Abstract
Purpose
To determine whether mTORC2 and RI-mTORC1 complexes are present in AML cells and to examine the effects of dual mTORC2/mTORC1 inhibition on primitive AML leukemic progenitors.
Experimental Design
Combinations of different experimental approaches were used, including immunoblotting to detect phosphorylated/activated forms of elements of the mTOR pathway in leukemic cell lines and primary AML blasts; cell proliferation assays; direct assessment of mRNA translation in polysomal fractions of leukemic cells; and clonogenic assays in methylcellulose to evaluate leukemic progenitor colony formation.
Results
mTORC2 complexes are active in AML cells and play critical roles in leukemogenesis. Rapamycin insensitive (RI) mTORC1 complexes are also formed and regulate the activity of the translational repressor 4E-BP1 in AML cells. OSI-027, blocks mTORC1 and mTORC2 activities and suppresses mRNA translation of cyclin D1 and other genes that mediate proliferative responses in AML cells. Moreover, OSI-027 acts as a potent suppressor of primitive leukemic precursors from AML patients and is much more effective than rapamycin in eliciting antileukemic effects in vitro.
Conclusions
Dual targeting of mTORC2 and mTORC1 results in potent suppressive effects on primitive leukemic progenitors from AML patients. Inhibition of the mTOR catalytic site with OSI-027 results in suppression of both mTORC2 and RI-mTORC1 complexes and elicits much more potent antileukemic responses than selective mTORC1 targeting with rapamycin.
Introduction
Despite recent advances in the field, acute myeloid leukemia (AML) remains highly fatal. Although a large number of AML patients achieve clinical remission with current treatment regimens, the majority of patients eventually relapse and die (1, 2). Undoubtedly, there is an urgent need to develop new therapeutic approaches to overcome the resistance that AML cells develop to chemotherapy (1, 2). Several mechanisms underlying the molecular pathogenesis of AML have been uncovered. It is now known that AML results from mutations in classes of genes that affect both proliferation/survival and differentiation/apoptosis (3). Changes in transcriptional control of hematopoietic cells and mutations involving different proto-oncogenes or tumor suppressors account for the deregulation of normal myelopoiesis (3). Such changes ultimately result in activation of cellular pathways that mediate signals that promote cell growth and/or maintain survival, leading to leukemogenesis (4, 5).
The phosphoinositide 3′-kinase (PI3′K)/AKT pathway is a critical network of signals that control key cellular functions, including gene transcription, differentiation and mRNA translation and survival (6-8). This pathway is frequently dysregulated in various tumors (6-8). Extensive work over the years has established that activation of PI3′K/AKT signaling results in a growth advantage for tumor cells by promoting cell proliferation and anti-apoptotic signals (6, 7, 9). There is evidence that PI3K/AKT activation contributes to tumor cell resistance to antineoplastic therapies and over-expression or constitutive activation of components of this pathway may contribute to a poorer prognosis in different malignancies (reviewed in 10, 11).
The mammalian target of rapamycin (mTOR) is a kinase activated downstream of PI3′K/AKT which plays critical and essential roles in the regulation of mRNA translation, cell cycle progression and growth of mammalian cells (6, 12-14). As mTOR mediates pro-growth signals in malignant cells, its pharmacological targeting is currently under extensive investigation for the treatment of various malignancies, including leukemias (11, 15). In fact, two rapamycin-related drugs, temsirolimus and everolimus, have significant activity in the treatment of renal cell carcinoma (16, 17) and were recently approved by the FDA for the treatment of this disease.
mTOR inhibitors currently approved for clinical use (rapamycin, temsirolimus, everolimus) are allosteric inhibitors of mTORC1 but not mTORC2 (18), however the precise functional roles of mTORC2 and other rapamycin-insensitive complexes in AML cells is unknown. In the present study we provide direct evidence for the presence and activity of mTORC2 complexes in AML cell lines and primary AML cells. We also establish that mTOR-mediated phosphorylation of rapamycin-insensitive (RI) sites on the translational repressor 4E-BP1 plays a key role in mRNA translation in AML cells and regulates cyclin D1 expression. We demonstrate that activation of mTORC2 and RI-mTORC1 complexes is selectively inhibited by a novel dual mTORC2/mTORC1 inhibitor OSI-027, a selective ATP-competitive catalytic site inhibitor of mTOR (19), but not by the allosteric inhibitor rapamycin. Our studies establish that OSI-027 induces potent antileukemic effects on a variety of AML cell lines, as well as primary leukemic progenitors (CFU-L) from AML patients. In addition, OSI-027-treatment enhances chemotherapy-induced antileukemic responses in vitro. Altogether, our data demonstrate critical roles for mTORC2 and RI-mTORC1 complexes in leukemic cell growth and survival, and provide a rationale for the clinical development of dual mTORC2/mTORC1 inhibitors for the treatment of AML.
Materials and Methods
Cells and Reagents
The KG1, HL-60, KBM-3B and U937 human leukemia cell lines were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and 1% L-Glutamine. ML-1 cells were grown in DMEM supplemented with 10% fetal calf serum (FCS) and 1% L-Glutamine. U937, KG1 and HL60 cells were obtained from ATCC. ML-1 and KBM-3B cells were generously provided by E. Harlow, Cold Spring Harbor Laboratories. Rapamycin was purchased from Calbiochem/EMD. Cytarabine was purchased from Sigma. All antibodies were purchased from Cell Signaling Technologies except the antibody against PDCD4, which was purchased from Rockland and the antibody against GAPDH which was purchased from Millipore.
