Abstract
Attempts to cure breast cancer by means of adoptive cellular therapy (ACT) have not been successful. This is primarily due to the presence of tumor-induced immune suppressive mechanisms as well as the failure of tumor-reactive T cells to provide long-term memory responses in vivo. In order to address these clinically important challenges we developed an ex vivo protocol for the expansion of tumor-reactive immune cells obtained from tumor-bearing animals prior to or after local radiation therapy. We used an antigen-free protocol which included bryostatin 1/ionomycin (B/I) and sequential common gamma-chain cytokines (IL-7/IL-15 + IL-2). The proposed protocol expanded tumor-reactive T cells as well as activated non-T cells, including NK T cells, NK cells and IFN-γ producing killer dendritic cells (IKDC). Anti-tumor efficacy of T cells depended on the presence of non-T cells. The effector non-T cells also rendered T cells resistant to myeloid-derived suppressor cells (MDSC). Radiation therapy altered phenotypic distribution and differentiation of T cells, as well as their ability to generate central memory T cells (TCM). ACT by means of the expanded cells protected animals from tumor challenge and generated long-term memory responses against the tumor, provided that leukocytes were derived from tumor-bearing animals prior to radiation therapy. The ex vivo protocol was also able to expand HER-2/neu-specific T cells derived from the PBMC of a single patient with breast carcinoma. These data suggest that the proposed ACT protocol should be studied further in breast cancer patients.
Keywords: adoptive cellular therapy, breast cancer, common gamma chain cytokines, adiation therapy of cancer, myeloid-derived suppressor cells
Introduction
The rationale for adoptive cellular therapy (ACT) for cancer is based on overcoming the low frequency of endogenous tumor-reactive T cells by ex vivo activation and expansion, and to direct differentiation of T cells of interest toward the most effective phenotype(s). Several groups have shown that ACT directed against melanoma-associated antigens results in objective responses in animal models as well as in some melanoma patients (1, 2). To improve objective responses of ACT, a number of strategies have been developed which include using genetically modified lymphocytes (3), highly effective T cell phenotypes (4) and use of common gamma-chain cytokines (5). However, unlike animal models, cancer patients usually receive ACT after conventional therapies which could interfere with the efficacy of the donor T cells. No comparative analysis has been performed to determine whether previous radiation therapy reduces or enhances the anti-tumor efficacy of ACT. ACT has also been tested against breast cancer both in mouse models and breast cancer patients (6, 7). However, unlike melanoma, ACT has not produced complete protection against breast tumors. Barriers to success include difficulty in the ex vivo expansion of tumor-reactive T cells (8), uncertainty as to the most relevant antigens, a lack of consensus as to the appropriate origin of the T cells to be used for expansion as well as phenotypic distribution of the most effective T cells, presence of myeloid-derived suppressor cells (MDSC) in cancer patients and during pre-malignant carcinogenesis which could abrogate anti-tumor efficacy of ACT (7, 9), and finally tumor stroma as a major barrier which prevents penetration of T cells into the solid tumor (10).
In the present study we addressed a number of key barriers listed above in order to produce objective responses against primary breast tumors and to generate long-term memory against recall tumor challenge. We took advantage of an antigen-free protocol for selective activation of tumor-primed immune cells by using bryostatin 1/ionomycin (B/I) as previously described by our group (7, 11). Bryostatin 1 activates protein kinase C and ionomycin increases intracellular calcium (12, 13). Together, B/I mimic signaling through the CD3/TcR complex and lead to activation and proliferation of tumor-primed T cells. Most recently, it was reported that bryostatin 1 can act as a TLR-4 ligand and activate innate immunity (14). We then developed a sequential common gamma-chain cytokine protocol for expansion of the B/I-activated tumor-primed immune cells. This ex vivo protocol induced expansion of tumor-reactive immune cells comprised of central memory T cells (TCM) and effector T cells (TE) as well as cells of the innate immune system or non-T cells. The expanded cells were found to be resistant to MDSC and were capable of generating long-term memory responses against the tumor in the FVBN202 transgenic mouse model of HER-2/neu overexpressing breast carcinoma. We also showed that HER-2/neu-specific T cells can be expanded from peripheral blood mononuclear cells (PBMC) of a breast cancer patient by using B/I activation and addition of the common gamma chain cytokines ex vivo.
Materials and Methods
Mouse model
FVBN202 transgenic female mice (Charles River Laboratories) were used between 8–12 weeks of age throughout these experiments. These mice overexpress an unactivated rat neu transgene under the regulation of the MMTV promoter (15). These mice develop premalignant mammary hyperplasia similar to ductal carcinoma in situ (DCIS) prior to the development of spontaneous carcinoma (16). Pre-malignant events in FVBN202 mice include increased endogenous MDSC (16). These studies have been reviewed and approved by the Institutional Animal Care and Use committee (IACUC) at Virginia Commonwealth University.
Tumor cell lines
The neu overexpressing mouse mammary carcinoma (MMC) cell line was established from a spontaneous mammary tumor harvested from FVBN202 mice as previously described by our group (17). Tumor cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS). Mice were challenged with 3–4×106 MMC cells subcutaneously (s.c.) in the lower part of the mammary region close to the groin area.
Flow cytometry
Flow cytometry analyses were performed as previously described by our group (16, 18). Briefly, spleens were disrupted into a single cell suspension and 106 cells were aliquoted into each sample tube. Non-specific binding of antibodies to Fc receptors was blocked by incubating the cells with anti-CD16/32 antibody (Biolegend). Cells were stained with surface antibodies towards various markers and incubated on ice in the dark for 20 minutes, then washed twice with cell staining buffer (PBS, 1% FBS, 0.1% Sodium Azide) and fixed with 1% paraformaldehyde. For intracellular staining of perforin (Prf) and FoxP3, we followed the FoxP3 staining protocol provided by the manufacturer (Biolegend). Cells stained for granzyme B (GrB) and IFN-γ were fixed with 2.5% paraformaldehyde for 10 minutes on ice, washed twice with 0.1% saponin cell staining buffer, and then stained with the indicated antibodies. Cells were then washed twice with normal cell staining buffer and fixed with 1% paraformaldehyde. For Annexin V staining, cells were stained for respective surface markers, washed with cell staining buffer, and then washed with 1X Annexin V buffer (BD Pharmingen). The Annexin V staining protocol given in the product data sheet was then followed. Antibodies used for flow cytometry were purchased from Biolegend (FITC-, PE-, PE/Cy5-CD3, FITC-, PE-, PE/Cy5-CD4, FITC-, PE-, PE/Cy5-, APC-CD8, FITC-, PE/Cy5-CD11b, PE-, PE/Cy5-Gr1, FITC-, PE-CD25, FITC-CD44, PE-CD62L, PE-, APC-CD49b, PE-CD69, FITC-, PE-CD122, FITC-, PE-CD127, FITC-, PE-B220, PE-IFN-γ, PE-Foxp3) or eBiosciences (FITC-, APC-, PE-Prf, PE-, PE/Cy5-GrB). All antibodies were used at the manufacture’s recommended concentrations. Multicolor data acquisition was performed on a BD FACSCanto II and analyzed using BD FACSDiva software.
Cell sorting
In order to sort distinct cellular populations of splenocytes, 107 cells were added to sample tubes in which Fc receptors were blocked and surface markers were stained with FITC-conjugated CD4 and PE-conjugated CD8 antibodies. Cells were then washed with sterile PBS supplemented with 2% FBS. T cells and non-T cells were sorted into 100% FBS using a BD FACSAria III cell sorter. Purity of sorted cells was greater than 98%.
Cytotoxicity assay
Freshly isolated tumor-primed splenocytes or the ex vivo expanded cells were cultured with MMC at a 10:1 E:T ratio in 3 ml complete medium (RPMI-1640 supplemented with 100 U/ml of penicillin, 100 µg/ml streptomycin, 10% FBS, 10mM L-glutamine and 5×10−5M 2-mercaptoethanol) with 20U/ml of IL-2 (Peprotech) in 6 well culture dishes. After 48 hs cells were harvested and stained for neu (anti-c-Erb2/c–Neu, Calbiochem), Annexin V and PI according to the manufacturer’s protocol (BD Pharmingen). Flow cytometry was used to analyze the viability of neu positive cells.
