growing in the same mouse as Par-4 overexpressing tumors compared to wild type tumors growing without Par-4 overexpressing tumors.
Conclusions
Par-4 overexpressing tumors exhibited a bystander effect on wild type tumors growing distally in the same mouse. This suggests that gene therapy need not achieve total penetration to have a positive effect on tumor treatment. Inhibition of Akt with ISC-4 inhibited tumor growth and had a greater effect on cells overexpressing Par-4. The data indicate ISC-4 alone or in combination with Par-4 can greatly reduce tumor growth.
Keywords: ISC-4, Par-4, colon cancer, Akt, 5-FU
Introduction
Colon cancer is the second most common cause of cancer deaths in both men and women in the US. With current therapeutic strategies, the 5-year survival rate of those with metastatic cancer is between 8% and 12% (1). To address this issue, a number of studies are focused on the search for new and more effective therapy targets. The Prostate apoptosis response protein-4 (Par-4) is a pro-apoptotic protein that was first identified in prostate cancer cells undergoing apoptosis. Par-4 can increase susceptibility of cancer cells to apoptotic agents such as doxorubicin, tumor necrosis factor alpha (TNF-α), and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (2–4). The down-regulation of Par-4 has been proposed to be a critical event in tumorigenesis (5). Par-4 is down-regulated in a number of human cancers, namely, endometrial (6), renal cell carcinoma (3), pancreatic (7), lung (8), and colon cancer (9). Furthermore, Par-4 has been shown to be inactivated by Akt1 in human cancers, as well as in normal lung embryonic epithelial cells (6, 10). In a number of cell lines, its overexpression is sufficient to induce apoptosis (10–12). In others, increasing Par-4 levels does not cause cell death but increases the apoptotic effect of cell death stimuli (4, 10, 13, 14).
Par-4 activity leads to apoptosis via both intrinsic and extrinsic pathways (15–17). Intrinsic pathways include inhibiting transcriptional regulation by NFκB (5, 11, 16). The extrinsic pathway involves the activation of TRAIL. In this case, Par-4 exhibits bystander effects, in that cells overexpressing Par-4 can secrete the protein and induce sensitivity to chemotherapy to nearby cancer cells that do not overexpress Par-4 (15). The phosphorylation of Par-4 by Akt1 enables the scaffolding protein 14-3-3 to bind Par-4, causing retention in the cytoplasm (18). Inhibition of Akt1 can result in activated Par-4 and sensitization to apoptotic stimuli.
The PI3K/Akt pathway, together with its associated negative regulator PTEN, is one important signal transduction pathway for chemoprevention and cancer treatment studies. Evidence supporting the importance of the PI3K/Akt signaling pathway in cancer chemoprevention and therapy has been well documented in literature (19–22), and has led to development of Akt signaling pathway inhibitors that are able to reduce tumor growth successfully. The entire pathway is deregulated in many human cancers, either by activating mutations, or by deletion of PTEN (23, 24). Specifically, in colon cancer, Akt over-expression has been shown in 57% of sporadic colon tumors, higher than in many cancers, and upregulation occurs at a pre-malignant stage (25). Moreover, activation of Akt has been shown in colon cancer cells but not in normal mucosa (25, 26). In this study we used a new inhibitor of Akt, phenylbutyl isoselenocyanate (Ph-(CH2)4-N=C=Se; ISC-4) (27, 28), alone and in combination with Par-4, to effect colon tumor regression. ISC-4 was recently developed in our laboratories (28) through extensive structure-activity studies based on naturally occurring phenylalkyl isothiocyanates (Ph-(CH2)n-N=C=S; ITCs), that have been shown to be effective at inhibiting Akt signaling pathways. In both epidemiological and laboratory investigations, naturally occurring and synthetic ITCs are well established anti-cancer agents for cancers at a variety of organ sites (29–37). The lead compounds were optimized and the best Akt inhibitors were obtained by the isosteric replacement of sulfur in ITCs by selenium leading to isoselenocyanate derivatives (Ph-(CH2)n-N=C=Se). The rationale for this modification was based on the observation that organoselenium compounds have been shown to be effective in retarding tumorigenesis of several cancer types, including colon cancer (38–42), in both animal models and epidemiological studies. In addition, it has been demonstrated that most cancer patients, including colon cancer patients (43, 44), have lower serum selenium levels than healthy controls. Hence, ISC compounds combined the anticancer properties of both selenium and ITCs. ISC-4 designed by increasing the alkyl chain length and replacing sulfur by selenium in naturally occurring ITCs was identified as the most potent drug-like PI3K/Akt inhibitor (27, 28).
We reported recently that Par-4 overexpression in human colon cancer cells resulted in reduced tumor growth in response to 5-fluorouracil (5-FU) when the cells were implanted into nude mice (45). As cells expressing Par-4 show a bystander effect in vitro, we examined the possibility that this effect may extend to tumor cells that are distally located in a nude mouse model of colon tumor growth. Mice were injected with wild type HT29 human colon cancer cells and half of the mice were injected distally with Par-4 overexpressing HT29 cells. Mice were then treated with ISC-4 to establish the efficacy of this drug on tumor growth either with or without the addition of 5-FU.
Materials and Methods
Reagents
ISC-4 was synthesized following a method recently developed by Sharma et al. (28). Other reagents obtained from: 5-FU Acros Organics (Geel, Belgium), API-2 (Tocris Biosciences, Ellisville, MO), and PBITC (LKT Laboratories, St. Paul, MN). Cell culture reagents: HT29, SW480, HCT116, and SW620 cells (American Type Culture Collection, Manassas, VA) and Fugene 6 reagent (Roche Diagnostics, Indianapolis, IN, USA). Antibodies were purchased from Santa Cruz Biotechnology, Santa Cruz, CA, Amersham, Piscataway, NJ, and Cell Signaling Technologies, Boston, MA.
Cell culture
Human colon cancer cells were cultured in RPMI containing 10% FBS and Pen/Strep at 37°C and 5% CO2. HT29 cells were transfected with either rat par-4 cDNA in pCB6+ (a kind gift of Dr. Vivek Rangnekar, University of Kentucky), with the human Par-4 clone in pCMVA6-AC (Origene, Rockville, MD), or with empty vector using Fugene 6. Human Par-4 was obtained from Origene. Transfectants were selected with G418 (Gibco, Carlsbad, CA) and colonies expanded and assayed for Par-4 expression.
