Abstract
Purpose
Salivary gland adenoid cystic carcinoma (ACC) is a rare malignancy that is poorly understood. In order to look for relevant oncogene candidates under the control of promoter methylation, an integrated, genome-wide screen was performed.
Experimental Design
Global demethylation of normal salivary gland cell strains using 5-aza-2′-deoxycytidine (5-Aza dC) and Trichostatin A (TSA), followed by expression array analysis was performed. ACC-specific expression profiling was generated using expression microarray analysis of primary ACC and normal samples. Next, the two profiles were integrated to identify a subset of genes for further validation of promoter demethylation in ACC versus normal. Finally, promising candidates were further validated for mRNA, protein, and promoter methylation levels in larger ACC cohorts. Functional validation was then performed in cancer cell lines.
Results
We found 159 genes that were significantly re-expressed after 5-Aza dC/TSA treatment and overexpressed in ACC. After initial validation, eight candidates showed hypomethylation in ACC: AQP1, CECR1, C1QR1, CTAG2, P53AIP1, TDRD12, BEX1, and DYNLT3. Aquaporin 1 (AQP1) showed the most significant hypomethylation and was further validated. AQP1 hypomethylation in ACC was confirmed with two independent cohorts. Of note, there was significant overexpression of AQP1 in both mRNA and protein in the paraffin-embedded ACC cohort. Furthermore, AQP1 was up-regulated in 5-Aza dC/TSA treated SACC83. Lastly, AQP1 promoted cell proliferation and colony formation in SACC83.
Conclusions
Our integrated, genome-wide screening method proved to be an effective strategy for detecting novel oncogenes in ACC. AQP1 is a promising oncogene candidate for ACC and is transcriptionally regulated by promoter hypomethylation.
Introduction
Adenoid cystic carcinoma (ACC) of the salivary gland is an uncommon malignancy, comprising only 1% of all head and neck tumors and 10% of salivary malignancies (1). Typically, ACCs are known for their persistent slow growth, proclivity for perineural invasion, high rate of recurrence, and distant metastasis. Surgical therapy with adjuvant radiation is the mainstay of treatment for all ACC and often provides excellent locoregional control (2, 3). Many types of combination chemotherapy and molecular therapy have been investigated. However, only limited response has been demonstrated with any regimen to this point (1). The basis of these disappointing results stems from a lack of understanding of the basic biologic mechanisms involved in the carcinogenesis of ACC.
Alterations in DNA promoter methylation, including both hypomethylation and hypermethylation, are widely accepted regulatory mechanisms for human carcinogenesis (4). Previous methylation analysis in ACC focused on selected candidate tumor suppressor genes, such as 14-3-3 sigma, p16, E-cadherin, RASSF1A, and DAPK, et al (5–11). In this study, we hoped to perform a comprehensive, genome-wide screen for methylation-regulated oncogene candidates in ACC.
Using a strategy of unmasking epigenetically silenced tumor suppressor genes (TSG) in cancer cell lines, our lab has successfully identified cancer associated TSGs in a variety of different human cancers (12, 13). However, there are essentially no well-established cancer cell lines that are presently available for ACC. In a recent report, most previously utilized ACC cell lines were proven to be contaminated with other cell lines upon genotyping (14). Due to lack of well-established ACC cell lines, we adapted this screening method to look for hypomethylation in normal salivary cell strains to look for oncogene candidates in ACC. This technique has been used with success in head and neck squamous cell carcinoma and lung cancer (15, 16).
In the current study, by using an integrated, oncogene discovery approach, we identified a list of 159 candidate oncogenes under control of promoter hypomethylation. After screening, we indentified eight candidate genes that showed relative hypomethylation in ACC compared to normal salivary gland tissues. The candidates included AQP1, CECR1, C1QR1, CTAG2, P53AIP1, TDRD12, BEX1, and DYNLT3. Among them, Aquaporin 1 (AQP1) was further explored in primary ACC because: 1) AQP1 was methylated in all normal samples (5/5) and 2) it was the most hypomethylated candidate in primary ACC (2/5) compared to other candidates. AQP1 was found to be hypomethylated and overexpressed in ACC relative to normal salivary tissue. The transcription of AQP1 in cell lines was found to be subject to promoter methylation control. AQP1 also promoted cell proliferation and anchorage-independent colony formation in SACC83. Therefore, we propose AQP1 to be a novel oncogene candidate in ACC.
Materials and Methods
Clinical samples
Primary ACC tissue was obtained via the Johns Hopkins Pathology Department under Johns Hopkins Institutional Review Board approved protocol IRB#92-07-21-01. Tumor tissues from 18 fresh surgical specimens were snap frozen in liquid nitrogen immediately after surgical resection and stored at −196°C until use. A separate cohort of 13 adjacent normal parotid samples were also freshly frozen and utilized for this study for controls. These patients had either benign or inflammatory disease, and the tissue used was distant from the benign lesion. For the paraffin-embedded samples, 16 blocks with high tumor yield were selected and confirmed to be ACC by a head and neck pathologist (WHW). Eight 10-micron slides were cut, and the tumors were manually microdissected to yield at least 80% tumor purity. Again, 18 separate normal parotid tissue samples that had been paraffin-embedded were also used after histologic confirmation that no tumor or inflammation was contained within those slides.
DNA and RNA extraction
DNA samples were isolated as described previously (17). The formalin-fixed, paraffin-embedded (FFPE) samples underwent deparaffinization using xylenes before proceeding to DNA extraction. RNA extraction from paraffin-embedded tissue specimens were performed with the RecoverAll Total Nucleic Acid Isolation for FFPE Kit (Ambion) according to the manufacturer’s instructions.
