Abstract
Background
Lonafarnib (LNF) is a protein farnesyl transferase (FTase) inhibitor that has shown synergistic activity with taxanes in preclinical models and early stage clinical trials. Preclinical findings suggested tubulin acetylation and FTase expression levels may be important determinants of drug sensitivity that would help identify patient populations more likely to benefit from this regimen. This pilot study evaluated the biological effects of LNF and docetaxel (DTX) combination therapy in refractory solid tumors by comparing pre- and post-treatment tumor biopsies.
Methods
Patients with histologically-confirmed locally advanced or metastatic solid malignancies refractory to standard therapies or with no effective therapies available were eligible. Patients were randomized to one of four dosing cohorts: (1) 30mg/m2, 100mg, (2) 36mg/m2, 100mg, (3) 30mg/m2, 150mg, or (4) 36mg/m2, 150mg of DTX IV weekly, LNF PO BID, respectively.
Results
Of the 38 patients enrolled, 36 were treated, and 29 were evaluable for toxicity and response assessment. The combination of LNF and DTX was tolerated in all cohorts with the exception of a 28% incidence of grade 3/4 diarrhea which was manageable with aggressive anti-diarrheal regimens. Seven patients derived clinically meaningful benefit from this combination treatment; these patients had significantly lower basal FTase-beta mRNA expression levels than the mean study population level (p<0.05). Correlation of clinical benefit with tubulin acetylation content as well as basal acetyl-tubulin content were evaluated. However, no significant correlation was found.
Conclusions
Despite the small number of patients, these findings support our preclinical mechanistic studies and warrant further clinical investigations using FTase-beta mRNA expression as a potential predictive biomarker to select for an enriched patient population to study the effects of taxane and FTase inhibitor combination therapies.
Introduction
Lonafarnib (SCH 66336) is a small molecule inhibitor of farnesyl transferase (FTase), which adds a 15-carbon farnesyl group to several G-proteins important in intracellular signaling involved in cell survival, including: Ras, RhoB, Pxf, Rap2, and cyclic GMP phosphodiesterase1-3. Mutations in the Ras family of oncogenes are common in human cancers4, and have been associated with shortened survival in several human tumor types5, 6. Since Ras farnesylation was found to be required for its membrane localization and thus its oncogene activation7-9, lonafarnib and other FTase inhibitors (FTIs) were developed as potential Ras inhibitors, and were shown to inhibit Ras function3, 10, 11. However, farnesylation of other proteins has also been shown to be involved in the anti-tumor effects of lonafarnib and other FTIs12-22. Despite its modest single-agent activity, lonafarnib has shown highly synergistic activity in combination with taxanes in preclinical models and early stage clinical trials23-34.
Members of our team reported the initial phase I trial of lonafarnib in combination with paclitaxel and determined the recommended phase II doses to be lonafarnib 100mg twice a day and paclitaxel 175mg/m2 every 21 days35. The subsequent phase II trial of lonafarnib and paclitaxel in taxane-refractory non-small cell lung carcinoma (NSCLC) reported promising anti-tumor activity: partial response (PR) rate of 10% (3 of 29 patients), and stable disease (SD) rate of 38% (11 of 29 patients)30. The ability to overcome taxane resistance would have significant clinical impact given the wide range of neoplastic diseases treated with taxane-based therapy. However, the phase II trial did not provide data that could be used to elucidate the biological basis for overcoming taxane resistance.
Our group also demonstrated lonafarnib not only synergizes with microtubule-stabilizing taxanes in vitro, but also is able to reverse taxane resistance in drug-resistant cancer models24, 25. Mechanistically, we have shown lonafarnib synergizes with taxanes through inhibition of tubulin deacetylase, histone deacetylase 6 (HDAC6), which leads to increased tubulin acetylation, microtubule stability and enhanced taxane binding to microtubules24, 25. In addition, we have shown FTase physically associates with HDAC6 and microtubules (docetaxel's target) and FTase knockdown sensitizes cells to lonafarnib/taxane drug combination36. Taken together, these preclinical findings suggested tubulin acetylation and basal FTase expression levels may be important determinants of drug sensitivity that would help us identify patients more likely to benefit from the combination of lonafarnib and docetaxel.
The primary goal of this study was to evaluate the biological effects of combination therapy with lonafarnib and docetaxel in refractory solid tumors by comparing pre-treatment and post-treatment tumor biopsies. Tumor specimens were evaluated for effective drug-target engagement, biologic interactions, surrogate markers of biologic activity, and potential predictive markers of benefit. Secondary goals were to determine safety, tolerability, toxicity profile, and preliminary evidence of anti-tumor activity of the combination of lonafarnib and docetaxel.
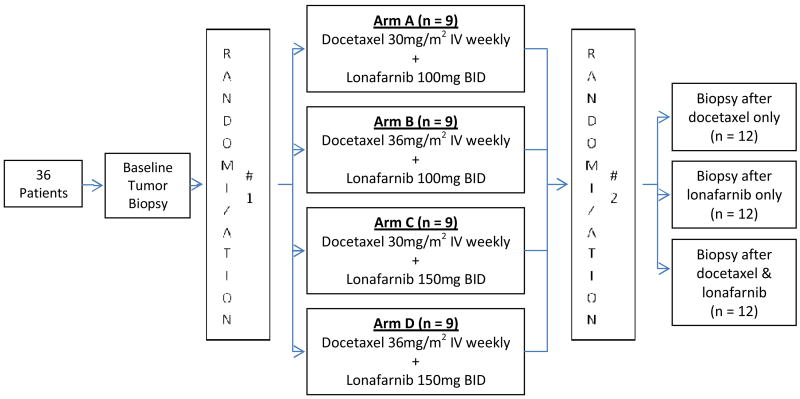
Patients were randomized into four cohorts of nine patients with different doses of lonafarnib and docetaxel. Within each cohort, patients were randomly subdivided into three separate subgroups receiving their post-treatment biopsy following docetaxel alone, lonafarnib alone, and docetaxel/lonafarnib combined. This randomization scheme would potentially allow us to identify what impact, if any, different doses of the FTI/taxane combination have on microtubule stabilization and farnesyl transferase inhibition.
