Abstract
A new flow cytometry method that uses an optimized DNA and RNA staining strategy to monitor the growth and development of the Plasmodium falciparum strain W2mef has been used in a pilot study and has identified Bay 43-9006 1, SU 11274 2, and TMC 125 5 as compounds that exhibit potent (<1 μM) overall and ring stage in vitro antimalarial activity.
Keywords: Cytometry, Malarial, Drug discovery
The World Health Organization’s World Malaria Report has focused attention on addressing the challenge of identifying and developing new treatments for drug resistant malaria.1 According to the report, some 3.2 billion people live in areas at risk of malaria transmission in 107 countries and territories. At least one million deaths occur every year due to malaria, and about 60% of the cases of malaria worldwide occur in sub-Saharan Africa. Treating the vast number of patients infected with malaria has resulted in wide-spread resistance to current antimalarial drugs and a limited number of effective drug therapies.2 Not only is the current antimalarial drug arsenal in a fragile state, there is also a very limited understanding of how existing antimalarial drugs work against Plasmodium species parasites. However, there are presently intense efforts ongoing to discover more structurally diverse antimalarials beyond the currently available four classes of compounds: antifolates, aminoquinolines, artemisinin derivatives, and the hydroxynaphthoquinone atovaquone. It is anticipated that these efforts will lead to antimalarial drug candidates that should not be primed for the rapid emergence of resistance. In conjunction, it has also become important to develop better methods to evaluate parasite drug susceptibility through in vitro studies. Efforts in this regard are intended to enable robust high throughput screening of large compound libraries of small molecules, to discover new antimalarial cellular targets, and to better understand the molecular mechanisms by which antimalarials work. Here, in a small pilot study, we demonstrate the effectiveness of our modified flow cytometry technique for screening small molecule compound libraries for overall antimalarial activity, highlight its usefulness to determine life cycle stage drug susceptibility of Plasmodium falciparum (P. falciparum) in vitro asynchronous cultures, and reveal the discovery of three new structurally diverse potent antimalarial compounds.
The two most common methods used to evaluate P. falciparum in vitro cultures in antimalarial drug susceptibility assays have been light microscopy of blood smears or tracking the incorporation of 3H-hypoxanthine.3 Both these techniques have well known drawbacks and limitations therefore, there has been a recent trend to switch from hypoxanthine uptake assays to fluorescent labeling assays such as SYBR Green I and DAPI.4–6 Our recently developed flow cytometry method that uses an optimized Hoechst 33342 (HO)-thiazole orange (TO) staining strategy for monitoring P. falciparum growth provides new potential for evaluating the activity of antimalarial drugs against the blood stage development program of P. falciparum.7 The method enables clear differentiation of parasitized erythrocytes (pRBC) from uninfected red blood cells (RBC) based upon the presence of DNA as indicated by the level of HO staining. The significant advantage of the new technique allows the identification of malarial developmental stages in pRBC and thus the drug susceptibility of these stages by simultaneously measuring DNA and RNA levels without damaging the red blood cells or killing the malaria parasites. Furthermore, the method can accurately obtain this information using asynchronous malaria parasite cultures simplifying the assay and increasing the speed with which screenings can be performed. The guiding premise behind the ability to identify the life cycle stages is the following: ring stage parasites (early G1 phase) contain primarily DNA and should stain with HO, but are in a resting state which expresses little or no detectable RNA. Early trophozoites (late G1 phase) begin to accumulate RNA as they express proteins to allow for erythrocyte digestion, which is inferred by increased TO staining. As parasite DNA replication proceeds (S phase) late trophozoites and schizonts (≥4 nuclei) increase both DNA and RNA contents which is characterized by both increased HO and TO specific fluorescence. When the parasite reaches its spore-like, pre-lytic segmenter stage, DNA synthesis has ceased (G2 phase) and the parasitized erythrocyte has reached maximum levels of HO staining.