Cell strains and cell lines
Normal salivary gland epithelial cell strains, HPAM1 and HPAF1 were obtained from ATCC (with the kind permission of Dr. Dharam Chopra) and grown according to ATCC instructions (18). Cells were grown in collagen IV coated flasks (BD Bioscience). The medium used was Keratinocyte Basal Medium (Lonza) supplemented with KGM SingleQuots (Lonza). The ACC cell line, SACC83, was a kind gift from Dr. Osamu Tetsu (personal communication), who performed the genotyping of the available ACC cell lines. SACC83 was cultured as described previously (17).
5-aza-2′-deoxycytidine (5-Aza dC) and Trichostatin A (TSA) treatment
We treated normal human salivary gland cell strains (HPAF1 and HPAM1) in triplicate with 5-Aza dC (Sigma-Aldrich) and TSA (Sigma-Aldrich) as described previously (12, 17). Total cellular RNA was isolated using the RNeasy kit (Qiagen) according to the manufacturer’s instructions.
Data Analysis
The 5-Aza dC/TSA experiments in two normal cell strains were performed using the Gene-Chip U133plus2.0 Affymetrix expression microarray (Affymetrix). Signal intensity and statistical significance was established for each transcript using dChip version 2005. Detailed data analyses were the same as described by Smith, et al (16). Briefly, the cutoff value after 5-Aza dC/TSA treated was two-fold increases based on the 90% confidence interval. We applied the Cancer Outlier Profile Analysis (COPA) (16, 19) to our Affymetrix expression microarray (Affymetrix) analysis of the primary ACC cohort of 18 patients and 8 normal samples from the University of Virginia.
We ranked target genes from the Affymetrix U133A mRNA expression microarray platform by COPA up-regulation at the 90th percentile (from 18 tumors and 8 normal tissues). A second rank list was produced by ranking genes in descending order of the degree of up-regulation upon 5-Aza dC/TSA treatment. These two sources of information (gene set demonstrating up-regulation with 5-Aza dC/TSA and COPA score) were combined by using a rank product. These two rankings were combined to rank all targets, and permutation of the data was used to establish significance with a threshold of p = 0.005. This resulted in 159 genes with the presence of CpG island(s) in the promoter region or the first intron, as determined by MethPrimer (20).
Bisulfite treatment and bisulfite genomic sequencing
The EpiTect Bisulfite Kit (Qiagen) was used to convert unmethylated cytosine in DNA to uracil according to the manufacturer’s instructions (17). Bisulfite primers, which contain no CG dinucleotides, were designed by MethPrimer to span areas of CpG island(s) in the promoter or first intron (20). Touch-down PCR, PCR products purification and sequencing conditions were described previously (17).
Quantitative methylation-specific PCR (qMSP)
QMSP was carried out in a 7900 sequence detector (Perkin-Elmer Applied Biosystems) and analyzed by a sequence detector system (SDS 2.3; Applied Biosystems), as previously described (11, 21). The AQP1 primer sequences were forward 5′-GGAGGGTAGTGGTGGTCGA-3′, and reverse 5′-CCTTCACGTTATCCTAAACCG-3′. The AQP1 probe was 6FAM 5′-AAAACCCAAAACAAAACCGATACTAAT-3′TAMRA. The β-actin primer sequences were forward 5′-TGGTGATGGAGGAGGTTTAGTAAGT-3′ and reverse 5′-AACCAATAAAACCTACTCCTCCCTTAA-3′. The β-actin probe was 6FAM 5′-ACCACCACCCAACACACAATAACAAACACA-3′ TAMRA (21). Amplifications conditions were: 95°C for 3 minutes followed by 50 cycles at 95°C for 15 seconds and 60°C for 1 minute. Leukocyte DNA from a healthy individual was methylated in vitro with excess SssI methyltransferase (New England Biolabs, Inc.) to generate completely methylated DNA, and serial dilutions (90–0.009 ng) of this bisulfite-treated DNA were used to construct a calibration curve for each plate. All samples were within the range of sensitivity and reproducibility of the assay based on the amplification of the internal reference standard (threshold cycle value for β-actin of 40). The relative level of methylated DNA in each sample was determined as a ratio of qMSP–amplified gene to β-actin (reference gene) and then multiplied by 100 for easier tabulation (average value of triplicates of the gene of interest divided by the average value of triplicates of β-actin × 100).
Transfecting AQP1 expression plasmid in SACC83 and cell proliferation and soft agar assays
An AQP1 expressing plasmid from our lab (22) and corresponding negative vectors were transfected by using Lipofectamine™ 2000 (Invitrogen). For the cell proliferation assay, cells numbers were measured at 0, 24, 48, and 72 hours by Cell Counting Kit-8 (CCK-8) (Dojindo) as described by Glazer et al (15). Absorbance (450 nm minus 650 nm) was measured by the Spectramax M2e 96-well fluorescence plate reader Molecular Devices (Sunnyvale). All growth experiments were performed in triplicate for all cell lines and vectors. For the soft agar assay, transfected cells were counted, and approximately 2000–5000 cells were added into each 6 well plate. The bottom layer was composed of 0.5% agar, RPMI +10% FBS, while the cells were suspended in a top layer of 0.35% agar, RPMI +10% FBS and G418 (500 ug/ml). Cells were cultured for 8–12 days before analysis.
Quantitative reverse transcription PCR (qRT-PCR)
CDNA synthesis was performed using random hexamer and oligo-dT with the SuperScript First-Strand Synthesis kit (Invitrogen). Subsequent qRT-PCR was performed with SYBR green and primers designed specifically for AQP1. The AQP1 RT primers are: forward. 5′-TCGCCACCGCCATCCTCTCA-3′ and reverse, 5′-CCGATGATCTCGATGCCCAGG-3′. PCR conditions were 95°C 5 minutes, 45 cycles of 95°C 15 seconds, 64°C 30 seconds. The β-actin RT primers are: forward, 5′-AGTCCTGTGGCATCCACGAAACTA-3′ and reverse, 5′-ACTGTGTTGGCGTACAGGTCTTTG-3′. For all primer sets used for qRT-PCR analysis with SYBR green, we confirmed the specificity of those primer sets in two ways: 1) the dissociation curve in qRT-PCR results was single peak and 2) the PCR products were single, dominant bands in agarose gel by electrophoresis.