Measurement of proliferation
Inhibition of proliferation was measured using the Cell Titer Glo Assay (Promega Corporation, Madison, WI) as noted in figure legends. To generate dose response curves, cell lines were seeded at a density of 5000 cells per well in a 96-well plate. 24 hours after plating cells were dosed with varying concentrations of either OSI-027 or rapamycin. The signal for Cell Titer Glo was determined 72 hours after dosing and normalized to vehicle-treated controls. Inhibition of proliferation, relative to vehicle-treated controls was expressed as a fraction of 1 and graphed using PRISM® software (Graphpad Software, San Diego, CA).
Cell lysis and Immunoblotting
For the immunoblotting experiments, cells were treated with rapamycin (20 nM) or OSI-027 or DMSO used control for untreated cells, for the indicated times and lysed in phosphorylation lysis buffer. Immunoblotting using an enhanced chemiluminescence (ECL) method was performed as in our previous studies (21, 22).
Isolation of Polysomal RNA and quantitative RT-PCR in polysomal fractions
U937 cells were treated with DMSO, rapamycin or OSI-027, polysomal fractionation and quantitative RT-PCR were performed with slight modifications of the protocol used in our previous studies (23, 24).
Studies with primary AML samples and hematopoietic progenitor cell assays
Peripheral blood was obtained from patients with acute myeloid leukemia (AML) after obtaining informed consent approved by the Institutional Review Board of Northwestern University. Cells were separated over Ficoll-Hypaque and cultured with the indicated concentrations of rapamycin, OSI-027, and/or cytarabine and leukemic progenitor (CFU-L) colony formation was assessed in clonogenic assays in methylcellulose (22, 25-28).
shRNA-mediated knockdown
U937 cells were transduced with lentiviral particles (29-31), expressing either control shRNA or S6K1 (p70 S6 kinase α) shRNAs, purchased from Santa Cruz Biotechnology, Inc., using the manufacturer's recommended procedure. Briefly, 5×104cells/ml were infected with above lentiviruses, and selected in puromycin, as recommended.
Results
In initial studies, we determined whether functional mTORC1 and mTORC2 complexes are present in AML cells and evaluated the effects of OSI-027 or rapamycin on their activation. Using the AML cell line U937 or circulating primary leukemic AML blasts, we examined the phosphorylation of AKT on serine 473, a marker of AKT/mTORC2 activity. As shown in Fig. 1, AKT phosphorylation was detectable in AML cells, and it was completely inhibited by treatment of cells with OSI-027 (Fig. 1A and B). On the other hand, rapamycin did not inhibit such phosphorylation (Fig. 1A and B) and, in fact, it had a slight stimulatory effect in U937 cells (Fig. 1A); a finding consistent with the previously described rapamycin-mediated inhibition of the S6K-IRS negative feedback loop which results in enhanced signaling through the mTORC2/Akt node (reviewed in 32). We also examined the phosphorylation of mTOR on Ser 2448, a marker for mTORC1 activity, as well as the phosphorylation of the protein on Ser 2481, a marker for mTORC2 activity (33). Phosphorylation of mTOR on both Ser 2448 and Ser 2481 was detectable in U937 cells (Fig. 1C and D), reflecting the presence of both mTORC1 and mTORC2 complexes. Ser 2448 phosphorylation was inhibited by treatment of cells with either rapamycin or OSI-027 (Fig. 1C), consistent with the expected sensitivity of mTORC1 complexes to both inhibitors. However, only OSI-027 inhibited phosphorylation on Ser 2481, demonstrating mTORC2 targeting (Fig. 1D). Similar results were obtained when the AML cell line, KG1, (Fig. 1E) or primary leukemic blasts from a patient with AML (Fig. 1F) were studied. mTORC1 kinase activity could be also directly demonstrated in in vitro kinase assays on anti-Raptor immunoprecipitates from U937 cell lysates, using S6K as a substrate (Fig. S1, supplemental data). These data clearly established the presence of mTORC1 kinase activity and demonstrated decreased phosphorylation of S6K substrate upon treatment with OSI-027 (Fig. S1A, supplemental data). Notably, in these studies it was also shown that, in contrast to rapamycin, the suppression of mTOR kinase activity occurs without disruption of the protein-protein interactions in the mTORC1 complex, a finding consistent with ATP-competitive catalytic inhibition of mTOR by OSI-027 (Fig. S1B, supplemental data).
Figure 1.
Presence of mTORC2 and mTORC1 complexes in AML cells and differential regulation by rapamycin or OSI-027. A. U937 cells were incubated with or without OSI-027 (10 μM) or rapamycin (20nM) for 90 minutes. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of AKT on serine 473. Subsequently, the same blot was re-probed with antibodies against AKT or GAPDH, to control for equal protein loading. B. Primary leukemic blasts from a patient with AML were incubated with or without OSI-027 (10 μM) or rapamycin (20 nM) for 90 minutes. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of AKT on serine 473. Subsequently, the same blot was re-probed with antibodies against AKT or GAPDH, to control for protein loading. C-D. U937 cells were incubated with or without OSI-027 (5 μM) or rapamycin (20 nM) for 90 minutes. Cell lysates were resolved by SDS-PAGE and immunoblotted with antibodies against the phosphorylated form mTOR on serine 2448 (C) or the phosphorylated form of mTOR on serine 2481 (D). The blots were then re-probed with an antibody against mTOR, to control for protein loading. E. KG1 cells were incubated with or without OSI-027 (10 μM) or rapamycin (20nM) for 24 hours and cell lysates were resolved and immunoblotted with an antibody against the phosphorylated form of mTOR on serine 2481. The same blot was subsequently re-probed with an antibody against mTOR. F. Leukemic blasts from a patient with AML were incubated with or without OSI-027 (10 μM) or rapamycin (20nM) for 90 minutes. Cell lysates were resolved by SDS-PAGE and then immunoblotted with an antibody against the phosphorylated form of mTOR on serine 2481. The blot was then re-probed with an antibody against mTOR.
