Abstract
Hormone therapy is an effective approach for the treatment of breast cancer. Although, the antiestrogen tamoxifen has had a major impact on the treatment of the disease, aromatase inhibitors, which reduce estrogen synthesis, have recently proved to be more effective. These agents are now used as first line therapy for postmenopausal breast cancer. Nevertheless despite their efficacy, resistance to treatment eventually may occur in some patients. In order to devise strategies to overcome resistance and extend the benefits of AIs, investigators have studied mechanisms involved in resistance to AIs and devised strategies to overcome the resistance. Using a xenograft or cell line models, adaptive changes that results in activation of alternate signaling pathways in tumors resistant to aromatase inhibitors have been identified. Expression of ERα and aromatase was decreased in the tumors after long-term treatment with AIs. In contrast, increased expression was observed of tyrosine kinase receptors such as HER-2 and IGFR as well as of downstream signaling proteins, including MAPK. Functional activation of the MAPK pathway and dependency on growth factor receptor signaling has been shown in AI resistant cells and tumors.
Keywords: aromatase inhibitors, estrogen, resistance, breast cancer
Background
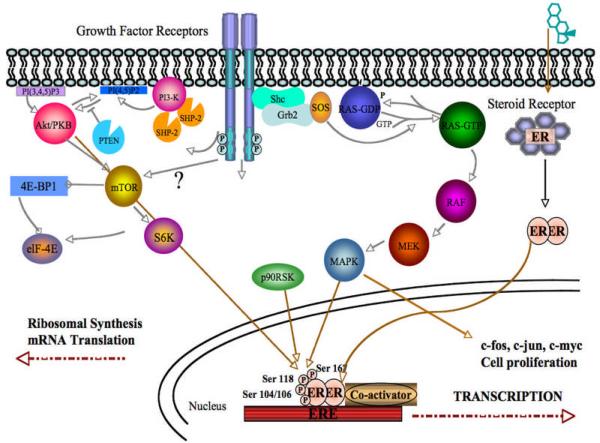
Estrogen is the major stimulus to breast cancer progression in both pre- and postmenopausal patients. The actions of estrogen on the tumor are mediated by the estrogen receptor alpha (ERα). Normally, in the absence of ligand, ERα resides in a large molecular complex with multiple heat shock proteins acting as chaperone proteins. The most potent ligand for ERα is 17-β estradiol (E2), with the weaker estrone (E1) and estriol (E3) activating ERα by similar mechanisms. Estrogens, owing to their fat-soluble nature, rapidly diffuse through the plasma membranes and bind to ERα at the hormone binding domain as depicted in Figure 1. This binding of estrogen to ERα results in dissociation of heat shock proteins, leading to conformational change in the receptor and its dimerization. This allows phosphorylation of ERα at several serine residues within its N-terminal portion. The receptor dimer then translocates to the nucleus and binds to estrogen response element located upstream of the proximal TATA box. The DNA bound dimer recruits co-activators such as Steroid Receptor Co-activator-1 (SRC-1), Amplified in Breast Cancer-1 (AIB-1), cAMP response element binding (CREB) protein (CBP) that allow the activation of estrogen responsive genes such as progesterone receptor (PgR), pS2, cyclin D1 and c-myc (Figure 1). These events lead to cell cycle entry and progression following expression of cell cycle regulating genes. Additionally, several genes associated with cell survival such as Bcl-2 and Tumor Growth Factor (TGFα) are also upregulated in estradiol treated cells in vitro and ERα positive tissues in vivo with increased estrogen induced cell survival contributing significantly to breast cancer growth in response to estrogens.
Figure 1.
