Abstract
Many anti-inflammatory strategies successful in healthy animals fail in clinical trials for sepsis, in part, because sepsis normally involves immunocompromised patients, and massive lymphocyte apoptosis prevents immunomodulation. Here we report a new set of regulatory lymphocytes able to reestablish the cholinergic anti-inflammatory modulation and to provide therapeutic advantages in sepsis. Vagus nerve controls inflammation in healthy, but not in septic mice. Likewise, vagus nerve and cholinergic agonists fail to control inflammation in splenectomized and nude animals. Unlike typical suppressor CD25+ cells, CD4+CD25− lymphocytes reestablish the anti-inflammatory potential of the vagus nerve and cholinergic agonists in immunocompromised and septic animals. These cholinergic lymphocytes reestablish splenic protection and the potential of cholinergic agonists to rescue immunocompromised animals from established sepsis. These results reveal these new regulatory lymphocytes as the first known physiological target for neuromodulation of the innate immune responses, and a potential therapeutic target for sepsis.
Introduction
Regulatory anti-inflammatory mechanisms are essential for survival and also a critical target in many clinical disorders from trauma to infectious diseases. One of the most dramatic examples of the need to control inflammation is sepsis, the third leading cause of death in the developed countries(1,2). There are very few treatment options and the mortality rate in sepsis remains extremely high ranging from 30–70% depending on the underlying cause and the organs affected(3). A challenge in sepsis is that the pathology is associated with two factors: the infection, and the inflammatory responses of the host. Sepsis is commonly originated by an infection and new antibiotics are being more effective controlling the infections(2). But, despite the advances in antibiotics and intensive care, sepsis remains the most common cause of death in hospitalized patients killing over 250,000 patients and accounting for 9.3% of the overall deaths in the United States annually(1,2). In addition to the infection, sepsis is characterized by detrimental inflammatory responses produced by the host. Overzealous cytokine production can become more detrimental than the original infection and causes tissue damage, multiple organ failure(4–7) and mortality(7–9). Many studies have shown the contribution of inflammatory cytokines to the pathogenesis of sepsis. Among others, inhibition of Tumor Necrosis Factor (TNF), Migration Inhibitory Factor (MIF) or High Mobility Group Box (HMGB)-1 have provided promising results in experimental sepsis(7–9). Yet, none of these cytokines is specific for sepsis and, so far, the inhibition of single cytokines has produced modest effects in clinical trials for sepsis(10). A potential suggestion is that sepsis is not produced by a single cytokine and hence successful treatments may require inhibiting several, rather than a single cytokine. Thus, alternative strategies focus on the physiological and cellular mechanisms modulating the immune system, and the potential alterations contributing to infectious or inflammatory disorders.
Physiological anti-inflammatory mechanisms represent efficient systems selected by evolution to control inflammation(11,12). The vagus nerve is the most characteristic nerve connecting the immune system with the peripheral organs, as it orchestrates the immune responses according to the physiological needs(11,12). Vagus nerve stimulation can prevent systemic inflammation and protect against experimental sepsis(13,14). The anti-inflammatory potential of the vagus nerve is mediated by the alpha7 nicotinic acetylcholine receptor (α7nR) (15–17). Alpha7nR-agonists,including acetylcholine (the principal neurotransmitter of the vagus nerve), nicotine, or choline, inhibit NF-kB and cytokine production in splenocytes(16). In vivo, treatment with cholinergic agonists attenuates systemic inflammation and protects mice from experimental sepsis(13–18). Different investigators have confirmed that electrical or pharmacological stimulation of the vagus nerve restrains the production of inflammatory cytokines in experimental ischemia and reperfusion(19–21), hemorrhage and resuscitation(21), pancreatitis(22), colitis(23), endotoxemia(13,24), and sepsis(16,25). Despite its clinical implications, the cellular mechanisms mediating the anti-inflammatory potential of the vagus nerve remain hitherto unknown. Our studies indicated that the vagus nerve and nicotinic agonists prevent systemic inflammation through a mechanism mediated by the spleen. Vagus nerve stimulation and α7nR-agonists fail to prevent systemic inflammation in splenectomized animals(24). From a clinical perspective, these results are particularly significant because experimental sepsis is normally studied in young, healthy animals. But, the clinical settings of sepsis typically involve immunocompromised patients. Indeed, most of the previous studies analyzing the vagus nerve focused on the potential of the vagus nerve to prevent TNF production when the stimulation is started before the endotoxic challenge. Little is known about the potential of the vagus nerve to modulate inflammation, when the stimulation is started after the pathological onset, in animals that are already septic. Here, we analyze the anti-inflammatory potential of the vagus nerve to rescue animals from established sepsis. Our results indicate that the vagus nerve and selective α7nR-agonists control of systemic inflammation in sepsis is not mediated by typical suppressor CD4+CD25+ cells, but through a set of CD3+CD4+CD25− regulatory lymphocytes. And, restitution of these regulatory lymphocytes reestablishes the anti-inflammatory potential of the vagus nerve and α7nR-agonists to rescue immunocompromised animals from established sepsis.
Material and Methods
Chemicals and reagents
Choline, Nicotine and LPS were purchased from Sigma-Aldrich Sant-Louis, MO) and fresh prepared in PBS (GIBCO, Invitrogen, CA) every experiment; LPS was dissolved in PBS stock 5 mg/ml. Pan T cell and CD4+CD25+ Modulatory T Cell Isolation kits were obtained from Miltenyi Biotec (Auburn, CA). Mouse CD4+ Selection kit was obtained from Stemcell Technologies (Vancouver.Ca). Anti-mouse CD3Ab (mCD3e) was obtained from BioXCell (West Lebanon, NH)(26,27) .