Western blotting
Whole protein extracts (~40 μg) were electrophoresed in NuPAGE 4–12% Bis-Tris gel (Invitrogen) and transferred to a PVDF membrane (BioRad), probed overnight at 4°C with the antibody against AQP1 (Abcam), and β-actin (Sigma-Aldrich). The second antibody was ECL Anti-mouse IgG, horseradish peroxidase linked whole antibody, from sheep, (GE healthcare).
Immunohistochemistry
Immunostaining (IHC) was performed on Bond-Leica autostaining system (Leica Microsystems, Bannockburn, IL.) using standard immunohistochemistry protocol. IHC protocol incorporated heat induced antigen retrieval with citrate buffer (pH 6.0) followed by peroxide blocking step and primary antibody incubation for 15 minutes with mouse monoclonal antibody AQP-1 (1/22) (Abcam, dil1:400). Reaction was developed with biotin free Bond-polymer detection system (Leica Microsystems). 3′,3′ diaminobenzidin (DAB) chromogen-substrate for used for visualization of reaction. Slides were counterstained with hematoxylin, dehydrated and cover slipped.
Clinical Correlation
Descriptive statistics were performed using STATA10 (College STation). Continuous variables were summarized using means, ranges and compared using a two-sided t-test. Categorical variables were presented as proportions. Differences in distribution of categorical variables were compared using a chi-square test. Differences were considered statistically significant if p was less than 0.05.
Results
1. Integrative epigenetic approach to screen for epigenetically regulated oncogenes in ACC
The purpose of this study was to screen for ACC-associated oncogenes silenced by DNA methylation in normal salivary gland cells. We hypothesized that normal cell strains contain methylated genes that are typically repressed in normal tissues, but that these genes can be re-expressed by pharmacologic manipulation. A subset of these genes would include candidate proto-oncogenes activated by demethylation in human cancers that could be further selected on the basis of primary tumor expression array analysis using integrative methods.
Figure 1 graphically depicts the experimental flow. An integrative rank product was calculated, and using a significance threshold (p = 0.005), we identified 254 genes that were significantly differentially up-regulated based on epigenetic screening and tissue microarray expression. Initially, an in silico approach utilizing MethPrimer was used to confirm the presence of CpG island(s) overlapping or close to the transcriptional start site (TSS) of our top candidates (20). The top 159 significant genes with CpG island(s) (Table S1) were selected to be screened via this approach. We then used five normal salivary gland tissues to study promoter methylation levels in these 159 targets. Twenty four out of 159 promoter regions demonstrated complete methylation at all sequenced CpG sites in all or nearly all of the normal tissues. These 24 candidates were further analyzed by bisulfite genomic sequencing in five primary ACCs to search for candidates with promoter hypomethylation. Eight candidates were found to have hypomethylation in primary ACCs, AQP1, CECR1, C1QR1, CTAG2, P53AIP1, TDRD12, BEX1, and DYNLT3 (Table S2). Among them, AQP1 was further explored in primary ACC because AQP1 was methylated in all normal samples (5/5), and it was the most hypomethylated candidate in ACC (2/5) compared to other candidates (Supplementary Table S2).
Figure 1. Integrative epigenomic screening strategy for oncogene candidates in ACC.
First, we treated normal salivary gland cell strains with 5-Aza dC/TSA to obtain a list of reactivated genes which were epigenetically silenced. In parallel, we performed COPA in primary ACC and normal controls to obtain a list of cancer-specific genes in ACC. With statistical integration, genes (by probeset) were ranked first by degree of up-regulation with 5-Aza dC/TSA treatment and second by COPA up-regulation at the 90th percentile. The product of these two ranks was used to rank all targets, and a significance threshold (p = 0.005) was chosen.
In this way, we narrowed the list to 254 genes, of which the top 159 genes with the presence of CpG islands were evaluated by bisulfite genomic sequencing in five normal salivary gland tissues. Only 24/159 genes showed promoter methylation in normal samples. Among these 24 targets, eight candidates showed demethylation in primary ACC samples. Finally, the selected candidate gene, AQP1, was chosen for further analysis because it is completely methylated (5/5) in normal tissues and the most demethylated (2/5) in ACC samples (Supplementary Table S2) among all eight candidates in our initial screening.
2. Validating AQP1 hypomethylation in ACC by qMSPwith two independent ACC cohorts
First, we validated DNA methylation changes in the CpG island of AQP1 in a cohort of 15 paraffin-embedded ACC samples and 18 normal salivary gland tissues (Table 1), using a highly efficient and sensitive method, qMSP. QMSP primers and probe were designed in a ~100 bp subregion of the CpG island (Supplementary Figs. 1A and 1B) containing CpG dinucleotides that showed significantly decreased methylation in ACC versus normals, according to the bisulfite genomic sequencing results (Supplementary Fig. 1B). The qMSP results confirmed significant hypomethylation in the AQP1 in ACC versus normals (Fig. 2A). The mean methylation level in primary ACCs was 27.9 (range 5.0 – 52.3), and in normal salivary gland tissue, the mean was 89.6 (range, 39.8 – 311.2), demonstrating that the ACC samples were significantly hypomethylated (p = 0.001).
Table 1.