Signaling pathways driving the growth of hormone therapy refractory cells
In young women, the main source of estrogen is the ovary. After menopause, ovarian production declines and extragonadal sites such as adipose tissue, which are not under the control of the pituitary, are the main source of circulating estrogen. However, tissue concentrations within the breast are comparable with those of premenopausal women as aresult of local estrogen synthesis and uptake. In breast cancer patients, tumor ERα concentrations are higher after menopause, resulting in cancers that are sensitive to even low levels of estrogens. About two thirds of the breast cancer patients are postmenopausal women with ER positive (ER+) tumors. Since estrogen signaling is of primary importance in the proliferation and progression of breast cancer, two types of breast cancer treatment have been developed to target this signaling pathway. The antiestrogens (AEs), such as tamoxifen, target the estrogen receptor alpha (ERα) whereas the more recent aromatase inhibitors target the biosynthesis of estrogen by directly interacting with the enzyme aromatase. Synthesis of estrogen is the last step in the steroid biosynthesis pathway and as such inhibition of aromatase does not affect the production of any other steroids.
Both antiestrogens and aromatase inhibitors (AIs) are effective treatments that are well tolerated compared to cytotoxic chemotherapy. Aromatase inhibitors (AIs) that reduce estrogen production have now been shown to be effective treatment for breast cancer. The triazole compounds such as letrozole and anastrozole as well as steroidal exemestane have been found to be superior to antiestrogen the (AE) tamoxifen and had few side effects (1–5). Furthermore, the benefits of hormone therapy are long lasting. Thus, patients receive hormone therapy for 5 years. Nevertheless, some patients may eventually relapse during treatment. Various studies have investigated the mechanisms involved as the tumor develops resistance to AIs, in order to gain a clearer understanding of how tumors adapt and survive the pressure of suppressive treatment with the ultimate goal of developing treatment strategies to overcome the resistance. Results to date indicate that over time tumors adapt to a low estrogen environment by switching to alternate signaling pathways thus allowing them to escape the antiproliferative effects of AIs.
Role of ER in acquired resistance to aromatase inhibitors
Although there is not change in the ability of AIs to inhibit aromatase, there is ample evidence suggesting that ER mediated pathways still play a role in growth of the breast cancer cells and tumors, despite showing resistance to endocrine agents such as tamoxifen or the AIs. Some tumors respond to another agent (switching to steroidal AI after non-steroidal AI); tamoxifen resistant tumors may respond to AIs.
Model systems developed by Santen et al and Brodie et al such as LTED, UMB-1Ca and LTLC exhibit upregulation of ER upon acquisition of resistance (6–8). Whereas the LTLT-Ca model developed by Brodie et al shows that ERα levels are down-regulated and Her-2/MAPK pathway upregulated (9–11). When LTLT-Ca cells were treated with MAPK inhibitor (PD98059), ER expression was increased to levels in MCF-7Ca cells. This suggests that hormone sensitivity may be restored by inhibiting the MAPK pathway (12). Similar results were also obtained with anti-Her-2 agent trastuzumab (11). El-Ashry et al have also shown that hyperactivation of MAPK results in loss of ERα and abrogating MAPK activity reverses ERα down-regulation (13–16). In addition, upon cessation of letrozole treatment, ER protein levels increase and response to letrozole is restored (17, 18). More recently, clinical studies have confirmed that Her-2 and ERα status changes in the secondary tumor or metastatic lesion compared to the primary tumor (19, 20).
Other mechanisms of resistance centering on ER signaling pathways include ERα mutation (21) and truncated ERα variant (ERα36) (22). Moreover, upregulation of the ER-related transcription factors like activator protein 1 (AP1) (23) and NF-κB (24–26) as well as co-activators of ER such as AIB1 (27) have also been described to confer resistant to endocrine therapy (25, 26). Nonetheless, these studies were described, as resistant mechanisms to tamoxifen and the role of these mechanisms in AI resistance remain unclear
Role of Growth Factor Receptor Mediated Pathways in Resistance
The Her-2 receptor is the protein product of the Her-2 proto-oncogene and a member of the epidermal growth factor (EGFR, Her-1) family of transmembrane receptor tyrosine kinases, which also includes Her-3 and Her-4. Her-2 is an orphan receptor because a ligand for Her-2 has not been identified. Amplification of Her-2 has been implicated as an important event in the genesis of human cancer. Extensive clinical studies have demonstrated that expression of high levels of Her-2 in patients with node positive breast cancer correlates with a poor prognosis. Upon binding of ligand to the EGFR, Her-3 (erbB3), or Her-4 (erbB4), Her-2 is recruited as the preferred partner of these ligand-bound receptors into an active, phosphorylated heterodimeric complex that activates several signaling pathways involved in the proliferation and enhanced survival of tumor cells. This occurs by inducing receptor autophosphorylation in several COOH-terminal tyrosines and the recruitment of signal transducers and adaptor molecules that promote cellular proliferation, differentiation, motility, adhesion, protection from apoptosis, and cellular transformation. In addition, overexpression of Her-2 on the cell surface also results in homodimerization and activation. Amplification and overexpression of Her-2 is observed in 20–30% of the human breast cancer patients and is inversely correlated with the survival of the patients. Therapeutic monoclonal antibody targeting extracellular domains of Her-2 (Trastuzumab, Herceptin®) has been approved for adjuvant treatment with chemotherapy for breast cancer patients with Her-2 positive disease.