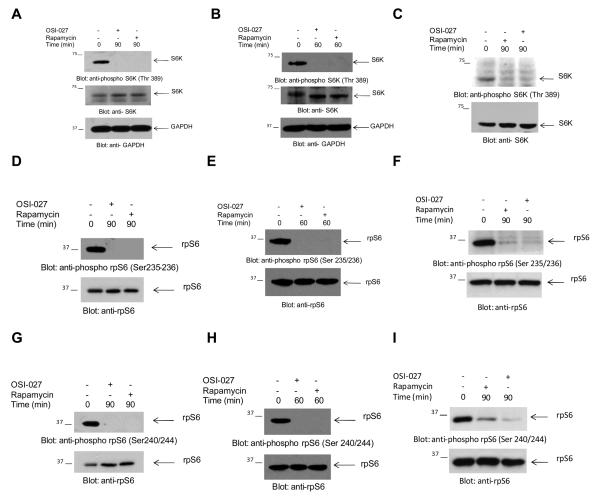
A major effector of activated mTORC1 complexes is the p70 S6 kinase (S6K), which in turn phosphorylates and activates several downstream effectors, including the S6 ribosomal protein (rpS6) and the eukaryotic initiation factor 4B (eIF4B) (6, 13). When different AML cell lines or primary AML blasts were treated with rapamycin or OSI-027, there was complete suppression of phosphorylation of S6K on Thr389, a site whose phosphorylation correlates with activation of its kinase domain (Fig. 2A-C). Consistent with the inhibitory effects of both mTOR inhibitors on S6K activity, phosphorylation of the downstream effector of S6K, rpS6, on both Ser 235/236 and Ser 240/244 was blocked by treatment of cells with either OSI-027 or rapamycin (Fig. 2D-I). We also determined the effects of OSI-027 or rapamycin on PDCD4, a tumor suppressor protein that acts as an inhibitor of cap-dependent translation by blocking the translation initiation factor eIF4A (34). This protein was recently identified as a target of the mTOR/S6K cascade, and it was shown that S6K-mediated phosphorylation of PDCD4 results in its degradation by ubiquitin ligases (35), while its upregulation in response to BCR-ABL kinase inhibitors appears to mediate generation of antileukemic responses in Ph+ cells (36). Consistent with the suppressive effects of both rapamycin and OSI-027 on S6K activity (Fig. 2A-C), there was upregulation of PDCD4 expression in response to treatment of AML cells with either inhibitor (Fig. 3A and B). Thus, both rapamycin and OSI-027 upregulate PDCD4, suggesting that this S6K target is a mediator of the antileukemic responses elicited by targeting mTORC1 complexes. To define the functional consequences of inhibition of S6K phosphorylation in leukemic progenitors, experiments were performed in which S6K1 was knocked down using S6K1-shRNA in a lentiviral vector (Fig. 3C). These studies demonstrated substantial inhibition in leukemic CFU-L colony formation by S6K1 knockdown (Fig. 3D), suggesting an important role for S6K1 activity and suppression of PDCD4 in the growth of AML progenitors.
Figure 2.
Phosphorylation/activation of S6K and S6 ribosomal protein in AML cells. A-C. U937 cells (A), KG1 cells (B), or primary leukemic blasts from an AML patient (C) were incubated with OSI-027 (10 μM) or rapamycin (20nM) for the indicated times. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of S6K on Thr 389, or with antibodies against S6K or GAPDH, as indicated. D-I. U937 cells (D, G) KG1 cells (E, H), or primary AML blasts (F, I) were incubated in the absence or presence of rapamycin (20nM) or OSI-027 (10 μM) as indicated. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of rpS6 on Ser235/236 (D, E, F) or against the phosphorylated form of rpS6 on Ser240/244 (G, H, I) or against rpS6, as indicated.
Figure 3.
Effects of mTOR inhibition on the expression of the S6K-dependent PDCD4 tumor suppressor in AML cells and functional consequences of S6K knockdown. A-B. U937 (A) or KG1 (B) cells were incubated in the absence (lane 1) or presence of OSI-027 at either 5 μM (lane 2) or 10 μM (lane 3) or rapamycin (20 nM) (lane 4) for 24 hours. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against PDCD4. The same blots were re-probed with an antibody against tubulin, to control for protein loading. C. Cell lysates from lentivirus transduced control-shRNA or S6K1-shRNA U937cells were resolved by SDS-PAGE and immunoblotted with an anti-S6K1 antibody. The same blot was then re-probed with an anti-GAPDH antibody to control for protein loading. D. Leukemic CFU-L colony formation derived from control-shRNA and S6K1-shRNA U937 cells is shown. Data are expressed as percentages of colony formation of untreated control-shRNA transduced cells and represent means ± S.E. of 5 independent experiments.