Animal Experiments
All animal experiments were performed in accordance with the National Institutes of Health Guidelines under protocols approved by the Institutional Animal Care and Use Committee of the UMDNJ-New Jersey Medical School. Male wild-type 6–8 week-old C57BL/6J mice (Jackson Laboratories, MA), nude mice B6.Cg-Foxn1<nu>/J (Jackson Laboratories, MA) or MC-deficient WBB6F1-KitW/KitW-v mice (Jackson Laboratories, MA) were randomly grouped and investigators were blinded to the experimental treatment. Endotoxemia and cecal ligation and puncture were performed as we previously described in Wang et al(16). Briefly, Endotoxemia: endotoxin (Escherichia coli LPS 0111:B4; Sigma) was dissolved in sterile, pyrogen-free PBS, and sonicated for 30 minutes immediately before used at the indicated concentration. Cecal ligation and puncture (CLP). Animals were anesthetized with Ketamine (75 mg/kg, i.m. Fort Dodge, Fort Dodge, Iowa) and xylazine (20 mg/kg; i.m.; Boehringer Ingelheim, St. Joseph, MO) and subjected to CLP with an average 50% natural mortality as we previously described (16). Briefly, animals are subjected to abdominal incision and ligation of the cecum at 5.0 mm from the cecal tip away from the ileocecal valve. The ligated cecal stump was punctured once with a 22-gauge needle, and stool was extruded (approx. 1mm) to ascertain patency of puncture. Abdominal wound was closed in two layers, peritoneum and fascia separately to prevent leakage of fluid. All animals received antibiotic (0.9mg/kg Enrofloxacine, s.c.) dissolved in 0.9% normal saline (20 ml/kg, s.c.) immediately after surgery and every twelve hours during 3 days. After collection, the organs were homogenized (Homogenizer; Omni International, GA) in lysis buffer (50 mM tris-HCl, 150 mM NaCl, 0.5% NPO4, 50mM NaF, 0.2 mM naPo4, 25 ug/ml aprotonin, 25ug/ml pepstatin A, 1mM phenylmethylsulfonyl fluoride). The resulting suspension was centrifuged (10,000g for 25 min at 4oC) and the supernatant was collected for analyses. The protein concentration was quantified by the Bradford method (Bio-rad, Hercules, CA). Vagus Nerve stimulation (VNS). Cervical vagus nerve stimulation was performed as we previously described (24). Briefly, a small incision was made to explore and identify the right cervical vagus nerve and a platinum electrode was then placed across. The platinum electrode was attached to the Stimulation Device (STM 150) controlled by the AcqKnowledge software (Biopac Sytems). Vagus stimulation was applied for 20 minutes at 5V. Sham operation was made on control animals with exception of stimulation.
CD3+CD4+CD25+ and CD3+CD4+CD25− T cell isolation
Spleens were harvested from mice and cell suspensions were prepared and filtered through a 70μm cell strainer. Red blood cells were lysed with ammonium chloride solution for 3 min. Splenic cells were washed twice before suspension in culture medium. Total CD3+ T cells were purified using a negative selection with the Pan T Cell isolation Kit (Miltenyi Biotec, Auburn, CA). Next, CD4+ cells were isolated using Mouse CD4 positive selection kit (Stemcell Technologies, Vancouver.Ca), and the CD25− and CD25+ cells were isolated using the CD3+CD4+CD25+ modulatory T cell isolation kit (Miltenyi Biotec, Auburn, CA) and, according to the manufacturer’s instructions. Cell purity was assessed by staining purified cell populations with Cy5-labeled anti-CD3 or CD4 Ab and PE-labeled anti-CD25 Ab followed by analysis using a FACSCalibur system (Becton Dickinson), and results were processed using the CellQuest pro software. The T cells fractions were consistently > 98% pure. T cell transfer. CD4+CD25+ and CD4+CD25− cells populations were resuspended in 0.9% sodium chloride solution and 1.5 x106 cells were injected intraperitoneally (i.p.) similar to that used for LPS. Cells were administered 24 hours previous to the experimental challenge.
In vitro cytokine Assay
Purified T cells populations (including CD3+CD4−, CD3+CD4+CD25+, CD3+CD4+CD25−, depending on the specific experiment and as described in the figure legends) were cultured in 24-well polystyrene culture plates at 1x106 cells/well in 1 ml of medium per well with serum free OPTIMEM I medium for the experimental procedure. Nicotine or Choline was added 30 minutes previous LPS stimulation. TNF in the culture cells was analyzed by using ELISA kit from eBioscience, (San Diego, CA), at three hours after LPS stimulation using a Versamax plate reader and SoftMax Pro software (Molecular Devices). In vivo, TNF levels in the serum were analyzed at 90 min after LPS stimulation.
Neutralizing antibody
Anti-CD3 neutralizing mCD3e antibody from BioXCell (West Lebanon, NH) was administeredintra peritoneal 3 days previous vagus nerve stimulation (20 mg/Kg; ip) similar as previously described(26,27).
Statistical Analyses
All data in the Figures and text are expressed as mean ± standard deviation (std). Statistical analyses were performed using the one way ANOVA with multiple pairwise comparisons with the Bonferroni’s adjustment for multiple hypothesis testing. Normality and homogeneity of variance were analyzed. ANOVA was used to compare all treatments and specific pair-wise comparisons as stated in the experiments. Statistical analyses of survival were determined using the Logrank test. Kaplan-Meier product-limit method was used for survival graphs. P values <0.05 were considered statistically significant.
Results
Vagus nerve stimulation prevents lethal endotoxemia
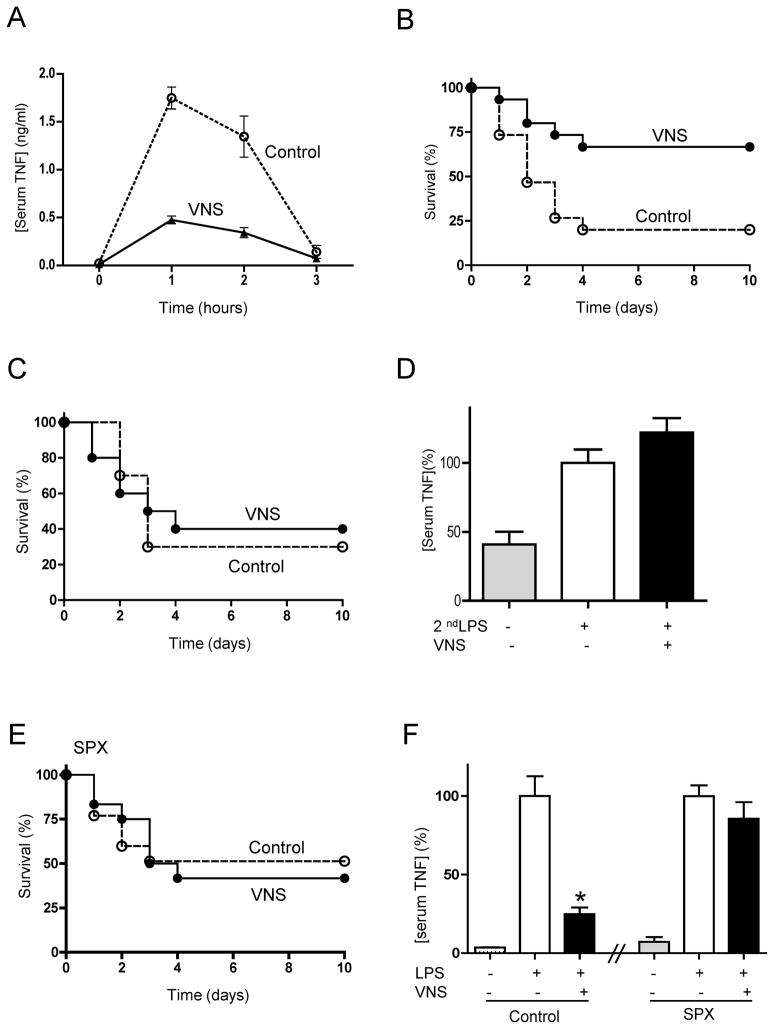
First, we analyzed whether vagus nerve stimulation inhibits or delays the onset of cytokine production in sepsis. Vagus nerve stimulation, started 20 mins prior the endotoxic challenge, inhibits serum TNF levels in endotoxemia by over 70% (Fig.1A). This mechanism induces a lasting inhibition of cytokine production and it prevents mortality in lethal endotoxemia (Fig.1B). Next, we analyzed whether vagus nerve stimulation can rescue animals from established endotoxemia when the treatment is started after the induction of lethal endotoxemia. Vagus nerve stimulation started 6 hrs after the endotoxic challenge fails to rescue animals and to improve survival in established endotoxemia (Fig.1C). Likewise, vagus nerve stimulation, started 6 hrs after the endotoxic challenge, also fails to attenuate serum TNF levels. Since serum TNF levels are very low at 6 hrs after the endotoxic challenge, we mimicked our original conditions with a secondary LPS challenge. All animals received a first dose of LPS, 6 hrs later they underwent sham surgery or vagus nerve stimulation and endotoxemia similar to that of our first experiments. A second LPS challenge induces serum TNF responses that are not inhibited by the vagus nerve, similar to that found in established endotoxemia (Fig.1D). Since previous studies indicated that sepsis is characterized by a massive apoptosis of splenocytes(28–30) and the spleen contributes to vagal modulation(24), we reasoned that the loss of splenocytes might abrogate vagal modulation. Thus, surgical splenectomy may mimic the loss of splenocytes during endotoxemia, and splenectomized animals may resemble the failure of vagal modulation in septic mice. Vagus nerve stimulation started 20 mins prior the endotoxic challenge improves survival in control but not in splenectomized animals (Fig.1E). Again, vagus nerve stimulation also fails to attenuate serum TNF levels in the splenectomized animals (Fig.1F) similar to that described in endotoxic animals. Together, these results suggest that the loss of splenocytes may abrogate the anti-inflammatory potential of the vagus nerve.