Letrozole Resistance Model
Studies done by Brodie et al involve the use of the xenograft mouse model which was previously developed as a model for postmenopausal breast cancer for studying the anti-tumor effects of sequencing and combining AIs and antiestrogens (28, 29). In this model, human estrogen receptor (ER) positive breast cancer cells (MCF-7) stably transfected with the aromatase gene (MCF-7Ca) (10) were inoculated into ovariectomized mice and grown as tumors under the influence of estrogen produced by aromatization of androstenedione (10). The mice were then treated with AI letrozole for an extended period of time (56 weeks) until tumor growth was no longer inhibited by the treatment and tumors were actively growing (30). Cells were then isolated from these resistant tumors and designated Long Term Letrozole Treated (LTLT-Ca) cells (31–34). The LTLT-Ca cells form tumors in immunosuppressed, ovariectomized mice without estrogen stimulation and are unresponsive to AI treatment. Western blot analysis of tumors collected during the course of letrozole treatment revealed that ERα expression was increased in tumors responding to letrozole but was progressively reduced as tumors became resistant. By 56 weeks of letrozole treatment, ERα expression decreased and Her-2 expression increased. In addition, levels of downstream proteins of Her-2 signaling including the MAPK pathway were all significantly increased (35–38).
Similar decreases in ERα and increases in Her-2 were also seen in the cells isolated from the resistant tumors. In addition, most of the proteins in the downstream signaling pathway were increased in the resistant tumors. When LTLT-Ca cells were incubated with a series of doses of MEK-1/2 inhibitor (UO126) and MAPK inhibitor (PD98059), there was a dose dependent inhibition of proliferation of LTLT-Ca cells, whereas there was no effect on the proliferation of parental MCF-7Ca cells. These results indicate that the LTLT-Ca cells are dependent on the MAPK pathway and no longer dependent on estrogen stimulation for proliferation. When LTLT-Ca cells were treated with MAPK inhibitor (PD98059), ER expression was increased to levels in MCF-7Ca cells. This suggests that inhibiting the MAPK pathway may restore hormone sensitivity. In addition, this finding also emphasizes the involvement of ERα in the growth of endocrine resistant cells and tumors.
Treatment with trastuzumab caused a marked dose response inhibition of proliferation of LTLT-Ca cells accompanied by inhibition of pHer-2 and p-MAPK expression, whereas ERα expression was increased. Further evidence for the restoration of ER was seen when LTLT-Ca cells were pretreated with trastuzumab for 72-hours followed by 1-hour of E2 treatment. ERα transcriptional activation was induced in the LTLT-Ca cells to the same extent as in parental MCF-7Ca stimulated with E2 only. This was also evident from the increase in recruitment of ERα to the pS2 promoter, and confirmed Her-2 regulation of ERα mediated transcription of downstream genes, such as pS2. These finding indicate that Her-2 is a negative regulator of ERα. In addition, treatment of LTLT-Ca cells with trastuzumab and E2 results in recruitment of ERα to the aromatase I.3/II promoter and leads to upregulation of aromatase, essential for response of the tumors to AIs. Similar results were also obtained in SKBr3 cells suggesting that these results were not unique to MCF-7Ca cells (16, 39–41). Thus, interaction between ERα and Her-2 and Her-2 regulation of ER is not limited only to MCF-7 cells and may be relevant to treating AI resistant breast cancer patients.