Clinical and pathologic characteristics of patient populations
| Category | Subcategory | Paraffin-embedded samples | Snap frozen samples | ACC | |
|---|---|---|---|---|---|
| Normal | ACC | Normal | |||
| Patients, n | 18 | 15 | 13 | 16 | |
| Age, yr, median (range) | 56 (42–77) | 58.7 (25–83) | 54 (29–81) | 47 (26–68) | |
| Sex, n (%) | Male | 11 (61.1%) | 8 (53.3%) | 10 (76.9%) | 8 (50%) |
| Female | 7 (38.9%) | 7 (46.7%) | 3 (23.1%) | 8 (50%) | |
| Smoking status, n (%) | Never | 9 (50%) | 5 (33.3%) | 6 (46.2%) | 6 (37.5%) |
| Former | 5 (27.8%) | 2 (13.3%) | 4 (30.8%) | 5 (31.3%) | |
| Current | 4 (23.2%) | 7 (46.7%) | 3 (23.1%) | 4 (25%) | |
| Unknown | 0 (0%) | 1 (6.7%) | 0 (0%) | 1 (6.3%) | |
| Tumor location, n (%) | Parotid | - | 4 (26.7%) | - | 2 (12.5%) |
| Submandibular | - | 0 (0%) | - | 5 (31.3%) | |
| Minor | - | 10 (66.7%) | - | 9 (56.3%) | |
| other | - | 1 (6.7%) | - | 0(0%) | |
| Stage, n (%) | I | - | 3 (20%) | - | 5 (31.3%) |
| II | - | 4 (26.7%) | - | 3 (18.8%) | |
| III | - | 1 (6.7%) | - | 2 (12.5%) | |
| IV | - | 6 (40%) | - | 3 (18.8%) | |
| Unknown | - | 0 (0%) | - | 3 (18.8%) | |
| Perineural invasion, n (%) | Yes | - | 8 (53.3%) | - | 5 (31.3%) |
| No | - | 2 (13.3%) | - | 5 (31.3%) | |
| Unknown | - | 5 (33.3%) | - | 6 (37.5%) | |
| Local recurrence, n (%) | Yes | - | 1 (6.7%) | - | 6 (37.5%) |
| No | - | 14 (93.3%) | - | 9 (56.3%) | |
| Unknown | - | 0 (0%) | - | 1 (6.3%) | |
| Metastasis, n (%) | Yes | 9 (60%) | 4 (25%) | ||
ACC - Adenoid cystic carcinoma
Figure 2. AQP1 is hypomethylated in ACC versus normal salivary gland tissues.
QMSP was conducted in two independent ACC cohorts: a paraffin-embedded cohort and a snap frozen cohort. A. in the paraffin-embedded ACC cohort, decreased methylation in AQP1 was found in ACC versus normal salivary gland tissue (p < 0.001, Student’s t-test). B. in the snap frozen ACC cohort, significantly decreased demethylation in AQP1 was found in ACC versus normal samples (p = 0.008, Student’s t-test). Error bars indicate the standard deviation.
We further validated this result in a separate freshly frozen ACC cohort (Table 1) with 16 ACC and 13 normal tissues by qMSP. As expected, significantly decreased methylation levels in AQP1 were found in ACC (mean, 43.8; range 5.4 ~ 114.4) versus normal tissues (mean, 73.8; range 50.6 ~ 111.8), p = 0.008 (Fig. 2B). Among this cohort, five ACCs and five normals were also used for the initial bisulfite genomic sequencing screening. The qMSP results were consistent with those from bisulfite genomic sequencing.
Of note, there was no significant difference in the qMSP results between the frozen and paraffin-embedded ACC cohorts, 57.2 versus 61.6 (p = 0.72). Therefore, when we combined the qMSP data from two ACC cohorts, the decreased methylation of the CpG island of AQP1 was still statistically significant, p < 0.001. In summary, with two independent cohorts, we concluded that quantitatively confirmed hypomethylation in the CpG island of AQP1 is a consistent finding for ACC.
3. AQP1 is overexpressed in ACC at both the mRNA and protein levels
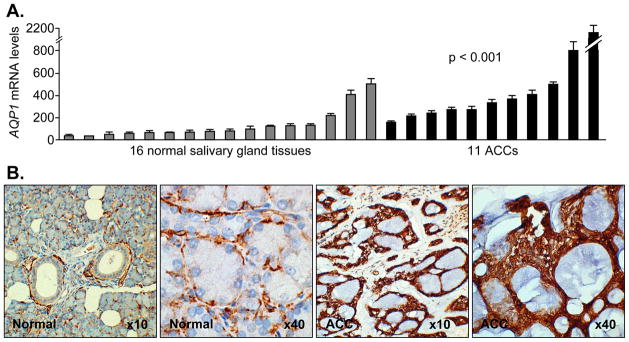
It is established that gene transcription can be regulated by promoter methylation at the CpG islands (promoter demethylation can reactivate gene transcription). Our qRT-PCR analysis with the paraffin-embedded ACC cohort showed that the AQP1 expression level in ACC showed a mean of 504.1 (range 158.7 – 2042.0), while that of the normal salivary gland tissues showed a mean of 132.8 (range 36.1 −498.1), p < 0.001. These data demonstrated significantly higher AQP1 mRNA levels in ACC compared to normal tissues (Fig. 3A). In this ACC cohort, six samples were not included due to insufficient amounts of RNA extracted from paraffin-embedded samples. These data were also consistent with the microarray results from an independent ACC cohort (from University of Virginia) that was initially used to define the gene lists.
Figure 3. AQP1 is overexpressed in ACC versus normal salivary gland tissues.
A. increased mRNA levels of AQP1 in ACC versus normal salivary gland tissue by qRT-PCR (p < 0.001, Student’s t-test). Error bars indicate the standard deviation. B. increased protein levels of AQP1 in ACC versus normal salivary gland tissues by immunohistochemistry. All ACC samples showed stronger staining than normal salivary gland tissues. Representative pictures in normal and ACC with both 10x and 40x magnification of originals are shown. In normal salivary gland tissue, a low level of AQP1 stained only in the myoepithelial cells. However, ACC samples showed consistently strong AQP1 staining throughout the entire tumor region in all 16 samples tested.