A key functional cellular response downstream of the mTOR pathway is the regulation of initiation of cap-dependent mRNA translation which occurs primarily via regulation of the 4E-BP1 repressor of mRNA translation (6, 13, 14). 4E-BP1 is phosphorylated in a hierarchical manner on Thr37/46, Ser65, Thr70; and such phosphorylation is essential for the de-activation of the protein and its subsequent dissociation from the eukaryotic initiation factor 4E (eIF4E), so cap-dependent mRNA translation can proceed (37, 38). Significant baseline levels of phosphorylation of 4E-BP1 on Thr37/46 and Ser65 were detectable in both U937 and KG1 cells (Fig. 4A and B). Such phosphorylation was completely blocked by treatment of cells with OSI-027, while treatment with rapamycin had no effect (Fig. 4A and B). Similar results were also seen when the phosphorylation of the protein on Thr70 was studied (Fig. 4C). OSI-027 was used at a maximal concentration of 10 μM which inhibits both mTORC1 and mTORC2 (19) and is readily exceeded in plasma in preclinical rodent models (20). Rapamycin was dosed at a maximal concentration of 20 nM, which is consistent with multiple published studies and exceeds the dose achievable in tumors, and that required to inhibit mTORC1 function (20, 39). In dose response experiments, we found that rapamycin failed to block Thr37/46 4E-BP1 phosphorylation even at very high, supra-normal concentrations (Fig. 4D), definitively establishing that 4E-BP1 phosphorylation at Thr 37/46 is a rapamycin-insensitive event. A similar pattern of 4E-BP1 dephosphorylation in response to OSI-027 or rapamycin was seen when in studies using primary AML leukemic blasts (Fig. 4E and F and Fig. S2, supplemental data). Consistent with the complete suppression of 4E-BP1 phosphorylation, OSI-027-treatment resulted in formation of 4E-BP1-eIF4E complexes that suppress cap-dependent mRNA translation, while it inhibited the formation of eIF4E-eIF4G complexes that are required for initiation of translation (Fig. 4G). On the other hand, rapamycin had much weaker effects in promoting formation of 4E-BP1-eIF4E complexes and it did not disrupt formation of eIF4E-eIF4G complexes (Fig. 4G). Thus, OSI-27 is a potent inhibitor of mTOR activity in AML cells and suppresses formation of complexes that regulate cap-dependent mRNA translation.
Figure 4.
OSI-027, but not rapamycin, inhibits phosphorylation of the 4E-BP1 repressor of mRNA translation and blocks formation of complexes required for cap-dependent mRNA translation. A-B. U937 or KG1 cells were incubated in the absence or presence of OSI-027 (10 μM) or rapamycin (20nM) for 90 minutes. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of 4E-BP1 on threonine 37/46 (A) or serine 65 (B). Subsequently, the same blots were re-probed with an antibody against 4E-BP1 or against GAPDH, to control for protein loading. C. KG1 cells were incubated in the absence or presence of OSI-027 (10 μM) or rapamycin (20nM) for 60 minutes. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against 4E-BP1 on threonine 70. The same blot was re-probed with an antibody against 4E-BP1 or against GAPDH. D. U937 cells were treated with increasing concentrations of rapamycin or OSI, as indicated for 90 minutes. Cell lysates were resolved by SDS-PAGE and immunoblotted with antibodies against the phosphorylated form of rpS6 at serine 240/244, the phosphorylated form of 4E-BP1 on threonine 37/46, or against GAPDH as indicated. E-F. Leukemic blasts from a patient with AML were incubated in the absence or presence of OSI-027 (10 μM) or rapamycin (20nM) for 90 minutes. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against 4E-BP1 on threonine 37/46 (E) or serine 65 (F). The same blot was re-probed with an antibody against 4E-BP1 and then GAPDH, to assess for equivalent protein loading. G. U937 cells were treated with OSI-027 (10 μM) or rapamycin (20nM) for 90 minutes. Cell lysates were immunoprecipitated with an anti-eIF4E antibody and immunoprecipitates were immunoblotted with antibodies against 4E-BP1, eIF4A, eIF4G or eIF4E, as indicated. A signal seen above the eIF4G band in the anti-eIF4G immunoblot in the OSI-027-treatment lane is an artifact and does not represent a slower migrating form of eIF4G.
The suppressive effects of OSI-027 on formation of eIF4E-eIF4G complexes prompted us to directly examine the effects of OSI-027 on mRNA recruitment to polysomal fractions in AML cells. OSI-027-treatment resulted in suppression of mRNA recruitment to polysomes, reflecting potent inhibitory effects on translation (Fig. 5A and B). Notably, the effects of OSI-027 were much more pronounced than the effects of rapamycin analyzed in parallel (Fig. 5A and B). In addition, when mRNA translation for cyclin D1 was directly analyzed in polysomal fractions from U937 cells, we found suppression by OSI-027, but not rapamycin (Fig. 5C). Consistent with the effects seen in the suppression of cyclin D1 mRNA translation, time-dependent suppression of cyclin D1 protein expression after OSI-027 treatment was documented (Fig. 5D). Thus, mTORC1-mediated phosphorylation of 4E-BP1 is a rapamycin-insensitive event that plays a key role in mRNA translation for cyclin D1 and possibly other genes whose products control cell- cycle progression in AML cells. These findings prompted us to directly examine the effects of dual mTORC1/mTORC2 inhibition in AML cells. Treatment of U937 cells with OSI-O27 resulted in G0/G1 arrest (Fig. S3, supplemental data), consistent with the suppressive effects of this agent on cyclin D1 mRNA translation and protein expression.
Figure 5.