Materials and Methods
Patient selection
Eligibility criteria included: histologically confirmed locally advanced or metastatic solid malignancies refractory to standard therapies; age ≥ 18 years; tumor accessible for repeat biopsy; ECOG performance status of ≤ 2; life expectancy ≥ 12 weeks; discontinue use of potent CYP3A4 inducers/inhibitors; adequate laboratory values including leukocyte count ≥ 3,000 cells/mm3, absolute neutrophil count ≥ 1,500 cells/mm3, platelet count ≥ 100,000/mm3, hemoglobin ≥ 9.0 g/dL, total bilirubin level ≤ upper limit of normal (ULN), albumin ≥ 2.5 g/dL, aspartate aminotransferase or alanine aminotransferase ≤ 2 × ULN, prothrombin time and partial thromboplastin time ≤ 1.5 × ULN. Women of child-bearing potential were required to have a negative pregnancy test. Exclusion criteria included: > grade 2 neuropathy; inability to swallow pills. All study subjects signed an informed consent form approved by the Emory University Institutional Review Board.
Study design
Patients were enrolled from April 2006 to April 2008. The primary objective was to determine the molecular interaction between docetaxel and lonafarnib in tumor samples. Secondary objectives included: (1) determine the safety and toxicity of docetaxel in combination with lonafarnib, (2) determine pharmacokinetic interactions, (3) determine molecular interactions between docetaxel and lonafarnib in peripheral blood mononuclear cells (PBMC).
Study subjects were randomly assigned (Figure 1) to one of four dosing cohorts: (1) 30mg/m2, 100mg, (2) 36mg/m2, 100mg, (3) 30mg/m2, 150mg, or (4) 36mg/m2, 150mg of docetaxel IV weekly, lonafarnib PO BID, respectively. Lonafarnib capsules were administered twice daily and docetaxel was administered weekly for three weeks every 28 days. Premedication for docetaxel included a 5-HT3 antagonist and dexamethasone.
Figure 1. Study Randomization Schema.
All patients underwent pre-treatment tumor biopsies one week prior to drug administration. Using a second randomization, patients were randomly assigned to one of three schedules for a second tumor biopsy: treatment with docetaxel alone on day 1, repeat biopsy on day 2, and then initiation of lonafarnib treatment (group 1); treatment with lonafarnib alone on day 1, repeat biopsy prior to dosing on day 5, and then docetaxel initiation (group 2); treatment with lonafarnib on day 1, docetaxel on day 4 and repeat biopsy on day 5, prior to lonafarnib (group 3).
Dose modification was permitted. A single 25% dose reduction of docetaxel due to toxicity was permitted and all subsequent treatments were administered at the reduced dose. Grade 3 or 4 neutropenia (without fever) with recovery prior to the next planned cycle did not require dose modification. Doses of docetaxel were also held for abnormal liver function: alkaline phosphatase > 2X ULN, bilirubin > ULN.
A single dose reduction of lonafarnib due to toxicity was permitted. All subsequent treatments were at the reduced dose (150 mg BID was reduced to 100 mg BID; 100 mg BID was reduced to 100 mg in the morning and 50 mg in the evening). Doses of lonafarnib were held for grade 3 or 4 thrombocytopenia until platelet counts returned to ≥ 100,000/mm3. Grade 3 or 4 nausea, vomiting, or diarrhea required discontinuation of lonafarnib until toxicities returned to grade 1 or better and subsequent doses were reduced by one level.
Study subjects were removed from the study for the following reasons: (1) patient request, (2) progressive disease, (3) unacceptable toxicity, (4) investigator judgment.
Baseline tumor evaluation with cross sectional imaging was performed within 4 weeks of therapy initiation and subsequent imaging was performed every 8 weeks. Tumor response was determined by utilizing the Response Evaluation Criteria In Solid Tumors (RECIST) criteria.
Pharmacokinetics
Sampling
Blood samples (6mL) were collected in sodium heparinized at times 0 (pre), 1, 1.25, 1.75, 4, 7.5, and 24 hours after docetaxel infusion. Lonafarnib maximum steady-state concentrations [Css(max)] were collected for each patient based on twice daily administration for 3 or 4 days prior to docetaxel administration. Samples were immediately centrifuged at 3000 rpm for 15 min at 4°C, plasma removed and separated into 3 aliquots and stored at -70°C.
Analytic Methods
Lonafarnib concentrations were analyzed at Taylor Technology, Inc. (Princeton, NJ) using liquid chromatographic method with tandem mass spectrometric detection (LC-MS/MS) with a lower limit of quantification (LLQ) of 5 ng/mL and a linear concentration range of 5–2,500 ng/mL. Docetaxel concentrations were assayed at Pharmaceutical Product Development (PPD, Richmond, VA) by LC-MS/MS with a LLQ of 10 ng/ml (10-5,000 ng/mL).
Data Analysis
Individual pharmacokinetic parameters for docetaxel were calculated from plasma concentration-time curves using WinNonlin® v 5.2 (Pharsight®, Mountain View, CA) using noncompartmental methods. Parameters reported included area under the concentration-time curve extrapolated to infinity (AUCinf), terminal half-life (t1/2), clearance (CL), and volume of distribution (Vd). Lonafarnib Css(max) values are reported due to inconsistent acquisition of other timepoints in the sampling scheme.
Tissue procurement
Each tumor biopsy specimen was divided into three portions and processed as follows: (1) flash frozen, (2)embedded in paraffin, and (3)the remainder saved for microtubule analysis. Samples for microtubule analysis were immediately fixed with complete PHEMO buffer37.
Tissue microtubule integrity and stability assessment by immunofluorescence staining followed by confocal microscopy
For immunofluorescence processing, tumor biopsies were processed, imaged and analyzed37. Images were acquired using a Zeiss 5LIVE confocal microscope with a 63X/1.3 NA objective.
Immunofluorescence of acetylated tubulin in PBMCs
PBMCs were centrifuged at 500 RPM for 5 mins onto poly-lysine–treated cover slips then fixed in PHEMO buffer (68 mM PIPES, 25 mM HEPES, pH 6.9, 15 mM EGTA, 3 mM MgCl2, 10% [vol/vol] DMSO)23-25, 38, 39. Confocal z-sections were acquired using a Zeiss LSM510 META microscope23, 38, 39 and analyzed using Metamorph 6.0.
Immunohistochemistry for acetylated tubulin
Immunohistochemistry for acetylated tubulin was performed at the Molecular Cytology Core Facility of Memorial Sloan Kettering Cancer Center using a Discovery XT processor (Ventana Medical Systems) and a monoclonal anti-acetylated tubulin antibody (Sigma clone 6-11B-1), followed by biotinylated mouse secondary antibody (Vector Labs), streptavidin-HRP D (Ventana Medical Systems) and DAB Detection Kit (Ventana Medical Systems). Stained tissue sections were mounted on glass slides and scanned using the ZEISS MIRAX SCAN. TIFF images of tissue sections were obtained using MIRAX Viewer Software. The tumor area in each slide was delineated by a pathologist and levels of tubulin acetylation were assessed in the defined tumor area using Metamorph. Acetylated tubulin was thresholded and the intensity of the thresholded image was expressed as percent of the total intensity in the tumor area.