IHC was performed with a monoclonal antibody against AQP1 (Abcam, Cambridge, MA) of each paraffin-embedded sample. As expected, we found clearly stronger staining of AQP1 in primary ACC than normal tissues (Fig. 3B). In normal salivary gland tissue, AQP1 was seen slightly staining the myoepithelial cells. However, ACC samples showed consistently strong AQP1 staining throughout the entire tumor region in all 16 samples tested.
4. AQP1 was up-regulated after 5-Aza dC/TSA treatment in an ACC cell line
Besides analyzing the ACC cohort samples, we also use the only available putative ACC-derived cell line, SACC83, to study AQP1 methylation and its transcription. SACC83 was suitable for this analysis because AQP1 was unexpectedly methylated and exhibited low expression in this cell line. After 5-Aza dC/TSA treatment, CpG dinucleotide methylation levels were decreased as confirmed by the chromatograph of sequencing results (Fig. 4A) and qMSP, decreased to 46.7% (±4.6%), p < 0.001, shown in Fig. 4B. The DNA methylation level of AQP1 in mock treated SACC83 DNA was assigned as 100% methylation.
Figure 4. AQP1 was up-regulated after 5-Aza dC/TSA treatment.
A. bisulfite genomic sequencing results are shown in the chromatograph for mock treated (upper panel) and 5-Aza dC/TSA treated SACC83 DNA (lower panel). In this chromatograph, the green peak stands for C; black, T; blue, G and red, A. Cytosines in CpG dinucleotides in AQP1 promoter region are indicated by rectangles. The bisulfite sequencing results validated complete promoter methylation (pure C peak) in AQP1 in mock treatment and demethylation (mixed C and T peaks) after 5-Aza dC/TSA treatment. B. confirmation that 5-Aza dC/TSA treatment can induce demethylation by qMSP (p < 0.001). C. by qRT-PCR, the mRNA level of AQP1 was significantly up-regulated due to 5-Aza dC/TSA induced demethylation in SACC83 (p < 0.001). D. by western blot, we confirmed the up-regulation of AQP1 at protein level after 5-Aza dC/TSA induced demethylation in SACC83. 5-Aza dC/TSA or mock treated samples were loaded in the same amount as indicated by β-actin. All statistical comparisons were performed with Student’s t-test. All error bars indicate the standard deviation.
We assigned the mRNA level of AQP1 in mock treated SACC83 DNA as “1”. The levels of AQP1 mRNA were significantly up-regulated to 26.0±1.1 in 5-Aza dC/TSA treated SACC83 DNA, p < 0.001 (Fig. 4C). As expected, the AQP1 protein was also re-expressed as shown by western blotting (Fig. 4D)
5. AQP1 promotes cell proliferation and colony formation in SACC83
Because the putative ACC-derived SACC83 expressed AQP1 at low levels (Supplementary Fig. S2), we transfected an AQP1 plasmid in this cell line to demonstrate the oncogenic function of AQP1. Firstly, we found that AQP1 could promote SACC83 proliferation after transient transfection of the AQP1 plasmid. The amount of cells was determined by CCK-8 counts at 0, 24, 48, and 72 hours: the absorbance for AQP1 was 0.44±0.03, 0.47±0.03, 1.13±0.08 and 2.61±0.16, respectively, while the absorbance for vector was 0.44±0.02, 0.48±0.03, 0.88±0.03, and 1.64±0.12, respectively (Fig. 5A). The mRNA and protein levels of AQP1 were confirmed to be overexpressed by qRT-PCR and western blot (Fig. 5B, C). Corresponding quantitative AQP1 mRNA levels (Fig 5B) and protein levels (Fig 5C) were also confirmed. Secondly, we observed that AQP1 promotes anchorage-independent growth of SACC83 by soft agar assay. After AQP1 transfection, SACC83 demonstrated larger size colonies than that of vectors (Fig. 5D). The number of colonies were also significantly increased than those of the control vector, 121.7±10.4 in AQP1 versus 7.3±1.5 in vector, p = 0.002 (Fig. 5E). Lastly, we demonstrated that transfection of AQP1 also significantly promoted anchorage-dependent growth in NCI-H1299 (Supplementary Fig. S8), which has low baseline expression of AQP1 (Supplementary Fig. S2)
Figure 5. AQP1 promotes cell growth and colony formation by soft agar assay in SACC83.
A. AQP1 promotes cell proliferation in SACC83 after transient transfection. CCK-8 absorbance indicates the amount of cells at each time point. B. AQP1 mRNA levels were increased after transfection by qRT-PCR. FC, fold change, calculated by the expression levels of AQP1 divided by those in empty vector transfection. C. AQP1 protein levels were increased after transfection by western blotting. D. AQP1 promotes anchorage-independent growth of SACC83 as shown by the size of colony in the soft agar assay. E. AQP1 increased the number of colonies formed in the soft agar assay. Error bars in A, B, E indicate the standard deviation of these triplicate assays.
Because no ACC cell lines with high expression of AQP1 were available, we used NSCLC cell lines with variable AQP1-expression levels to confirm the function of AQP1. We investigated the oncogenic function of AQP1 by silencing its expression in NSCLC cell lines with high AQP1 expression, e.g. NCI-H1437 and NCI-H1703 (Supplementary Fig. S2). By transiently silencing the expression of AQP1 with siRNAs mixtures, we found inhibited cell proliferation in both NCI-H1437 (Supplementary Fig. S3A) and NCI-H1703 (Supplementary Fig. S5A), compared to scramble siRNA treated samples. Each single AQP1 siRNA also displayed similar inhibition of cell proliferation (Supplementary Figs. S3C and S5C). However, in NSCLC cell lines with low AQP1 expression, e.g. NCI-H1299 and NCI-H1944 (Supplementary Fig. S2), transient silencing of AQP1 did not show proliferation inhibition (Supplemenray Fig. S7A, B). The mRNA levels of AQP1 were confirmed by qRT-PCR at each time point after silencing (Supplementary Figs. S3B, S3D, S5B, S5D, S7C and S7D).