Immunoprecipitation and Western blotting
Antibodies used were: Par-4 rabbit polyclonal, Caspase 9 rabbit polyclonal, Caspase 8 mouse monoclonal (Cell Signaling, Danvers, MA), and β-actin mouse monoclonal (Sigma, Saint Louis, MO). Cells were grown to 80% confluence. Plates were washed with PBS and the cells were lysed into lysis buffer (50 mm HEPES, 100 mm NaCl, 10 mm EDTA, 0.5 % NP40, 10% glycerol, 0.0001% Tween 20, supplemented with 0.1 mm PMSF, 0.1 mm NaVO4, 0.5 mm NaF, 5 µg/ml leupeptin, 0.1 mm DTT). In the case of mouse tissues, snap-frozen tissues were homogenized in lysis buffer using a Fisher Scientific PowerGen homogenizer (Fisher Scientific, Pittsburg, PA). The proteins were quantified according to the Bradford Assay and loaded equally onto 10% polyacrylamide gels. For immunoprecipitation, 100 µg protein were incubated with 50 µl Dynabeads (Invitrogen, Carlsbad, CA) conjugated to 14-3-3 goat polyclonal antibody (Santa Cruz Biotechnologies, Santa Cruz, CA). Beads were washed and proteins eluted. Proteins were electrophoresed at 150 v and transferred to nitrocellulose membranes using a semi-dry blotter (BioRad, Hercules, CA). Membranes were blocked with 5% non-fat dry milk for 2 h and incubated with primary antibody overnight. The blots were washed 3X in TBS-Tween and incubated for 1 h in appropriate HRP-conjugated secondary antibodies (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Blots were washed and developed using the ECL chemiluminescent kit (Amersham, Piscataway, NJ, USA). The blots were exposed to autoradiography film and scanned.
Cell viability assay
Human colon cancer cells were seeded in a 96 well plate were treated with 3.15 to 50 µM ISC-4, PBITC, or API-2 for 48 hours, or according to text. In addition, HT29 cells, transfected with either Par-4 or empty vector, were treated with ISC-4. In vitro cytotoxic efficacy was measured using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole (MTT) cell viability assay (Chemicon, Temecula, CA).
Nude mouse experiments
All mice were treated according to the guidelines set forth by the Association for Assessment and Accreditation of Laboratory Animal Care. Forty 6-week old female athymic nude mice (Harlan Sprague Dawley, Indianapolis, IN) were injected in the right flank with 1 ×107 wild type HT29 cells and 20 of the mice were also injected in the left flank with 1 ×107 HT29 cells transfected to overexpress Par-4. Starting at 7 days post injection, tumors were measured weekly with calipers. Tumor volume was calculated using the equation; (l × w2)/2. Half of the mice were treated 3X weekly with ISC-4, at 3 PPM in 50 µl DMSO by intraperitoneal (IP) injection. Half of the ISC-4 treated mice were additionally treated by IP injection with 5-FU (30 mg/kg in PBS) (based on maximum tolerated dose of 30 mg/kg/day (46)) on day 5, 7, 10, 14, 21, and 28 after injection of cells. Once the tumors reached a size of 2 cm in the largest diameter, mice were euthanized, tumors were removed, counted, weighed, and the tissue snap frozen in liquid nitrogen and stored at −80°C. The experiment was repeated with an additional 40 mice. All animal work was performed with the full approval of the Penn State Hershey’s Institutional Animal Care and Use Committee.
Fluorescent tissue staining
Frozen tumor tissue from mice was sliced and fixed in 4% paraformaldehyde solution for 20 min at room temperature. Slides were washed in PBS and tissue sections permeabilized in 0.2% Triton-X-100 then blocked with 5% FBS, 0.1% Triton-X-100 in PBS for 1h. Samples were then incubated overnight at 4°C in primary antibody against Par-4, 1:200 dilution in 1% BSA and 0.05% Triton-X-100 in PBS. Sections were washed in PBS and secondary antibody conjugated to Cy2 was applied and incubated in the dark for 1h at room temperature. Slides were mounted with mounting medium (60% glycerol) and stored in the dark. The images were collected using a Leica TCS SP2 AOBS confocal microscope with ×63 oil immersion optics. (Leica Microsystems Inc., Bannockburn, IL). To avoid crosstalk between the two channels, sequential scanning of the tissue sample mounts was done.
Statistical methods
The statistical software program R version 2.11.1 (http://www.r-project.org/) was used to perform the statistical analysis. A repeated measures analysis of variance was used to test for an overall significance in treatment effect as well as at individual concentrations of the treatments for the in vivo studies. A two-sample t-test with unequal variances was used to test two individual treatments at specified concentrations. The robust Mann-Whitney two-sample nonparametric test was calculated for comparisons. The IC50 values were computed with the drc (Analysis of dose-response curves), package (version 2.1.1) using R (version 2.12.2) (47, 48). Samples were normalized to the WT cells treated with DMSO only in each experiment.
Results
ISC-4 induces cell death in Human colon cancer cells
Akt inhibitors have been well studied as therapeutic options for cancer treatment. As a downstream target of Akt1, Par-4 may play a role in this process. ISC-4 (Fig. 1A) induces apoptosis at very low concentrations in cancer cells but not in normal cells (27). We investigated the relative potency of ISC-4 and the sulfur analog, phenylbutyl isothiocyanate (PBITC), with a commercially available Akt inhibitor, API2, in HT29 cells (Fig. 1B). The human colon cancer cell line, HT29, was used for the experiments in this study for its high tumorigenicity in nude mice. The results show ISC-4, with an IC50 = 6.57 µM, to be more potent than either PBITC or API-2 with IC50 of 38.1 µM and >50 µM, respectively (Fig. 1B).
Fig. 1.
ISC-4 reduces cell viability more potently than other Akt inhibitors. A, structure of ISC-4. B, HT29 cells were treated for 48 hours with increasing concentrations of Akt inhibitors, or with DMSO vehicle. ISC-4 was significantly more potent than were API2 and PBITC at doses of 12.5 and 25 µM. Both PBITC and ISC-4 compounds were more potent than API2 at 50 µM (p=0.000585). The table depicts IC50 values of compounds from MTT assay. C, A panel of colon cancer cell lines was treated with increasing concentrations of ISC-4. Data show a dose dependent response in each cell line. All cells were dead at 50 µM ISC-4. The Western blot analysis shows Par-4 and phosphoAkt (pAkt) protein expression in all cell lines. The upper band in the pAkt lane represents Akt1, the isoform responsible for Par-4 inhibition. D, ISC-4 treatment of wild type or Par-4 transfected cells with increasing doses of ISC-4 or DMSO vehicle for 48 hours showed that mock transfected cells and cells transfected with rat par-4 were less sensitive to treatment than were human Par-4 transfected cells (clones 12 and 17) (p=0.014177 - overall treatment effect). Compare Par-4 transfected cells to Mock transfected cells at specific dose levels (hPar-4 cl 12, p=0.0304 and 0.015, hPar-4 cl 17, p=0.0014 and 0.0038 for 6.25 µM and 12.5 µM ISC-4, respectively). Asterisks indicate cells with significantly increased sensitivity to ISC-4 over Mock transfected cells.
Relative absorbance in the MTT assay was analyzed with a repeated measures analysis of variance that included the predictor variables treatment, concentration, and a treatment by concentration interaction effect. Both treatment and concentration had a significant effect on cellular response. An analysis of variance at individual concentrations shows no significant difference among the DMSO groups (p=.684) or at concentrations less than 12.5 µM, but a significant difference is observed between ISC-4 and the other two treatments at concentrations of 12.5 µM (p=.0017), 25 µM (p<.001), and 50 µM (p=.0035). The differences among the three treatment groups as varied by concentration are graphed in Figure 1B along with standard error bars. The higher concentrations of ISC-4 treatment yielded the smallest absorbances, and individual comparisons of ISC-4 to the two other treatments yielded statistically significant differences.