Effects of OSI-027 on polysomal assembly and mRNA translation of the cyclin D1 gene in AML cells. A. U937 cells were treated with solvent control (DMSO), rapamycin (20nM) or OSI-027 (10 μM) for 24 hours and the lysates were layered on a 10-50% sucrose gradient. The gradients were subjected to ultracentrifugation and fractions were collected by continuous monitoring of OD at 254nm. The OD 254nm is shown as a function of gradient depth for each treatment and the polysomal peaks are indicated. B. The area under the polysome peaks and polysome (PS) + monosome (MS) peaks was quantified for each treatment using Image J software. The ratio of area under the polysomal and polysomal plus monosomal peaks was calculated for each treatment and is represented as percent control (DMSO). The data represent means + SE from 2 independent experiments. C. The polysomal fractions were pooled and the total RNA was isolated. Quantitative real time RT-PCR assay to determine the Cyclin D1 mRNA expression in polysomal fractions was carried out using HPRT as control. Data are expressed as fold change as compared to DMSO treated samples and represent means + SE of 2 independent experiments. D. U937 cells were incubated with or without OSI-027 (1 μM) for the indicated times. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against cyclin D1. Subsequently, the same blot was re-probed with antibodies against GAPDH, to control for equal protein loading.
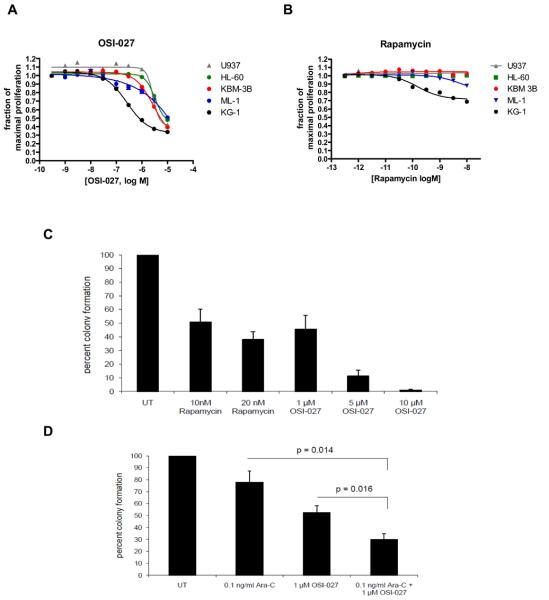
In subsequent studies the effects of dual mTORC2/mTORC1 inhibition on cell proliferation of several AML cell lines were examined. As shown in Fig. 6A, OSI-027 inhibited the growth of several acute leukemia cell lines of myeloid/megakaryocytic origin in a dose-dependent manner, including U937, KG1, KBM-3B, ML-1, HL-60 and MEG-01 cells (Fig. 6A). On the other hand, rapamycin-treatment of such AML cell lines had minimal effects on proliferation (Fig. 6B).
Figure 6.
Inhibitory effects of OSI-027 on leukemic cell lines and primary leukemic progenitor colony formation from AML patients. A-B. The sensitivity of 5 leukemic cell lines to growth inhibition by OSI-027 (A) or rapamycin (B) is shown. Effects of varying concentrations of each drug at 72 hours are expressed as dose-response curves, and error bars, where visible, show the standard error of the mean for three replicates per data point. Results shown are typical of three or more independent experiments. C. Peripheral blood mononuclear cells from 5 patients with AML were plated in methylcellulose culture assay system with increasing concentrations of rapamycin or OSI-027, as indicated. Data are expressed as percent control of CFU-L colony numbers for untreated cells. Means +/− S.E. of the values from 5 experiments using different patient samples are shown. Paired t test analysis, comparing the effects of OSI–027 (5 μM) to the effects of rapamycin (20 nM), showed p value = 0.0034. D. Peripheral blood mononuclear cells from three patients with AML were plated in methylcellulose culture assay system with Ara-C (0.1 ng/ml) and/or OSI-027 (1 μM), as indicated. Data are expressed as percent control of leukemic colonies for untreated cells and represent means +/− S.E. of 3 experiments. Paired t test analysis of the combination of Ara-C plus OSI-027 compared with Ara-C alone, showed a p= 0.014 and as compared with OSI-027 alone, p= 0.016.
To determine the effects of OSI-027 in a more functionally relevant system, experiments were performed in which its effects on primary leukemic progenitor colony formation (CFU-L) from patients with AML were examined in clonogenic survival assays in methylcellulose, using standard approaches (25-28). OSI-027 exhibited very potent dose-dependent suppressive effects on primary leukemic progenitors (Fig. 6C), and such effects were clearly more potent than the effects of rapamycin (Fig. 6C). We also assessed whether OSI-027 exhibits enhancing effects on the antileukemic properties of very low concentrations of cytarabine (AraC). OSI-027 was combined with very low concentrations of cytarabine that have minimal antileukemic effects when used alone. As shown in Fig. 6D, combined addition of low dose cytarabine and OSI-027 resulted in more suppressive effects than each agent alone (Fig. 6D), suggesting that combinations of OSI-027 with chemotherapeutic agents may ultimately provide an approach to improve generation of antileukemic responses.