Figure 1. Vagus nerve stimulation prevents lethal endotoxemia.
Mice underwent sham surgery (Control) or vagus nerve stimulation (VNS) started 20 mins before LPS (6mg/Kg, ip). (A) Serum TNF was analyzed by ELISA (n=4; One-way ANOVA with Bonferroni’s corrections). (B) Survival was analyzed for 2 weeks and no late deaths were observed (*p<0.05 vs. control, Logrank survival test; n=15/group). (C) Mice underwent sham surgery (Control) or vagus nerve stimulation (VNS) started 6 hrs after the LPS challenge. Survival was analyzed for 2 weeks and no late deaths were observed (*p<0.05 vs. control, Logrank survival test; n=15/group). (D) Mice underwent sham surgery (Control) or vagus nerve stimulation (VNS) started 6 hrs after the original LPS challenge. Then, animals received a second dose of LPS (2ndLPS), and serum TNF concentrations were measured at 90 mins later (n=4; One-way ANOVA with Bonferroni’s corrections). (E) Splenectomized (SPX) animals underwent sham (Control) or vagus nerve stimulation (VNS) started 20 mins before LPS (6mg/Kg, ip). Survival was analyzed for 2 weeks and no late deaths were observed (*p<0.05 vs. control, Logrank survival test; n=15/group). (F) Control (laparotomy) or splenectomized (SPX) animals underwent sham surgery or vagus nerve stimulation (VNS) and LPS (n=4; One-way ANOVA with Bonferroni’s corrections).
Vagus nerve immunomodulation requires splenic T lymphocytes
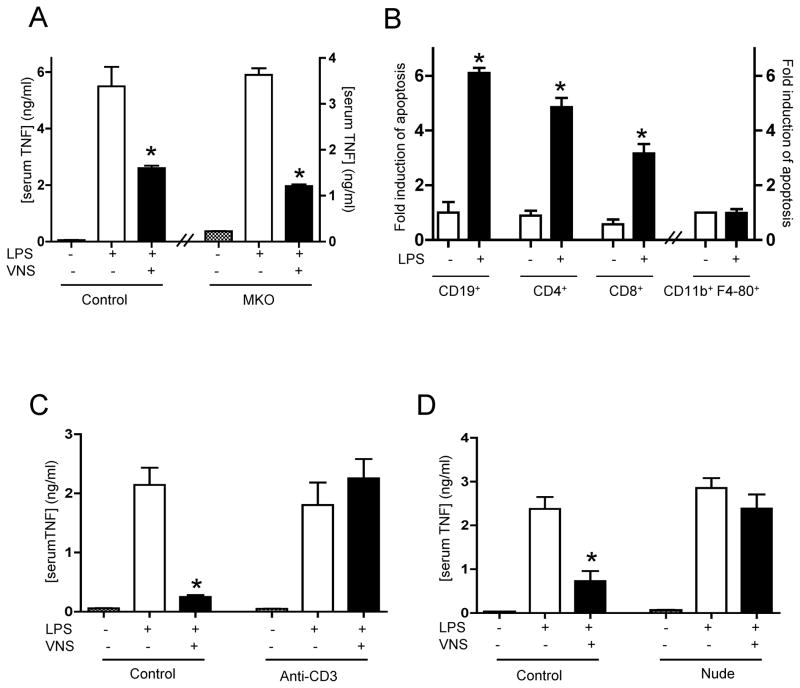
The specific contribution of particular splenic cell types to the anti-inflammatory potential of the vagus nerve was analyzed using knockout mice. Since our previous studies indicate that mast cells contribute to sepsis(31) and they can be in the proximity of the vagus nerve(32), we analyzed their potential role by performing vagus nerve stimulation in mast cell-knockout animals. Mast cell-deficient mice have normal phenotype, but they have higher mortality to sepsis(33) and limited bacterial clearance(34). Vagus nerve stimulation inhibits serum TNF responses in both control wild-type and the mast cell-knockout animals (Fig.2A). Since our results suggested the implication of other cell types, we analyzed apoptosis in splenic lymphocytes and macrophages during endotoxemia. Endotoxemia is characterized by a dramatic increase in apoptosis and a progressive loss of B and T lymphocytes but not of macrophages (Fig.2B). According to these results, we analyzed whether the loss of T lymphocytes may abrogate the anti-inflammatory potential of the vagus nerve. T lymphocytes were inhibited by using anti-CD3 neutralizing antibody as previously described(26,27). Inhibition of T lymphocytes does not affect endotoxin-induced TNF responses but it specifically blunts the anti-inflammatory potential of the vagus nerve (Fig.2D). To further confirm our studies we used T cell-deficient nude mice and their counterpart wild-type mice. Bacterial endotoxin induces similar TNF responses in both wild-type and nude mice, and yet vagus nerve stimulation fails to modulate serum TNF levels in the nude mice (Fig.2E). Together, these results indicate that the vagus nerve requires T lymphocytes to inhibit the innate immune responses to bacterial endotoxin.
Figure 2. Vagus nerve immunomodulation requires splenic T lymphocytes.
