Abstract
A common denominator among the multiple damage-inducing agents that ultimately lead to the activation of NLRP3 has not yet been identified. Recently, the production of reactive oxygen species (ROS) has been suggested to act as a common event upstream of the NLRP3 inflammasome machinery. Since de novo translation of NLRP3 is an essential step in the activation of NLRP3, we investigated the role of substances that either inhibit ROS production or its oxidative activity. While we observe that NLRP3 inflammasome activation is unique amongst other known inflammasomes due to its sensitivity to ROS inhibition, we have found that this phenomenon is attributable to the fact that NLRP3 strictly requires priming by a pro-inflammatory signal, a step that is blocked by ROS inhibitors. While these data do not exclude a general role of ROS production in the process of NLRP3-triggered inflammation, they put ROS upstream of NLRP3 induction, but not activation.
Introduction
IL-1β driven inflammation plays a pivotal role both in antimicrobial immunity and in many sterile inflammatory conditions. Due to its highly pro-inflammatory potential, release of bioactive IL-1β is a tightly controlled process, in which caspase-1-mediated cleavage of pro-IL-1β is a rate-limiting step (1). Inflammasome complexes control the regulated cleavage of pro-IL-1β and also other pro-cytokines by assembling a multi-component protein platform that leads to the activation of pro-caspase-1. In addition, the activation of inflammasome pathways leads to a special type of inflammatory cell death that is commonly referred to as pyroptosis. So far, several proteins have been described that can initiate the formation of inflammasome complexes: the NLR (nucleotide-binding domain leucine-rich repeat) proteins NLRP1, NLRP3, NLRC4 and the PYHIN (pyrin and HIN200 domain-containing) protein AIM2. Up to now, only AIM2 has been shown to directly bind to its activating stimulus (double stranded DNA) (2–4), whereas the NLR inflammasome proteins have not been established as bona fide receptors. Of all of the NLR Proteins, NLRP3 has attracted particular attention due to the fact that it seems to sense a large variety of stimuli of diverse physiochemical nature (e.g. ATP, pore forming toxins or crystalline material (5–7)) and also because it plays a pivotal role in many inflammatory diseases. Prior to the discovery of NLRP3 as an upstream component of caspase-1 activation, it was already known that ATP critically requires a pro-inflammatory priming step (e.g. LPS) for caspase-1 activation (8, 9). Moreover, priming cells is also necessary for caspase-1 cleavage after exposure to pore forming toxins and crystalline inflammasome activators. We have recently shown that induction of NLRP3 expression is the only critical factor that determines the necessity of this priming step (10, 11). In fact, this requirement for priming can be solely overcome by constitutive NLRP3 expression, as macrophages expressing heterologous NLRP3 do not require pro-inflammatory priming for their responsiveness towards ATP or other NLRP3 activators (10). As trivial as this necessity for priming might appear, it is important to consider when studying mechanisms of NLRP3 activation or when exploring strategies to specifically inhibit NLRP3 activation.
Various models of activation have been proposed for NLRP3, and, most recently, the concept of reactive oxygen species (ROS) being upstream of NLRP3 activation has gained particular attention. Previous studies using RNA interference and pharmacological inhibitors suggested that NADPH oxidase (NOX)-dependent ROS production, which is observed upon phagocytosis of crystalline material, would be upstream of NLRP3 inflammasome activation (12). However, we and others found that macrophages deficient in NOX subunits p47phox, p91phox orp22phox (essential for functional NOX1–4) responded normally to NLRP3 stimulation (Supplemental Fig. 1A–D and (13–15)). Nevertheless, inhibitors of ROS production or scavengers of ROS exhibit a strong inhibition of NLRP3 inflammasome activation (12, 16). Indeed, in line with the notion that mitochondria constitute the biggest source of cellular ROS, it was subsequentlyshown that mitochondria are in fact the site of ROS production during NLRP3 inflammasome activation (17, 18). To this effect, it has also been demonstrated that inhibitors of mitochondrial ROS production (17) and the knocking down of mitochondrial respiration by targeting the expression of voltage-dependent anion channels (18) down modulate NLRP3-mediated inflammasome activation. Furthermore, there is also independent evidence that ROS activate pro-inflammatory transcription factors (19, 20) and that ROS production positively regulates pro-inflammatory gene expression in various innate immune signaling pathways (14, 21). Based on these findings, we hypothesized that ROS inhibition does not directly affect theactivation of the NLRP3 inflammasome, but, instead, negatively regulates the priming step of NLRP3 inflammasome activation.
Materials and Methods
Mice
Wild type C57BL6/J, Ncf1m1J/J, and Cybb mutant mice in C57BL6/J background were purchased from Jackson Laboratories, whereas bones from Cyba mutant mice were kindly provided by Dr. David Bergstrom (Jackson Laboratories) and have been previously described (22). All animal studies were approved by the University of Michigan Committee on Use and Care of Animals.