Discussion
Because of its critical role in regulation of malignant cell growth and survival, the Akt/mTOR pathway has been identified as an attractive target for the treatment of various malignancies. Over the last few years, there has been a significant effort to precisely define the role of this pathway in leukemogenesis and to incorporate its targeting in the development of novel approaches for the treatment of AML. Previous work has shown that there is constitutive phosphorylation and activation of AKT in primary AML blasts, while normal human CD34+ cells have only low levels of AKT activation (40, 41). A role for the PI 3′ kinase in such regulation of AKT activity has been suggested based on the fact pharmacological inhibition of PI 3′K using LY294002 blocks growth of primary leukemic blasts (40). Interestingly, phosphorylation of AKT on the PDK1 phosphorylation site, Thr 308, has been associated with high risk cytogenetics and poor overall survival in AML (42). Other studies have demonstrated that mTOR inhibition using rapamycin increases the cytotoxicity of etoposide chemotherapy against AML blasts in vitro (43). On the other hand, more recent work has shown that mTORC1 targeting using the rapamycin derivative everolimus (RAD001) fails to inhibit eIF4F assembly and c-Myc mRNA translation in AML cells and does not induce apoptosis of leukemic blasts (44). A phase I dose escalation study of rapamycin in combination with the chemotherapy regimen MEC (mitoxantrone, etoposide, and cytarabine) in AML was recently reported (45). That study demonstrated that combination of rapamycin and chemotherapy was a feasible regimen, but failed to demonstrate synergistic effects between that mTOR inhibitor and chemotherapy in vivo (45).
Although the role of mTORC1-mediated signaling in AML is well established, the precise functional relevance of mTORC2 complexes has been unknown. As rapalogs have only limited antileukemic effects, this has suggested that mTORC2 or other rapamycin-insensitive mTOR signals may be of importance in the regulation of leukemic cell growth and survival. mTORC1 and mTORC2 are distinct protein complexes that both have mTOR as their central catalytic subunit (46). In the case of mTORC1, mTOR is associated with Raptor and mLST8, while in mTORC2 it is associated with Rictor, mLST8, and SIN1 (45, 47). There have been some reports of that rapamycin or related rapalogs are capable of inhibiting mTORC2 activity under certain conditions in vitro and in vivo (48, 49). However, it appears that such effects occur in a cell type-, time- and dose-dependent manner (48), while generally rapamycin exhibits specificity towards mTORC1 (46, 47). Similarly, other FDA approved rapalogs (temsirolimus, everolimus) selectively block mTORC1, but have minimal activity against mTORC2 (46, 47). This is highly relevant, as engagement of mTORC2 during inhibition of mTORC1 leads to AKT activation and generation of anti-apoptotic signals (46, 47). In addition, other negative feedback regulatory mechanisms are induced during treatment of AML cells with classic mTOR inhibitors, resulting in AKT activation. For instance, inhibition of mTOR in AML cells by RAD001 also upregulates expression of IRS2 (50), providing an alternative mechanism for activation of AKT and induction of anti-apoptotic responses.
In the present study we provide evidence for the existence of mTORC2 complexes in AML cells, and demonstrate that such mTORC2 activity is selectively blocked by the novel dual mTORC2/mTORC1 inhibitor OSI-027 (19, 20). In addition, our studies establish the presence of rapamycin-insensitive mTORC1 complexes that regulate phosphorylation of the translational repressor 4E-BP1 at sites required for its de-activation and dissociation from the eukaryotic initiation factor 4E (eIF4E). In contrast to rapamycin, OSI-027 acts as a potent inhibitor of phosphorylation of 4E-BP1 on Thr37/46, Ser65, and Thr70 and this results in dissociation of eIF4E-eIF4G complexes and enhanced formation of eIF4E-4E-BP1 complexes that negatively control mRNA translation. Consistent with this, OSI-027- treatment results in potent suppression of polysomal assembly and mRNA translation and this correlates with generation of much more potent antileukemic responses than rapamycin. It should be noted that a previous study had suggested that rapalogs can inhibit phosphorylation of 4E-BP1 in AML cells and also reduce mTORC2 signaling (49). However, in that study (49) the concentrations of CCI-779 that were used for the in vitro experiments demonstrating inhibition of 4E-BP1 phosphorylation were very high, when taken in context with previous pharmacokinetic studies of CCI-779 in patients with cancer (51).
The precise relationship of pathways targeted by dual mTORC1/mTORC2 inhibition to other cellular events that may be contributing to cap-dependent mRNA translation in AML cells remains to be precisely defined. A recently published study (44) demonstrated that phosphorylation of 4E-BP1 on Ser65 and assembly of eIF4F complexes is resistant to RAD001 in AML cells. In this study it was also shown that Pim-2 regulates phosphorylation of 4E-BP1 on Ser65 and may ultimately control cap-dependent mRNA translation in an mTORC1-independent manner (44). In that study siRNA-knockdown of Pim2 blocked phosphorylation of 4E-BP1 on Ser65, but not Thr37/46 (44). It is possible that OSI-027 also affects the function of Pim2 in AML cells, and this should be examined in future studies. However, as OSI-027 blocks phosphorylation of all key phosphorylation sites of 4E-BP1 (Thr37/46, Ser65, Thr70 ), its major effects on cap-dependent translation are consistent with inhibition of RI-mTORC1 complexes (52).