A number of human colon cancer cell lines, HCT116, HT29, KM12C, SW480, and SW620, were compared for relative sensitivity to ISC-4. In all cases ISC-4 inhibited cell growth in a dose dependent manner at the concentrations tested, with IC50s of 9.15, 8.05, 13.07, 11.79, and 9.31, respectively (Fig. 1C), indicating that the effect of ISC-4 is not specific to only one or two colon cancer cell lines. The levels of Par-4 and phospho-Akt (pAkt) proteins were compared by Western blot analysis between cell lines, and correlated to the sensitivity of the cells to ISC-4. While there is little variation in the Par-4 levels of these cells, the amount of pAkt varies more widely. The upper band present most notably in HT29 and SW620 represents the Akt1 isoform. (Fig. 1C). Inhibition of this protein would be expected to result in activation of Par-4, sensitizing the cells to apoptosis. However, it is difficult to say from this data that the pAkt levels affect sensitivity to ISC-4.
ISC-4 was shown previously to increase the binding of Par-4 to NFκB and decrease the binding to 14-3-3, indicating that ISC-4 causes inhibition of Akt1 and subsequent activation of Par-4 (45). As our earlier data on Par-4 was collected using the rat par-4 gene, the in vivo experiments in this study were performed using the same cells transfected for continuity. We transfected HT29 cells with the human PAR-4 gene for comparison with the rat par-4 gene. HT29 cells transfected with the plasmid for expression of either rat or two selected clones of human Par-4, or with an empty vector (Mock), were incubated with ISC-4. The overexpression of human Par-4 in the cells resulted in a reduction of the IC50 to half that of the mock transfected cells in this experiment, with IC50 values of 11.0 for Mock cells and 5.64 and 4.6 for hPar-4 clones 12 and 17, respectively (Fig. 1D).
A repeated measures analysis of variance was used to compare overall effects of the Mock and Par-4 treatments yielding a statistically significant effect due to treatment (p=.0141) and concentration level (p=.0017), with no significant interaction effect (p=.7686). The individual significant differences between clones were analyzed with a two sided T-Test, and were only observed at the higher concentrations of 12.5 µM and 6.25 µM for the two human Par-4 clones.
ISC-4 reduces tumor growth in nude mice
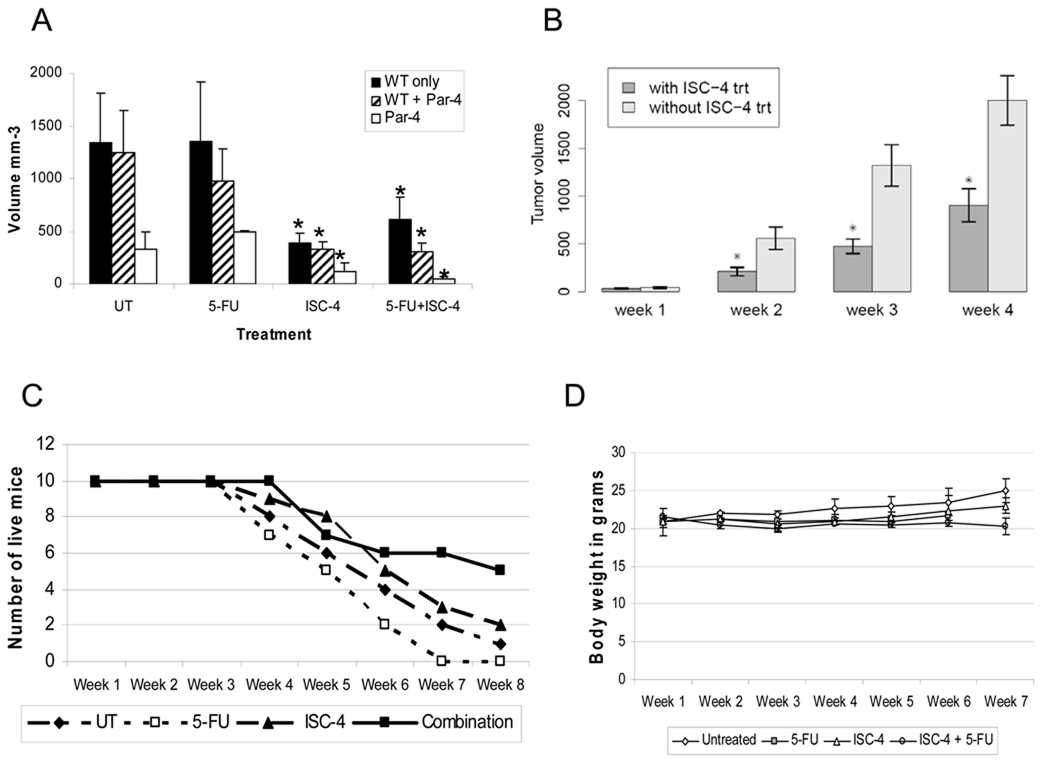
As ISC-4 inhibits tumor cell viability but not normal cell viability in vitro (27), both the effects of ISC-4 on colon tumor growth and the toxicity of ISC-4 in mice were tested. Mice were injected with wild type (WT) HT29 tumor cells only or with WT cells plus Par-4 overexpressing cells in opposite flanks. Mice were treated by IP injection 3 times weekly for 5 weeks with 3 ppm ISC-4 in DMSO, or with DMSO only. Table 1 outlines the experimental groups. Tumors were measured weekly, and tumor volumes calculated. The tumor growth rate was assessed in two ways. One assessment was a comparison of tumor volumes at a time point when all of the mice were still alive, i.e. week 3 (Fig. 2A). Expectedly, tumors formed by Par-4 overexpressing HT29 cells were smaller than tumors formed by wild type HT29 cells. This is consistent with our previous findings that Par-4 overexpressing tumors grew more slowly than did WT tumors (49). Par-4 tumors showed a great response to ISC-4, particularly in conjunction with 5-FU. In 20% of the cases, the Par-4 tumors treated with ISC-4 disappeared altogether. In these cases, the WT tumors in those mice grew as rapidly as WT tumors in other mice that had not been injected with Par-4 overexpressing tumor cells. The rate of tumor growth both with and without ISC-4 treatment was determined through week 4 (Fig. 2B). After week 4, the number of mice remaining in the treatment groups was not large enough for statistically valid comparisons of tumor volumes. Results showed that mice treated with ISC-4 showed significantly retarded tumor growth compared with mice receiving no ISC-4 (p=0.042). The second assessment was a comparison of the length of time it took for the tumors to exceed a maximum allowable diameter of 2 cm (Fig. 2C). The growth rate, including both tumor volume and time to a size of 2 cm diameter indicated that tumors in mice treated with ISC-4 grew more slowly than did tumors in mice that did not receive ISC-4. The drug had no severe systemic effects on the mice, as no mice sickened and died as a result of treatment and no mice demonstrated weight loss during the experiment, although those mice treated with the combination of ISC-4 and 5-FU showed a lack of weight gain (Fig. 2D). Interestingly, the mice treated with 5-FU alone had the fastest WT tumor growth, indicating that 5-FU had no positive effect on WT tumor regression or growth inhibition. This trend was repeatable when the experiment was repeated, as mice with the combination treatment presented the slowest growing tumors and those with 5-FU treatment had the fastest growing tumors. Finally, for the mice with combination treatment, 5-FU was stopped after week 6, and the tumors did not seem to increase in growth significantly. In the future, treatment can be stopped earlier to detect more variation. Potentially, HT29 cells are resistant to 5-FU, although the reason for a growth stimulatory effect is not clear. However, 5-FU alone did retard the growth of Par-4 overexpressing tumors.