(A) Wild-type (Control) or Mast cell-knockout (MKO) mice received sham or vagus nerve stimulation (VNS) and LPS(6mg/Kg, ip). (B) BALB/c mice received vehicle or LPS (6mg/Kg, ip), and apoptosis in different cell types was analyzed by double-staining in FACS. (C) BALB/c mice were treated with vehicle (control) or the neutralizing anti-CD3 antibody (Anti-CD3, 20 mg/kg) 24 hrs before endotoxemia. (D) Control or nude mice received LPS, and sham surgery or vagus nerve stimulation (VNS). In all the experiments, serum TNF concentrations were analyzed at 90 mins and represented as percent of the LPS response. # represents p<0.05 vs. LPS (n=4; One-way ANOVA with Bonferroni’s corrections).
CD3+CD4+CD25− lymphocytes reestablish nicotinic modulation in culture of splenocytes
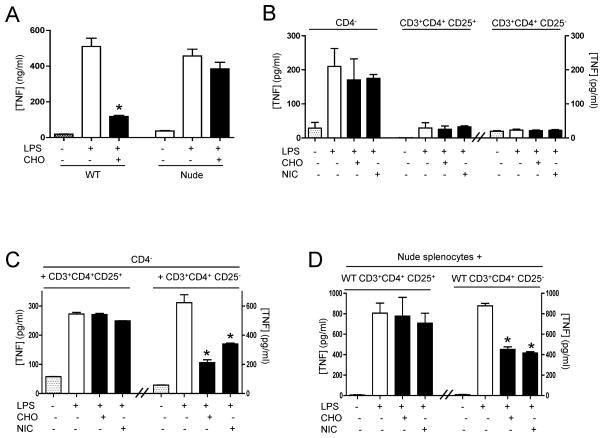
Since acetylcholine, the principal neurotransmitter of the vagus nerve, inhibits cytokine production in splenocytes via the alpha7 nicotinic acetylcholine receptor (α7nR)(15,16,35), we analyzed whether α7nR-agonists require T cells to control cytokine production in splenocytes. Primary culture of splenocytes from nude or wild-type mice were activated with endotoxin and treated with choline, a well-characterized specific α7nR-agonist(35,36). Similar to described for the vagus nerve, cultures of splenocytes from nude mice have similar TNF responses to endotoxin than those from wild-type mice. α7nR-agonist inhibits TNF production in splenocytes from wild-type but not those from nude mice (Fig. 3A). These results were further analyzed in specific splenic cell populations by fractioning splenocytes in CD4−, CD3+CD4+CD25+, or CD3+CD4+CD25− cells. The most significant production of TNF was found in the CD4− splenocytes (Fig.3B). α7nR-agonists (both choline and nicotine) fail to inhibit TNF production in any isolated population of splenocytes. The potential interaction between different cell types was analyzed by combining CD4− splenocytes with CD3+CD4+CD25+ or CD3+CD4+CD25− splenocytes. α7nR-agonists (both choline and nicotine) inhibit TNF production in co-cultures of CD4− and CD3+CD4+CD25− splenocytes but not in CD4− and CD3+CD4+CD25+ splenocytes (Fig.3C). Similar results were confirmed in the splenocytes from nude mice co-cultured with particular populations of splenocytes from wild-type mice (Fig.3D). Both choline and nicotine fail to attenuate TNF production in either splenocytes from nude mice or splenocytes from nude mice co-culture with CD3+CD4+CD25+ splenocytes isolated from wild-type mice. However, the addition of wild-type CD3+CD4+CD25− splenocytes reestablishes cholinergic modulation in splenocytes isolated from nude mice. FACS studies confirmed that our CD3+CD4+CD25− lymphocytes were neither CD25+ nor Foxp3+ with >98% purity and do not have major production of IL-10. Together, these results suggest that TNF responses to endotoxin are mainly produced by CD4− splenocytes, but the anti-inflammatory potential of cholinergic agonists (to both nicotine and choline) specifically requires CD3+CD4+CD25− splenocytes. Thus, T lymphocytes are required for both the anti-inflammatory potential of the vagus nerve and cholinergic agonists in primary culture of splenocytes.
Figure 3. CD3+CD4+CD25− lymphocytes reestablish the anti-inflammatory potential of α7nR-agonists in splenocytes.
(A) Primary cultures of splenocytes from wild-type (WT) or nude mice were treated with α7nR-agonist choline (CHO) and/or LPS. (B) Splenocytes from wild-type mice were fractionated to isolate CD4−, CD3+CD4+CD25+ or CD3+CD4+CD25− cells, stimulated with endotoxin, and treated with choline (CHO, 20mM) or nicotine (NIC,2uM). (C) Purified CD4− splenocytes were co-cultured with CD3+CD4+CD25+ or CD3+CD4+CD25− splenocytes and treated with choline (CHO, 20mM) or nicotine (NIC,2uM). (D) Splenocytes from nude mice were co-cultured with CD3+CD4+CD25+ or CD3+CD4+CD25− splenocytes from wild-type animals, stimulated with endotoxin, and treated with the indicated agonists. In all the experiments, splenocytes were pretreated with α7nR-agonists 30 mins prior LPS (100ng/mL). TNF concentrations were analyzed by ELISA in the conditioned media at 3 hours after stimulation. Graph represents the data of three different experiments in mean ± STD. * represents p<0.01 vs. LPS (n=4; One-way ANOVA with Bonferroni’s corrections).