IFN-γ ELISA
Freshly isolated tumor-primed splenocytes or the ex vivo expanded cells were cultured in complete medium at a 10:1 ratio with irradiated MMC cells (14,000 rad) for 24 hs. Supernatants were then collected and stored at −80°C until assayed. IFN-γ was detected using a Mouse IFN-γ ELISA set (BD Pharmingen) according to the manufacture’s protocol.
Expansion of effectors T cells from FVBN202 mice
FVBN202 transgenic mice were inoculated with 4×106 MMC cells and splenocytes were harvested after 21–25 days. Splenocytes (106 cells/ml) were then stimulated in complete medium containing 15% FBS as well as Bryostatin 1 (5 nM)/Ionomycin (1µM), and 80 U/ml of IL-2 for 16 hs, as previously described by our group (12). Cells were then washed three times and cultured at 106cells/ml in complete medium with 10–20 ng/ml each of IL-7 and IL-15 (Peprotech). After 24 hs, 20U/ml of IL-2 was added to the culture. On the next day cells were washed three times and cultured at 106cells/ml in complete medium with 40 U/ml of IL-2. Cells were split and cultured at 106cells/ml in complete medium with 40 U/ml of IL-2 every other day for a total of 6 days. After day 6, cells were then used for ACT or in vitro studies.
Adoptive cellular therapy (ACT)
Twenty four hours prior to ACT, FVBN202 mice were injected i.p. with CYP (100 mg/Kg) in order to induce lymphopenia. Mice were challenged with 3×106 MMC cells and then received 70×106 of the expanded cells by tail vein injection later the same day. Tumor growth was monitored by digital caliper and tumor volumes were calculated by: Volume (v) = [L (length) × W (width) 2]/2.
Isolation of MDSC in vitro
The Gr1+ MDSC population was isolated using an EasySep-FITC selection kit from StemCell Technologies, as previously described by our group (7, 19). We have previously shown that endogenous MDSC isolated from bone marrow or secondary lymphoid tissues can inhibit T cell responsiveness to anti-CD3/anti-CD28 antibodies (7, 19–22). Also, the Ly6C+Ly6G- subset but not the Ly6G+Ly6C+ subset isolated from FVBN202 mice was found to be suppressive (19). Because of the higher proportion of Ly6C+Ly6G- subset in tumor-bearing mice compared to tumor-free mice, the suppressive effects of MDSC isolated from tumor-bearing mice was greater using an optimal 2:1 ratio of T cells to MDSC (19). Therefore, in this study we used an optimal 2:1 ratio of splenic MDSC isolated from tumor-bearing FVBN202 mice.
Expansion of HER-2/neu-specific T cells from PBMC
Peripheral blood mononuclear cells (PBMC) were harvested from a breast cancer patient under Institutional Review Board (IRB) protocol HM10920. PBMC were cultured at 37°C for 2 hs; adherent cells were used for the generation of monocyte-derived DCs in the presence of GM-CSF and IL-4, as previously described by our group (17). Floater cells were split into two groups. One group was maintained with IL-2 (40 U/ml/106 cells) for 6–7 days until autologous DCs became available. Another group was activated with B/I and expanded with common gamma-chain cytokines. The expanded cells or IL-2 maintained cells were cultured with autologous DCs (4:1) in the presence or absence of recombinant HER-2/neu (100 ug/ml) or LPS (10 ug/ml). After 24 hs, supernatants and cells were collected and subjected to IFN-γ ELISA and flow cytometry analysis, respectively.
Recombinant HER-2/neu protein
Extracellular domain (ECD) and intracellular domain (ICD) of human HER-2/neu protein were expressed in E-coli and purified by Ni-NTA-Agarose (Qiagen, Valentica, CA), as previously described by our group (23). Concentration of the recombinant proteins was determined using the Bradford assay.
Statistical analysis
Graphical data are presented as means with standard errors. Statistical comparisons between groups were made using Student’s t test with P< 0.05 being statistically significant.
Role of the funding source
The study sponsors provided funding for the study and had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Results
Sequential common gamma-chain cytokines expand B/I-activated T cells derived from tumor-bearing FVBN202 mice
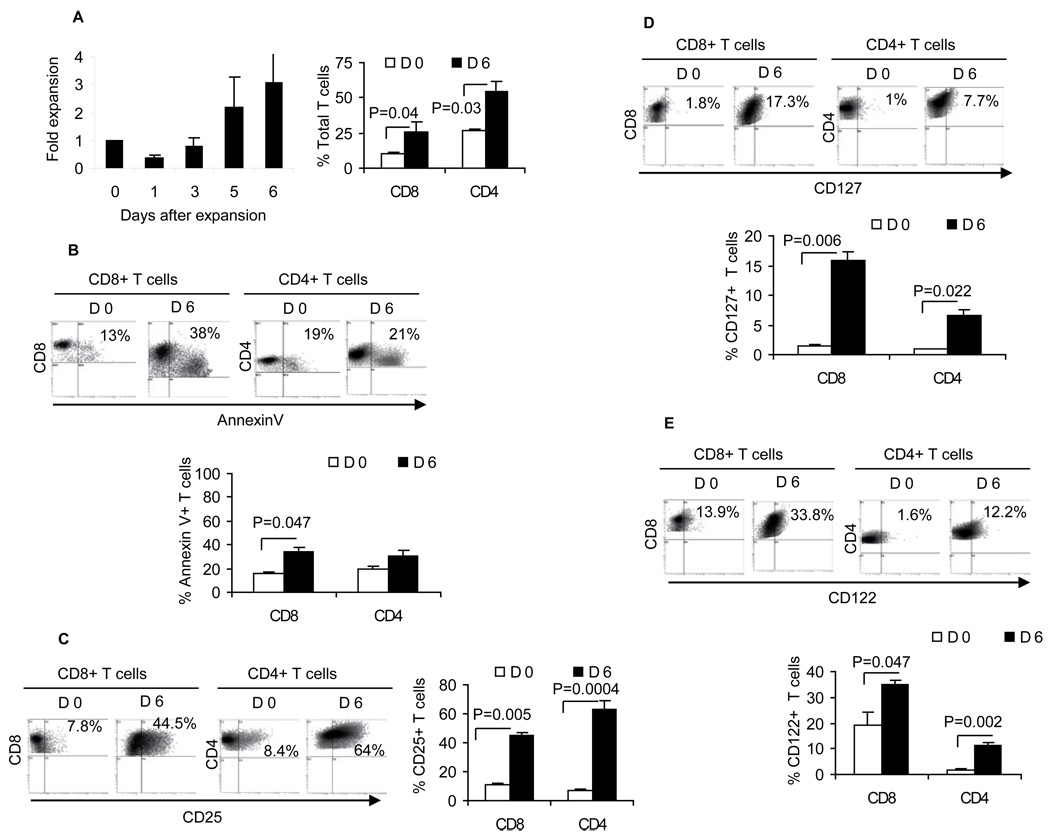
We have previously shown that activation of splenocytes (7) or tumor-draining lymphocytes with B/I could mimic T cell receptor signaling and selectively activate tumor-primed T cells (7, 20–22). Expansion of the B/I-activated T cells would then result in differential phenotype distribution depending on the cytokine formulation used during the expansion (7). We have previously tested different combinations of IL-2, IL-7, IL-15 (7, 21, 22) and showed superiority of an alternating sequence of common gamma-chain cytokines (IL-7+IL-15 => IL-2 => IL-7+IL-15). However, antitumor efficacy of cells grown in alternating cytokines was limited in the neu transgenic tumor model because of a high level of endogenous MDSC in the FVBN202 transgenic mice, which was further increased during tumor challenge (7, 16). Therefore, we sought to determine whether a sequential common gamma-chain cytokine regimen (IL-7+IL-15 followed by IL-2) would improve anti-tumor efficacy of the expanded cells in FVBN202 mice. We first examined the composition of cells that were expanded with the sequential common gamma-chain cytokine formulation, and showed a three-four fold expansion of CD8+ and CD4+ T cells during a 6-day culture ex vivo (Fig. 1A). Although expanded CD8+ T cells showed reduced viability during the ex vivo culture (Annexin V+ CD8+ T cells: 13% to 38%, p= 0.047; Fig. 1B), the proportion of CD25+, CD127+ and CD122+ T cells was significantly increased (6–8 fold increases of CD25+ cells, Fig. 1C; 7–9 fold increases of CD127+ cells, Fig. 1D; and 2–6 fold increases of CD122+ cells, Fig. 1E). There were marginal increases in the number of CD4+CD25+Foxp3+ T cells, accounting for only 2% of the gated CD4+ T cells after a 6-day expansion (Fig. S1).