Cross-talk between the pathways
A number of previous studies demonstrated that other signaling pathways like MAPK and PI3K/Akt pathways can cross-talk and activate ERα signaling pathways in a ligand-independent manner [97–99]. MAPK has been shown to phosphorylate ERα directly or indirectly via Elk-1 and p90RSK and result in the transcription of genes involved in growth regulation and tumor progression [97–99]. As depicted in Figure 1, both MAPK and Akt can directly phosphorylate ERα within the AF-1 domain (hormone independent activation function domain) at serine 118 and serine 167, respectively (42). Besides phosphorylation of ERα itself, these two pathways can also stimulate ER signaling pathway by phosphorylate ER co-activator like AIB1 [101, 102].
Several reports have suggested that translocation of ERα to the membrane (43–46) may be responsible for the crosstalk with EGFR family members in endocrine resistant pheynotype whereas a few reports have also suggested that EGFR family transmembrane receptors such as Her-2 can translocate to the nucleus and act as transcription factors (47–49).
A strong inverse correlation has been reported between the ERα and Her-2 proteins and pathways activated by these proteins (50). Stable transfection of hormone dependent cells with members of growth factor signaling pathway such as Her-2, EGFR, Raf etc has led to loss of ER– and the estrogen refractory phenotype (51). Interestingly, resistance to trastuzumab is associated with upregulation of genes such as VEGF, and TGFα, which are also known ERα target genes (52). Figure 1 shows how these pathways may interact.
Translational Studies
Multiple emerging clinical data support the observation that there is an upregulation of Her-2/MAPK pathway corresponding to the AI resistance and that dual targeting of ER signaling pathway with AIs and other growth factor receptor signaling cascade may confer better clinical outcome. Lipton et al. demonstrated that approximately 26% of patients treated with letrozole converted from serum Her-2 negative to positive at the time of disease progression (42). Serum Her-2 which is the extracellular domain of Her2 protein that is shed into serum has been shown to correlate with the overexpression of Her2 protein in tumor cells and can be used to predict the response to trastuzumab. The dynamic interaction between ER and Her2/MAPK signaling pathway is illustrated in a small clinical study of 10 patients with ER-negative/HER2-positive advanced breast cancer treated with trastuzumab and chemotherapy. Intriguingly, the tumors of 3 patients on this trial converted from ER negative to ER positive after the treatment with trastuzumab and subsequently responded to letrozole (53–55). For dual inhibition with AIs and Her2 inhibitors, three clinical trials have demonstrated better clinical outcome when combining Her2 targeted therapy to AIs. A phase II trial of letrozole and trastuzumab in ER-positive/Her2-negative metastatic breast cancer patients demonstrated that the combination was well tolerated with a clinical benefit rate of 50% (53). In another randomized phase III trial (TAnDEM trial), addition of trastuzumab to anastrozole showed a significant improvement in PFS compared with patients receiving anastrozole alone (HR = 0.63; 95% CI, 0.47 to 0.84; median PFS, 4.8 v 2.4 months; log-rank P = .0016) (56). Furthermore, lapatinib, which is a dual tyrosine kinase inhibitor of Her-2 and EGFR, also showed superiority when combining with letrozole in a randomized phase III trial of ER-positive metastatic breast cancer. In this trial, there was a significant improvement in PFS from 3.0 months to 8.2 months with the hazard ratio (HR) of 0.71 (p = 0.019) with an addition of lapatinib to letrozole in patients with Her-2-positive breast cancer (57). There was no significant improvement in PFS in patients with Her-2-negative tumors. Interestingly, the preplanned Cox regression analysis of patients with Her-2-negative tumors who relapse less than 6 months after tamoxifen discontinuation demonstrated a non-significant trend toward improvement in PFS for the combination (HR 0.78; p = 0.117) (57). This result highlights the significance of dual inhibition of the Her-2 and ER signaling pathways in tumors that have been resistant to endocrine therapy even though the initial tumors may lack Her-2 overexpression. Furthermore, given that there was no benefit of combining letrozole and lapatinib upfront in ER-positive and Her-2-negative tumors, this implies that co-blockage of Her-2 and estrogen deprivation from the beginning may not delay the emergence of resistance. In addition, co-targeting the downstream signaling of PI3K/Akt pathway with mTOR inhibitors has also been under investigation in multiple clinical trials. A recent randomized phase II study of letrozole ± everolimus for 4 months in the preoperative setting demonstrated a significant increased in tumor shrinkage as determined by ultrasound (58% vs. 47%; p = 0.03) and a greater reduction in cell proliferation index, Ki67 (57% vs. 30%) (58).