By soft agar assay, we also demonstrated that AQP1 inhibited anchorage-independent colony formation in NCI-H1437 and NCI-H1703 in both colony size (Supplementary Figs. S4A and S6A, respectively) and colony number (Supplementary Figs. S4B and S6B, respectively). For the anchorage-dependent colony formation assay, although the number of colonies was significantly decreased with AQP1 shRNA transfected NCI-H1437 and NCI-H1703, the size change of colonies was not obvious (Supplementary Figs. S6C and data not shown).
6. Clinical correlation
As discussed, there was no significant difference in the qMSP data between the paraffin-embedded and freshly frozen cohort. Thus, these groups were combined for subsequent analyses. There were significant differences for the AQP1 expression levels and the qMSP data between tumor and normal (p < 0.001). AQP1 hypomethylation did not correlate with smoking status, margin status, perineural invasion, or stage.
Overall, neither AQP1 expression nor AQP1 methylation status were associated with differences in overall survival. Because of the small sample size and relatively few numbers of events, it was not statistically viable to draw accurate conclusions for the risk of locoregional recurrence or the development of distant metastasis. Larger cohort studies would be necessary to better address this question.
Discussion
Adenoid cystic carcinoma of the salivary gland is an unusual malignancy with little known about its carcinogenesis. Here, we employed a new screening strategy by using normal salivary gland cell strains and primary ACC tumors to search for novel, methylation-regulated oncogene candidates in ACC. This comprehensive epigenomic screening method has been well-established and successfully applied for screening novel candidate genes in many types of human cancers (12, 13, 15, 16). Using this technique, we found eight oncogene candidates for ACC.
Among those oncogene candidates, some of them had been previously correlated with human cancers. C1QR1 was associated with breast cancer risk (23). CTAG2 (cancer/testis antigen 2) was expressed in a wide variety of cancers including melanoma (24), breast cancer (25), bladder cancer (26) and prostate cancer (27). P53AIP1 is a component of P53 mediated apoptosis (28); its expression was correlated with better survival in patients with NSCLC (29). BEX1 was proposed to be a tumor suppressor gene candidate in malignant glioma and its transcription was found to be influenced by promoter methylation (30). In this study, we focused on AQP1, as it appeared to be the most promising candidate for ACC.
AQP1 is a water channel protein that belongs to the water channel family of transmembrane proteins. AQP1 has been known to be involved in cell migration and proliferation, and overexpressed in various types of human cancers (31), such as cholangiocarcinoma (32), glioma (33), laryngeal carcinoma (34), hemangioblastoma (35), choroid plexus tumors (36), colorectal cancer (37), lung cancer (22) and mammary carcinoma (38). Similar to previous reports, we found significant over-expression of AQP1 in ACC versus normal salivary gland tissues at both the mRNA and protein levels. AQP1 is also thought to play a role in promoting tumor angiogenesis, tumor cell migration, tumor cell extravasation and metastasis (39). AQP1 was shown to stimulate cell proliferation and anchorage-independent growth in a lung cancer model (22). This is consistent with our observations for the lung cancer cell lines tested. We demonstrated that forced overexpression of AQP1 could enhance both cell proliferation and colony formation in SACC83. We also showed that silencing AQP1 could inhibit cell proliferation and colony formation in NSCLC cell lines with high expression of AQP1. Due to the toxicity of transfection reagents, we were unable to demonstrate the oncogenic effects of AQP1 in promoting cell proliferation in the normal salivary gland epithelial cell strains, HPAM1 and HPAF1.
Besides its function as a water channel, AQP1 has been recently suggested as an O2 transporter (40). AQP1 could facilitate loss of O2 in cytoplasm and stabilize O2-dependent hypoxia-inducible transcription factor (HIF). Therefore, HIF-dependent pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), would be upregulated (41–43). Tumor hypoxia has been one of the most intensively studied features of the tumor microenvironment (44). As AQP1 is not known to be one of the classical oncogenic drivers, its upregulation in ACC might fit the picture of a slow-growing and relatively indolent tumor.
Our data showed that hypomethylation in the CpG island of AQP1 was characteristic of ACC, as validated by two independent ACC cohorts. This characteristic of ACC was supported by overexpression of AQP1, which has been shown to promote carcinogenesis in many types of tumors. These observations were consistent with the widely accepted regulatory scheme: promoter hypomethylation can up-regulate the transcription of the downstream gene. We confirmed that after genome wide demethylation of the SACC83 cell line, AQP1 is significantly re-expressed, implying that the promoter status of AQP1 plays a significant role in modulating its expression. This finding is consistent with a recent report that suggested the transcription of AQP1 is epigenetically regulated (45).
The methylation levels or expression levels of AQP1 might be promising, non-invasive diagnostic markers for ACC. As we had a relatively small ACC cohort in this study, further studies with larger and independent ACC cohorts would be helpful to validate the value of this potential biomarker for ACC. Further statistical analyses in a larger cohort would also help clarify its association with local recurrence and/or the development of distant metastases.
One of the limitations of this study is the lack of well-established ACC cell lines, which is due to the rare nature of this disease, as well as its slow growth pattern. In a recent report, most previously used ACC cell lines were proven to be contaminated with other cell lines upon genotyping (14). The only available putative ACC-derived cell line, SACC83, showed unexpectedly low expression of AQP1. It is unclear whether ACC is of myoepithelial origin, and it is not proven whether SACC83 could represent the myoepitheIial origin for ACC. Therefore, our current SACC83 cell line data could tentatively shed light on AQP1’s potential oncogenic roles in ACC carcinogenesis, though not necessarily in an ACC specific fashion. In depth functional analysis can only be performed once viable cell line models have been produced and validated.
In summary, by using a genome-wide screening for oncogene candidates under the control of promoter hypomethylation, we identified AQP1 as a novel oncogene candidate in salivary gland ACC. We found hypomethylation and overexpression of AQP1 is hallmarks of ACC. Furthermore, we demonstrated that silencing of AQP1 inhibited cancer cell growth and that the expression of AQP1 was regulated by promoter demethylation. These findings support AQP1 as having a mechanistic role in the development of ACC and offer a potential novel therapeutic target.