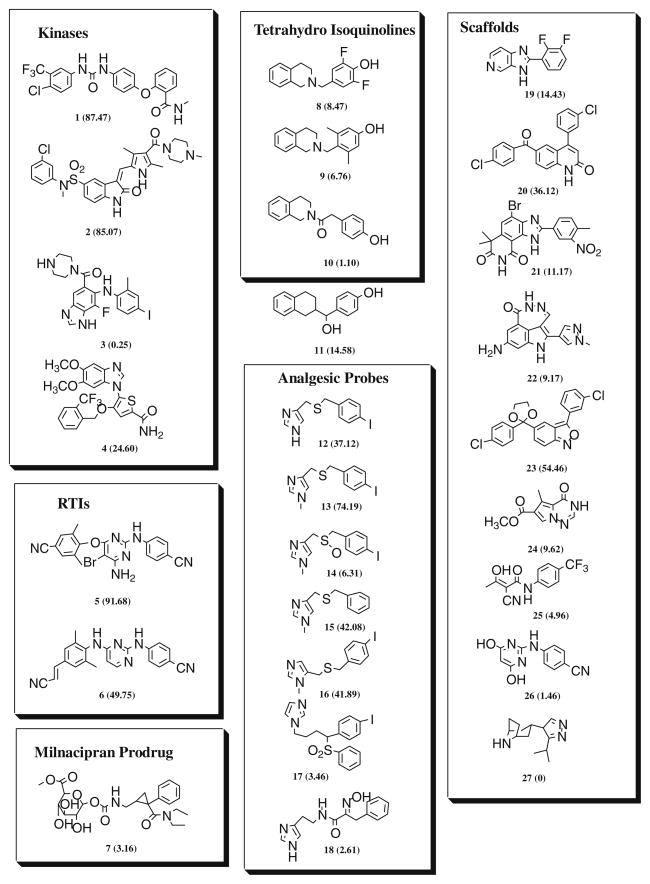
Given this new potential to monitor the growth and development of the P. falciparum malaria parasite, we evaluated 27 structurally diverse compounds with various previously defined therapeutic potential for their overall and life cycle stage antimalarial activity.8 Although this pilot study was quite small, there are numerous similar acting derivatives of these compounds available and it was our intention that they would be examined subsequently for antimalarial activity in a high throughput manner as our new screening methodology develops its full potential. Presently, the assay can accommodate the study of 60 compounds per run in a 96-well microtiter plate format. The assay is in the process of being optimized for a 384 plate format which can accommodate over 300 compounds at once. In addition, for completeness and further validation, we also examined the three currently used antimalarials: artemisinin, mefloquine, and chloroquine for overall and life cycle stage antimalarial activity. The new compounds (1–27) included in our study are shown in Figure 1 and represent several chemotypes and include kinase inhibitors (1–4),9,10 non-nucleoside reverse transcriptase inhibitors (5–6),11 a prodrug of milnacipran (7),12 1,2,3,4-Tetrahydro-isoquinolines (8–10), 1,2,3,4-Tetrahydro-naphthalene (11), mechanistic probes for analgesic and anti-analgesic activity (12–18),13 and scaffolds useful for the preparation of anti-viral and anti-cancer drugs (19–27).14
Figure 1.
Compound library screened for antimalarial activity. Parentheses show percent overall inhibition of parasite growth at 10 μM.
The antimalarial activity of the compounds studied was determined using highly resistant P. falciparum W2mef asynchronous cultures in continuous exposure 48 h experiments.15 Our flow cytometry method determines the overall growth inhibition demonstrated by each compound by measuring the number of DNA positive cells (HO) using an univariate analysis.16 At the same time, we measured growth inhibition of the 3 main life cycle stages by measuring the levels of HO (DNA) and TO (RNA) staining using bivariate analysis to identify and quantify rings, trophozoites, and schizonts in the presence of each drug and drug concentration. 16 Complex bivariate data patterns are analyzed by manual cluster gating. The IC50 values determined for overall and stage growth inhibition of P. falciparum for the currently available antimalarials: chloroquine, mefloquine, and artemisinin are shown in Table 1. The overall growth inhibition data generated by our flow cytometry method are consistent with the same data disclosed in the general literature from assays that track the incorporation of 3H-hypoxanthine.17–19
Table 1.