CD3+CD4+CD25− lymphocytes reestablish the anti-inflammatory mechanism of the vagus nerve in nude mice
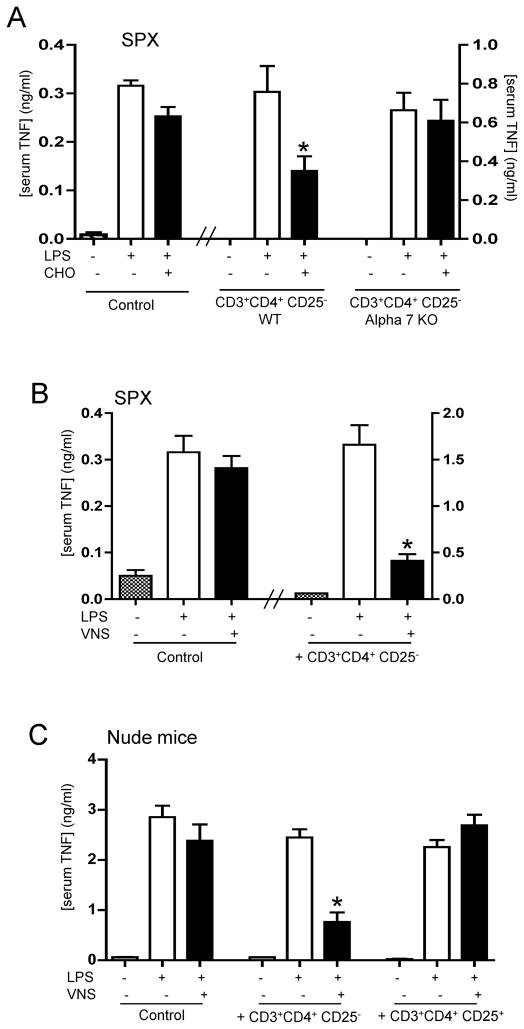
We also analyzed whether CD4+CD25− splenocytes can reestablish cholinergic modulation in vivo. Similar as described above for the vagus nerve stimulation, α7nR-agonist, choline, fails to inhibit serum TNF levels in splenectomized animals. But, restitution of CD4+CD25− splenocytes into splenectomized mice reestablishes the anti-inflammatory potential of the α7nR-agonist (Fig.4A). This mechanism is specifically mediated by the α7nR located in the lymphocytes because α7nR-null CD3+CD4+CD25− splenocytes from α7nR-knockout animals fail to reestablish the anti-inflammatory potential of choline (Fig.4A). Since the anti-inflammatory potential of the vagus nerve is also mediated by the α7nR, we also analyzed whether CD3+CD4+CD25− splenocytes could reestablish the anti-inflammatory potential of the vagus nerve in splenectomized animals. Vagus nerve stimulation fails to inhibit serum TNF levels in control splenectomized animals. But, the transfer of CD4+CD25− splenocytes into splenectomized mice reestablishes the anti-inflammatory potential of the vagus nerve (Fig.4B). According to our results, we reasoned that CD3+CD4+CD25− lymphocytes could also reestablish the anti-inflammatory potential of vagus nerve stimulation in nude mice. Vagus nerve stimulation fails to modulate serum TNF levels in nude mice. However, the transfer of CD4+CD25− wild-type splenocytes into nude mice reestablishes the anti-inflammatory potential of the vagus nerve (Fig.4C). This effect is specific for CD3+CD4+CD25− lymphocytes, because the transfer of typical regulatory CD25+ wild-type lymphocytes into nude mice did not reestablish vagal modulation. Together, these results indicate that CD3+CD4+CD25− splenocytes reestablish the anti-inflammatory potential of the vagus nerve and α7nR-agonists in vivo.
Figure 4. CD3+CD4+CD25− lymphocytes reestablish vagal anti-inflammatory mechanism in Nude mice.
(A) Splenectomized (SPX) animals received CD3+CD4+CD25− lymphocytes from wild-type (WT) or α7nR-knockout (Alpha7KO) animals. 24 hrs later, animals were treated with α7nR-agonist, choline (CHO, 400ug/kg; ip) and endotoxemia (LPS). (B) Splenectomized (SPX) animals received vehicle (control) or CD4+CD25− splenocytes from control animals, 24 hrs before vagus nerve stimulation (VNS) and endotoxemia (LPS). (C) Nude mice received vehicle (control), CD3+CD4+CD25+ or CD3+CD4+CD25− lymphocytes from control wild-type animals, 24 hrs before vagus nerve stimulation (VNS) and endotoxemia (LPS). In all the experiments, serum TNF levels were analyzed by ELISA at 90 mins after the endotoxic challenge. Graphs represent the results of four different animals in mean ± STD. * represents p<0.01 vs. LPS (n=4; One-way ANOVA with Bonferroni’s corrections).
CD3+CD4+CD25− lymphocytes reestablish splenic protection against sepsis
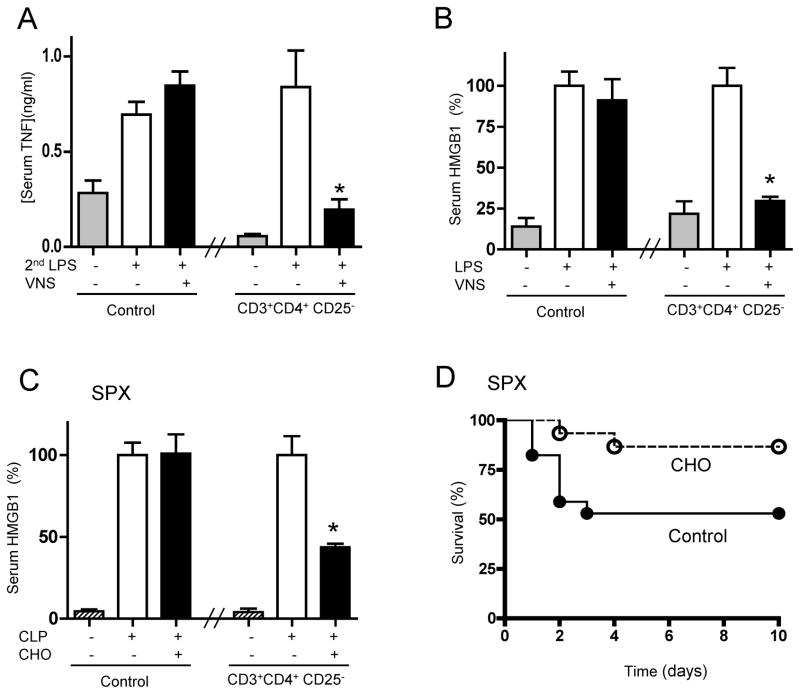
Since vagus nerve stimulation in splenectomized animals resembles that observed in endotoxemic mice, and CD3+CD4+CD25− lymphocytes reestablish vagal modulation in splenectomized and endotoxemic animals, we reasoned that CD3+CD4+CD25− lymphocytes could reestablish vagal modulation in animals with established endotoxemia. Vagus nerve stimulation fails to modulate serum TNF levels in endotoxemic mice, when the treatment is started after the endotoxic challenge. However, restitution of CD3+CD4+CD25− lymphocytes reestablishes the anti-inflammatory potential of vagus nerve stimulation started at 6 hrs after the endotoxic challenge (Fig.5A). Transfer of CD3+CD4+CD25− lymphocytes can also reestablish vagal modulation of serum HMGB1, a late mediator of sepsis, even when the vagal stimulation was started at 24 hrs after the endotoxic challenge (Fig.5B). Similar to vagus nerve stimulation, nicotinic agonists also fail to control inflammation in splenectomized animals, when the treatment is started after the onset of sepsis (24). Since vagus nerve stimulation signals via α7nR, we reasoned that CD3+CD4+CD25− lymphocytes could also reestablish the anti-inflammatory potential of nicotinic agonists in animals immunocompromised by splenectomy. From a clinical perspective, many pharmacological strategies modulate inflammation in healthy but not septic patients. Likewise, nicotinic agonists modulate inflammation in healthy but not in splenectomized animals(24). The effects of α7nR-agonist were analyzed in polymicrobial sepsis induced by cecal ligation and puncture (CLP), a more clinically relevant model with polymicrobial peritonitis induced by the cecal puncture and the necrotic tissue induced by the cecal ligation. Treatment with α7nR-agonist, started 24 hrs after cecal ligation and puncture, fails to inhibit serum HMGB1 levels in splenectomized animals with polymicrobial peritonitis (24). Restitution of CD3+CD4+CD25− lymphocytes reestablishes the splenic protection and the potential of choline to inhibit serum HMGB1 levels in splenectomized animals with established polymicrobial peritonitis (Fig.5C). Restitution of CD3+CD4+CD25− lymphocytes also reestablishes the potential of choline to rescue splenectomized animals from polymicrobial peritonitis, even when the treatment was started 24 hrs after the cecal ligation and puncture (Fig.5D). Together, these results indicate that CD3+CD4+CD25− lymphocytes reestablish the splenic protection and the anti-inflammatory potential of cholinergic agonists to rescue animals from polymicrobial peritonitis.