Supplementary Material
References
- 1.Dodd RL, Slevin NJ. Salivary gland adenoid cystic carcinoma: a review of chemotherapy and molecular therapies. Oral Oncol. 2006;42:759–69. doi: 10.1016/j.oraloncology.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Fordice J, Kershaw C, El-Naggar A, Goepfert H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999;125:149–52. doi: 10.1001/archotol.125.2.149. [DOI] [PubMed] [Google Scholar]
- 3.Garden AS, Weber RS, Morrison WH, Ang KK, Peters LJ. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32:619–26. doi: 10.1016/0360-3016(95)00122-F. [DOI] [PubMed] [Google Scholar]
- 4.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–54. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 5.Uchida D, Begum NM, Almofti A, Kawamata H, Yoshida H, Sato M. Frequent downregulation of 14-3-3 sigma protein and hypermethylation of 14-3-3 sigma gene in salivary gland adenoid cystic carcinoma. Br J Cancer. 2004;91:1131–8. doi: 10.1038/sj.bjc.6602004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruya S, Kurotaki H, Shimoyama N, Kaimori M, Shinkawa H, Yagihashi S. Expression of p16 protein and hypermethylation status of its promoter gene in adenoid cystic carcinoma of the head and neck. ORL J Otorhinolaryngol Relat Spec. 2003;65:26–32. doi: 10.1159/000068658. [DOI] [PubMed] [Google Scholar]
- 7.Maruya S, Kurotaki H, Wada R, Saku T, Shinkawa H, Yagihashi S. Promoter methylation and protein expression of the E-cadherin gene in the clinicopathologic assessment of adenoid cystic carcinoma. Mod Pathol. 2004;17:637–45. doi: 10.1038/modpathol.3800104. [DOI] [PubMed] [Google Scholar]
- 8.Li J, El-Naggar A, Mao L. Promoter methylation of p16INK4a, RASSF1A, and DAPK is frequent in salivary adenoid cystic carcinoma. Cancer. 2005;104:771–6. doi: 10.1002/cncr.21215. [DOI] [PubMed] [Google Scholar]
- 9.Zhang CY, Mao L, Li L, Tian Z, Zhou XJ, Zhang ZY, et al. Promoter methylation as a common mechanism for inactivating E-cadherin in human salivary gland adenoid cystic carcinoma. Cancer. 2007;110:87–95. doi: 10.1002/cncr.22758. [DOI] [PubMed] [Google Scholar]
- 10.Williams MD, Chakravarti N, Kies MS, Maruya S, Myers JN, Haviland JC, et al. Implications of methylation patterns of cancer genes in salivary gland tumors. Clin Cancer Res. 2006;12:7353–8. doi: 10.1158/1078-0432.CCR-06-1272. [DOI] [PubMed] [Google Scholar]
- 11.Durr ML, Mydlarz WK, Shao C, Zahurak ML, Chuang AY, Hoque MO, et al. Quantitative methylation profiles for multiple tumor suppressor gene promoters in salivary gland tumors. PLoS One. 2010;5:e10828. doi: 10.1371/journal.pone.0010828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita K, Upadhyay S, Osada M, Hoque MO, Xiao Y, Mori M, et al. Pharmacologic unmasking of epigenetically silenced tumor suppressor genes in esophageal squamous cell carcinoma. Cancer Cell. 2002;2:485–95. doi: 10.1016/s1535-6108(02)00215-5. [DOI] [PubMed] [Google Scholar]
- 13.Hoque MO, Kim MS, Ostrow KL, Liu J, Wisman GB, Park HL, et al. Genome-wide promoter analysis uncovers portions of the cancer methylome. Cancer Res. 2008;68:2661–70. doi: 10.1158/0008-5472.CAN-07-5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phuchareon J, Ohta Y, Woo JM, Eisele DW, Tetsu O. Genetic profiling reveals cross-contamination and misidentification of 6 adenoid cystic carcinoma cell lines: ACC2, ACC3, ACCM, ACCNS, ACCS and CAC2. PLoS One. 2009;4:e6040. doi: 10.1371/journal.pone.0006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glazer CA, Smith IM, Ochs MF, Begum S, Westra W, Chang SS, et al. Integrative discovery of epigenetically derepressed cancer testis antigens in NSCLC. PLoS One. 2009;4:e8189. doi: 10.1371/journal.pone.0008189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith IM, Glazer CA, Mithani SK, Ochs MF, Sun W, Bhan S, et al. Coordinated activation of candidate proto-oncogenes and cancer testes antigens via promoter demethylation in head and neck cancer and lung cancer. PLoS One. 2009;4:e4961. doi: 10.1371/journal.pone.0004961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao C, Bai W, Junn JC, Uemura M, Hennessey PT, Zaboli D, et al. Evaluation of MYB promoter methylation in salivary adenoid cystic carcinoma. Oral Oncol. 2011 doi: 10.1016/j.oraloncology.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chopra DP, Xue-Hu I, Bacchetti S, Gupta J. Extended abstract: Unlimited life span of human diploid normal epithelial cells. Radiation Oncology Investigations. 1995;3:363–367. [Google Scholar]
- 19.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 20.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–31. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 21.Kim MS, Yamashita K, Baek JH, Park HL, Carvalho AL, Osada M, et al. N-methyl-D-aspartate receptor type 2B is epigenetically inactivated and exhibits tumor-suppressive activity in human esophageal cancer. Cancer Res. 2006;66:3409–18. doi: 10.1158/0008-5472.CAN-05-1608. [DOI] [PubMed] [Google Scholar]