These results suggest the possibility that inhibition of both the estrogen pathway as well as growth factor signaling may be an effective strategy to overcome resistance of breast cancer to AI therapy. A number of trials are currently underway based on these pre-clinical findings. Currently, there are multiple novel targeted therapies under clinical development that can also be used to target MAPK and PI3K/Akt signaling pathway besides Her-2 with trastuzumab and lapatinib. These agents include MEK inhibitors, Raf inhibitors, PI3K inbitors, mTOR inhibitors, and Akt inhibitors.
Conclusion
Above data suggests that an inverse and compensatory relationship exists between growth factor receptors and ERα, and that inhibition of one pathway leads to activation of the other. Blocking both estrogen signaling and growth factor receptor signaling may have application to treating breast cancer patients by reversing resistance, restoring sensitivity to AI therapy and delaying the need for more toxic chemotherapy. However, most of the results obtained suggest that careful pre-selection of patients based on specific biomarkers is necessary for the success of these newer strategies.
Delay in the use of chemotherapy by sequential use of endocrine therapies offers significant quality-of-life advantages due to good tolerability of the agents, especially in elderly patients or those with advanced disease.
Acknowledgements
This work was supported by grant CA-62483 to Dr. Brodie from the National Cancer Institute, National Institute of Health.
Abbreviations used
- ER
Estrogen Receptor
- Δ4A
Androstenedione
- E2
Estradiol
- Her-2
Human Epidermal Growth factor Receptor- 2
- MAPK
Mitogen Activated Protein Kinase
- AIs
Aromatase Inhibitors
- AEs
Antiestrogens
- TRZ
Trastuzumab
- Let
Letrozole
References
- 1.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. Journal of the National Cancer Institute. 2005;97:1262–71. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 2.Goss PE, Ingle JN, Martino S, et al. Efficacy of Letrozole Extended Adjuvant Therapy According to Estrogen Receptor and Progesterone Receptor Status of the Primary Tumor: National Cancer Institute of Canada Clinical Trials Group MA.17. J Clin Oncol. 2007 doi: 10.1200/JCO.2006.09.4482. [DOI] [PubMed] [Google Scholar]
- 3.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 4.Buzdar A. Anastrozole as adjuvant therapy for early-stage breast cancer: implications of the ATAC trial. Clinical breast cancer. 2003;4(Suppl 1):S42–8. doi: 10.3816/cbc.2003.s.014. [DOI] [PubMed] [Google Scholar]
- 5.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–9. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 6.Sabnis GJ, Jelovac D, Long B, Brodie A. The role of growth factor receptor pathways in human breast cancer cells adapted to long-term estrogen deprivation. Cancer Res. 2005;65:3903–10. doi: 10.1158/0008-5472.CAN-04-4092. [DOI] [PubMed] [Google Scholar]
- 7.Santen RJ, Song RX, Zhang Z, et al. Adaptive hypersensitivity to estrogen: mechanisms and clinical relevance to aromatase inhibitor therapy in breast cancer treatment. J Steroid Biochem Mol Biol. 2005;95:155–65. doi: 10.1016/j.jsbmb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Belosay A, Brodie AM, Njar VC. Effects of novel retinoic acid metabolism blocking agent (VN/14-1) on letrozole-insensitive breast cancer cells. Cancer Res. 2006;66:11485–93. doi: 10.1158/0008-5472.CAN-06-2168. [DOI] [PubMed] [Google Scholar]