FTase alpha and beta mRNA expression
Total RNA was isolated from fresh frozen tumor biopsies using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was prepared from 1 μg total RNA using random primers and High-Capacity cDNA Reverse Transcription Kit from Applied Biosystems. Real-Time PCR reactions were prepared using SYBR Green Supermix (BioRad). Primers for FTase alpha were 5′-TGATCGTGCTGTATTGGAGAG-3′ and 5′-CTGTGCTGTGTTTGCTTTGAA-3′; primers for FTase beta were 5′-TACTATTGCCCTCCATCTTCCTCC-3′ and 5′-GACCTCTTGGATCTTTTCTTCTAC-3′; primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were 5′-GGAGTCAACGGATTTGGTCG-3′ and 5′-CTTGATTTTGGAGGGATCTCG-3′. Quantitation was performed using Applied Biosystems (ABI) Prism 7700 Real-Time qPCR system. under the following cycling conditions: (step 1) 50°C (2 min); (step 2) 95°C (10 min); (step 3) 40 cycles of 95°C (15s) and 60°C (1min). The fluorescence threshold value was determined using SDS and RQ Manager software. The relative expression level of FTase alpha or FTase beta was normalized using GAPDH as an internal standard. The Ct value for each FTalpha and FTbeta expression was determined and normalized to the Ct value obtained with GAPDH from the same samples. The mean of all normalized FTase alpha or beta expression values from all samples was set to 1.
Statistical Analysis
FTase expression was dichotomized into low or high expression for each patient based on the median of sample delta Ct value. Kaplan-Meier product limit estimators were generated and plotted for progression free survival (PFS) stratified by FTase expression levels (low VS high). Log rank test was performed. PFS is defined as the time interval from the start of treatment to disease progression.
For each patient, the change in acetylated tubulin staining was categorized as having either increased or decreased based on the change of acetylated tubulin levels before and after treatment. Kaplan-Meier product limit estimators stratified by change in acetylated tubulin were generated for PFS. The log-rank test was performed.
Results
Of 38 patients enrolled, 36 were treated and 29 were evaluable for toxicity and response assessment (Table 1). All patients underwent tumor biopsies, however, due to technical reasons (e.g. insufficient tumor sample in biopsy specimen), several patients did not have adequate paired biopsies for molecular analysis
Table 1. Patient Characteristics.
| Patient Characteristics | |||
|---|---|---|---|
| Sex | No. | Malignancy | No. |
| Male | 19 | Head & Neck | 8 |
| Female | 17 | Lung | 6 |
| Ethnicity | Colorectal | 4 | |
| Neuroendocrine | 4 | ||
| Caucasian | 27 | Sarcoma | 2 |
| African American | 7 | Breast | 2 |
| Other | 2 | Gynecological | 2 |
| Age | Esophageal | 1 | |
| Small Bowel Adenocarcinoma | 1 | ||
| Range (yr) | 40 - 80 | Anus Squamous Cell Carcinoma | 1 |
| Median (yr) | 58 | Unknown Primary | 1 |
| Melanoma | 1 | ||
| Mesothelioma | 1 | ||
| Papillary Thyroid | 1 | ||
| Prostate | 1 | ||
Toxicity
With the exception of diarrhea, which was manageable with aggressive anti-diarrheal medications, the combination of lonafarnib and docetaxel was tolerated in all four cohorts (Table 2). The most common drug-related clinical adverse events were: diarrhea (all grades – 69%, grade 3/4 – 28%), nausea (all grades – 61%, grade 3/4 – 14%), vomiting (all grade – 56%, grade 3/4 – 8%), fatigue (all grades – 47%, grade 3/4 – 22%), neuropathy (all grades – 38%, grade 3/4 – 3%), weight loss (all grades – 37%, grade 3/4 – 0%), rash (all grades – 17%, grade 3/4 – 3%).
Table 2. Grade 3/4/5 Toxicities.
| All Arms | |||
|---|---|---|---|
| Toxicity | Incidence (No.) per CTC Grade | ||
| 3 No. | 4 No. | 5 No. | |
| Clinical | |||
| Alopecia | 1 | 0 | 0 |
| Anorexia | 1 | 0 | 0 |
| Bowel Perforation | 0 | 1 | 0 |
| Dehydration | 2 | 1 | 0 |
| Diarrhea | 8 | 2 | 0 |
| Dizziness | 1 | 0 | 0 |
| Fatigue | 8 | 0 | 0 |
| Infection | 1 | 3 | 3 |
| Mucositis | 1 | 0 | 0 |
| Nausea/Vomiting | 5 | 0 | 0 |
| Neuropathy | 0 | 1 | 0 |
| Pain | 4 | 0 | 0 |
| Rash | 1 | 0 | 0 |
| Serum Chemistries | |||
| Low Sodium | 4 | 0 | 0 |
| Low Potassium | 1 | 1 | 0 |
| Elevated Glucose | 6 | 2 | 0 |
| ALT | 0 | 1 | 0 |
| AST | 0 | 1 | 0 |
| Low Albumin | 3 | 0 | 0 |
| Elevated Amylase | 2 | 0 | 0 |
| Low Calcium | 2 | 2 | 0 |
| Hematology | |||
| Low WBC | 4 | 3 | 0 |
| Low Hgb | 3 | 2 | 0 |
| Low Hct | 3 | 1 | 0 |
| Low Plts | 2 | 1 | 0 |
The most common drug-related laboratory abnormalities were: hyperglycemia (all grades – 92%, grade 3/4 – 23%), hyponatremia (all grades – 53%, grade 3/4 – 11%), hypoalbulinemia (all grades – 53%, grade 3/4 – 16%), leukopenia (all grades – 53%, grade 3/4 – 19%), anemia (all grades – 50%, grade 3/4 – 14%), hypocalcemia (all grades – 47%, grade 3/4 – 12%), hypokalemia (all grades – 39%, grade 3/4 – 6%), creatinine elevation (all grades – 34%, grade 3/4 – 3%), elevated amylase (all grades – 30%, grade 3/4 – 6%), hypophosphatemia (all grades – 25%, grade 3/4 – 0%), thrombocytopenia (all grades – 25%, grade 3/4 – 9%). Hyperglycemia was a common finding and most likely due to protocol mandated dexamethasone premedication prior to docetaxel infusion. All episodes of hyperglycemia were treated with either oral agents (i.e. glyburide, metformin) or subcutaneous insulin. Drug induced leukopenia resolved within 1 week of delaying treatment.