- 22.Hoque MO, Soria JC, Woo J, Lee T, Lee J, Jang SJ, et al. Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3 cell proliferation and anchorage-independent growth. Am J Pathol. 2006;168:1345–53. doi: 10.2353/ajpath.2006.050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JY, Park AK, Lee KM, Park SK, Han S, Han W, et al. Candidate gene approach evaluates association between innate immunity genes and breast cancer risk in Korean women. Carcinogenesis. 2009;30:1528–31. doi: 10.1093/carcin/bgp084. [DOI] [PubMed] [Google Scholar]
- 24.Vaughan HA, Svobodova S, Macgregor D, Sturrock S, Jungbluth AA, Browning J, et al. Immunohistochemical and molecular analysis of human melanomas for expression of the human cancer-testis antigens NY-ESO-1 and LAGE-1. Clin Cancer Res. 2004;10:8396–404. doi: 10.1158/1078-0432.CCR-04-0809. [DOI] [PubMed] [Google Scholar]
- 25.Mashino K, Sadanaga N, Tanaka F, Yamaguchi H, Nagashima H, Inoue H, et al. Expression of multiple cancer-testis antigen genes in gastrointestinal and breast carcinomas. Br J Cancer. 2001;85:713–20. doi: 10.1054/bjoc.2001.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma P, Gnjatic S, Jungbluth AA, Williamson B, Herr H, Stockert E, et al. Frequency of NY-ESO-1 and LAGE-1 expression in bladder cancer and evidence of a new NY-ESO-1 T-cell epitope in a patient with bladder cancer. Cancer Immun. 2003;3:19. [PubMed] [Google Scholar]
- 27.Fossa A, Alsoe L, Crameri R, Funderud S, Gaudernack G, Smeland EB. Serological cloning of cancer/testis antigens expressed in prostate cancer using cDNA phage surface display. Cancer Immunol Immunother. 2004;53:431–8. doi: 10.1007/s00262-003-0458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura Y. Isolation of p53-target genes and their functional analysis. Cancer Sci. 2004;95:7–11. doi: 10.1111/j.1349-7006.2004.tb03163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamashita S, Chujo M, Miyawaki M, Tokuishi K, Anami K, Yamamoto S, et al. Combination of p53AIP1 and survivin expression is a powerful prognostic marker in non-small cell lung cancer. J Exp Clin Cancer Res. 2009;28:22. doi: 10.1186/1756-9966-28-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foltz G, Ryu GY, Yoon JG, Nelson T, Fahey J, Frakes A, et al. Genome-wide analysis of epigenetic silencing identifies BEX1 and BEX2 as candidate tumor suppressor genes in malignant glioma. Cancer Res. 2006;66:6665–74. doi: 10.1158/0008-5472.CAN-05-4453. [DOI] [PubMed] [Google Scholar]
- 31.Verkman AS, Hara-Chikuma M, Papadopoulos MC. Aquaporins--new players in cancer biology. J Mol Med. 2008;86:523–9. doi: 10.1007/s00109-008-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazal PR, Susani M, Wrba F, Haitel A. Diagnostic significance of aquaporin-1 in liver tumors. Hum Pathol. 2005;36:1226–31. doi: 10.1016/j.humpath.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 33.McCoy E, Sontheimer H. Expression and function of water channels (aquaporins) in migrating malignant astrocytes. Glia. 2007;55:1034–43. doi: 10.1002/glia.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan B, Zhu D, Dong Z, Yang Z. Expression and distribution of aquaporin 1 in laryngeal carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;21:269–72. [PubMed] [Google Scholar]
- 35.Chen Y, Tachibana O, Oda M, Xu R, Hamada J, Yamashita J, et al. Increased expression of aquaporin 1 in human hemangioblastomas and its correlation with cyst formation. J Neurooncol. 2006;80:219–25. doi: 10.1007/s11060-005-9057-1. [DOI] [PubMed] [Google Scholar]
- 36.Longatti P, Basaldella L, Orvieto E, Dei Tos A, Martinuzzi A. Aquaporin(s) expression in choroid plexus tumours. Pediatr Neurosurg. 2006;42:228–33. doi: 10.1159/000092359. [DOI] [PubMed] [Google Scholar]
- 37.Moon C, Soria JC, Jang SJ, Lee J, Obaidul Hoque M, Sibony M, et al. Involvement of aquaporins in colorectal carcinogenesis. Oncogene. 2003;22:6699–703. doi: 10.1038/sj.onc.1206762. [DOI] [PubMed] [Google Scholar]
- 38.Endo M, Jain RK, Witwer B, Brown D. Water channel (aquaporin 1) expression and distribution in mammary carcinomas and glioblastomas. Microvasc Res. 1999;58:89–98. doi: 10.1006/mvre.1999.2158. [DOI] [PubMed] [Google Scholar]
- 39.Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch. 2008;456:693–700. doi: 10.1007/s00424-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Echevarria M, Munoz-Cabello AM, Sanchez-Silva R, Toledo-Aral JJ, Lopez-Barneo J. Development of cytosolic hypoxia and hypoxia-inducible factor stabilization are facilitated by aquaporin-1 expression. J Biol Chem. 2007;282:30207–15. doi: 10.1074/jbc.M702639200. [DOI] [PubMed] [Google Scholar]
- 41.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 42.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 43.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. Faseb J. 1999;13:9–22. [PubMed] [Google Scholar]
- 44.Sun W, Liu Y, Glazer CA, Shao C, Bhan S, Demokan S, et al. TKTL1 is activated by promoter hypomethylation and contributes to head and neck squamous cell carcinoma carcinogenesis through increased aerobic glycolysis and HIF1alpha stabilization. Clin Cancer Res. 2010;16:857–66. doi: 10.1158/1078-0432.CCR-09-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huysseune S, Kienlen-Campard P, Hebert S, Tasiaux B, Leroy K, Devuyst O, et al. Epigenetic control of aquaporin 1 expression by the amyloid precursor protein. Faseb J. 2009;23:4158–67. doi: 10.1096/fj.09-140012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.