- 9.Brodie A, Jelovac D, Sabnis G, Long B, Macedo L, Goloubeva O. Model systems: mechanisms involved in the loss of sensitivity to letrozole. J Steroid Biochem Mol Biol. 2005;95:41–8. doi: 10.1016/j.jsbmb.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Jelovac D, Sabnis G, Long BJ, Macedo L, Goloubeva OG, Brodie AM. Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res. 2005;65:5380–9. doi: 10.1158/0008-5472.CAN-04-4502. [DOI] [PubMed] [Google Scholar]
- 11.Sabnis G, Schayowitz A, Goloubeva O, Macedo L, Brodie A. Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res. 2009;69:1416–28. doi: 10.1158/0008-5472.CAN-08-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jelovac D, Macedo L, Goloubeva OG, Handratta V, Brodie AM. Additive antitumor effect of aromatase inhibitor letrozole and antiestrogen fulvestrant in a postmenopausal breast cancer model. Cancer Res. 2005;65:5439–44. doi: 10.1158/0008-5472.CAN-04-2782. [DOI] [PubMed] [Google Scholar]
- 13.Creighton CJ, Hilger AM, Murthy S, Rae JM, Chinnaiyan AM, El-Ashry D. Activation of mitogen-activated protein kinase in estrogen receptor alpha-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor alpha-negative human breast tumors. Cancer Res. 2006;66:3903–11. doi: 10.1158/0008-5472.CAN-05-4363. [DOI] [PubMed] [Google Scholar]
- 14.Oh AS, Lorant LA, Holloway JN, Miller DL, Kern FG, El-Ashry D. Hyperactivation of MAPK induces loss of ERalpha expression in breast cancer cells. Molecular endocrinology. 2001;15:1344–59. doi: 10.1210/mend.15.8.0678. [DOI] [PubMed] [Google Scholar]
- 15.Bayliss J, Hilger A, Vishnu P, Diehl K, El-Ashry D. Reversal of the estrogen receptor negative phenotype in breast cancer and restoration of antiestrogen response. Clin Cancer Res. 2007;13:7029–36. doi: 10.1158/1078-0432.CCR-07-0587. [DOI] [PubMed] [Google Scholar]
- 16.Brinkman JA, El-Ashry D. ER re-expression and re-sensitization to endocrine therapies in ER-negative breast cancers. Journal of mammary gland biology and neoplasia. 2009;14:67–78. doi: 10.1007/s10911-009-9113-0. [DOI] [PubMed] [Google Scholar]
- 17.Sabnis G, Goloubeva O, Gilani R, Macedo L, Brodie A. Sensitivity to the aromatase inhibitor letrozole is prolonged after a “break” in treatment. Molecular cancer therapeutics. 2010;9:46–56. doi: 10.1158/1535-7163.MCT-09-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabnis GJ, Macedo LF, Goloubeva O, Schayowitz A, Brodie AM. Stopping treatment can reverse acquired resistance to letrozole. Cancer Res. 2008;68:4518–24. doi: 10.1158/0008-5472.CAN-07-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown M, Bauer K, Pare M. Tumor marker phenotype concordance in second primary breast cancer, California, 1999–2004. Breast Cancer Res Treat. 2010;120:217–27. doi: 10.1007/s10549-009-0469-z. [DOI] [PubMed] [Google Scholar]
- 20.Simmons C, Miller N, Geddie W, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol. 2009;20:1499–504. doi: 10.1093/annonc/mdp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Yau C, Gray JW, et al. Enhanced NF kappa B and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007;7:59. doi: 10.1186/1471-2407-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11:643–58. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- 23.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–43. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 24.Brodie A, Jelovac D, Long BJ. Predictions from a preclinical model: studies of aromatase inhibitors and antiestrogens. Clin Cancer Res. 2003;9:455S–9S. [PubMed] [Google Scholar]
- 25.Yue W, Brodie A. MCF-7 human breast carcinomas in nude mice as a model for evaluating aromatase inhibitors. J Steroid Biochem Mol Biol. 1993;44:671–3. doi: 10.1016/0960-0760(93)90278-5. [DOI] [PubMed] [Google Scholar]