Table 1. Number of mice in each treatment group.
Treatment groups in the in vivo tumor growth experiment. Table 1 outlines the tumor injections and drug treatments of each of the 8 mouse groups.
| Compound | WT only | WT + Par-4 |
|---|---|---|
| Vehicle | 5 | 5 |
| 5-FU | 5 | 5 |
| ISC-4 | 5 | 5 |
| 5-Fu + ISC-4 | 5 | 5 |
Fig. 2.
Treatment of mice with ISC-4 to determine effects on tumor volume. A, mice were treated with ISC-4, 5-FU, combination, or neither starting one week after injection of tumor cells. Three weeks after tumor cell injection, tumors were measured and volumes shown for each tumor type across all treatment levels. Tumors in mice treated with ISC-4 were smaller than those in mice without ISC-4 (WT alone tumors, p=0.005; WT + Par-4 tumors, p=0.0038; Par-4 tumors, p=0.0193). Tumor volumes were compared for growth rates up through week 4. Nonparametric values at weeks 2, 3, and 4 are: 0.008, 0.0003, and 0.0006, respectively. C, Measurement of time until tumors reached 2 cm at the largest diameter, resulting in euthanasia. D, Mice were weighed once weekly prior to drug injection and weights recorded.
Par 4 tumors had a bystander effect on WT tumors growing in the same mice
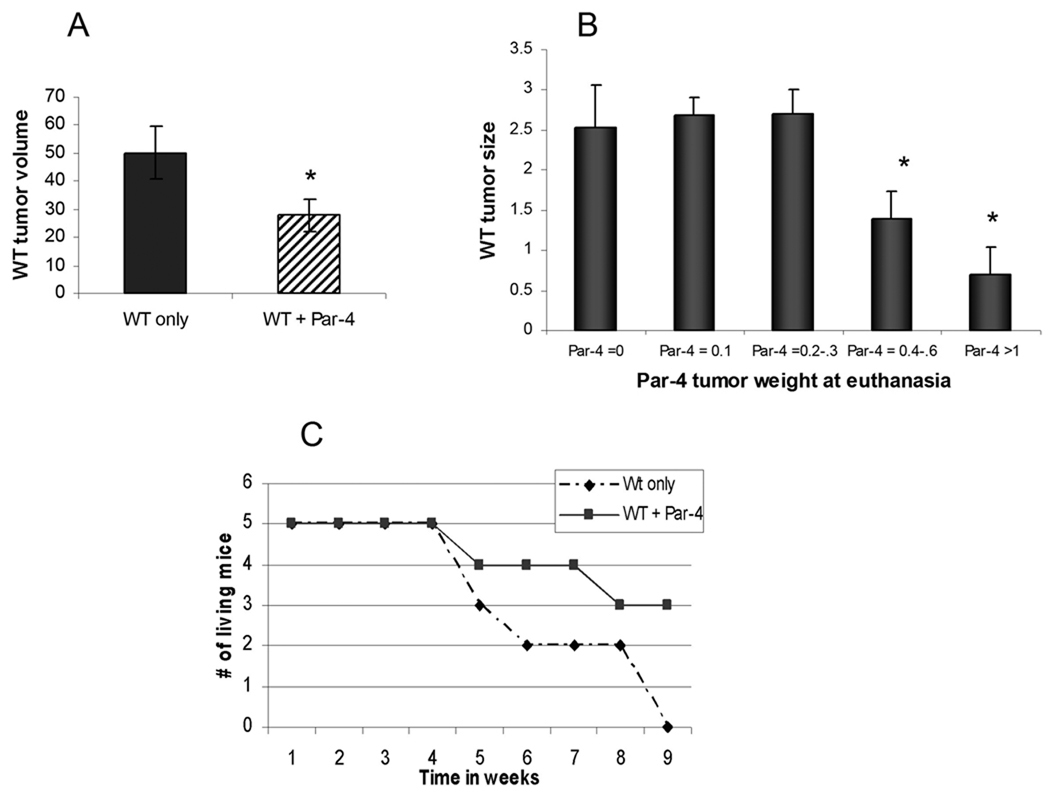
Wild type tumors in mice were examined prior to administration of therapeutic drugs. At 7 days after injection of cells, the tumors were measured and volumes calculated. All tumors growing from WT cells in mice with no other tumor were larger than every WT tumor growing in a mouse that had also been implanted with Par-4 overexpressing cells (Fig. 3A). Similar results were obtained when the experiment was repeated. The tumor volume ratio of WT only/WT with Par-4 in the same mouse in the first experiment was 1.8, while in the second experiment the ratio was 2.0. Furthermore, at the time of euthanasia, the size of the WT tumors growing in the mice was inversely proportional to the size of the Par-4 tumor growing in the same mouse, indicating a dose dependent bystander effect of Par-4 overexpressing cells on WT cells (Fig. 3B). This also indicates that the bystander effect functions effectively in distally growing tumors.
Fig. 3.
Bystander effects of Par-4. A, volume of WT tumors one week after cell injection, prior to treatment. p=0. B, comparison of the wild type tumor weights at euthanasia with the Par-4 tumor weight in the same mouse. Tumors were excised and weighed. The larger Par-4 tumors were present in mice with smaller wild type tumors in an inverse proportional scale. C, time to maximum tumor size of wild type tumors growing alone compared with wild type tumors growing in mice that also had Par-4 tumors. The comparison is made in animals receiving both 5-FU and ISC-4. At the end of 9 weeks, 60% of mice with Par-4 tumors growing in them had wild type tumors less than 2 cm in diameter, while all the tumors growing in mice without Par-4 tumors had reached maximum diameter.
To examine the role of Par-4 with both treatment elements, ISC-4 and 5-FU, the wild type tumors in all mice with both treatments were compared. The wild type tumors in mice that also had Par-4 tumors grew significantly more slowly than did the wild type tumors growing alone in mice (Fig. 3C). 5-FU alone did not show a growth reduction of tumors. This suggests that the apoptotic inducement of 5-FU alone was not sufficient to fully induce Par-4 mediated apoptosis in WT cells as Par-4 may still have been inhibited by Akt1 activity. However, with both agents together, tumor growth was significantly slowed. On the other hand, the growth of Par-4 overexpressing tumors was retarded by treatment with 5-FU as compared to vehicle treated tumors (data not shown).