Cells and cell culture
C57BL/6 or NLRP3-deficient macrophage cell lines (Fig. 1 and 2 and Supplemental Fig. 1E, F and 2) were cultured and stimulated as previously described (10). Macrophages stably overexpressing NLRP3 were obtained through lentiviral transduction as previously described (10).
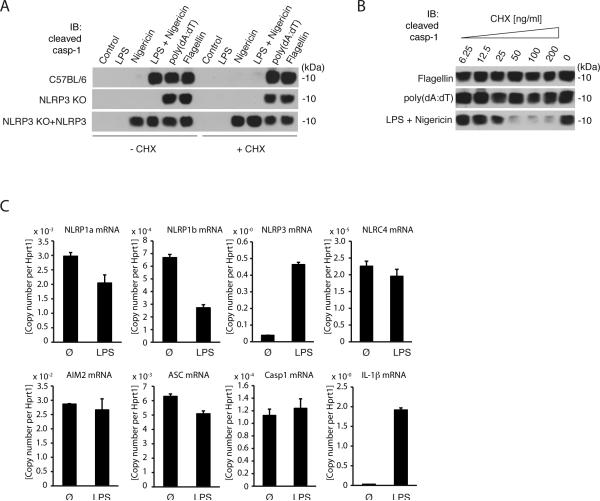
Figure 1. The requirement of priming is a distinctive feature of the NLRP3 inflammasome.
A, immunoblot of cleaved caspase-1 from supernatants of wild type (C57BL/6), NLRP3-deficient or NLRP3-deficient macrophages reconstituted with NLRP3 (NLRP3-KO + NLRP3) treated with 100 ng/ml cycloheximide (CHX) or left untreated. Stimulation was performed as indicated. B, immunoblotting of caspase-1 from supernatants of wild-type macrophages pretreated with CHX for 1 h and stimulated as indicated. C, Messenger RNA expression in LPS primed (200 ng/ml) or untreated macrophages. Relative expression data per Hprt1 are shown. Readouts were performed 6 h (A and B) or 3h (C) after stimulation and data are from one representative experiment of three (A and B) or of four (C) experiments.
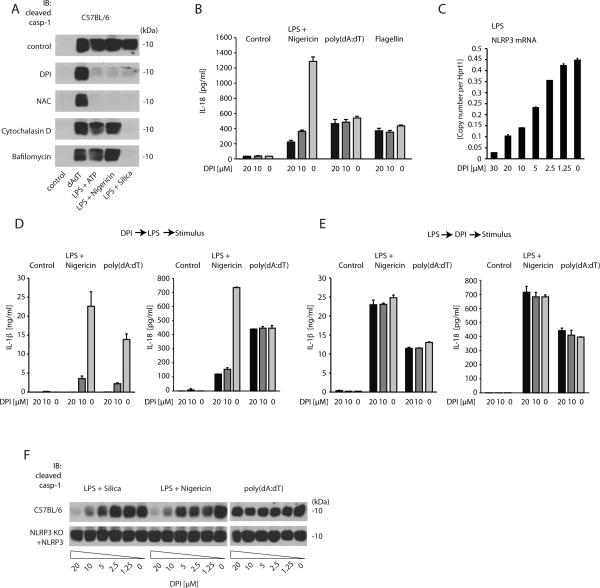
Figure 2. Inhibitors of the ROS system block NLRP3-mediated caspase-1 activation byinhibiting cell priming.
A, wild type macrophages were pretreated for 1 h with DPI (20 μM), NAC (20 mM), Cytochalasin D (5 μM) or Bafilomycin (250 nM), thenstimulated as indicatedand subsequently assessed for cleavage ofcaspase-1. B, wild type macrophages were pretreated for 1 h with 0, 10 or 20 μM DPI, then stimulated as indicated and subsequently IL-18 release was measured by ELISA. C, wild type macrophages were treated with ascending doses of DPI, subsequently primed with LPS and then assessed for NLRP3 mRNA expression. D and E, wild type macrophages were treated with DPI (1h) and then primed with LPS (3h) or alternatively, macrophages were primed with LPS (3h), then treated with DPI (1h) and subsequently stimulated as indicated. 6 h following stimulation IL-1β and IL-18 release were assessed in the supernatant. F, Cleaved caspase-1 of wild type and NLRP3-deficient macrophages reconstituted with NLRP3 is depicted. Data from one representative experiment of two (A, B, D and E) or three (C and F) are presented.