Figure 5.
CD3+CD4+CD25− lymphocytes reestablish cholinergic protection against sepsis and rescue animals from established sepsis. (A) Mice received vehicle (control) or CD3+CD4+CD25− lymphocytes 6 hrs after endotoxemia (LPS; 6mg/Kg; ip). One hour later, animals underwent sham surgery (Control) or vagus nerve stimulation (VNS) started 20 mins before a second dose of LPS (2ndLPS) (n=4; One-way ANOVA with Bonferroni’s corrections). (B) Mice received vehicle (control) or CD4+CD25− lymphocytes, one day after endotoxemia (LPS; 6mg/Kg; ip). Mice underwent sham surgery (Control) or vagus nerve stimulation (VNS), and serum HMGB1 levels were analyzed at 44 hrs (n=4; One-way ANOVA with Bonferroni’s corrections). (C, D) Splenectomized (SPX) underwent polymicrobial sepsis induced by cecal ligation and puncture (CLP). One day later, mice received vehicle (control) or CD3+CD4+CD25− lymphocytes. Mice were treated with vehicle (control) or choline (CHO, 400ug/kg;ip), and serum HMGB1 levels were analyzed at 44 hrs (n=4; One-way ANOVA with Bonferroni’s corrections). (D) Survival was analyzed for 2 weeks and no late deaths were observed (*p<0.05 vs. control, Logrank survival test; n=15/group).
Discussion
Immune homeostasis does not arise passively from an absence of inflammatory stimuli; rather, maintenance of health requires of specific mechanisms to restrain reactions to inflammatory stimuli that do not warrant a full response(9). In this sense, the regulation of inflammation appears to be proportional to the complexity of the immune system, and it can be considered in terms of checkpoints(9). Among these mechanisms, the nervous system is the main regulator of the immune system. We reported that vagus nerve controls systemic inflammation, including both serum TNF and HMGB1 levels, by regulating cytokine production in the spleen during endotoxemia(24). But, the cellular mechanisms mediating vagal modulation and controlling inflammation in sepsis are unknown. Our current results now indicate that this mechanism involves two specific cellular responses, including an extrinsic cellular mechanism to control innate immune responses. First, CD4− TNF-producing cells, representing splenic macrophages of the red pulp and the marginal zone as previously described(37). Second, the cholinergic regulatory CD3+CD4+CD25− cells, required by both the vagus nerve and cholinergic agonists to inhibit cytokine production both in vitro (culture of splenocytes) and in vivo. It is important to note that our results mimic the histological organization of the spleen(38); where the nerve fibers are mainly located in the periarterial lymphoid sheath (PALS) making synaptic-like connections with T lymphocytes located in the vicinity of macrophages (39–41). Previous studies described typical T suppressor lymphocytes as CD3+CD4+CD25+ lymphocytes that constitutively inhibit cytokine production from macrophages(42–44). Unlike typical T suppressor lymphocytes characterized by the expression of the IL-2Rα (CD25) and Foxp3+ our results are particularly significant showing that neuro-immunomodulation requires a set of regulatory CD3+CD4+CD25− lymphocytes that are neither CD25+ nor Foxp3+ and do not have major production of IL-10. The requirement of these cells appears specific because the typical regulatory CD3+CD4+CD25+ lymphocytes failed to induce any significant effect to either the vagus nerve or cholinergic agonists in vitro or in vivo. Indeed, the transfer of CD3+CD4+CD25− lymphocytes is sufficient to reestablish anti-inflammatory modulation in nude mice even in the absence of the classic CD3+CD4+CD25+ T suppressor cells. The claim for regulatory T cells is usually associated with modulation of a variety of in vitro immune responses of T cells to antigenic stimuli. Our studies suggest that regulatory CD3+CD4+CD25− lymphocytes mediate vagal and cholinergic modulation of cytokine production in resident macrophages. Our studies included both cholinergic agonists and vagus nerve stimulation to control TNF and HMGB1 as analyzed in vitro and in vivo. However, future studies analyzing other cytokines and the potential effects of these cells on activation, maturation and proliferation of typical immune cells will be required to fully determine the regulatory potential of these cholinergic CD3+CD4+CD25− lymphocytes. To our knowledge, these results reveal regulatory CD3+CD4+CD25− lymphocytes as the first known physiological target for neuromodulation of the innate immune responses.
Pharmacological studies allowed the identification of specific cholinergic agonists to mimic the anti-inflammatory potential of the vagus nerve while avoiding the limitation of the surgical nerve manipulation(17,45). These studies focused on identifying the receptors mediating the anti-inflammatory potential of acetylcholine, the principal neurotransmitter of the vagus nerve. Cholinergic agonists (acetylcholine and nicotine) inhibit NF-kB and cytokine production in splenocytes through the alpha7 nicotinic receptor (α7nR)(15,16,24,46). In vivo, both vagus nerve stimulation and cholinergic agonists fail to modulate serum TNF levels in α7nR-deficient animals(15,47). From a pharmacologic perspective, α7nR-agonists mimic the anti-inflammatory potential of the vagus nerve, prevent organ damage, and improve survival in experimental sepsis(15,16,36,48–50). However, both vagus nerve stimulation and cholinergic agonists fail to control inflammation and improve survival in septic animals immunocompromised by surgical splenectomy(24). Indeed, treatment with nicotine worsens survival in splenectomized animals with sepsis(24). This is an important consideration as the clinical settings of sepsis typically involve immunocompromised patients, and overwhelming sepsis is the most typical risk of splenectomy and asplenia(51,52). Our current results indicate that cholinergic agonists require regulatory CD3+CD4+CD25− lymphocytes both in vitro and in vivo. In vitro, restitution of CD3+CD4+CD25− lymphocytes reestablishes the anti-inflammatory potential of nicotinic agonists in primary culture of splenocytes from nude mice. In vivo, restitution of CD3+CD4+CD25− α7nR+ lymphocytes reestablishes the anti-inflammatory potential of the vagus nerve and α7nR-agonists in nude mice. This effect was dependent on the α7nR because the CD3+CD4+CD25− lymphocytes from the α7nR-knockout animals failed reestablish cholinergic modulation. Moreover, these cholinergic regulatory lymphocytes can reestablish neuromodulation in splenectomized animals. This is a significant result considering the clinical implications of sepsis and asplenia(51,52). These results also suggest that these cholinergic regulatory lymphocytes do not require the splenic structure, and therefore, they can control other inflammatory responses and not only those reported in the spleen. Future studies are required to determine how these cholinergic regulatory T cells respond to the vagus nerve and reestablish vagal modulation in the absence of the spleen.