Our data establish that dual targeting of mTORC2 and mTORC1 with a catalytic site inhibitor is much more effective than rapamycin in blocking the growth of AML cell lines and primitive leukemic progenitors from AML patients in vitro. In addition, we demonstrate that OSI-027 enhances the suppressive effects of cytarabine on leukemic precursors, raising the possibility of future clinical-translational efforts involving OSI-027, or combinations of chemotherapy with OSI-027, for the treatment of AML. Notably, recently published studies demonstrated potent antileukemic effects of another dual mTORC2/mTORC1 inhibitor, PP242 (53) or OSI-027 (54), on BCR-ABL expressing leukemia cells including Ph+ ALL cells. This suggests that beyond AML, dual mTORC2/mTORC1 inhibition may provide an approach for the treatment of other leukemias as well. Future studies should examine the antileukemic potential of combined AML cell targeting using OSI-027 together with other agents that target cap-dependent translation or with inhibitors of pathways activated in a negative feedback regulatory manner to counteract the antileukemic effects of mTOR inhibition. For instance, it would be interesting to test combinations of OSI-027 with the 4E-BP1 mimetic 4EGI-1 that blocks mRNA translation in AML cells via competing with 4E-BP1 binding (44). There is also evidence that in certain solid tumor cells mTORC1 inhibition results in activation of Mek/Erk via an S6K-PI3′K-Ras feedback loop (55, 56). Based on this, studies to determine whether the Mek/Erk pathway is activated in AML cells and whether it can be exploited to further enhance the antileukemic effects of mTORC2/mTORC1 inhibition are warranted.
Statement of Translational Relevance.
mTOR provides an attractive cellular target for the development of new therapeutic approaches for hematological malignancies, but a major limitation of the classic mTOR inhibitor rapamycin and related rapalogs is their selectively for mTORC1 and their inability to effectively inhibit mTORC2. In the present study we provide evidence for constitutive formation of mTORC2 and rapamycin-insensitive (RI) mTORC1 complexes in AML cells and establish that dual mTORC2/mTORC1 inhibition using a novel catalytic inhibitor of mTOR, OSI-027, has greater antileukemic effects than rapamycin on AML progenitors. These findings provide the basis for the development of clinical-translational efforts involving mTORC1/mTORC2 inhibitors such as OSI-027 or combinations of such agents with chemotherapeutic agents for the treatment of AML.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grants R01CA121192, R01CA77816, KL2RR025740-01, a VA Merit review grant, and Leukemia and Lymphoma society grant LLS-6166-09.
Footnotes
Conflict of interest disclosure: SB and SR are employees of OSI Pharmaceuticals
References
- 1.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–62. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–63. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 3.Gilliland DG, Tallman MS. Focus on acute leukemias. Cancer Cell. 2002;1:417–20. doi: 10.1016/s1535-6108(02)00081-8. [DOI] [PubMed] [Google Scholar]
- 4.Cammenga J. Gatekeeper pathways and cellular background in the pathogenesis and therapy of AML. Leukemia. 2005;19:1719–28. doi: 10.1038/sj.leu.2403894. [DOI] [PubMed] [Google Scholar]
- 5.Fathi AT, Grant S, Karp JE. Exploiting cellular pathways to develop new treatment strategies for AML. Cancer Treat Rev. 2010;36:142–50. doi: 10.1016/j.ctrv.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–83. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Martelli AM, Nyakern M, Tabellini G, et al. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–28. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- 8.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 9.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 10.Kim D, Dan HC, Park S, et al. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci. 2005;10:975–87. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- 11.Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Manzoli L, McCubrey JA. Targeting the PI3′K/AKT/mTOR signaling network in acute myelogenous leukemia. Expert Opin Investig Drugs. 2009;18:1333–49. doi: 10.1517/14728220903136775. [DOI] [PubMed] [Google Scholar]
- 12.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 14.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signaling. Nat Rev Immunol. 2005;5:375–86. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 15.Altman JK, Platanias LC. Exploiting the mammalian target of rapamycin (mTOR) pathway in hematologic malignancies. Curr Opin Hematol. 2008;15:88–94. doi: 10.1097/MOH.0b013e3282f3deaa. [DOI] [PubMed] [Google Scholar]
- 16.Hudes G, Carducci M, Tomczak P, et al. Global ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 17.Oudard S, Medioni J, Aylllon J, et al. Everolimus (RAD001): an mTOR inhibitor for the treatment of metastatic renal cell carcinoma. Expert Rev Anticancer Ther. 2009;9:705–17. doi: 10.1586/era.09.27. [DOI] [PubMed] [Google Scholar]
- 18.Shor B, Gibbons JJ, Abraham RT, Yu K. Targeting mTOR globally in cancer: thinking beyond rapamycin. Cell Cycle. 2009;8:3831–37. doi: 10.4161/cc.8.23.10070. [DOI] [PubMed] [Google Scholar]
- 19.Falcon BL, Barr S, Gokhale PC, Cho J, Fogarty J, Depeille P, Miglarese M, Epstein DM, McDonald DM. Dual mTORC1/mTORC2 inhibitors reduce tumor growth, VEGF production, angiogenesis, and vascular re-growth. Cancer Research. 2011 doi: 10.1158/0008-5472.CAN-10-3126. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gokhale PC, Bhagwat SV, Crew AP, et al. OSI-027, a selective dual mTORC1/TORC2 kinase inhibitor displays broad spectrum anti-tumor activity in preclinical models of human cancer. Proc AACR. 2010 abstr 4486. [Google Scholar]
- 21.Alsayed Y, Uddin S, Mahmud N, et al. Activation of Rac1 and the p38 mitogen-activated protein kinase pathway in response to all-trans-retinoic acid. J Biol Chem. 2001;276:4012–19. doi: 10.1074/jbc.M007431200. [DOI] [PubMed] [Google Scholar]
- 22.Altman JK, Yoon P, Katsoulidis E, et al. Regulatory effects of mammalian target of rapamycin-mediated signals in the generation of arsenic trioxide responses. J Biol Chem. 2008;283:1992–2001. doi: 10.1074/jbc.M705227200. [DOI] [PubMed] [Google Scholar]