Figure 1. Ex vivo expansion of tumor-primed T cells with the sequential common gamma-chain cytokines.
A) Ex vivo expansion of splenocytes harvested from MMC-primed (tumor volume ≤ 500–2000mm3) female FVBN202 mice before and after activation with B/I and expansion with sequential common gamma-chain cytokines as determined by trypan blue exclusion (left panel) or flow cytometry analysis of gated CD8+ or CD4+ T cells (right panel). B) Viability of freshly isolated (D 0) and ex vivo expanded (D 6) T cells was determined by flow cytometry analysis of gated CD8+ or CD4+ T cells. Expression of CD25 (C), CD127 (D) and CD122 (E) were determined on gated CD8+ or CD4+ T cells before and after a 6-day expansion. Data represent five independent experiments.
T cells expanded with sequential common gamma-chain cytokines are highly responsive to neu+ MMC cells
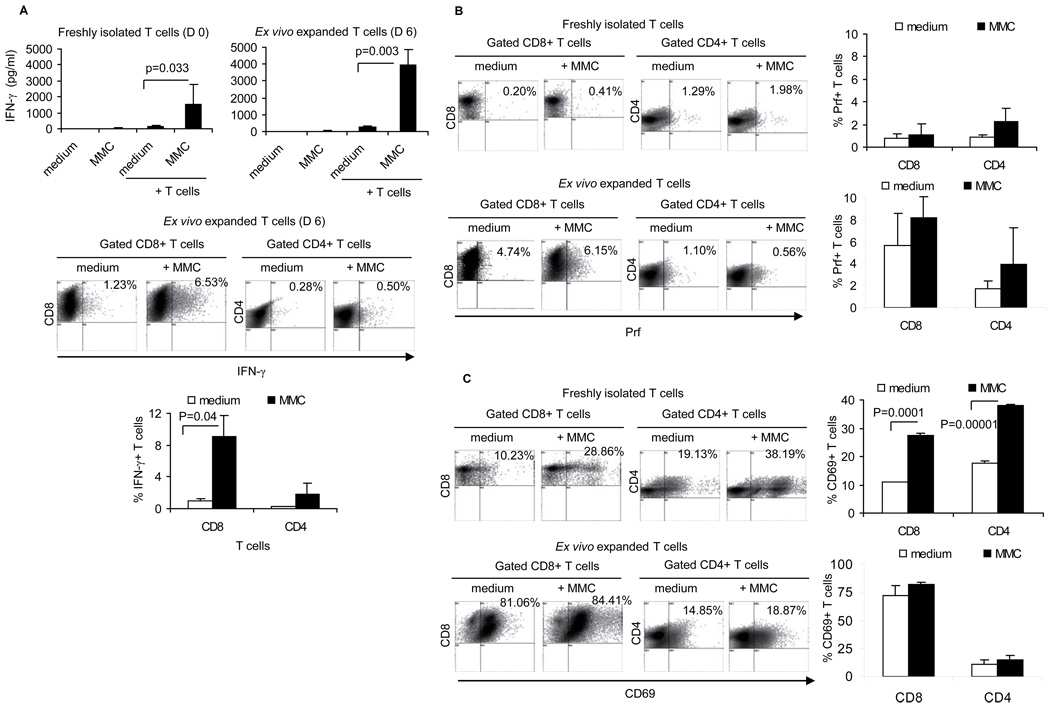
In order to determine whether the ex vivo expansion of T cells enriched tumor-reactive T cells, production of IFN-γ, Prf, GrB as well as the expression of the CD69 early activation marker was determined in the presence or absence of irradiated neu+ MMC in vitro. Compared to freshly isolated splenocytes, the ex vivo expanded T cells showed greater IFN-γ production upon stimulation with MMC (average 1500 pg/ml vs. 4000 pg/ml, p= 0.042; Fig. 2A, upper panel). T cells isolated from naive FVBN202 mice did not show IFN-γ production upon MMC stimulation in vitro (data not shown). Flow cytometry analysis of the ex vivo expanded cells determined that CD8+ T cells were the source of tumor-specific IFN-γ production (Fig. 2A, lower panel). Although no significant increases of Prf+ T cells was detected upon MMC stimulation, the proportion of Prf+CD8+ T cells was greater among the ex vivo expanded cells than freshly isolated splenocytes (average 1% vs. 7%, p= 0.028; Fig. 2B). The presence of tumor-reactive T cells among freshly isolated splenocytes was further confirmed by detecting an increased proportion of CD69+ T cells upon MMC stimulation (Fig. 2C). The ex vivo expansion of CD8+ T cells resulted in an increased proportion of CD69+ early effector cells prior to MMC stimulation (average 10% vs. 81%, p= 0.011; Fig. 2C) and after MMC stimulation (average 28% vs. 84%, p= 0.001; Fig. 2C). Almost all T cells expressed GrB prior to and after a 6-day expansion ex vivo (Fig. S2A).
Figure 2. Ex vivo expanded, MMC-primed T cells respond to MMC cells in vitro.
A) Tumor reactivity of freshly isolated (D 0) and ex vivo expanded (D 6) T cells was determined by a 24-hs culture of T cells in the presence or absence of irradiated MMC cells followed by the detection of IFN-γ in the supernatant (upper panel). Medium alone and MMC alone were used as negative controls for IFN-γ production. The MMC-specific IFN-γ production in gated CD8+ and CD4+ T cells was determined by flow cytometry analysis (lower panel). The MMC-specific expression of Prf (B) and CD69 (C) were determined in gated T cells. Data represent five independent experiments.