ISC-4 downregulates Akt1 in mouse tumors
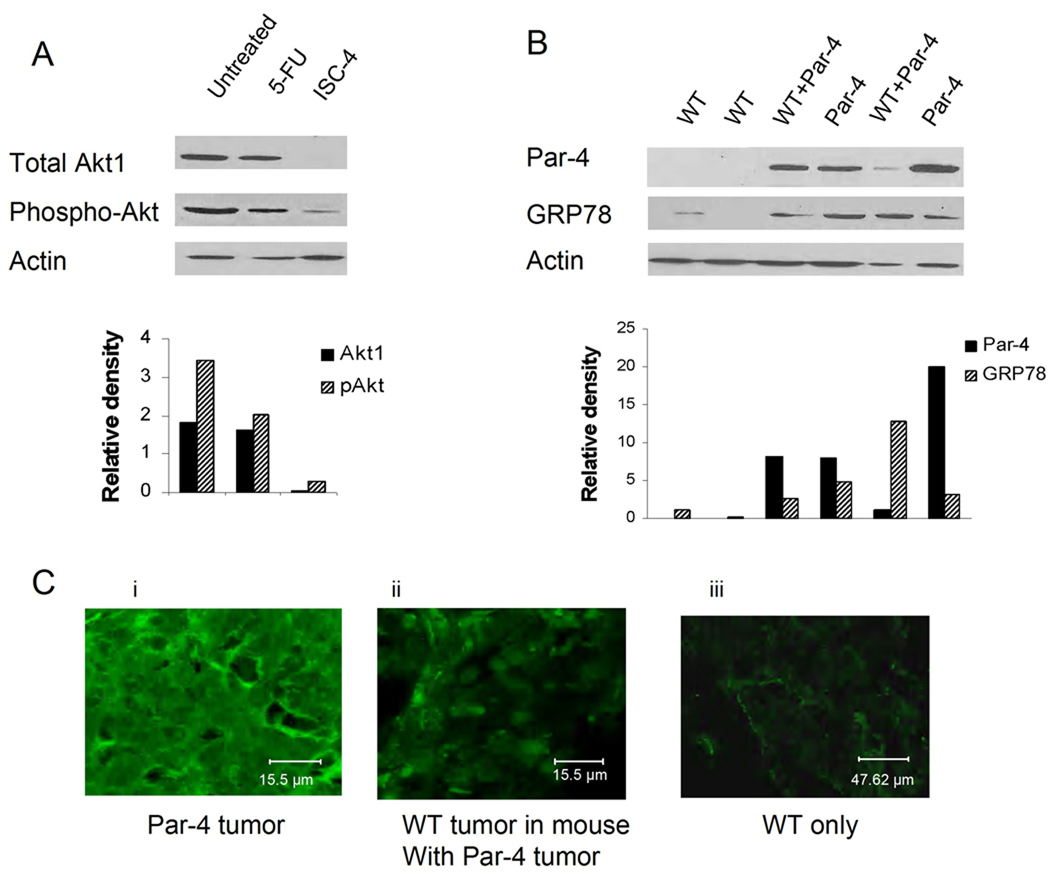
As ISC-4 downregulates Akt activity and Akt1 activity is important for the inhibition of Par-4 activity, the effects of ISC-4 on Akt1 expression and Akt phosphorylation in tumor tissues was examined. Lysates were made from tumor tissue taken from mice at euthanasia. The tumor lysates were assayed by Western blot for expression of Par-4, Akt1, phospho Akt, and β-actin for control. Figure 4A shows that administration of ISC-4 to the mice downregulates both the protein levels and the phosphorylation levels of Akt1 in mouse tumors. Potentially the faint band in the phospho-Akt lane under ISC-4 treatment is the result of Akt 2 or 3, which are present in small amounts in these cells. Shown beneath the Western blots are densitometric analyses of the band densities.
Fig. 4.
Analysis of proteins expressed in mouse tumors. A, western blot analysis of total Akt1 and phospho Akt (pan-Akt) in tumors treated with 5-FU, ISC-4, or neither. Akt1 expression is reduced to negligible levels in ISC-4 treated tumors (top panel). B, Par-4 (upper panel) and GRP78 (middle panel) expression in wild type tumors growing alone and paired wild type and Par-4 tumors growing in the same mouse. Lower panels show β-actin for loading control. Densitometric analysis of banding is shown, normalized against β-actin. C, fluorescent staining of tumor thin slices for Par-4 (green): (i) Par-4 overexpressing tumors. (ii) WT tumor growing in mouse with Par-4 tumor. (iii) WT tumors growing alone.
Par-4 protein levels can increase in WT tumors growing in mice with Par-4 tumors
GRP78 is a protein expressed in the endoplasmic reticulum of cells. However, GRP78 is also present on cell surfaces where it acts as a receptor for soluble ligands (50), including exogenous Par-4 (15). Under conditions of ER stress, Par-4 mediates translocation of GRP78 to the cell surface. When GRP78 is present on the cell surface, it can be bound by exogenous Par-4, activating the apoptotic machinery within the cell (15). Therefore, we asked the question of whether GRP78 is present in the tumor cells, and whether the presence of Par-4 alters GRP78 expression. We examined the WT tumors from mice with only WT tumors and WT tumors from mice with paired Par-4 tumors, as well as Par-4 tumors themselves. Fig. 4B, upper panel, shows Par-4 levels in tumors excised from mice at euthanasia. Lanes 1 and 2 are WT tumors from mice with only WT tumors (labeled WT), lanes 3 and 4 are WT and Par-4 tumors from the same mouse (labeled WT + Par-4 and Par-4, respectively), and lanes 5 and 6 paired WT + Par-4 and Par-4 tumors from a different mouse. The western blot shows that WT tumors growing in mice with only WT tumors have very little Par-4 or GRP78. However, when Par-4 is overexpressed in tumors (lanes 4 and 6), GRP78 is increased. Likewise, in WT tumors growing in mice that also have Par-4 tumors (lanes 3 and 5) GRP78 is also increased.
Fluorescence microscopy was used to determine subcellular localization of Par-4 in tumor cells, as well as to validate the results of western blotting. Sections were made from frozen tumor samples and stained with a primary antibody against Par-4. The secondary antibody contained a Cy-2 fluorescent tag and the images were collected using a Leica TCS SP2 AOBS confocal microscope. Results showed that Par-4 was highest in tumors overexpressing Par-4 (Fig. 4C, panel i) and was also increased in WT tumors growing in the same mouse (panel ii) as compared to WT tumors growing in mice that had no Par-4 tumors (panel iii).