Three deaths occurred while on study. One patient died due to aspiration pneumonia in the setting of chemotherapy-induced neutropenia. A second patient died due to complications of urosepsis in the setting of chemotherapy-induced neutropenia. The third patient death was due to rapidly progressive community-acquired pneumococcal sepsis, which was not felt to be related to study treatment as neutrophil counts were in the normal range.
Efficacy
Clinical benefit was defined as SD, PR, or complete response (CR). Seven patients clinically benefitted from treatment with docetaxel and lonafarnib (Table 3). One patient with parotid carcinoma had a CR and 6 patients had SD lasting from 6 to 10 months. Of note, several of the patients who benefitted from protocol therapy had documented disease progression when previously treated with taxanes; 1 patient with prior docetaxel treatment, 5 patients with prior paclitaxel treatment.
Table 3. Efficacy.
| Subject No. | Disease | Best Response | Duration of Benefit (mo) | Prior Taxane (dose) | Best Response to Prior Taxane |
|---|---|---|---|---|---|
| 5 | Adenoid Cystic Carcinoma of Parotid | SD | 10 | Paclitaxel (175mg/m2q21days) | SD |
| 12 | MFH | SD | 8 | Docetaxel (75mg/m2q21 days) | PD |
| 20 | SCC Lung | SD | 4 | Paclitaxel (175mg/m2q21days) | SD |
| 22 | Adenoid Cystic Carcinoma of Nasopharynx | SD | 8 | Paclitaxel (175mg/m2q21days) | PD |
| 27 | Carcinoid | SD | 10 | None | NA |
| 28 | Parotid Carcinoma | CR | 10 | Paclitaxel (60mg/m2 qweek) | SD |
| 30 | Bronchoalveolar | SD | 6 | Paclitaxel (175mg/m2q21days) | PD |
Pharmacokinetic data
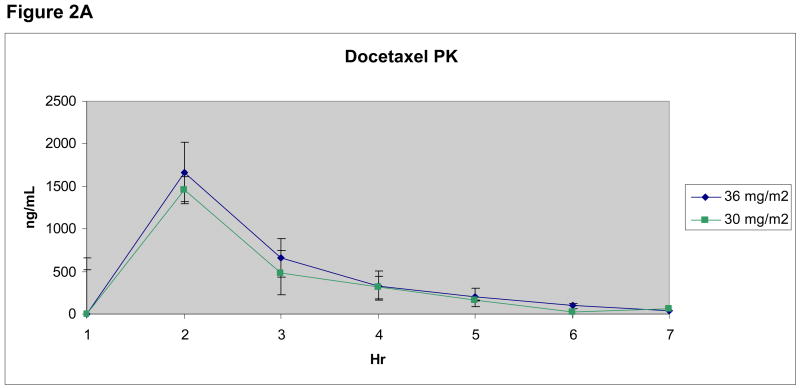
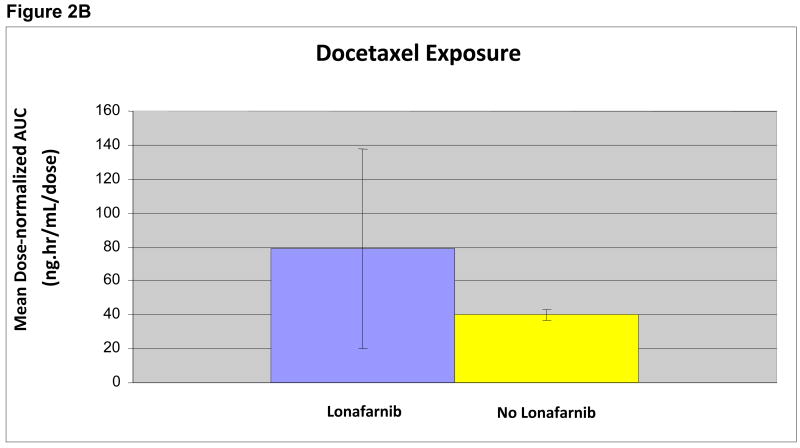
For docetaxel, the AUC0-inf and t1/2 values were only reported for patients in whom concentrations were above the LLQ at 24 hours, allowing for a full set of values for parameter calculations (n=13) (Figure 2A). Comparing docetaxel exposure in the presence and absence of lonafarnib at steady-state concentrations, a trend toward increased docetaxel exposure as measured by Cmax and AUC0-inf values in patients receiving the combination was seen, however, this did not correspond with greater clinical efficacy or toxicity. Median docetaxel dose-normalized AUC0-inf was compared between groups receiving and not receiving lonafarnib (Figure 2B), and showed no difference in mean AUC0-inf values, despite a numeric trend (p=0.46). Lonafarnib Cssmax values were variable, but mean values increased in a dose-dependent fashion.
Figure 2. Figure 2A. Median Docetaxel Concentrations.
Docetaxel plasma concentration-time curves for patients receiving 30 mg/m2 (n=3) and 36 mg/m2 (n=10) are shown.
Figure 2B. Effect of Lonafarnib on Docetaxel AUC
Docetaxel exposure as measured by mean AUC with lonafarnib (n=10) and without (n=3) was compared following dose-normalization between the 30 and 36 mg/m2 groups.
P = 0.46 (2 sided Wilcoxon rank sum)
Biomarker data
Tubulin acetylation analysis in pre- and post-treatment biopsies
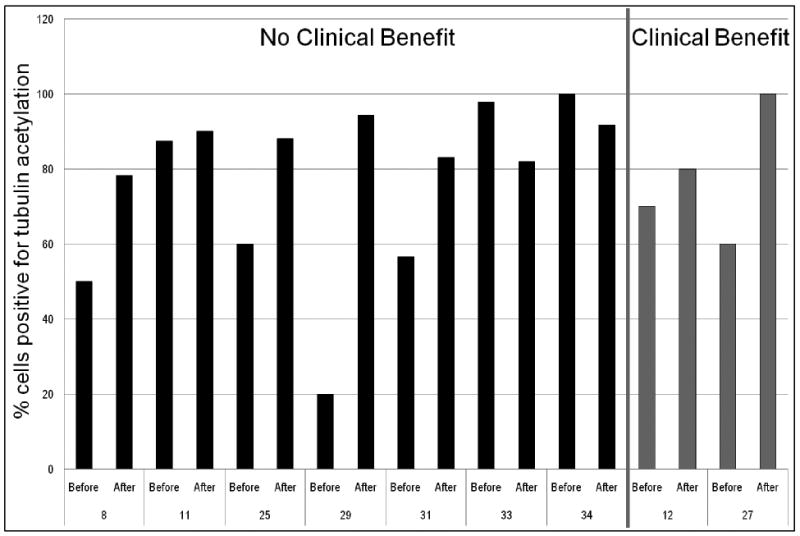
Tubulin acetylation content was determined as the percentage of cells staining positive for tubulin acetylation. Due to the need to validate multiple biomarkers and limited tissue supply, we were only able to identify evaluable tumor areas with matched pre- and post-treatment biopsies in 9 patients. In 2 patients who clinically benefitted, one showed a robust increase in tubulin acetylation (60% at baseline to 100% post-treatment) while the second did not show a significant change (70% to 80%) (Figures 3 and 4). Among the 7 patients who did not clinically benefit, 3 patients showed no increase in the levels of tubulin acetylation, while 4 patients had a 20-70% increase. We also investigated whether basal acetyl-tubulin content could serve as a predictive biomarker of benefit. However, all evaluable pre-treatment samples from patients who benefitted had levels of tubulin acetylation of greater than 40%, as did those patients who did not benefit.