Ex vivo expanded T cells are enriched for CD44+CD62L- effector (TE) and CD44+CD62Lhigh central memory (TCM) phenotypes and provide complete protection against primary as well as recall tumors
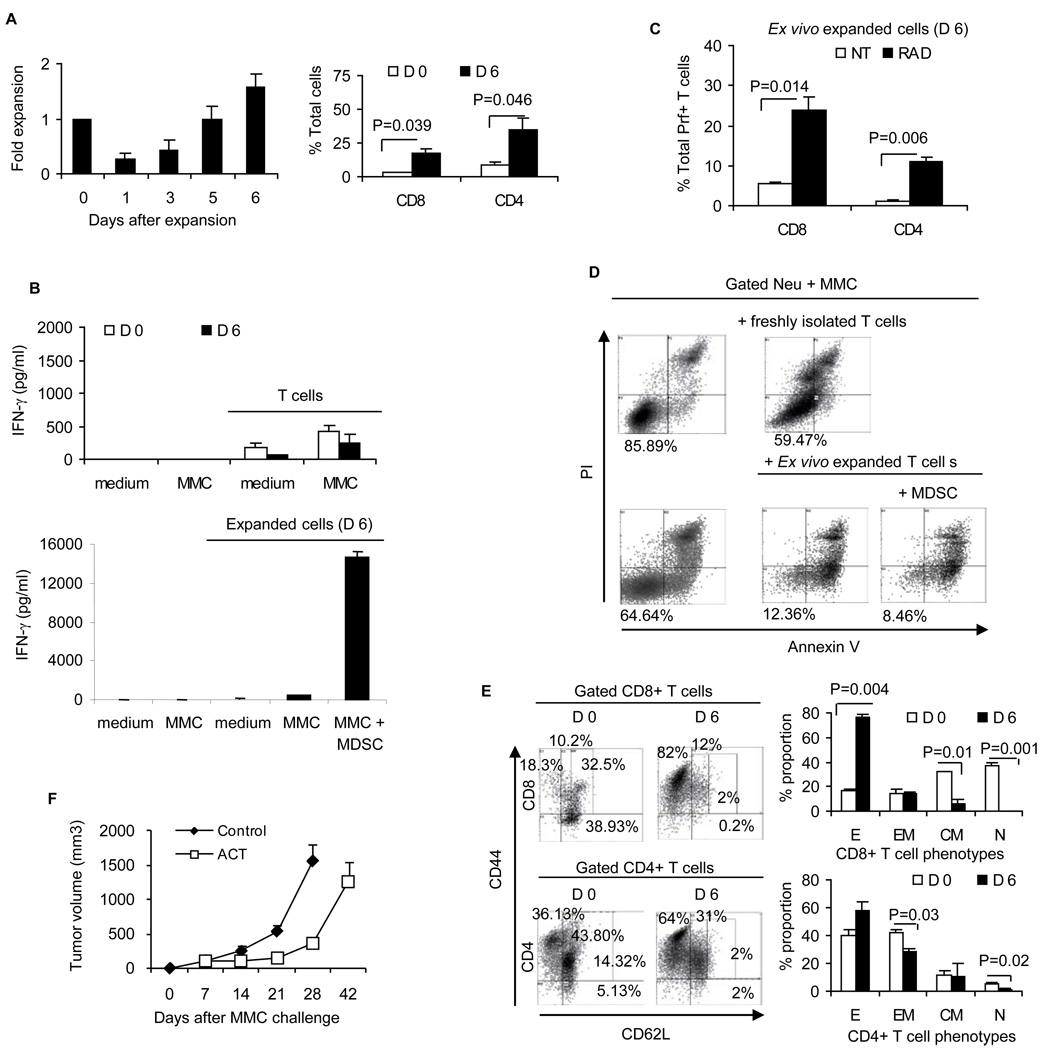
CD44+CD62L- effector T cells (TE) and CD44+CD62Llow effector memory T cells (TEM) provide immediate protection against tumors whereas CD44+CD62Lhigh central memory T cells (TCM) are important for generating long-term protection. TCM are particularly important during recall tumor challenge. Ideally, the presence of the both phenotypes can provide protection against primary and recall tumor challenges. Therefore, we sought to determine the phenotypic distribution of CD8+ and CD4+ T cells prior to and following the 6-day ex vivo expansion with sequential common gamma chain cytokines. Freshly isolated CD8+ T cells contained roughly equal proportions of TE (30%), TEM (26%) and CD44-CD62L+ naive T cells (TN: 33%). Ex vivo expanded CD8+ T cells were enriched for TE (D 6: 55.6% vs. D 0: 30%, p= 0.02) and TCM (D 6: 26% vs. D 0: 7.2%, p= 0.008) (Fig. 3A). Ex vivo expanded CD4+ T cells showed an unchanged proportion of TE (D 6: 31.9% vs. D 0: 26.3%) but were enriched for TCM (D 6: 61.3% vs. D 0: 6.6%, p= 0.002). TN phenotypes almost disappeared in the expanded CD8+ T cells (D 6: 1.8% vs. D 0: 33.7%, p= 0.009) and CD4+ T cells (D 6: 2% vs. D 0: 14.1%, p= 0.003). Such a phenotypic distribution towards CD8+ TE and TCM suggests the potential for immediate as well as long-term memory responses against the tumor. We then performed in vitro cytotoxicity assays and in vivo tests of tumor growth inhibition in order to determine the anti-tumor efficacy of the expanded cells. Freshly isolated splenocytes or expanded T cells were cultured with viable neu+ MMC tumor cells in an effector:target (E:T) ratio of 10:1 for 2 days. Gated neu+ MMC cells were then analyzed for the detection of apoptosis as determined by Annexin V+/PI+ cells. As shown in Fig. 3B, freshly isolated T cells reduced viability of neu+ MMC from 87.5% to 50.79% (1.7 fold) while the ex vivo expanded T cells displayed greater cytotoxic function, reducing the viability of MMC from 68.8% to 17.6% (3.9 fold).
Figure 3. Phenotypic distribution of tumor-reactive T cells and their anti-tumor efficacy in vitro and in vivo.
A) Phenotypic distribution of freshly isolated (D 0) and ex vivo expanded (D 6) splenic T cells was determined by flow cytometry analysis of gated CD8+ or CD4+ T cells. Distribution of T cell phenotypes including CD44+CD62L- effector (E: TE), CD44+CD62Llow effector memory (EM: TEM), CD44+CD62Lhigh central memory (CM: TCM) and CD44-CD62L+ naive (N: TN) was determined. B) Gated neu positive MMC cells were analyzed for apoptosis (Annexin V+/PI+) in the absence or presence of freshly isolated or ex vivo expanded T cells. C) CYP-treated FVBN202 mice (n=3) were inoculated with MMC cells and received no further treatment (left panel) or received ACT (middle panel). Animals that had rejected MMC following ACT were given rest for 2 months and then were challenged with MMC cells on the contralateral side (right panel). Data represent five independent experiments.
To test in vivo efficacy of expanded T cells, we used FVBN202 mice, which harbor increased MDSC because of premalignant mammary hyperplasia preceding spontaneous mammary tumors. Endogenous MDSCs were further increased upon MMC tumor challenge (7, 16). Here, we injected FVBN202 mice with cyclophosphamide (CYP) one day prior to ACT in order to generate a semi-lymphopenic condition. Animals were then challenged with MMC followed by i.v. injection of the ex vivo expanded cells 6–8 hs after the MMC challenge. Recipients of ACT rejected the neu+ MMC (Fig. 3C), despite the presence of MDSC before and 7 days after MMC challenge (Fig. S2B). All control mice that had received CYP alone developed tumors. In order to determine memory responses, ACT-treated mice were challenged on the contralateral side with MMC two months after the rejection of primary MMC cells. During recall tumor challenge, animals received neither CYP nor ACT, yet all the mice rejected the recall tumors (Fig. 3C). In order to determine which T cell phenotypes were effective in vivo, we sorted T cells into CD62L−/low (TE/TEM) and CD62Lhigh (TCM), and performed ACT with the sorted cells. No protection was observed against the tumors (data not shown). These data suggests critical interactions among tumor-reactive T cell phenotypes which requires further investigation.
The ex vivo expanded T cells acquire resistance to inhibitory function of MDSC
Since the ex vivo expanded cells protected FVBN202 mice against primary and recall tumor challenges even in the absence of MDSC depletion (Fig. 3C), we sought to determine whether the ex vivo expanded cells were resistant to MDSC in vitro. We have previously reported that MDSC isolated from bone marrow or spleens of tumor-bearing FVBN202 mice can inhibit T cell responsiveness to CD3/CD28 stimulation (7, 19). MDSC can also inhibit MMC tumor-specific IFN-γ production by T cells expanded with alternating common gamma-chain cytokines (Fig. S2C). Therefore, splenocytes expanded with sequential common gamma-chain cytokines were cultured for 24 hr in the presence or absence of irradiated MMC (10:1 ratio of expanded cells to MMC) to show their reactivity with MMC in the presence or absence of splenic MDSC (2:1 ratio of expanded cells to MDSC). Supernatants were collected and subjected to IFN-γ ELISA. T cells were also analyzed for the expression of IFN-γ, Prf, GrB, and CD69. As shown in Fig. 4A (upper panel), the ex vivo expanded cells produced IFN-γ in the presence of MMC (p= 0.004) as expected. Importantly, addition of MDSC not only failed to suppress MMC-specific IFN-γ secretion by the expanded cells, but also increased the IFN-γ response (p= 0.012). Flow cytometry analysis of the expanded cells determined that CD8+ T cells were the main source of MMC-specific IFN-γ production (Fig. 4A, lower panel). Addition of MDSC increased MMC-induced production of IFN-γ by both CD8+ and CD4+ T cells. Increasing the dose of MDSC did not increase MMC-specific production of IFN-γ by the expanded T cells (data not shown). The presence of MDSC also resulted in an increased production Prf in CD8+ T cells upon MMC stimulation (p= 0.035, Fig. 4B). There was no IFN-γ or Prf production by MMC as determined by flow cytometry analysis of gated neu+ MMC in the co-culture (Fig. S3A). In addition, MDSC did not suppress production of GrB or expression of the CD69 early activation marker in the expanded T cells (Figs. S3B–C). PBMC from naive FVBN202 mice that was depleted of Gr1+ cells had no suppressive effect or supportive effect on tumor-reactive T cells (data not shown). These data suggest that MDSC did not inhibit anti-tumor responses of the T cells expanded with the sequential gamma-chain cytokine regimen. We next determined whether MDSC could inhibit cytotoxicity of these T cells against MMC tumor cells in vitro. Expanded cells were cultured with viable neu+ MMC (10:1 ratio) in the presence of absence of MDSC (2:1 ratio). Control MMC cells were cultured with medium alone. As shown in Fig. 4C, expanded cells induced apoptosis in MMC cells in a 2-day culture, and the presence of MDSC did not alter the cytotoxic function of the tumor-reactive cells.