Reagents
ATP, Poly(dA:dT), Nigericin, cycloheximide (CHX), and N-Acetyl-L-cysteine (NAC) were from Sigma-Aldrich. DPI was from Alexis. Flagellin and ultra pure LPS from E. coli were from Invivogen. Silica (U.S. Silica) was used at a final concentration of 500 μg/ml. Poly(dA:dT) and Flagellin were transfected using Lipofectamine 2000 (Invitrogen) and DOTAP (Roche Applied Science) respectively. If indicated, 5 □M Nigericin or 5 mM ATP was added 1 h before supernatants were collected.
Immunoblot analysis
Caspase-1 cleavage was detected by immunoblotas previously described (Fig. 1 and 2 and Supplemental Fig. 1E and 2 (10) or Supplemental Fig. 1C–D (11)). NLRP3 expression was assessed using the Cryo-2 antibody from Axxora.
Quantitative real-time PCR analysis
RNA from macrophages was reverse transcribed and quantitative PCR analysis was performed on a Roche LC480. All gene expression data are presented as relative expression to HPRT1. Primer sequences are available upon request.
Results and Discussion
Only the NLRP3 inflammasome requires priming by a pro-inflammatory signal
NLRP3 inflammasome activation is tightly controlled by a priming step that requires de novo translation10,11. To address whether other inflammasome pathways also require de novo translation, we carried out experiments in murine macrophages treated with the translation inhibitor cycloheximide. These cells were then treated with prototypical stimuli of the NLRP3 inflammasome (LPS/Nigericin), the AIM2 inflammasome (poly(dA:dT)) or the NLRC4 inflammasome (Flagellin) and then monitored for caspase-1 activation as a direct readout for inflammasome activation. As previously shown, NLRP3 inflammasome activation was critically dependent on the presence of LPS and abrogated by cycloheximide treatment (Fig. 1A, upper panel). Macrophages transduced to constitutively express NLRP3 at levels that equal LPS-primed macrophages (Supplemental Fig. 1E) did not require LPS-priming and inhibition of de novo translation had no impact on NLRP3 activation in these cells (Fig. 1A, lower panel). Conversely, activation of the AIM2 or the NLRC4 inflammasome was independent of LPS priming. Moreover, even though AIM2 and NLRC4 ligands can serve as pro-inflammatory priming signals themselves, also complete blockage ofde novo translation by cycloheximide did not inhibit their activation of caspase-1. Similarly, IL-18, which is constitutively expressed in macrophages, was released in cycloheximide-treated macrophages when stimulated via NLRC4, but not when stimulated via NLRP3 (Supplemental Fig. 1F). Careful titration of cycloheximide indicated that the inhibitory effect on NLRP3 activation was dose dependent (Fig. 1B) and that AIM2 or NLRC4 were responsive even at high concentrations of cycloheximide. The specific role of priming for NLRP3 inflammasome activation was reflected by the fact that NLRP3 was highly inducible in response to pro-inflammatory stimuli such as LPS. Interestingly, LPS-priming enhanced neither the expression of NLRP1a, nor of NLRP1b, NLRC4, AIM2, caspase-1 or ASC. As expected, IL-1β was highly inducible upon LPS priming (Fig. 1C). Similar results were obtained for other TLR ligands such as Pam3Cys (TLR2) or R848 (TLR7/8) (data not shown). Altogether, these data indicated that NLRP3 is unique amongst the known inflammasome pathways due to its specific requirement of a pro-inflammatory priming signal.