Figure 3. Percent Tubulin Acetylation in Tumor Biopsies Before and After Treatment.
Immunohistochemical analysis of acetylated tubulin in formalin-fixed paraffin-embedded.
Figure 4. Immunohistochemistry Analysis of Acetylated Tubulin in Pre- and Post-Treatment Biopsies from Responder and Non-Responder Patients.
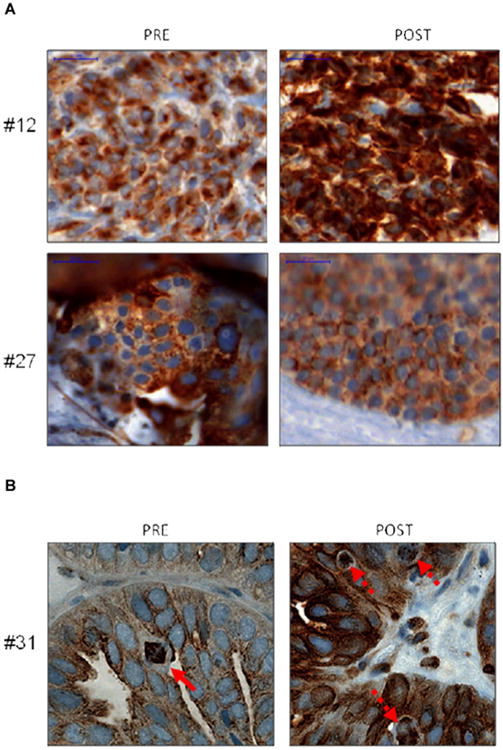
Immunohistochemistry was performed using an anti-acetylated tubulin antibody (brown staining) in tumor biopsy samples from responder patients (#12 and #27, panel A) and a non-responder patient (#31, panel B) taken before and after treatment. Hematoxylin was used as a nuclear stain (blue). Scale bar = 20 μM. In panel B, the solid arrow indicates normal metaphase in the pre-treatment specimen, and the dashed arrows indicate abnormal mitotic figures in the post-treatment sample.
FTase expression in pre-treatment biopsies
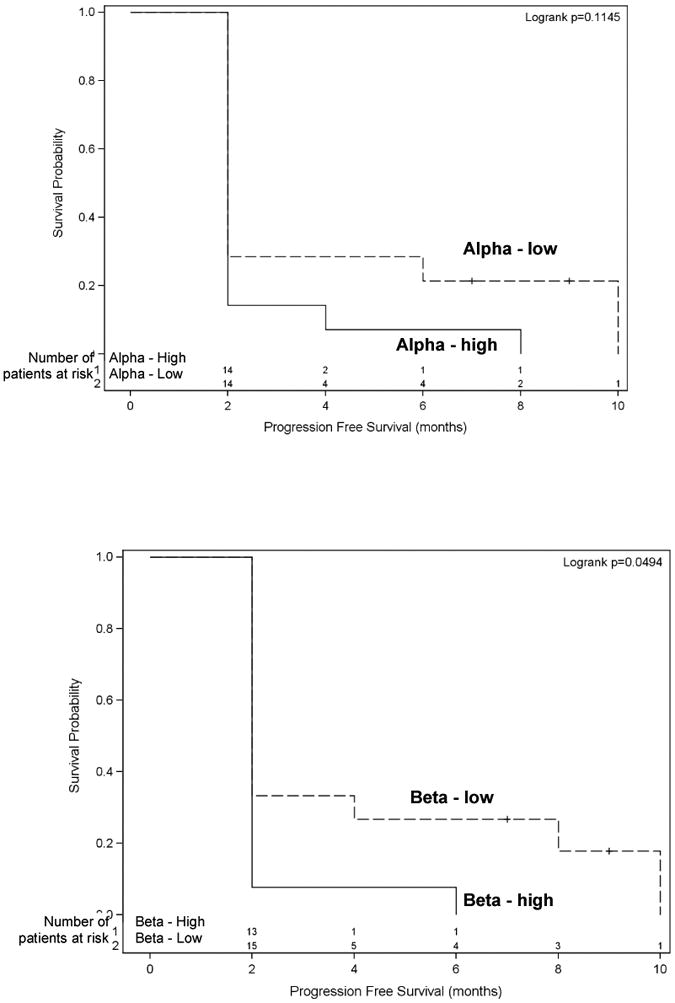
Quantitative RT-PCR for FTase-alpha and FTase-beta mRNA was performed in 28 baseline tumor samples. Results were normalized to GAPDH expression and categorized into low or high expression for each patient based on the median of sample delta CT values. Kaplan-Meier analysis indicated that patients with low FTase-alpha expression had a trend toward improved survival, albeit not significant (p = 0.1145) (Figure 5A). Log-rank test showed the difference in PFS between patients with low versus high FTase-beta expression was statistically significantly different (p < 0.05) (Figure 5B).
Figure 5. PFS stratified by basal FTase alpha expression (A: top figure) and PFS stratified by basal FTase beta expression (B: bottom figure).
Tubulin acetylation analysis in PBMCs
Pre- and post-treatment levels of acetylated tubulin in PBMCs did not correlate with PFS (p = 0.4986).