Severe sepsis remains a scientific challenge with more than 30 unsuccessful clinical trials(3). Recombinant human activated protein C (Drotrecogin-α), is the only treatment approved by the FDA. And yet, this treatment is approved only for a small subset of patients with severe sepsis due to the risk of hemorrhage(53). A critical challenge is that many anti-inflammatory strategies that control inflammation in healthy animals fail to modulate inflammation in septic patients(42,54,55). One potential explanation is that many experimental strategies are effective to prevent systemic inflammation when the treatment is started before the pathogenesis, but not when started after the pathological onset. Thus, these strategies fail to provide any therapeutic effects on animals or patients that are already sick. A feasible possibility for the failure of some of these strategies is the immunological changes induced during sepsis. Sepsis is characterized by a massive apoptosis of lymphocytes in the thymus and the spleen(29,30,56). Indeed, clinical studies indicate that leukocytes derived from septic patients exhibit T cell anergy(42,57). And, septic patients have shown a dramatic decrease of CD3+CD4+CD25− cells, but a significant increase of CD3+CD4+CD25+ T cells that persisted only in nonsurviving patients(58,59). Similar results were confirmed in trauma patients(60) and in mice after polymicrobial sepsis and stroke(61–63). The prevention of lymphocyte apoptosis by using caspase inhibitors can prevent mortality in experimental sepsis(28,30). The mechanism by which lymphocyte apoptosis contributes to sepsis remains controversial. Caspase inhibitors can enhance immunity by preventing lymphocyte apoptosis and lymphocytes act rapidly to control infection(28). On the other hand, apoptotic cells can activate macrophages to release inflammatory cytokines such as HMGB1(30). Our results now suggest that the lymphocyte apoptosis can exacerbate inflammatory responses by preventing the anti-inflammatory potential of the vagus nerve and cholinergic regulatory lymphocytes. We confirmed that vagus nerve stimulation fail to control serum TNF levels in splenectomized and nude mice, experimental models that mimic splenic apoptosis during sepsis. The restitution of cholinergic regulatory lymphocytes reestablishes the anti-inflammatory potential of the vagus nerve in splenectomized, nude, and septic mice. In addition to its physiological implications, the vagus nerve mediates the effect of multiple clinical treatments and anti-inflammatory compounds including some non-steroidal anti-inflammatory drugs, semapimod (CNI-1493, currently in clinical trials for Crohn’s disease31), melanocortin peptides(64), cholecystokinin (CCK) (65,66) and leptin(17,45). So, inhibition of the vagal immunomodulation renders these drugs inefficient to control inflammation. Among other examples, semapimod and ACTH-(1–24) activate the efferent vagus nerve and limit circulating serum TNF levels during endotoxemia through a mechanism that requires functional vagus nerve(64,67). Inactivation of the vagus nerve abrogates the anti-inflammatory potential of these drugs(64,67). Our study suggests a previously unrecognized role of splenic CD3+CD4+CD25− cholinergic lymphocytes in mediating the anti-inflammatory potential of the vagus nerve and clinical drugs(66). And thus, restitution of these regulatory lymphocytes might provide pharmacological advantages to reestablish the therapeutic potential of anti-inflammatory compounds in septic patients. One significant result is that the restitution of these cholinergic regulatory lymphocytes rescued the animals from ‘established’ polymicrobial peritonitis, and it improved survival even when the cells were transferred 24 hrs after the cecal ligation and puncture. The therapeutic window for the restitution of regulatory lymphocytes is significantly wider than that described for caspase inhibitors and typical inflammatory targets. Caspase inhibitors were used at 90 mins after cecal ligation and puncture(42,57) . By comparison with other targets, administration of anti-TNF antibodies increased mortality when administered after cecal perforation(68). Anti-macrophage migration inhibitory factor antibodies(69) or lysophosphatidylcholine(70) are ineffective if administered more than 8 or 10 hours after the induction of peritonitis(69). These results suggest the potential of harnessing regulatory lymphocytes for the treatment of sepsis. However, future studies are warranted to determine their potential clinical translation, because as opposed to the experimental models using healthy animals, the clinical settings of sepsis typically involve elderly patients with previous disorders.
Acknowledgments
LR is funded by the Spanish Department of Health (CM05/00055). LU is supported by the faculty program of the Department of Surgery of the New Jersey Medical School, and grants from the American Heart Association [AHA06352230N], and the NIH [RO1-GM084125].
References
- 1.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, Parsonnet J, Panzer R, Orav EJ, Snydman DR. Epidemiology of sepsis syndrome in 8 academic medical centers. Academic Medical Center Consortium Sepsis Project Working Group. Jama. 1997;278:234–240. [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC. Caring for the critically ill patient: challenges and opportunities. Jama. 2007;298:456–458. doi: 10.1001/jama.298.4.456. [DOI] [PubMed] [Google Scholar]
- 4.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello CA. Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr Opin Pharmacol. 2004;4:378–385. doi: 10.1016/j.coph.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 7.Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Ulloa L, Brunner M, Ramos L, Deitch EA. Scientific and clinical challenges in sepsis. Curr Pharm Des. 2009;15:1918–1935. doi: 10.2174/138161209788453248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 10.Abraham E, Laterre PF, Garbino J, Pingleton S, Butler T, Dugernier T, Margolis B, Kudsk K, Zimmerli W, Anderson P, Reynaert M, Lew D, Lesslauer W, Passe S, Cooper P, Burdeska A, Modi M, Leighton A, Salgo M, Van der Auwera P. Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit Care Med. 2001;29:503–510. doi: 10.1097/00003246-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 12.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 14.Tracey KJ. Understanding immunity requires more than immunology. Nat Immunol. 2010;11:561–564. doi: 10.1038/ni0710-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 17.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 18.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 19.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Susarla S, Czura CJ, Tracey KJ. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 20.Altavilla D, Guarini S, Bitto A, Mioni C, Giuliani D, Bigiani A, Squadrito G, Minutoli L, Venuti FS, Messineo F, De Meo V, Bazzani C, Squadrito F. Activation of the cholinergic anti-inflammatory pathway reduces NF-kappab activation, blunts TNF-alpha production, and protects againts splanchic artery occlusion shock. Shock. 2006;25:500–506. doi: 10.1097/01.shk.0000209539.91553.82. [DOI] [PubMed] [Google Scholar]