ROS inhibitors block the priming step of NLRP3 inflammasome activation
Diphenyliodonium (DPI) is a competitive inhibitor of flavin-containing cofactors and is thus a potent inhibitor of NOX-dependent ROS production (23). At the same time, DPI also blocks mitochondria-derived ROS production, although higher concentrations are required for this effect (21, 24). N-acetylcysteine (NAC), on the other hand, functions as a scavenger of ROS regardless of the source of production. ROS inhibitors have been reported to potently inhibit NLRP3 activation (12, 16) and, in line with this finding, we observed that both DPI and NAC potently inhibited caspase-1 activation in response to various NLRP3 stimuli (LPS/ATP, LPS/Nigericin or LPS/Silica), yet not in response to AIM2 activation (poly(dA:dT)). NLRP3 stimuli that require phagosomal uptake and acidification such as silica were also inhibited by cytochalasin D or bafilomycin A (Fig. 2A). Moreover, IL-18 release in response to NLRP3, but not AIM2 or NLRC4 stimulation was inhibited by DPI as well (Fig. 2B). Measuring IL-1β release in response to NLRP3 activation confirmed the caspase-1 and IL-18 activation data. However, ROS inhibition blocked AIM2-mediated IL-1β release equally potent (Supplemental Fig. 2A). In fact, assessing IL-1β expression at the protein level in cell lysates or at the mRNA level using real-time PCR revealed that ROS inhibitors down modulated LPS-mediated IL-1β expression per se (Supplemental Fig. 2B). At the same time, the expression of other pro-inflammatory genes such as TNF was also blocked by ROS inhibitors (Supplemental Fig.2C). In line with this observation, ROS inhibition dose-dependently inhibited the expression of NLRP3, which is also induced in response to pro-inflammatory signals (Fig. 2C). Based on these findings, we speculated that ROS inhibitors block NLRP3 inflammasome activation due to the fact that NLRP3 upregulation is inhibited. To address this assumption, we performed experiments in which we added DPI before or after LPS priming. Given its constitutive and thus priming-independent expression, only IL-18 as a read out of inflammasome activation suggested a NLRP3-specific inhibitory effect of DPI pretreatment. IL-1β release was again completely blocked in DPI pretreated macrophages stimulated with either Nigericinor poly(dA:dT) (Fig. 2D). Moreover, we only observed an inhibition of NLRP3 activation when macrophages were treated with DPI before, but not after prolonged LPS priming (Fig. 2E). To further address the hypothesis of DPI blocking NLRP3 inflammasome activation by inhibiting its upregulation, were-evaluated ROS inhibitors in macrophages stably expressing NLRP3. Indeed, while DPI blocked NLRP3 activation in wild type macrophages stimulated with LPS/Silica or LPS/Nigericin, no inhibition of caspase-1 activation was observed, when NLRP3 expression was uncoupled from the priming signal by stable overexpression (Fig. 2F). Analogous data were obtained when NAC was used to block ROS (Supplemental Fig. 2D–G). Altogether, these data indicate that ROS inhibitors block NLRP3 inflammasome activation by interfering with the priming step that is required to induce NLRP3 expression, while direct NLRP3 activation is not affected. In this regard, the specificity of ROS inhibitors for the NLRP3 inflammasome can be explained by the fact that the NLRP3 inflammasome is critically dependent on priming because NLRP3 is expressed at limiting levels in un-primed macrophages. AIM2- or NLRC4-mediated capase-1 activation, on the other hand, are not affected by ROS inhibition given their constitutive expression and thus independence of de novo translation. These findings are not only important for our understanding of the mechanistics of NLRP3 activation, but they also have critical implications for the development of drugs that specifically block NLRP3-mediated inflammation without affecting pro-inflammatory transcription in general. In this regard, we would favor approaches that explore inhibitory strategies in the setting of constitutive NLRP3 expression.
Supplementary Material
Acknowledgements
We thank Millennium Pharmaceuticals for providing NLRP3-deficient mice and E. Latz for discussion.
This work was supported by grants from the German Research Foundation (SFB704 and SFB670) and the European Research Council (ERC-2009-StG 243046) to V.H., from the National Institutes of Health (R01AI063331) to G. N., and L. F. was supported by a Career Development Award from the Crohn's and Colitis Foundation of America.
Footnotes
Disclosures The authors have no financial conflicts of interest.
References
- 1.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 2.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 6.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 7.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 8.Sanz JM, Di Virgilio F. Kinetics and mechanism of ATP-dependent IL-1 beta release from microglial cells. Journal of immunology (Baltimore, Md: 1950) 2000;164:4893–4898. doi: 10.4049/jimmunol.164.9.4893. [DOI] [PubMed] [Google Scholar]
- 9.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappa B-driven protein synthesis. Journal of immunology (Baltimore, Md: 1950) 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 10.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappa B activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Bruggen R, Koker MY, Jansen M, van Houdt M, Roos D, Kuijpers TW, van den Berg TK. Human NLRP3 inflammasome activation is Nox1–4 independent. Blood. 2010;115:5398–5400. doi: 10.1182/blood-2009-10-250803. [DOI] [PubMed] [Google Scholar]
- 14.van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, van der Meer JW, Netea MG. Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci U S A. 2010;107:3030–3033. doi: 10.1073/pnas.0914795107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116:1570–1573. doi: 10.1182/blood-2010-01-264218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 19.Kamata H, Honda S-I, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. The Journal of biological chemistry. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 21.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakano Y, Longo-Guess CM, Bergstrom DE, Nauseef WM, Jones SM, Banfi B. Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J Clin Invest. 2008;118:1176–1185. doi: 10.1172/JCI33835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock JT, Jones OT. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem J. 1987;242:103–107. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holland PC, Sherratt HS. Biochemical effects of the hypoglycaemic compound diphenyleneiodonnium. Catalysis of anion-hydroxyl ion exchange across the inner membrane of rat liver mitochondria and effects on oxygen uptake. Biochem J. 1972;129:39–54. doi: 10.1042/bj1290039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.