Discussion
This NCI P01-funded, translational biomarker-driven clinical trial investigated the interaction between docetaxel and lonafarnib, evaluating molecular predictors of outcome, as well as pharmacokinetic and pharmacodynamic interactions. Our previous clinical and mechanistic studies have suggested lonafarnib could reverse taxane resistance24, 30. However, the biological basis of this finding was not well understood. Mechanistically, we have shown that the synergy between taxanes and FTIs could be explained by FTIs ability to inhibit the tubulin deacetylase function of HDAC6, leading to microtubule stabilization, enhanced tubulin acetylation and taxane binding24, 25, 28, 36.
Of the 36 patients treated, 7 patients derived benefit from protocol treatment. Remarkably, six of these patients had previously failed taxane-based therapy, and thus the lonafarnib/docetaxel combination was able to overcome this resistance. The pharmacokinetic analysis of both agents was consistent with previous reports40-42. Although dose-normalized docetaxel exposure in patients receiving lonafarnib was numerically higher, this difference was not significant (p=0.46). In the 7 patients who benefitted, no difference in exposure was seen compared to those who did not benefit, suggesting factors other than plasma drug concentrations contributed to the likelihood of benefit.
Tubulin acetylation was assessed as a biomarker indicative of effective drug-target engagement and predictive of a patient's response to this combination therapy. Inconsistencies were noted between the increases observed in tubulin acetylation in post-treatment biopsies and the actual clinical response of individual patients, which were attributable to the small number of biopsies with analyzable tumors areas, and the high baseline level of tubulin acetylation. Since most subjects had failed prior taxane chemotherapy, it is possible that tubulin acetylation analysis was biased by prior treatment. When examining tumor biopsy specimens as well as PBMCs for changes in levels of acetylated tubulin, immunofluorescence did not reveal a statistically significant correlation with PFS. Interestingly, microtubule disruption, evidenced by microtubule bundling or aberrant mitosis, did not always lead to cell death nor was it associated with clinical response (data not shown). This suggests additional pathways downstream of microtubule targeting are dysregulated, potentially through the overexpression of anti-apoptotic proteins like Bcl2 or deficient spindle assembly checkpoints43.
Recent studies from our group have revealed cells with a stable knockdown of FTase were sensitized to taxane and lonafarnib alone or in combination36. Similar results were obtained with FTase-beta knockdown, leading us to hypothesize that patients with lower FTase expression at baseline could potentially show a better response to this combination. Indeed, patients that benefitted from treatment had statistically significantly lower basal mRNA expression levels of FTase-beta compared to the mean mRNA expression levels for the study population. Lower basal mRNA expression levels of FTase-alpha were also seen in the seven patients who clinically benefited; however this association did not reach statistical significance. These clinical observations are in agreement with our previous studies in which knockdown of FTase-alpha resulted in concomitant downregulation of FTase-beta subunit and vice versa, since the two subunits are co-translationally regulated. However, in the clinical samples we did not observe such a coordinated expression for the two subunits, suggesting that in patients, additional pathways may affect the expression of each subunit individually. Our data from the current trial also show FTase-beta may be a more accurate biomarker than FTase-alpha for predicting clinical benefit to lonafarnib/docetaxel, possibly due to the fact that FTase and geranylgeranyl transferase exist as alpha and beta heterodimers and share a common alpha subunit but a homologous beta subunit (25% sequence identity). Therefore, FTase-beta would be expected to be a more specific biomarker for FTase activity than FTase-alpha44, 45. Moreover, mutations in FTase that confer resistance to lonafarnib have been described at residue betaY361 both in vitro and in patients46; the presence of such a mutation could explain the lack of clinical benefit from the lonafarnib/docetaxel combination.
During the conception of this trial, we set the ambitious goal of prospectively collect tumor samples from all participants before and after treatment. Although we had a well thought out plan, unforeseen technical issues precluded us from obtaining adequate amounts of viable tumor tissue for all our planned correlative studies. Many of our samples contained necrotic debris or little malignant tissue limiting our ability to complete all planned testing. Given the small number of paired biopsies, we were unable to investigate the impact of administering lonafarnib versus docetaxel, versus the combination had on molecular end points. Nor were we able to definitively correlate pre and post treatment acetylation levels with clinical benefit. In future studies investigating molecular end point we would suggest having a pathologist at bedside during biopsy to confirm the presence of adequate tumor cells so as to improve tumor collection rates and quality of specimens collected.
Overall, the regimen of docetaxel and lonafarnib appears to be tolerable. The most common toxicities of diarrhea, nausea, and vomiting were mostly grade 1/2 and manageable with oral regimens. We do acknowledge a moderate (28%) incidence of grade 3/4 diarrhea, but with aggressive anti-diarrheal medications, we found the trial regimen tolerable. Hyperglycemia was most likely related to dexamethasone premedication but was manageable with oral hypoglycemics or insulin. Of the three deaths that occurred while on study, two were study-related (sepsis in the setting of chemotherapy-induced neutropenia), the third death was felt to be unrelated to study treatment (rapidly progressive community-acquired pneumococcal sepsis). There is no evidence that lonafarnib enhanced docetaxel-induced neutropenia; it bears mentioning that the study population was extensively pre-treated. During the course of the trial, enrollment was suspended after each patient death and the clinical data were reviewed by the Emory University, Winship Cancer Institute Data Safety Monitoring Board (WCI-DSMB). Accrual was re-opened only after the WCI-DSMB found the risks to participants were acceptable.
This study highlights the feasibility and potential utility of incorporating serial tumor biopsies into clinical trials. As in previous trials investigating the combination of lonafarnib and taxanes, we observed several patients with clinical benefit despite pretreatment with taxanes in prior regimens. However, unlike previous studies, we were able to identify mRNA expression levels of FTase-beta and FTase-alpha as potential predictive biomarkers. This study also stresses the importance of National Cancer Institute funding through the P01 mechanism to support correlative translational research to go from bedside to bench (early clinical trials of taxanes and lonafarnib), and then back to bedside (mechanistic studies on lonafarnib and taxane synergy) to confirm preclinical observation of mechanisms of FTI-enhanced effects of taxanes3, 24, 25, 30, 35, 36, 47, 48. Even though the agent lonafarnib is unlikely to be developed further by the pharmaceutical industry, the data gathered during the conduct of this study may prove to be a valuable step forward in the personalization of cancer care.
Acknowledgments
Dr. Harvey received the PPD Bioanalytical Fluid and Tissue Sample Grant Award from the American College of Clinical Pharmacy (ACCP) Research Institute and PPD, for analysis of docetaxel concentrations. We thank Dr. Anthea Hammond for assistance in editing, Dr. Wade M. Smith for protocol writing, Dr. Mourad for assistance in data analysis, and Dr. Selwyn Hurwitz for critical reading of the manuscript. This study was supported by NIH P01 CA116676 to FRK, funds from the Georgia Cancer Coalition to GCC scholars FRK, SSR, DMS and AM, and a grant from Sanofi-Aventis to MPF and FRK.