Figure 4. Ex vivo expanded T cells are resistant to MDSC.
The ex vivo expanded T cells were cultured with irradiated MMC in the presence or absence of MDSC. MMC-specific IFN-γ was detected in the supernatant of a 24-hs culture (A; upper panel) and in gated CD8+ or CD4+ T cells (A; lower panels). B) MMC-specific Prf production in gated CD8+ or CD4+ T cells was also determined. C) The ex vivo expanded T cells were cultured with viable MMC cells in the presence or absence of MDSC for 48 hs. Viability (Annexin V-/PI-) of gated neu positive MMC cells was determined by flow cytometry analysis. Data represent 3 independent experiments.
Presence of non-T cells in the ex vivo expanded cells overcomes MDSC and enhances T cell responses to MMC cells
The ex vivo expanded cells showed a significantly reduced proportion of CD4-CD8- cells compared to that of freshly isolated splenocytes (D 6: 20 % vs. D 0: 57%, p= 0.0001, Fig. 5A). The expanded cells contained 17–20% CD4-CD8- cells. As shown in Fig. 5B, gated CD4-CD8- cells contained a significantly higher proportion of CD3+ cells in the expanded cells compared to freshly isolated cells (D 6: 60.8% vs. D 0: 5.5%, p= 0.002). Then, we sought to determine the cellular composition of these CD4-CD8- cells. CD49b is a common marker for NK cells, NK T cells and IFN-γ producing killer DC (IKDC) (24). As shown in Fig. 5C, NK T cells (CD49b+CD3+) and NK cells (CD49b+CD3-) showed significant increases in the expanded CD4-CD8- cells. The proportion of IKDC (CD49b+CD3-B220+) in the gated CD3-CD11b+ cells was also significantly increased after the expansion (Fig. 5D, p= 0.001). The CD3+CD49+ NK T cells and CD3-CD49b+ NK cells showed higher expression of the activation marker CD25 after a 6-day expansion (D 6) compared to freshly isolated cells (D 0) (Fig. S3D). The expanded CD3+ non-T cells (NK T cells) showed higher viability compared to the expanded CD3- non-T cells (NK cells and IKDC) (Fig. 5E: 71.9% vs. 37.5% on day 6, p= 0.004).
Figure 5. Ex vivo expanded T cells include non-T cells (NK T cells, NK cells and IPKDC) that are responsible for rendering T cells resistant to MDSC.
Freshly isolated ( D 0) and ex vivo expanded (D 6) splenic cells were analyzed for the presence of CD4-CD8- non-T cells and CD4+ or CD8+ T cells (A). Expression of CD3 on non-T cells was determined (B). Non-T cells were also analyzed to determine the proportion of NK T cells and NK cells (C). Cells were gated on CD3-CD11b+ DC population and analyzed for the expression of CD49b and B220 as well as IFN-γ to identify IKDC population (D). Viability (Annexin V-/PI-) of CD3+ and CD3- non-T cells was determined (E). Ex vivo expanded splenocytes from MMC-primed FVBN202 mice were sorted into non-T cells (CD4-CD8-) and T cells (CD4+ plus CD8+). The sorted cells were cultured with irradiated MMC in the presence or absence of MDSC for 24 hs and IFN-γ production was determined in the supernatants (F). The sorted cells were used for ACT (n=3) at doses proportional to the unsorted cells, and tumor growth was determined (G). Data represent three independent experiments.
In order to determine whether the presence of non-T cells renders the tumor-reactive T cells resistant to MDSC, in vitro and in vivo studies were performed on the sorted cells. The ex vivo expanded cells were sorted into CD4+ plus CD8+ T cells and CD4-CD8- non-T cells. Sorted cells were then cultured for 24 hs in the presence or absence of irradiated MMC (10:1 ratio of expanded cells to MMC) to show their reactivity with MMC in the presence or absence of MDSC (2:1 ratio of expanded cells to MDSC). Supernatants were collected and subjected to IFN-γ ELISA. As shown in Fig. 5F, MDSC induced secretion of IFN-γ by CD4-CD8- non-T cells in the presence of MMC but not in CD4+ plus CD8+ T cells (p= 0.031). Lower amounts of IFN-γ were secreted by sorted CD4+ plus CD8+ T cells compared to unsorted cells (107 pg/ml in Fig. 5F compared to 2642 pg/ml in Fig. 4A). This suggests that the presence of non-T cells boosts the tumor-reactivity of T cells. In addition, the presence of MDSC significantly increased MMC-induced IFN-γ production by non-T cells (p= 0.006). However, CD4+ plus CD8+ T cells in the absence of non-T cells lost their ability to secrete MMC-specific IFN-γ while MDSC were present. This suggests that MDSC-stimulated, MMC-activated non-T cells render T cells resistant to MDSC. ACT with sorted T cells or non-T cells failed to protect FVBN202 mice from challenge with MMC cells (Fig. 5G). Since IL-12 induces the expression of IFN-γ by NK cells and T cells we sought to determine whether CD4-CD8-CD49b+ cells produce IL-12 in the presence of MMC and MDSC, resulting in the induction of enhanced IFN-γ production by T cells. No IL-12 production was detected in non-T cells or T cells (data not shown).
Radiation therapy of tumor-bearing mice prior to the isolation of donor T cells results in failure of the expanded T cells to generate objective responses upon ACT despite sustained anti-tumor responses of the T cells in vitro
Cancer patients who participate in clinical trials of ACT have usually received conventional therapies, often including radiation therapy. Therefore, it is important to determine whether tumor-primed T cells that were isolated following radiation therapy can also be expanded, and can generate objective responses against the tumors following ACT. In order to test this, FVBN202 mice were inoculated with MMC cells and as soon as tumors reached 75–150 mm3, animals received three doses of local radiation therapy to the tumor site (5 Gy) in a 3-day interval. Animals were then sacrificed one week after the last radiation treatment and their splenocytes subjected to B/I activation and a 6-day expansion with sequential common gamma-chain cytokines. The frequency of freshly isolated T cells was significantly lower after radiation therapy (Fig. 6A) compared to that without radiation therapy (Fig. 1A) (CD8+ T cells, p= 0.002; CD4+ T cells, p= 0.0002). However, radiation therapy did not alter the ability of T cells to grow after B/I activation, such that after six days in culture, the cells showed similar rates of expansion compared to those from mice that did not receive any radiation (Fig. 6A and Fig. 1A). The frequency of apoptotic T cells did not increase during the ex vivo expansion (Fig. S4A). However, expanded T cells from mice subjected to radiation failed to increase CD127 (Fig. S4B), and increased expression of CD122 was evident only in CD8+ T cells (Fig. S4C).
Figure 6. In vitro and in vivo efficacy of the ex vivo expanded and MMC-primed T cells harvested from FVBN202 mice after radiation therapy.