- 26.Yue W, Zhou D, Chen S, Brodie A. A new nude mouse model for postmenopausal breast cancer using MCF-7 cells transfected with the human aromatase gene. Cancer Res. 1994;54:5092–5. [PubMed] [Google Scholar]
- 27.Zhou DJ, Pompon D, Chen SA. Stable expression of human aromatase complementary DNA in mammalian cells: a useful system for aromatase inhibitor screening. Cancer Res. 1990;50:6949–54. [PubMed] [Google Scholar]
- 28.Long BJ, Jelovac D, Handratta V, et al. Therapeutic strategies using the aromatase inhibitor letrozole and tamoxifen in a breast cancer model. Journal of the National Cancer Institute. 2004;96:456–65. doi: 10.1093/jnci/djh076. [DOI] [PubMed] [Google Scholar]
- 29.Long BJ, Jelovac D, Thiantanawat A, Brodie AM. The effect of second-line antiestrogen therapy on breast tumor growth after first-line treatment with the aromatase inhibitor letrozole: long-term studies using the intratumoral aromatase postmenopausal breast cancer model. Clin Cancer Res. 2002;8:2378–88. [PubMed] [Google Scholar]
- 30.Sabnis G, Brodie A. Trastuzumab sensitizes ER negative, Her-2 positive breast cancer cells (SKBr-3) to endocrine therapy. Endorine Society's Annual Meeting; Washington DC. 2009.2009. p. Abstract OR38-04. [Google Scholar]
- 31.Fox EM, Andrade J, Shupnik MA. Novel actions of estrogen to promote proliferation: integration of cytoplasmic and nuclear pathways. Steroids. 2009;74:622–7. doi: 10.1016/j.steroids.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Molecular and cellular biology. 2003;23:1633–46. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shupnik MA. Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene. 2004;23:7979–89. doi: 10.1038/sj.onc.1208076. [DOI] [PubMed] [Google Scholar]
- 34.Song RX, Fan P, Yue W, Chen Y, Santen RJ. Role of receptor complexes in the extranuclear actions of estrogen receptor alpha in breast cancer. Endocrine-related cancer. 2006;13(Suppl 1):S3–13. doi: 10.1677/erc.1.01322. [DOI] [PubMed] [Google Scholar]
- 35.Hsu SC, Hung MC. Characterization of a novel tripartite nuclear localization sequence in the EGFR family. J Biol Chem. 2007;282:10432–40. doi: 10.1074/jbc.M610014200. [DOI] [PubMed] [Google Scholar]
- 36.Lo HW, Hsu SC, Hung MC. EGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalization. Breast Cancer Res Treat. 2006;95:211–8. doi: 10.1007/s10549-005-9011-0. [DOI] [PubMed] [Google Scholar]
- 37.Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2007;96(Suppl):R16–20. [PubMed] [Google Scholar]
- 38.Xie Y, Hung MC. Nuclear localization of p185neu tyrosine kinase and its association with transcriptional transactivation. Biochemical and biophysical research communications. 1994;203:1589–98. doi: 10.1006/bbrc.1994.2368. [DOI] [PubMed] [Google Scholar]
- 39.Sharma AK, Horgan K, Douglas-Jones A, McClelland R, Gee J, Nicholson R. Dual immunocytochemical analysis of oestrogen and epidermal growth factor receptors in human breast cancer. Br J Cancer. 1994;69:1032–7. doi: 10.1038/bjc.1994.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science (New York, NY. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 41.van Agthoven T, Timmermans M, Foekens JA, Dorssers LC, Henzen-Logmans SC. Differential expression of estrogen, progesterone, and epidermal growth factor receptors in normal, benign, and malignant human breast tissues using dual staining immunohistochemistry. The American journal of pathology. 1994;144:1238–46. [PMC free article] [PubMed] [Google Scholar]