References
- 1.Gibbs JB, Graham SL, Hartman GD, et al. Farnesyltransferase inhibitors versus Ras inhibitors. Curr Opin Chem Biol. 1997;1:197–203. doi: 10.1016/s1367-5931(97)80010-5. [DOI] [PubMed] [Google Scholar]
- 2.Haluska P, Dy GK, Adjei AA. Farnesyl transferase inhibitors as anticancer agents. Eur J Cancer. 2002;38:1685–1700. doi: 10.1016/s0959-8049(02)00166-1. [DOI] [PubMed] [Google Scholar]
- 3.Basso AD, Mirza A, Liu G, Long BJ, Bishop WR, Kirschmeier P. The farnesyl transferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR signaling. Role in FTI enhancement of taxane and tamoxifen anti-tumor activity. J Biol Chem. 2005;280:31101–31108. doi: 10.1074/jbc.M503763200. [DOI] [PubMed] [Google Scholar]
- 4.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 5.Slebos RJ, Kibbelaar RE, Dalesio O, et al. K-ras oncogene activation as a prognostic marker in adenocarcinoma of the lung. N Engl J Med. 1990;323:561–565. doi: 10.1056/NEJM199008303230902. [DOI] [PubMed] [Google Scholar]
- 6.Malats N, Porta M, Corominas JM, Pinol JL, Rifa J, Real FX. Ki-ras mutations in exocrine pancreatic cancer: association with clinico-pathological characteristics and with tobacco and alcohol consumption. PANK-ras I Project Investigators. Int J Cancer. 1997;70:661–667. doi: 10.1002/(sici)1097-0215(19970317)70:6<661::aid-ijc6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Jackson JH, Cochrane CG, Bourne JR, Solski PA, Buss JE, Der CJ. Farnesol modification of Kirsten-ras exon 4B protein is essential for transformation. Proc Natl Acad Sci U S A. 1990;87:3042–3046. doi: 10.1073/pnas.87.8.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato K, Cox AD, Hisaka MM, Graham SM, Buss JE, Der CJ. Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc Natl Acad Sci U S A. 1992;89:6403–6407. doi: 10.1073/pnas.89.14.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willumsen BM, Christensen A, Hubbert NL, Papageorge AG, Lowy DR. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984;310:583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]
- 10.Li T, Sparano JA. Inhibiting Ras signaling in the therapy of breast cancer. Clin Breast Cancer. 2003;3:405–416. doi: 10.3816/CBC.2003.n.005. discussion 417-420. [DOI] [PubMed] [Google Scholar]
- 11.Rowinsky EK, Windle JJ, Von Hoff DD. Ras protein farnesyltransferase: A strategic target for anticancer therapeutic development. J Clin Oncol. 1999;17:3631–3652. doi: 10.1200/JCO.1999.17.11.3631. [DOI] [PubMed] [Google Scholar]
- 12.Cox AD, Der CJ. Farnesyltransferase inhibitors and cancer treatment: targeting simply Ras? Biochim Biophys Acta. 1997;1333:F51–71. doi: 10.1016/s0304-419x(97)00011-5. [DOI] [PubMed] [Google Scholar]
- 13.Du W, Liu A, Prendergast GC. Activation of the PI3′K-AKT pathway masks the proapoptotic effects of farnesyltransferase inhibitors. Cancer Res. 1999;59:4208–4212. [PubMed] [Google Scholar]
- 14.Ashar HR, James L, Gray K, et al. The farnesyl transferase inhibitor SCH 66336 induces a G(2) --> M or G(1) pause in sensitive human tumor cell lines. Exp Cell Res. 2001;262:17–27. doi: 10.1006/excr.2000.5076. [DOI] [PubMed] [Google Scholar]
- 15.Crespo NC, Ohkanda J, Yen TJ, Hamilton AD, Sebti SM. The farnesyltransferase inhibitor, FTI-2153, blocks bipolar spindle formation and chromosome alignment and causes prometaphase accumulation during mitosis of human lung cancer cells. J Biol Chem. 2001;276:16161–16167. doi: 10.1074/jbc.M006213200. [DOI] [PubMed] [Google Scholar]
- 16.Ashar HR, James L, Gray K, et al. Farnesyl transferase inhibitors block the farnesylation of CENP-E and CENP-F and alter the association of CENP-E with the microtubules. J Biol Chem. 2000;275:30451–30457. doi: 10.1074/jbc.M003469200. [DOI] [PubMed] [Google Scholar]
- 17.Morgillo F, Lee HY. Lonafarnib in cancer therapy. Expert Opin Investig Drugs. 2006;15:709–719. doi: 10.1517/13543784.15.6.709. [DOI] [PubMed] [Google Scholar]
- 18.End DW, Smets G, Todd AV, et al. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 2001;61:131–137. [PubMed] [Google Scholar]
- 19.Nagasu T, Yoshimatsu K, Rowell C, Lewis MD, Garcia AM. Inhibition of human tumor xenograft growth by treatment with the farnesyl transferase inhibitor B956. Cancer Res. 1995;55:5310–5314. [PubMed] [Google Scholar]
- 20.Sepp-Lorenzino L, Ma Z, Rands E, et al. A peptidomimetic inhibitor of farnesyl:protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Res. 1995;55:5302–5309. [PubMed] [Google Scholar]
- 21.Petit T, Izbicka E, Lawrence RA, Bishop WR, Weitman S, Von Hoff DD. Activity of SCH 66336, a tricyclic farnesyltransferase inhibitor, against human tumor colony-forming units. Ann Oncol. 1999;10:449–453. doi: 10.1023/a:1008313232381. [DOI] [PubMed] [Google Scholar]
- 22.Liu M, Bryant MS, Chen J, et al. Antitumor activity of SCH 66336, an orally bioavailable tricyclic inhibitor of farnesyl protein transferase, in human tumor xenograft models and wap-ras transgenic mice. Cancer Res. 1998;58:4947–4956. [PubMed] [Google Scholar]
- 23.Schafer-Hales K, Iaconelli J, Snyder JP, et al. Farnesyl transferase inhibitors impair chromosomal maintenance in cell lines and human tumors by compromising CENP-E and CENP-F function. Mol Cancer Ther. 2007;6:1317–1328. doi: 10.1158/1535-7163.MCT-06-0703. [DOI] [PubMed] [Google Scholar]