A) Ex vivo expansion of splenocytes harvested from MMC-primed female FVBN202 mice who received 3 cycles of local radiation therapy (5 Gy) in 3-day intervals, before and after activation with B/I and expansion with sequential common gamma-chain cytokines as determined by trypan blue exclusion (left panel) or flow cytometry analysis of gated CD8+ or CD4+ T cells (right panel). B) Tumor reactivity of freshly isolated (D 0) and ex vivo expanded (D 6) T cells was determined by a 24-hs culture of T cells in the presence or absence of irradiated MMC followed by the detection of IFN-γ in the supernatant. Medium alone and MMC alone were used as negative controls for IFN-γ production. C) The MMC-specific IFN-γ production in gated CD8+ and CD4+ T cells derived from donor mice with no radiation treatment (NT) and radiation treatment (RAD) was determined by flow cytometry analysis. D) Gated neu positive MMC cells were analyzed for apoptosis (Annexin V+/PI+) in the absence of presence of freshly isolated or ex vivo expanded T cells. E) Phenotypic distribution of freshly isolated (D 0) and ex vivo expanded (D 6) splenic T cells was determined by flow cytometry analysis of gated CD4+ or CD8+ T cells. Data represent 3 independent experiments. F) CYP-treated FVBN202 mice (n=3) were inoculated with MMC and received no further treatment (control) or received the expanded cells derived from donors after the local radiation therapy (ACT). Data represent three independent experiments.
In order to determine the tumor-reactivity of the expanded T cells from mice whose tumors had been irradiated, in vitro studies were performed. As shown in Fig. 6B, freshly isolated T cells (D 0) and ex vivo expanded cells (D 6) failed to produce significant amounts of IFN-γ upon MMC stimulation. However, the addition of MDSC resulted in the induction of IFN-γ by the expanded T cells (Fig. 6B). The expanded cells were also comprised of 19.2% non-T cells (Fig. S4D). Interestingly, the proportion of Prf+ T cells in the expanded T cells (D 6) was markedly higher in this group that had received prior radiation therapy (RAD) compared to T cells that were isolated from donors with no prior radiation therapy (NT) (Fig. 6C). Expanded CD8+ T cells were highly positive for the expression of CD69 and GrB (Fig. S4E–F). Importantly, lack of IFN-γ production by the expanded T cells did not alter the ability of these cells to kill neu+ MMC cells in vitro such that viability (Annexin V-/PI-) of MMC was reduced from 64.64% to 12.35% in the presence of the expanded T cells (Fig. 6D). In addition, expanded T cells were able to kill neu+ MMC cells even in the presence of MDSC in vitro, as shown by a reduced viability from 64.64% to 12.35% and 8.46% (Fig. 6D).
Results of in vivo studies presented in Fig. 3 suggest a correlation between phenotypic distribution of T cells and objective responses following ACT such that high proportions of TCM were associated with the rejection of primary and recall tumor challenge. Therefore, we performed phenotype analysis of post-radiation T cells before proceeding with ACT. As shown in Fig. 6E, phenotypic distribution of CD8+ and CD4+ T cells was different from those isolated from animals with no prior radiation therapy (Fig. 3A). Freshly isolated CD8+ T cells (D 0) were mainly of TCM and TN phenotypes whereas CD4+ T cells contained TE and TEM (Fig. 6E). After six days of ex vivo expansion, CD8+ T cells were enriched for TE whereas CD4+ T cells contained TE and TEM phenotypes. After a 6-day expansion, CD8+ TCM and TN phenotypes had almost disappeared. An increased proportion of CD8+ TE cells may account for the in vitro efficacy of the expanded T cells against MMC cells, though anti-tumor efficacy in vivo may require tumor-specific TCM cells. To test this possibility ACT studies were performed as described above. As shown in Fig. 6F, ACT using the ex vivo expanded cells from mice whose tumors had been irradiated showed minimal tumor inhibitory effects compared to the control group. The rate of tumor growth was the same in the two groups (p< 0.21), though the change from day to day was significant (p< 0.0001).
HER-2/neu-specific T cells can be expanded from PBMC of a patient with breast cancer
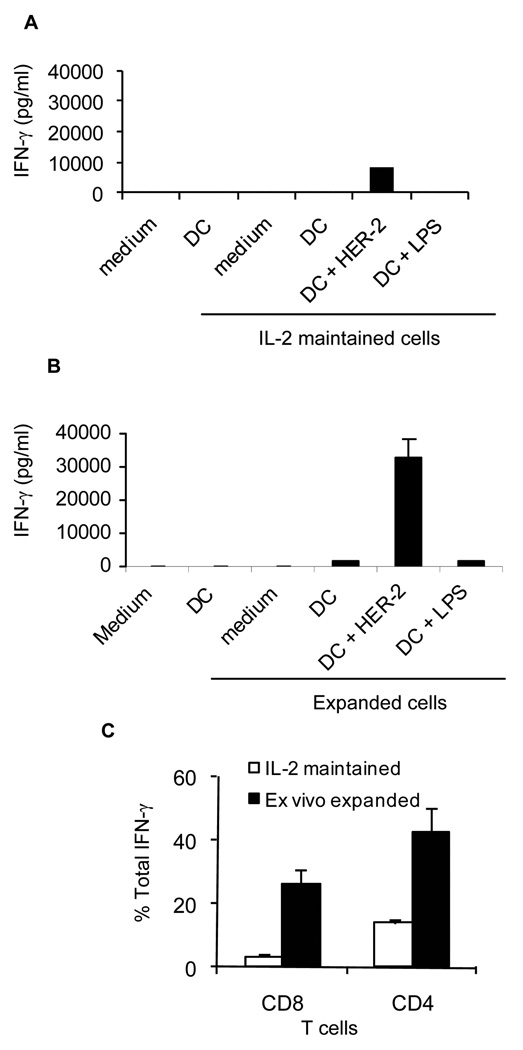
Splenocytes isolated from tumor-bearing mice with no radiation therapy were effective against the neu+ MMC cells despite the lack of splenic tumor metastasis. This suggests that tumor-reactive T cells may be present in the circulation. To test this, PBMC were collected from a breast cancer patient after Ficoll-Paque gradient centrifugation of blood and split into two fractions. Adherent cells were selected by 2 hs culture and used for generating autologous DCs by a 6-day culture in the presence of GM-CSF and IL-4, as described elsewhere (25). Non-adherent cells were split into two fractions. One fraction was maintained in complete RPMI1640 supplemented with 10% FBS and IL-2 (40 U/ml/106 cells) at 37°C/5% CO2 for 6 days (IL-2 maintained cells). The remaining cells were subjected to B/I activation and expansion with the common gamma-chain cytokines (expanded cells with IL-7, IL-15, IL-2). Viability of the T cells (Annexin V-) after a 6-day culture or ex vivo expansion was greater than 85% (data not shown). Cells were then co-cultured with autologous DCs in the presence or absence of recombinant HER-2/neu protein for 24 hs. In order to rule out nonspecific IFN-γ production by CD4+ T cells as a result of low endotoxin levels in the HER-2/neu recombinant protein we pulsed control wells with LPS. As shown in Fig. 7A–B, compared to IL-2 maintained T cells the B/I-activated and expanded cells produced significantly higher amounts of IFN-γ when stimulated with HER-2/neu (average 8600 vs. 32500 pg/ml, p= 0.001). Flow cytometry analysis of IL-2 maintained and ex vivo expanded T cells determined that both CD8+ T cells and CD4+ T cells were sources of HER-2/neu-stimulated IFN-γ production (Fig. 7C). It is yet to be determined whether consistently similar results can be obtained in a large group of breast cancer patients.
Figure 7. Patient with breast cancer harbors peripheral HER-2/neu-specific T cell precursors which can be activated by B/I activation and expanded with common gamma-chain cytokines.
A) T cells maintained with low dose IL-2 (40 U/ml) for 6–7 days were cultured in the presence or absence of autologous DC and presence or absence of recombinant human HER-2/neu for 24 hs. IFN-γ production was detected in the supernatant of triplicate wells. B) B/I activated and common gamma-chain cytokine expanded T cells were cultured in the presence or absence of autologous DC and presence or absence of recombinant human HER-2/neu for 24 hs. IFN-γ production was detected in the supernatant of triplicate wells. C) IL-2 maintained T cells (white bars) and ex vivo expanded T cells (black bars) were stained with anti-CD4, anti-CD8 and anti IFN-γ antibodies in order to determine cellular source of HER-2/neu-specific IFN-γ production. Data represent two independent experiments.