- 21.Cai B, Chen F, Ji Y, Kiss L, DeJonge W, Szabo C, Deitch E, Ulloa L. Alpha7 cholinergic-agonist prevents systemic inflammation and improves survival during resuscitation. J Cell Mol Med. 2009;13:3774–3785. doi: 10.1111/j.1582-4934.2008.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Westerloo DJ, I, Giebelen A, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, Tracey KJ, van der Poll T. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Pullan RD, Rhodes J, Ganesh S, Mani V, Morris JS, Williams GT, Newcombe RG, Russell MA, Feyerabend C, Thomas GA, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 24.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Westerloo DJ, I, Giebelen A, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF, Tracey KJ, van der Poll T. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005;191:2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 26.Henrickson M, Reid J, Bellet JS, Sawchuk SS, Hirsch R. Comparison of in vivo efficacy and mechanism of action of antimurine monoclonal antibodies directed against TCR alpha beta (H57-597) and CD3 (145-2C11) Transplantation. 1995;60:828–835. [PubMed] [Google Scholar]
- 27.Cretney E, Uldrich AP, McNab FW, Godfrey DI, Smyth MJ. No requirement for TRAIL in intrathymic negative selection. Int Immunol. 2008;20:267–276. doi: 10.1093/intimm/dxm144. [DOI] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 30.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, Larosa G, Newman W, Tracey KJ, Yang H. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos L, Pena G, Cai B, Deitch EA, Ulloa L. Mast cell stabilization improves survival by preventing apoptosis in sepsis. J Immunol. 2010;185:709–716. doi: 10.4049/jimmunol.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Downing JE, Miyan JA. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today. 2000;21:281–289. doi: 10.1016/s0167-5699(00)01635-2. [DOI] [PubMed] [Google Scholar]
- 33.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 34.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 35.Pena G, Cai B, Deitch EA, Ulloa L. JAK2 inhibition prevents innate immune responses and rescues animals from sepsis. J Mol Med. doi: 10.1007/s00109-010-0628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, Hudson L, Lin X, Patel N, Johnson SM, Chavan S, Goldstein RS, Czura CJ, Miller EJ, Al-Abed Y, Tracey KJ, Pavlov VA. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14:567–574. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 39.Felten DL, Felten SY, Bellinger DL, Carlson SL, Ackerman KD, Madden KS, Olschowki JA, Livnat S. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev. 1987;100:225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 40.Felten SY, Madden KS, Bellinger DL, Kruszewska B, Moynihan JA, Felten DL. The role of the sympathetic nervous system in the modulation of immune responses. Adv Pharmacol. 1998;42:583–587. doi: 10.1016/s1054-3589(08)60818-2. [DOI] [PubMed] [Google Scholar]
- 41.Straub RH, Westermann J, Scholmerich J, Falk W. Dialogue between the CNS and the immune system in lymphoid organs. Immunol Today. 1998;19:409–413. doi: 10.1016/s0167-5699(98)01297-3. [DOI] [PubMed] [Google Scholar]
- 42.Venet F, Chung CS, Monneret G, Huang X, Horner B, Garber M, Ayala A. Regulatory T cell populations in sepsis and trauma. J Leukoc Biol. 2008;83:523–535. doi: 10.1189/jlb.0607371. [DOI] [PubMed] [Google Scholar]
- 43.O'Mahony C, van der Kleij H, Bienenstock J, Shanahan F, O'Mahony L. Loss of vagal anti-inflammatory effect: in vivo visualization and adoptive transfer. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1118–1126. doi: 10.1152/ajpregu.90904.2008. [DOI] [PubMed] [Google Scholar]
- 44.Shevach EM. The resurrection of T cell-mediated suppression. J Immunol. 2011;186:3805–3807. doi: 10.4049/jimmunol.1100364. [DOI] [PubMed] [Google Scholar]
- 45.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pena G, Cai B, Liu J, van der Zanden EP, Deitch EA, de Jonge WJ, Ulloa L. Unphosphorylated STAT3 modulates alpha 7 nicotinic receptor signaling and cytokine production in sepsis. Eur J Immunol. 2010;40:2580–2589. doi: 10.1002/eji.201040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 49.Peña G, Cai B, Deitch EA, Ulloa L. JAK2 inhibition prevents innate immune responses and rescues animals from sepsis. J Mol Med. 2010;88:851–859. doi: 10.1007/s00109-010-0628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peña G, Cai B, Deitch EA, Ulloa L. Unphosphorylated STAT3 Modulates alpha7nAChR-signaling and Cytokine Production In Sepsis. Eur J Immunol. doi: 10.1002/eji.201040540. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reid MM. Splenectomy, sepsis, immunisation, and guidelines. Lancet. 1994;344:970–971. doi: 10.1016/s0140-6736(94)91635-7. [DOI] [PubMed] [Google Scholar]
- 52.Morgan MS, Cruickshank JG. Prevention of postsplenectomy sepsis. Lancet. 1993;341:700–701. doi: 10.1016/0140-6736(93)90474-u. [DOI] [PubMed] [Google Scholar]
- 53.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 54.Vincent JL, Sun Q, Dubois MJ. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin Infect Dis. 2002;34:1084–1093. doi: 10.1086/339549. [DOI] [PubMed] [Google Scholar]
- 55.Graf J, Doig GS, Cook DJ, Vincent JL, Sibbald WJ. Randomized, controlled clinical trials in sepsis: has methodological quality improved over time? Crit Care Med. 2002;30:461–472. doi: 10.1097/00003246-200202000-00032. [DOI] [PubMed] [Google Scholar]
- 56.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Hotchkiss RS, I, Karl E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 58.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31:2068–2071. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 59.Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, Monneret G. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25− lymphocytes. Crit Care Med. 2004;32:2329–2331. doi: 10.1097/01.ccm.0000145999.42971.4b. [DOI] [PubMed] [Google Scholar]
- 60.MacConmara MP, Maung AA, Fujimi S, McKenna AM, Delisle A, Lapchak PH, Rogers S, Lederer JA, Mannick JA. Increased CD4+ CD25+ T regulatory cell activity in trauma patients depresses protective Th1 immunity. Ann Surg. 2006;244:514–523. doi: 10.1097/01.sla.0000239031.06906.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scumpia PO, Delano MJ, Kelly KM, O'Malley KA, Efron PA, McAuliffe PF, Brusko T, Ungaro R, Barker T, Wynn JL, Atkinson MA, Reeves WH, Salzler MJ, Moldawer LL. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J Immunol. 2006;177:7943–7949. doi: 10.4049/jimmunol.177.11.7943. [DOI] [PubMed] [Google Scholar]
- 62.Wisnoski N, Chung CS, Chen Y, Huang X, Ayala A. The contribution of CD4+ CD25+ T-regulatory-cells to immune suppression in sepsis. Shock. 2007;27:251–257. doi: 10.1097/01.shk.0000239780.33398.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 64.Guarini S, Cainazzo MM, Giuliani D, Mioni C, Altavilla D, Marini H, Bigiani A, Ghiaroni V, Passaniti M, Leone S, Bazzani C, Caputi AP, Squadrito F, Bertolini A. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc Res. 2004;63:357–365. doi: 10.1016/j.cardiores.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 65.Flood JF, Smith GE, Morley JE. Modulation of memory processing by cholecystokinin: dependence on the vagus nerve. Science. 1987;236:832–834. doi: 10.1126/science.3576201. [DOI] [PubMed] [Google Scholar]
- 66.Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202:1023–1029. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, Sudan S, Czura CJ, Ivanova SM, Tracey KJ. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- 69.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, Heumann D, Mannel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 70.Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004;10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]