Par-4 causes apoptosis in tumors through both intrinsic and extrinsic pathways
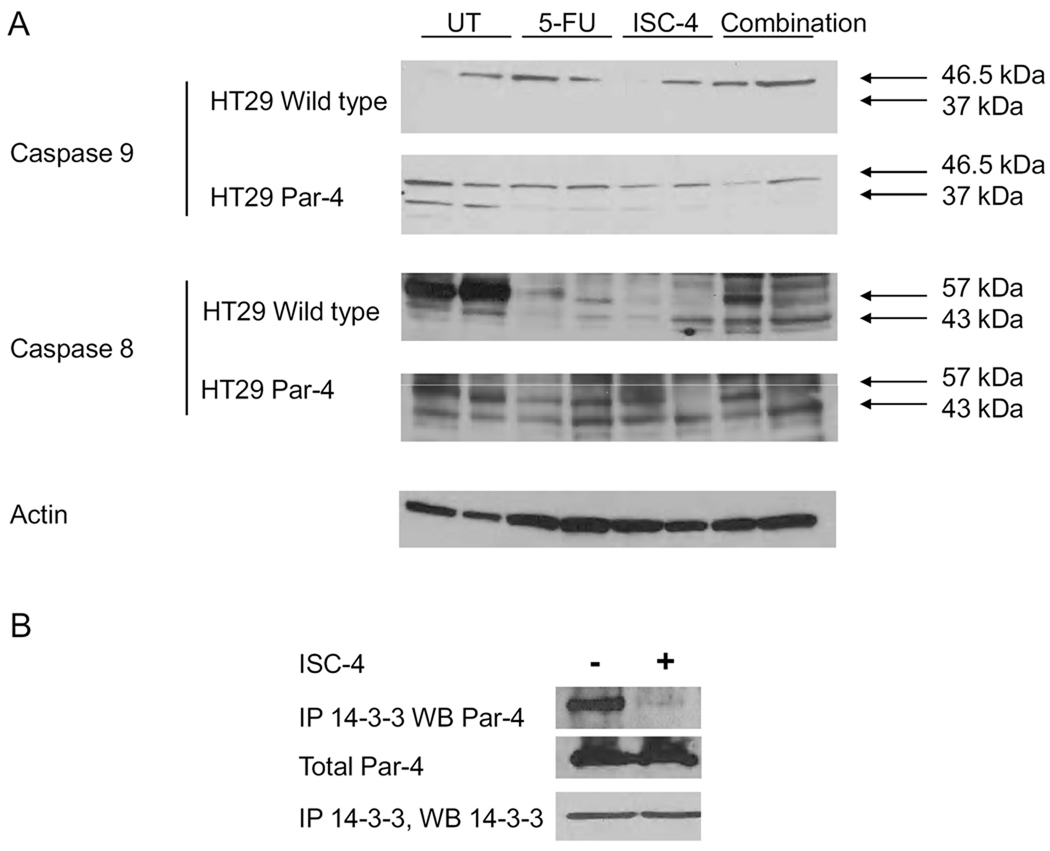
Par-4 protein in cells acts through both intrinsic and extrinsic pathways. To examine which pathway plays a role in apoptosis in the mouse tumors, the cleavage of caspase 8 and caspase 9 were examined. In wild type tumors, no caspase 9 was cleaved, yet in Par-4 overexpressing tumors caspase 9 was cleaved (Figure 5A, note band at 37 kDa in second panel), particularly when no chemotherapy treatment was administered. This indicates that Par-4 alone can induce apoptosis through the intrinsic pathway. However, when apoptotic stimuli is added, potentially the extrinsic pathway takes over apoptotic activities, as evidenced by the fact that caspase 8 is cleaved in both WT and Par-4 overexpressing tumors that were treated with either 5-FU, ISC-4, or both (Figure 5A, note band at 43 kDa). Finally, ISC-4 given to mice results in release of Par-4 from 14-3-3 in the tumors, allowing it to become active for induction of apoptosis (Fig. 5B).
Fig. 5.
Both extrinsic and intrinsic pathways are active in mouse tumors. A, Caspase 9 cleavage in WT and Par-4 tumors indicates activation of the intrinsic pathway by Par-4 overexpressing, but not in WT HT29 tumors. Caspase 8 cleavage increases with chemotherapeutic treatment in all tumors, possibly overcoming the activity of caspase 9 in Par-4 overexpressing cells. B, Addition of chemotherapy causes release of Par-4 from 14-3-3.
Discussion
5-FU has been used as a component of the therapeutic regimen for colon cancer patients for decades (51). However, there is a need for a more effective regimen, as even when using a combination of 5-FU with other chemotherapeutic agents, the clinical response rate for patients with metastatic disease remains at 20–39% (52). Recent studies have shown that the tumor suppressor, Par-4, may play a role in response to colon cancer treatment. Par-4 levels have been shown to be reduced in human colon cancer cells as compared to normal colon tissue. However, although Par-4 with no chemotherapy appears to retard tumor growth, simply increasing Par-4 protein levels may not provide optimal desired therapeutic effects. Maintaining Par-4 in an active state is important to the apoptotic activity of Par-4 in tumor cells. As Akt1 results in inactivation of Par-4, it is necessary to inhibit Akt1. This allows not only for the activation of Par-4, but also for inhibition of additional pro-survival downstream targets of Akt1. ISC-4 is an Akt inhibitor that has been shown to cause apoptosis in cancer cells, but not in normal cells and reduce tumor growth with no toxicity in mice at effective doses (27, 28), and is, therefore, a suitable compound to use for in vivo inhibition of Akt1. A comparison of ISC-4 with other Akt inhibitors showed ISC-4 to be more effective in cultured cells.
The only effect of Akt inhibition that we tested in this study was the activity of Par-4. However, ISC-4 is a pan-Akt inhibitor, so it inhibits Akt 2 and Akt 3 as well as Akt 1. Inhibition of all Akt isoforms can have an effect on tumor growth, regardless of Par-4 status. While Western blot analysis showed very little Akt 2 or 3 in these cells, there may still be an effect of inhibiting their activity. In addition, Akt 1 affects additional pathways that regulate apoptosis and survival. This may explain why the use of ISC-4 had a similar effect on WT tumors growing alone in mice as WT tumors growing in mice that also had Par-4 tumors growing in them.
Tumors from Par-4 overexpressing cells grew more slowly from the beginning than did wild type tumors, although equal numbers of viable cells were injected. This suggests that Par-4 affects tumor growth from the point of initiation even without chemotherapy, and may, therefore, be a natural inhibitor of the formation of metastatic lesions. One confounding factor of the rapid tumor regression of Par-4 overexpressing tumors is that once those tumors shrank, the wild type tumors in those mice began to grow. For this reason, a method of reintroducing Par-4 into tumor cells needs to be developed. The significance of the bystander effect is that there need not be 100% transfection efficiency to elicit a profound effect on the tumor. This laboratory is exploring those possibilities. The finding that the bystander effect functions distally to the cells overexpressing Par-4 has great significance for offering a therapeutic value of gene therapy using Par-4, in that transfected cells need not be proximally located to have an effect on untransfected tumor cells. Not only known tumor burden but also distant metastases can be affected by systemically released Par-4. In this study, as Par-4 overexpressing tumors decreased in size, the WT tumors in the same mice grew more rapidly. Therefore, to be effective in long-term therapy outcome, the Par-4 must continue to be released, meaning that a method of in vivo transfection of cells with Par-4 must be repeated periodically. The use of nanotechnology to deliver Par-4 to cells has been and continues to be explored.
In conclusion, ISC-4 alone is a potent and safe inhibitor of colon tumor growth in a xenograft model when used as a single therapy. The addition of the current standard of care, 5-FU, enhances the growth inhibition of ISC-4. This suggests that tumors that are resistant to 5-FU treatment can be alternately treated with ISC-4 alone or can be sensitized to 5-FU through combination with ISC-4. Finally, when Par-4 is added to the cells, either from overexpression within the tumor or exogenously applied, tumor growth is further slowed.
Statement of Translational Relevance.