Discussion
Development of an ex vivo protocol that can expand highly efficient populations of tumor-reactive immune cells, which include cells of the adaptive and innate immune systems, may be the key to successful ACT in breast cancer model. Others have reported that rejection of mammary carcinoma in HER-2/neu transgenic mice depends on the stimulation of both innate and adaptive immunity (25). In addition, NK T cells have been shown to be involved in secondary anti-tumor T cell responses (26).
We demonstrated that activation of tumor-primed lymphoid cells with B/I followed by ex vivo expansion with sequential common gamma-chain cytokines can activate and expand tumor-reactive T cells and non-T cells including NK T cells, NK cells and IKDC. We have tested a number of the cytokine combinations (7, 21, 22) and found that IL-7+IL-15 followed by IL-2 was the best sequence for the expansion of the most effective cells. The presence of activated non-T cells in the expanded cells was critical not only for the in vivo anti-tumor efficacy of T cells but also for their resistance to MDSC. The absence of such activated non-T cells in freshly isolated splenocytes or depletion of these non-T cells in the expanded cells resulted in susceptibility to MDSC-induced suppression of tumor-reactive T cells. Neither tumor-reactive T cells nor these non-T cells alone were able to protect FVBN202 mice against tumor challenge when they were used separately in ACT. Since the viability of NK cells was very low (37.5%) as opposed to the high viability (72%) of NK T cells, it is likely that NK T cells are the key component of the supportive cells. Our findings are consistent with recent reports showing that cells of the innate and adaptive immune system work together to produce objective responses against tumors (25, 26). Our results also showed that MDSC can further activate the ex vivo expanded non-T cells, as shown by an increased CD25 expression, thereby enhancing the supportive function of non-T cells for tumor-reactive T cells. This is the first report showing a cellular mechanism by which T cells may become resistant to MDSC. Because of the T cell inhibitory role of MDSC in a variety of cancers, the proposed protocol could be applicable to a variety of carcinomas.
Although the presence of T cells and cells of the innate immune system were critical for anti-tumor efficacy of the immune response, long-term protection against the tumor depended on the presence of TCM cells. Our data suggest that B/I activation and ex vivo expansion with sequential common gamma-chain cytokines may have improved the quality of neu-specific cells for tumor rejection, and it was not just because of an increase in frequency of neu-specific T cells. For instance, freshly isolated T cells from tumor-bearing but not from tumor-free FVBN202 mice produced IFN-γ upon stimulation with MMC in vitro; yet such increased frequency of endogenous neu-specific T cells did not induce tumor rejection in donor mice. In addition, ACT with an increased numbers of freshly isolated T cells derived from tumor-bearing donors (2 × 109 cells/mouse) did not protect mice against tumor challenge (data not shown). These data suggest that an increase in neu-specific T cells without ex vivo expansion/differentiation using the proposed protocol cannot provide protection against the tumor. Whereas T cells from non-irradiated and post-radiation donors showed comparable levels of anti-tumor efficacy in vitro (Fig. 6D), T cells obtained from non-irradiated donors provided long-term memory responses against recall tumor challenge in vivo, likely because of the phenotypic distribution of T cells toward TCM cells. Local radiation therapy of the tumors in donor mice altered the phenotypic distribution of freshly isolated T cells as well as the capacity of T cells to differentiate into TCM cells during the ex vivo expansion. These data suggest that CD8+ TE and TEM cells may be more susceptible to radiation therapy than previously established TCM cells, as has been reported by others (27). Our data suggest that local radiation therapy could alter the differentiation of tumor-reactive CD8+ TE and TEM cells toward TCM cells. However, we performed local radiation therapy of primary tumors whereas breast cancer patients usually receive radiation therapy after surgery to destroy residual microscopic disease. Also, patients with advanced breast cancer have undergone multiple radiation treatments followed by a period of recovery prior to ACT. These scenarios are somewhat different from the treatment protocol that we used in our study. In order for T cell to be effective for ACT we had to isolate T cells from tumor-bearing animals. Therefore, we had to perform radiation therapy on primary tumors. Application of the proposed approach may be limited to patients with early stage breast cancer (stage I–III), provided that PBMC are harvested and cryopreserved prior to radiation therapy for ACT in future. Feasibility of this strategy remains to be determined. The importance of TCM against cancer has also been reported by others (4). Our data showing a greater anti-tumor efficacy of ACT in association with the presence of TCM are consistent with other reports showing that effector cells derived from TCM rather than TEM possess greater ability to survive and establish immunologic memory following infusion (28). However, naïve T cells have been reported to convey more anti-tumor activity than memory cells (28). Such contradictory results may be due to the use of a mouse model harboring a transgenic T cell receptor for gp100 tumor antigen, which is different from the FVN202 mouse model of spontaneous breast carcinoma with no transgenic TcR against the tumor antigen.
IL-7 has been shown to support viability and homeostatic proliferation of T cells and enhance NK cell function (29). IL-15 supports differentiation of memory T cells and activation of quiescent NK cells more efficiently than IL-2 (30). IL-2 is T cell growth factor and is also involved in NK cell activation and proliferation. Therefore, culture of tumor-primed T cells initially with IL-7+IL-15 followed by IL-2 can support differentiation of T cells as well as non-T cells. The presence of non-T cells in this model appears to be critical for rendering T cells resistant to MDSC, regardless of whether T cells were obtained before or after radiation therapy. A similar observation has been made in mice and humans with hematologic malignancies undergoing allogeneic stem cell transplantation. In the animal model, depletion of donor NK cells abrogated anti-leukemia effects of donor T cells (31). In humans, early donor derived NK cell recovery has been shown to be associated with a lower relapse risk in the non-myeloablative setting in the recipients of T cell replete allografts (32). Earlier observations in the same clinical model had demonstrated a significant impact of NK cell dose in the graft on day 28 T cell chimerism (33). Similarly our group has shown a trend towards a higher level donor T cell chimerism at 12 weeks post transplant, in patients with superior NK cell recovery at 4 weeks. (unpublished data). Together these data provide intriguing evidence of T cell- NK cell interactions in the clinical transplant setting and suggesting interdependence between innate and adaptive immunity.
Altogether, these data suggest that lymph nodes (11) or PBMC of breast cancer patients may be explored as a source of tumor-reactive immune cells for ex vivo expansion and use in ACT, provided that immune cells are obtained prior to radiation therapy and expanded with the sequential common gamma-chain cytokines. Expanded T cells and non-T cells obtained prior to radiation therapy may be able to be cryopreserved and used for experimental ACT protocols after the completion of conventional therapies in an attempt to eliminate residual disease and prevent tumor relapse. In fact, the majority of patients with breast cancer die from tumor metastases rather than from the primary cancer. Despite the fact that tumor stroma in breast cancer patients makes regression of established solid tumors difficult, regression of residual disease is possible (6, 10). Therefore, the proposed ACT protocol should be further explored in breast cancer patients. We have previously tested anti-tumor efficacy of ACT using different common gamma-chain cytokine regimens (22). The efficacy of cells expanded with sequential common gamma-chain cytokines remains to be determined in melanoma and other tumor models.
Supplementary Material
Acknowledgements
We gratefully acknowledge the support of VCU Massey Cancer Center and the Commonwealth Foundation for Cancer Research.
Footnotes
This work was supported by NIH R01 CA104757 Grant (M. H. Manjili) and VCU Presidential Research Incentive Program (M. H. Manjili).
Supplemental materials are available online.
Authors have no financial interests to disclose.