- 23.Kaur S, Sassano A, Dolniak B, et al. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc Natl Acad Sci U S A. 2008;105:4808–13. doi: 10.1073/pnas.0710907105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joshi S, Kaur S, Redig AJ, et al. Type I Interferon (IFN)-dependent activation of Mnk1 and its role in the generation of growth inhibitory responses. Proc Natl Acad Sci USA. 2009;106:12097–102. doi: 10.1073/pnas.0900562106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parmar S, Smith J, Sassano A, et al. Differential regulation of the p70 S6 kinase pathway by interferon alpha (IFNalpha) and imatinib mesylate (STI571) in chronic myelogenous leukemia cells. Blood. 2005;106:2436–43. doi: 10.1182/blood-2004-10-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blair A, Hogge DE, Ailles LE, Lansdorp PM, Sutherland HJ. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood. 1997;89:3104–12. [PubMed] [Google Scholar]
- 27.Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, Arber DA, Slovak ML, Forman SJ. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–7. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 28.La Rosée P, Johnson K, Corbin AS, Stoffregen EP, Moseson EM, Willis S, Mauro MM, Melo JV, Deininger MW, Druker BJ. In vitro efficacy of combined treatment depends on the underlying mechanism of resistance in imatinib-resistant Bcr-Abl-positive cell lines. Blood. 2004;103:208–15. doi: 10.1182/blood-2003-04-1074. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Li F, Cardelli JA, Martin KA, Blenis J, Huang S. Rapamycin inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene. 2006;25:7029–40. doi: 10.1038/sj.onc.1209691. [DOI] [PubMed] [Google Scholar]
- 30.Armour SM, Baur JA, Hsieh SN, Land-Bracha A, Thomas SM, Sinclair DA. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging. 2009;1:515–28. doi: 10.18632/aging.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fritz RD, Varga Z, Radziwill G. CNK1 is a novel Akt interaction partner that promotes cell proliferation through the Akt-FoxO signalling axis. Oncogene. 2010;29:3575–82. doi: 10.1038/onc.2010.104. [DOI] [PubMed] [Google Scholar]
- 32.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser 2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–27. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang HS, Jansen AP, Komar AA, et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol. 2003;23:26–37. doi: 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–71. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 36.Carayol N, Katsoulidis E, Sassano A, Altman JK, Druker BJ, Platanias LC. Suppression of programmed cell death 4 (PDCD4) protein expression by BCR-ABL-regulated engagement of the mTOR/p70 S6 kinase pathway. J Biol Chem. 2008;283:8601–10. doi: 10.1074/jbc.M707934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt (PKB) signaling pathway. Genes Dev. 1998;12:502–13. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gingras AC, Raught B, Gygi SP, et al. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–64. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhn JG, Chang SM, Wen PY, et al. North american brain tumor consortium and the National Cancer Institute. Pharmacokinetic and tumor distribution characteristics of temsirolimus in patients with recurrent malignant glioma. Clin Cancer Res. 2007;13:7401–06. doi: 10.1158/1078-0432.CCR-07-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Q, Simpson S, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia required PI3 kinase activation. Blood. 2003;102:972–80. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 41.Grandage VL, Gale RE, Linch DC, Khwaja A. PI3-kinase/Akt is constitutively active in primary acute myeloid leukaemia cells and regulates survival and chemoresistance via NF-κB, Mapkinase and p53 pathways. Leukemia. 2005;19:586–94. doi: 10.1038/sj.leu.2403653. [DOI] [PubMed] [Google Scholar]
- 42.Gallay N, Dos Santos C, Cuzin L, et al. The level of AKT phosphorylation on threonine 308 but not on serine 473 is associated with high-risk cytogenetics and predicts poor overall survival in acute myeloid leukaemia. Leukemia. 2009;23:1029–38. doi: 10.1038/leu.2008.395. [DOI] [PubMed] [Google Scholar]
- 43.Xu Q, Thompson JE, Carroll M. mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood. 2005;106:4261–68. doi: 10.1182/blood-2004-11-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamburini J, Green AS, Bardet V, et al. Protein synthesis is resistant to rapamycin and constitutes a promising therapeutic target in acute myeloid leukemia. Blood. 2009;114:1618–27. doi: 10.1182/blood-2008-10-184515. [DOI] [PubMed] [Google Scholar]
- 45.Perl AE, Kasner MT, Tsai DE, et al. A Phase I Study of the mammalian target of rapamycin Inhibitor Sirolimus and MEC Chemotherapy in Relapsed and Refractory Acute Myelogenous Leukemia. Clin Cancer Res. 2009;15:6732–39. doi: 10.1158/1078-0432.CCR-09-0842. [DOI] [PubMed] [Google Scholar]
- 46.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 47.Cybulski N, Hall MN. TOR complex 2: a signaling pathway of its own. Trends Biochem Sci. 2009;34:620–27. doi: 10.1016/j.tibs.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 49.Zeng Z, Sarbassov dos D, Samudio IJ, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–12. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamburini J, Chapuis N, Bardet V, et al. Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: rationale for therapeutic inhibition of both pathways. Blood. 2008;111:379–82. doi: 10.1182/blood-2007-03-080796. [DOI] [PubMed] [Google Scholar]
- 51.Raymond E, Alexandre J, Faivre S, et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22:2336–47. doi: 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 52.Thoreen CC, Kang SA, Chang JW, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–32. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janes MR, Limon JJ, So L, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–13. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carayol N, Vakana E, Sassano A, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci U S A. 2010;107:12469–74. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3′K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–74. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, et al. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone- refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;18:3051–64. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.