Par-4 is a tumor suppressor that induces apoptosis in cancer but not in normal cells, and has been shown to reduce tumor growth in mice. A second treatment, inhibition of Akt, reduces tumor growth in mice. The use of ISC-4 to inhibit Akt shows toxicity to cancer cells at low concentrations, but is not toxic to normal cells. ISC-4 has a two fold effect: through inhibition of Akt, it results in activation of Par-4 to induce apoptosis and inhibits survival pathways. This two-pronged approach, systemically applied for cancer treatment, can reduce tumor growth significantly in primary tumors as well as in both occult and frank metastases without damaging normal tissue.
Acknowledgments
Grant support:
The Barsumian Trust and W. W. Smith (R.B. Irby), Elsa U. Pardee Foundation and R03 # CA143999-01 (A.K. Sharma), and NCI Contract #. NO2-CB-81013-74 (S. Amin).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Boehrer S, Nowak D, E EP, Ruthardt M, Sattler N, Trepohl B, et al. Prostate-apoptosis-response-gene-4 increases sensitivity to TRAIL-induced apoptosis. Leuk Res. 2006;30:597–605. doi: 10.1016/j.leukres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Cook J, Krishnan S, Ananth S, Sells SF, Shi Y, Walther MM, et al. Decreased expression of the pro-apoptotic protein Par-4 in renal cell carcinoma. Oncogene. 1999;18:1205–1208. doi: 10.1038/sj.onc.1202416. [DOI] [PubMed] [Google Scholar]
- 4.Sells SF, Han SS, Muthukkumar S, Maddiwar N, Johnstone R, Boghaert E, et al. Expression and function of the leucine zipper protein Par-4 in apoptosis. Mol Cell Biol. 1997;17:3823–3832. doi: 10.1128/mcb.17.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barradas M, Monjas A, Diaz-Meco MT, Serrano M, Moscat J. The downregulation of the pro-apoptotic protein Par-4 is critical for Ras-induced survival and tumor progression. EMBO J. 1999;18:6362–6369. doi: 10.1093/emboj/18.22.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno-Bueno G, Fernandez-Marcos PJ, Collado M, Tendero MJ, Rodriguez-Pinilla SM, Garcia-Cao I, et al. Inactivation of the candidate tumor suppressor par-4 in endometrial cancer. Cancer Res. 2007;67:1927–1934. doi: 10.1158/0008-5472.CAN-06-2687. [DOI] [PubMed] [Google Scholar]
- 7.Qiu SG, Krishnan S, el-Guendy N, Rangnekar VM. Negative regulation of Par-4 by oncogenic Ras is essential for cellular transformation. Oncogene. 1999;18:7115–7123. doi: 10.1038/sj.onc.1203199. [DOI] [PubMed] [Google Scholar]
- 8.Joshi J, Fernandez-Marcos PJ, Galvez A, Amanchy R, Linares JF, Duran A, et al. Par-4 inhibits Akt and suppresses Ras-induced lung tumorigenesis. Embo J. 2008;27:2181–2193. doi: 10.1038/emboj.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang BD, Kline CL, Pastor DM, Olson TL, Frank B, Luu T, et al. Prostate apoptosis response protein 4 sensitizes human colon cancer cells to chemotherapeutic 5-FU through mediation of an NF kappaB and microRNA network. Mol Cancer. 2010;9:98. doi: 10.1186/1476-4598-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goswami A, Burikhanov R, de Thonel A, Fujita N, Goswami M, Zhao Y, et al. Binding and phosphorylation of par-4 by akt is essential for cancer cell survival. Mol Cell. 2005;20:33–44. doi: 10.1016/j.molcel.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Gurumurthy S, Goswami A, Vasudevan KM, Rangnekar VM. Phosphorylation of Par-4 by protein kinase A is critical for apoptosis. Mol Cell Biol. 2005;25:1146–1161. doi: 10.1128/MCB.25.3.1146-1161.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JW, Lee KF, Hsu HY, Hsu LP, Shih WL, Chu YC, et al. Protein expression and intracellular localization of prostate apoptosis response-4 (Par-4) are associated with apoptosis induction in nasopharyngeal carcinoma cell lines. Cancer Letters. 2007;257:252–262. doi: 10.1016/j.canlet.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Boehrer S, Chow K, Beske F, Kukoc-Zivojnov N, Puccetti E, Ruthardt M, et al. In lymphatic cells par-4 sensitizes to apoptosis by down-regulating bcl-2 and promoting disruption of mitochondrial membrane potential and caspase activation. Cancer Research. 2002;62:1768–1775. [PubMed] [Google Scholar]
- 14.Ahmed MM, Sheldon D, Fruitwala MA, Venkatasubbarao K, Lee EY, Gupta S, et al. Downregulation of PAR-4, a pro-apoptotic gene, in pancreatic tumors harboring K-ras mutation. Int J Cancer. 2008;122:63–70. doi: 10.1002/ijc.23019. [DOI] [PubMed] [Google Scholar]
- 15.Burikhanov R, Zhao Y, Goswami A, Qiu S, Schwarze SR, Rangnekar VM. The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell. 2009;138:377–388. doi: 10.1016/j.cell.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakraborty M, Qiu SG, Vasudevan KM, Rangnekar VM. Par-4 drives trafficking and activation of Fas and Fasl to induce prostate cancer cell apoptosis and tumor regression. Cancer Res. 2001;61:7255–7263. [PubMed] [Google Scholar]
- 17.Bergmann M, Kukoc-Zivojnov N, Chow KU, Trepohl B, Hoelzer D, Weidmann E, et al. Prostate apoptosis response gene-4 sensitizes neoplastic lymphocytes to CD95-induced apoptosis. Ann Hematol. 2004;83:646–653. doi: 10.1007/s00277-004-0922-3. [DOI] [PubMed] [Google Scholar]
- 18.Goswami A, Ranganathan P, Rangnekar V. The Phosphoinositide 3-Kinase/Akt1/Par-4 Axis: A Cancer-Selective Therapeutic Target. Cancer Res. 2006;66:2889–2892. doi: 10.1158/0008-5472.CAN-05-4458. [DOI] [PubMed] [Google Scholar]
- 19.Kwon KH, Barve A, Yu S, Huang MT, Kong AN. Cancer chemoprevention by phytochemicals: potential molecular targets, biomarkers and animal models. Acta Pharmacol Sin. 2007;28:1409–1421. doi: 10.1111/j.1745-7254.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- 20.Sporn MB, Liby KT. Cancer chemoprevention: scientific promise, clinical uncertainty. Nat Clin Pract Oncol. 2005;2:518–525. doi: 10.1038/ncponc0319. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Jin B, Huang C. The PI3K/Akt pathway and its downstream transcriptional factors as targets for chemoprevention. Curr Cancer Drug Targets. 2007;7:305–316. doi: 10.2174/156800907780809741. [DOI] [PubMed] [Google Scholar]
- 22.Kada F, Saji M, Ringel MD. Akt: a potential target for thyroid cancer therapy. Curr Drug Targets Immune Endocr Metabol Disord. 2004;4:181–185. doi: 10.2174/1568008043339857. [DOI] [PubMed] [Google Scholar]