- 42.Johnston SR. Enhancing the efficacy of hormonal agents with selected targeted agents. Clin Breast Cancer. 2009;9(Suppl 1):S28–36. doi: 10.3816/CBC.2009.s.003. [DOI] [PubMed] [Google Scholar]
- 43.El-Ashry D, Miller DL, Kharbanda S, Lippman ME, Kern FG. Constitutive Raf-1 kinase activity in breast cancer cells induces both estrogen-independent growth and apoptosis. Oncogene. 1997;15:423–35. doi: 10.1038/sj.onc.1201198. [DOI] [PubMed] [Google Scholar]
- 44.Kasid A, Lippman ME, Papageorge AG, Lowy DR, Gelmann EP. Transfection of v-rasH DNA into MCF-7 human breast cancer cells bypasses dependence on estrogen for tumorigenicity. Science (New York, NY. 1985;228:725–8. doi: 10.1126/science.4039465. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, el-Ashry D, Chen D, Ding IY, Kern FG. MCF-7 breast cancer cells overexpressing transfected c-erbB-2 have an in vitro growth advantage in estrogen-depleted conditions and reduced estrogen-dependence and tamoxifen-sensitivity in vivo. Breast Cancer Res Treat. 1995;34:97–117. doi: 10.1007/BF00665783. [DOI] [PubMed] [Google Scholar]
- 46.Miller DL, el-Ashry D, Cheville AL, Liu Y, McLeskey SW, Kern FG. Emergence of MCF-7 cells overexpressing a transfected epidermal growth factor receptor (EGFR) under estrogen-depleted conditions: evidence for a role of EGFR in breast cancer growth and progression. Cell Growth Differ. 1994;5:1263–74. [PubMed] [Google Scholar]
- 47.du Manoir JM, Francia G, Man S, et al. Strategies for delaying or treating in vivo acquired resistance to trastuzumab in human breast cancer xenografts. Clin Cancer Res. 2006;12:904–16. doi: 10.1158/1078-0432.CCR-05-1109. [DOI] [PubMed] [Google Scholar]
- 48.Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11:865s–70s. [PubMed] [Google Scholar]
- 49.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977–84. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 50.Lipton A, Leitzel K, Ali SM, et al. Serum HER-2/neu conversion to positive at the time of disease progression in patients with breast carcinoma on hormone therapy. Cancer. 2005;104:257–63. doi: 10.1002/cncr.21202. [DOI] [PubMed] [Google Scholar]
- 51.Munzone E, Curigliano G, Rocca A, et al. Reverting estrogen-receptor-negative phenotype in HER-2-overexpressing advanced breast cancer patients exposed to trastuzumab plus chemotherapy. Breast Cancer Res. 2006;8:R4. doi: 10.1186/bcr1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackey J, Kaufman B, Clemens M. Trastuzumab prolongs progression-free survival in hormone-dependent and HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2006;100(suppl 1) abstract 3. [Google Scholar]
- 53.Johnston SR, Leary A, Martin LA, Smith IE, Dowsett M. Enhancing endocrine response with novel targeted therapies: why have the clinical trials to date failed to deliver on the preclinical promise? Cancer. 2008;112:710–7. doi: 10.1002/cncr.23190. [DOI] [PubMed] [Google Scholar]
- 54.Leary A, Johnston SR. Small molecule signal transduction inhibitors for the treatment of solid tumors. Cancer investigation. 2007;25:347–65. doi: 10.1080/07357900701259694. [DOI] [PubMed] [Google Scholar]
- 55.Leary AF, Sirohi B, Johnston SR. Clinical trials update: endocrine and biological therapy combinations in the treatment of breast cancer. Breast Cancer Res. 2007;9:112. doi: 10.1186/bcr1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–37. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 57.Johnston S, Pippen J, Jr., Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–46. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 58.Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–7. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]