References
- 1.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA SA. Gene therapy with human and mouse T-cell receptors mediate cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Riley J, Rosenberg S, Parkhurst M. Comparison of common gamma-chain cytokines, interleukin-2, interleukin-7, and interleukin-15 for the in vitro generation of human tumor-reactive T lymphocytes for adoptive cell transfer therapy. J. Immunother. 2006;29:284–293. doi: 10.1097/01.cji.0000190168.53793.6b. [DOI] [PubMed] [Google Scholar]
- 6.Bernhard H, Neudorfer J, Gebhard K, Conrad H, Hermann C, Nährig J J, Fend F, Weber W, Busch DH, Peschel C C. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol. Immunother. 2008;57:271–280. doi: 10.1007/s00262-007-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales JK, Kmieciak M, Graham L, Feldmesser M, Bear HD, Manjili MH. Adoptive transfer of HER2/neu-specific T cells expanded with alternating gamma chain cytokines mediate tumor regression when combined with the depletion of myeloid-derived suppressor cells. Cancer Immunol. Immunother. 2009;58:941–953. doi: 10.1007/s00262-008-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho WY, Yee C, Greenberg PD. Adoptive therapy with CD8(+) T cells: it may get by with a little help from its friends. J. Clin. Invest. 2002;110:1415–1417. doi: 10.1172/JCI17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blankenstein T. The role of tumor stroma in the interaction between tumor and immune system. Curr. Opin. Immunol. 2005;17:180–186. doi: 10.1016/j.coi.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Bear HD, Roberts J, Cornell D, Tombes MB, Kyle B. Adoptive immunotherapy of cancer with pharmacologically activated lymph node lymphocytes: a pilot clinical trial. Cancer Immunol. Immunother. 2001;50:269–274. doi: 10.1007/s002620100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazanietz MG, Lewin NE, Gao F, Pettit GR, Blumberg PM. Binding of [26-3H] bryostatin 1 and analogs to calcium-dependent and calcium-independent protein kinase C isozymes. Mol. Pharmacol. 1994;46:374–379. [PubMed] [Google Scholar]
- 13.Chatila T, Silverman L, Miller R, Geha R. Mechanisms of T cell activation by the calcium ionophore ionomycin. J. Immunol. 1989;143:1283–1289. [PubMed] [Google Scholar]
- 14.Ariza ME, Ramakrishnan R, Singh NP, Chauhan A, Nagarkatti PS, Nagarkatti M. Bryostatin-1, a naturally occurring antineoplastic agent, acts as a Toll-like Receptor 4 (TLR-4) ligand and induces unique cytokines and chemokines in dendritic cells. J. Biol. Chem. 2010;286:24–34. doi: 10.1074/jbc.M110.135921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kmieciak M, Morales JK, Morales J, Bolesta E, Grimes M, Manjili MH. Danger signals and nonself entity of tumor antigen are both required for eliciting effective immune responses against HER-2/neu positive mammary carcinoma: implications for vaccine design. Cancer Immunol. Immunother. 2008;57:1391–1398. doi: 10.1007/s00262-008-0475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kmieciak M, Gowda M, Graham L, Godder K, Bear HD, Marincola FM, Manjili MH. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. J. Transl. Med. 2009;7:89. doi: 10.1186/1479-5876-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Worschech A, Kmieciak M, Knutson KL, Bear HD, Szalay AA, Wang E, Marincola FM, Manjili MH. Signatures associated with rejection or recurrence in HER-2/neu-positive mammary tumors. Cancer Res. 2008;68:2436–2446. doi: 10.1158/0008-5472.CAN-07-6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales JK, Kmieciak M, Knutson KL, Bear HD, Manjili MH. GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1- bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res. Treat. 2010;123:39–49. doi: 10.1007/s10549-009-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid-derived suppressor cells in Balb/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int. Immunopharmacol. 2009;9:900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Cha E, Graham L, Manjili MH, Bear HD. IL-7 + IL-15 are superior to IL-2 for the ex vivo expansion of 4T1 mammary carcinoma-specific T cells with greater efficacy against tumors in vivo. Breast Cancer Res. Treat. 2010;122:359–369. doi: 10.1007/s10549-009-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le HK, Graham L, Miller CH, Kmieciak M, Manjili MH, Bear HD. Incubation of antigen-sensitized T lymphocytes activated with Brayostatin-1 + Ionomycin in IL-7 + IL-15 increases yield of cells capable of inducing regression of melanoma metastasis compared to culture in IL-2. Cancer Immunol. Immunother. 2009;58:1565–1576. doi: 10.1007/s00262-009-0666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manjili MH, Henderson R, Wang X-Y, Chen X, Li Y, Repasky E. E, Kazim EL, Subjeck HJR. Development of a recombinant HSP110-HER-2/neu vaccine using the chaperoning properties of HSP110. Cancer Res. 2002;62:1737–1742. [PubMed] [Google Scholar]
- 24.Terme M, Mignot G, Ullrich E, Bonmort M, Minard-Colin V, Jacquet A, Schultze JL, Kroemer G, Leclerc C, Chaput N, Zitvogel L L. The dendritic cell-like functions of IFN-producing killer dendritic cells reside in the CD11b+ subset and are licensed by tumor cells. Cancer Res. 2009;69:6590–6597. doi: 10.1158/0008-5472.CAN-08-4473. [DOI] [PubMed] [Google Scholar]
- 25.Spadaro M, Ambrosino E, Iezzi M, Di Carlo E, Sacchetti P, Curcio C, Amici A, Wei W-Z, Musiani P, Lollini PL, Cavallo F, Forni G. Cure of mammary carcinomas in Her-2 transgenic mice through sequential stimulation of innate (neoadjuvant interleukin-12) and adaptive (DNA vaccine electroporation) immunity. Clin. Cancer Res. 2005;11:1941–1952. doi: 10.1158/1078-0432.CCR-04-1873. [DOI] [PubMed] [Google Scholar]
- 26.Hong C, Lee H, Park YK, Shin J, Jung S, Kim H, Hong S, Park SH SH. Regulation of secondary antigen-specific CD8(+) T-cell responses by natural killer T cells. Cancer Res. 2009;69:4301–4308. doi: 10.1158/0008-5472.CAN-08-1721. [DOI] [PubMed] [Google Scholar]
- 27.De Ruysscher D, Waer M, Vandeputte M, Aerts R, Vantongelen K, van der Schueren E. Changes of lymphocyte subsets after local irradiation for early stage breast cancer and seminoma testis: long-term increase of activated (HLA-DR+) T cells and decrease of "naive" (CD4-CD45R) T lymphocytes. Eur. J. Cancer. 1992;28A:1729–1734. doi: 10.1016/0959-8049(92)90079-h. [DOI] [PubMed] [Google Scholar]
- 28.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, Palmer DC, Ji Y, Reger RN, Leonard WJ, Danner RL, Rosenberg SA, Restifo NP NP. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl. Acad. Sci. U.S.A. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pillet AH, Bugault F, Thèze J, Chakrabarti LA, Rose T. A programmed switch from IL-15- to IL-2-dependent activation in human NK cells. J. Immunol. 2009;182:6267–6277. doi: 10.4049/jimmunol.0801933. [DOI] [PubMed] [Google Scholar]
- 31.De Somer L, Sprangers B, Fevery S, Rutgeerts O, Lenaerts C, Waer M, Billiau AD. Recipient lymphocyte infusion in MHC-matched bone marrow chimeras induces a limited lymphohematopoietic host-versus-graft reactivity but a significant antileukemic effect mediated by CD8+ T-cells and natural killer cells. Haematologica. 2011 doi: 10.3324/haematol.2010.035329. 2011; Jan 12 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baron F, Petersdorf EW, Gooley T, Sandmaier BM, Malkki M, Chauncey TR, Maloney DG, Storb R. What is the role for donor natural killer cells after nonmyeloablative conditioning? Biol. Blood Marrow Transplant. 2009;15:580–588. doi: 10.1016/j.bbmt.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panse JP, Heimfeld S, Guthrie KA, Maris MB, Maloney DG, Baril BB, Little MT, Chauncey TR, Storer BE, Storb R, Sandmaier BM. Allogeneic peripheral blood stem cell graft composition affects early T-cell chimaerism and later clinical outcomes after non-myeloablative conditioning. Br. J. Haematol. 2005;128:659–667. doi: 10.1111/j.1365-2141.2005.05363.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.