- 23.Moser AR, Luongo C, Gould KA, McNeley MK, Shoemaker AR, Dove WF. ApcMin: a mouse model for intestinal and mammary tumorigenesis. Eur J Cancer. 1995;31A:1061–1064. doi: 10.1016/0959-8049(95)00181-h. [DOI] [PubMed] [Google Scholar]
- 24.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 25.Roy HK, Olusola BF, Clemens DL, Karolski WJ, Ratashak A, Lynch HT, et al. AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis. 2002;23:201–205. doi: 10.1093/carcin/23.1.201. [DOI] [PubMed] [Google Scholar]
- 26.Shtilbans V, Wu M, Burstein DE. Current overview of the role of Akt in cancer studies via applied immunohistochemistry. Ann Diagn Pathol. 2008;12:153–160. doi: 10.1016/j.anndiagpath.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Sharma A, Sharma AK, Madhunapantula SV, Desai D, Huh SJ, Mosca P, et al. Targeting Akt3 signaling in malignant melanoma using isoselenocyanates. Clin Cancer Res. 2009;15:1674–1685. doi: 10.1158/1078-0432.CCR-08-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma AK, Sharma A, Desai D, Madhunapantula SV, Huh SJ, Robertson GP, et al. Synthesis and anticancer activity comparison of phenylalkyl isoselenocyanates with corresponding naturally occurring and synthetic isothiocyanates. J Med Chem. 2008;51:7820–7826. doi: 10.1021/jm800993r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung FL, Jiao D, Conaway CC, Smith TJ, Yang CS, Yu MC. Chemopreventive potential of thiol conjugates of isothiocyanates for lung cancer and a urinary biomarker of dietary isothiocyanates. J Cell Biochem Suppl. 1997;27:76–85. [PubMed] [Google Scholar]
- 30.Stoner GD, Adams C, Kresty LA, Amin SG, Desai D, Hecht SS, et al. Inhibition of N'-nitrosonornicotine-induced esophageal tumorigenesis by 3-phenylpropyl isothiocyanate. Carcinogenesis. 1998;19:2139–2143. doi: 10.1093/carcin/19.12.2139. [DOI] [PubMed] [Google Scholar]
- 31.Traka M, Mithen R. Glucosinolates, isothiocyanates and human health. Phytochem Rev. 2009;8:269–282. [Google Scholar]
- 32.Beecher CW. Cancer preventive properties of varieties of Brassica oleracea: a review. Am J Clin Nutr. 1994;59:1166S–1170S. doi: 10.1093/ajcn/59.5.1166S. [DOI] [PubMed] [Google Scholar]
- 33.Chung KY, Saltz LB. Adjuvant therapy of colon cancer: current status and future directions. Cancer J. 2007;13:192–197. doi: 10.1097/PPO.0b013e318074d26e. [DOI] [PubMed] [Google Scholar]
- 34.Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DK, Botero-Omary M, et al. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer. 2000;38:168–178. doi: 10.1207/S15327914NC382_5. [DOI] [PubMed] [Google Scholar]
- 35.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 36.Yu R, Mandlekar S, Harvey KJ, Ucker DS, Kong AN. Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity. Cancer Res. 1998;58:402–408. [PubMed] [Google Scholar]
- 37.Zhang Y, Talalay P. Anticarcinogenic activities of organic isothiocyanates: chemistry and mechanisms. Cancer Res. 1994;54:1976s–1981s. [PubMed] [Google Scholar]
- 38.Reddy BS, Wynn TT, el-Bayoumy K, Upadhyaya P, Fiala E, Rao CV. Evaluation of organoselenium compounds for potential chemopreventive properties in colon cancer. Anticancer Res. 1996;16:1123–1127. [PubMed] [Google Scholar]
- 39.Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Jama. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 40.Combs GFGW., Jr Chemopreventive Agents: Selenium. Pharmacol Ther. 1998;79:179–192. doi: 10.1016/s0163-7258(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 41.El-Bayoumy K. The role of selenium in cancer prevention. In: DeVita VTHS, Rosenberg SS, editors. Cancer Principles and Practice of Oncology. 4th ed. Philadelphia, PA: J B Lippincott; 1991. pp. 1–15. [Google Scholar]
- 42.Jacobs ET, Jiang R, Alberts DS, Greenberg ER, Gunter EW, Karagas MR, et al. Selenium and colorectal adenoma: results of a pooled analysis. J Natl Cancer Inst. 2004;96:1669–1675. doi: 10.1093/jnci/djh310. [DOI] [PubMed] [Google Scholar]
- 43.Mikac-Devic M, Vukelic N, Kljaic K. Serum selenium level in patients with colorectal cancer. Biol Trace Elem Res. 1992;33:87–94. doi: 10.1007/BF02783996. [DOI] [PubMed] [Google Scholar]
- 44.Milde D, Novak O, Stu ka V, Vyslou il K, Macha ek J. Serum levels of selenium, manganese, copper, and iron in colorectal cancer patients. Biol Trace Elem Res. 2001;79:107–114. doi: 10.1385/bter:79:2:107. [DOI] [PubMed] [Google Scholar]
- 45.Wang BD, Kline CL, Pastor DM, Olson TL, Frank B, Luu T, et al. Prostate apoptosis response protein 4 sensitizes human colon cancer cells to chemotherapeutic 5-FU through mediation of an NFkappaB and microRNA network. Mol Cancer. 9:98. doi: 10.1186/1476-4598-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guichard S, Cussac D, Hennebelle I, Bugat R, Canal P. Sequence-dependent activity of the irinotecan-5FU combination in human colon-cancer model HT-29 in vitro and in vivo. Int J Cancer. 1997;73:729–734. doi: 10.1002/(sici)1097-0215(19971127)73:5<729::aid-ijc20>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 47.Ritz C, JC S. Bioassay Analysis using R. J. Statist. Software. 2005;12 [Google Scholar]
- 48.Team RDC. R: A language and environment for statistical computing. Vienna Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 49.Kline CL, Shanmugavelandy SS, Kester M, Irby RB. Delivery of PAR-4 plasmid in vivo via nanoliposomes sensitizes colon tumor cells subcutaneously implanted into nude mice to 5-FU. Cancer Biol Ther. 2009;8:1831–1837. doi: 10.4161/cbt.8.19.9592. [DOI] [PubMed] [Google Scholar]
- 50.Misra UK, Gonzalez-Gronow M, Gawdi G, Hart JP, Johnson CE, Pizzo SV. The role of Grp 78 in alpha 2-macroglobulin-induced signal transduction. Evidence from RNA interference that the low density lipoprotein receptor-related protein is associated with, but not necessary for, GRP 78-mediated signal transduction. J Biol Chem. 2002;277:42082–42087. doi: 10.1074/jbc.M206174200. [DOI] [PubMed] [Google Scholar]
- 51.Monga DK, O'Connell MJ. Surgical adjuvant therapy for colorectal cancer: current approaches and future directions. Ann Surg Oncol. 2006;13:1021–1034. doi: 10.1245/ASO.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 52.Matsuyama R, Togo S, Shimizu D, Momiyama N, Ishikawa T, Ichikawa Y, et al. Predicting 5-fluorouracil chemosensitivity of liver metastases from colorectal cancer using primary tumor specimens: three-gene expression model predicts clinical response. Int J Cancer. 2006;119:406–413. doi: 10.1002/ijc.21843. [DOI] [PubMed] [Google Scholar]