- 24.Marcus AI, O'Brate AM, Buey RM, et al. Farnesyltransferase inhibitors reverse taxane resistance. Cancer Res. 2006;66:8838–8846. doi: 10.1158/0008-5472.CAN-06-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcus AI, Zhou J, O'Brate A, et al. The synergistic combination of the farnesyl transferase inhibitor lonafarnib and paclitaxel enhances tubulin acetylation and requires a functional tubulin deacetylase. Cancer Res. 2005;65:3883–3893. doi: 10.1158/0008-5472.CAN-04-3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moasser MM, Sepp-Lorenzino L, Kohl NE, et al. Farnesyl transferase inhibitors cause enhanced mitotic sensitivity to taxol and epothilones. Proc Natl Acad Sci U S A. 1998;95:1369–1374. doi: 10.1073/pnas.95.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen LL, Shi B, Hajian G, et al. Combination therapy with the farnesyl protein transferase inhibitor SCH66336 and SCH58500 (p53 adenovirus) in preclinical cancer models. Cancer Res. 1999;59:5896–5901. [PubMed] [Google Scholar]
- 28.Shi B, Yaremko B, Hajian G, et al. The farnesyl protein transferase inhibitor SCH66336 synergizes with taxanes in vitro and enhances their antitumor activity in vivo. Cancer Chemother Pharmacol. 2000;46:387–393. doi: 10.1007/s002800000170. [DOI] [PubMed] [Google Scholar]
- 29.Adjei AA. An overview of farnesyltransferase inhibitors and their role in lung cancer therapy. Lung Cancer. 2003;41 1:S55–62. doi: 10.1016/s0169-5002(03)00143-0. [DOI] [PubMed] [Google Scholar]
- 30.Kim ES, Kies MS, Fossella FV, et al. Phase II study of the farnesyltransferase inhibitor lonafarnib with paclitaxel in patients with taxane-refractory/resistant nonsmall cell lung carcinoma. Cancer. 2005;104:561–569. doi: 10.1002/cncr.21188. [DOI] [PubMed] [Google Scholar]
- 31.Sun J, Blaskovich MA, Knowles D, et al. Antitumor efficacy of a novel class of non-thiol-containing peptidomimetic inhibitors of farnesyltransferase and geranylgeranyltransferase I: combination therapy with the cytotoxic agents cisplatin, Taxol, and gemcitabine. Cancer Res. 1999;59:4919–4926. [PubMed] [Google Scholar]
- 32.Brunner TB, Hahn SM, Gupta AK, Muschel RJ, McKenna WG, Bernhard EJ. Farnesyltransferase inhibitors: an overview of the results of preclinical and clinical investigations. Cancer Res. 2003;63:5656–5668. [PubMed] [Google Scholar]
- 33.Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- 34.Checchi PM, Nettles JH, Zhou J, Snyder JP, Joshi HC. Microtubule-interacting drugs for cancer treatment. Trends Pharmacol Sci. 2003;24:361–365. doi: 10.1016/S0165-6147(03)00161-5. [DOI] [PubMed] [Google Scholar]
- 35.Khuri FR, Glisson BS, Kim ES, et al. Phase I study of the farnesyltransferase inhibitor lonafarnib with paclitaxel in solid tumors. Clin Cancer Res. 2004;10:2968–2976. doi: 10.1158/1078-0432.ccr-03-0412. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Vos CC, Gjyrezi A, et al. The protein farnesyltransferase regulates HDAC6 activity in a microtubule-dependent manner. J Biol Chem. 2009;284:9648–9655. doi: 10.1074/jbc.M808708200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mabjeesh NJ, Escuin D, LaVallee TM, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 38.Marcus AI, Peters U, Thomas SL, et al. Mitotic kinesin inhibitors induce mitotic arrest and cell death in Taxol-resistant and -sensitive cancer cells. J Biol Chem. 2005;280:11569–11577. doi: 10.1074/jbc.M413471200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S, Schafer-Hales K, Khuri FR, Zhou W, Vertino PM, Marcus AI. The tumor suppressor LKB1 regulates lung cancer cell polarity by mediating cdc42 recruitment and activity. Cancer Res. 2008;68:740–748. doi: 10.1158/0008-5472.CAN-07-2989. [DOI] [PubMed] [Google Scholar]
- 40.Baker SD, Zhao M, Lee CK, et al. Comparative pharmacokinetics of weekly and every-three-weeks docetaxel. Clin Cancer Res. 2004;10:1976–1983. doi: 10.1158/1078-0432.ccr-0842-03. [DOI] [PubMed] [Google Scholar]
- 41.Baker SD, Sparreboom A, Verweij J. Clinical pharmacokinetics of docetaxel : recent developments. Clin Pharmacokinet. 2006;45:235–252. doi: 10.2165/00003088-200645030-00002. [DOI] [PubMed] [Google Scholar]
- 42.Eskens FA, Awada A, Cutler DL, et al. Phase I and pharmacokinetic study of the oral farnesyl transferase inhibitor SCH 66336 given twice daily to patients with advanced solid tumors. J Clin Oncol. 2001;19:1167–1175. doi: 10.1200/JCO.2001.19.4.1167. [DOI] [PubMed] [Google Scholar]
- 43.Chanel-Vos C, Giannakakou P. CENP-E checks in microtubule-drug resistance. Cell Cycle. 2010;9 doi: 10.4161/cc.9.8.11382. [DOI] [PubMed] [Google Scholar]
- 44.Basso AD, Kirschmeier P, Bishop WR. Lipid posttranslational modifications. Farnesyl transferase inhibitors. J Lipid Res. 2006;47:15–31. doi: 10.1194/jlr.R500012-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Lane KT, Beese LS. Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res. 2006;47:681–699. doi: 10.1194/jlr.R600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Raz T, Nardi V, Azam M, Cortes J, Daley GQ. Farnesyl transferase inhibitor resistance probed by target mutagenesis. Blood. 2007;110:2102–2109. doi: 10.1182/blood-2006-12-064907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loprevite M, Favoni RE, De Cupis A, et al. In vitro study of farnesyltransferase inhibitor SCH 66336, in combination with chemotherapy and radiation, in non-small cell lung cancer cell lines. Oncol Rep. 2004;11:407–414. [PubMed] [Google Scholar]
- 48.Ready NE, Lipton A, Zhu Y, et al. Phase I study of the farnesyltransferase inhibitor lonafarnib with weekly paclitaxel in patients with solid tumors. Clin Cancer Res. 2007;13:576–583. doi: 10.1158/1078-0432.CCR-06-1262. [DOI] [PubMed] [Google Scholar]