Overall and stage growth inhibitory activity of currently used antimalarialsa
| Compound | Overall IC50 hypoxanthine uptake | Overallb IC50 cytometry | Ringsc IC50 cytometry | Trophozoitesc IC50 cytometry | Schizontsc IC50 cytometry |
|---|---|---|---|---|---|
| Chloroquine | 311d | 123 ± 1.2 | 219 ± 1.2 | 111 ± 1.6 | 70 ± 1.6 |
| Mefloquine | 142e | 91 ± 1.1 | 147 ± 1.1 | 74 ± 1.1 | 44 ± 1.0 |
| Artemisinin | 12f | 11 ± 1.1 | 20 ± 1.2 | 7 ± 1.1 | 8 ± 1.0 |
Each flow cytometry assay determination was done in triplicate.
Overall IC50 values were determined based upon the number of DNA positive erythrocytes.
Ring, trophozoite, and schizont IC50 values were calculated based upon the number of each life cycle stages present at each dose of drug. Life cycle stages were determined based upon the level of DNA and RNA within the cell.
Saliba et al.17
Cowman et al.18
Peel et al.19
The flow cytometry IC50 values determined for the life cycle stages show a trend for all three of the known antimalarial compounds to be slightly more active against the trophozoite stages (approximately twofold) and the schizont stages (approximately 2–3-fold) as compared to the ring stage. Consistent with several previous reports is our finding that the trophozoite stage is approximately twofold more sensitive to chloroquine relative to rings.20,21 The IC50 data determined by our flow cytometry method found the schizont stage to be more sensitive to chloroquine than the trophozoite stage. Although the difference is less than a factor of two, the values are in slight contrast to data detailed in some previous reports. Most malarial pharmacologists have concluded to date that chloroquine action must be fairly trophozoite specific because of the drug’s effect on hemozoin crystal formation. However, it must be emphasized that few studies have quantified the stage specificity of chloroquine action, and not all studies support that notion. A recent study evaluating chloroquine toxicity using spinning disk confocal microscopy demonstrates the trophozoite stage to be approximately twofold more sensitive, but relatively small differences between sensitivity of the three stages.22 These studies suggest that the small discrepancy (schizonts slightly more sensitive to chloroquine than trophozoites) found in our study using flow cytometry may be due to the use of asynchronous cultures and type and time of exposure of our compounds to this highly resistant P. falciparum strain. These experimental issues will be examined in more detail in subsequent studies.
In Figure 1 shown in parentheses next to the compound number is the percent growth inhibition of P. falciparum when continuously exposed to 10 μM concentration of each compound for 48 h. The selective MEK kinase inhibitor 3,23 the Polo like kinase 1 inhibitor 4,24 and all the scaffolds useful for the preparation of known anti-virals and anti-cancer drugs were not active at 10 μM against the malaria parasite. Most of the compounds of the imidazole series that have been previously tested for analgesic activity showed little or no activity except the N-methyl-4-substituted imidazole 13. The derivative 13 along with compounds 1, 2, 5, and 6 that showed approximately 50% overall growth inhibitory activity against the malaria parasite at 10 μM was further evaluated. Their IC50 values for overall growth inhibition and life cycle stages were determined and are shown in Table 2. The data generated for the imidazole 13 are quite unique, and show approximately 1 μM selective activity against rings, twofold less activity against schizonts, and 15-fold less activity against trophozoites. Although the antimalarial mechanisms of action of this compound are undetermined at this time, the identification of its unique stage activity profile illustrates the point that our new flow cytometry assay may be beneficial in helping discover and characterize novel antimalarials.25
Table 2.
Overall and stage growth inhibitory activity of compounds from Figure 1 against Plasmodium falciparum strain W2mef
| Compound # | % Inhibition at 10 μMa | IC50 nMb overall | IC50 nM ringsc | IC50 nM trophozoitesc | IC50 nM schizontsc |
|---|---|---|---|---|---|
| 1 | 87.48 | 384 ± 1.1 | 328 ± 1.3 | 509 ± 1.1 | 376 ± 1.6 |
| 2 | 85.07 | 320 ± 1.1 | 228 ± 1.2 | 620 ± 1.6 | 317 ± 1.1 |
| 5 | 91.69 | 506 ± 1.3 | 409 ± 1.3 | 726 ± 1.4 | 716 ± 1.5 |
| 6 | 49.75 | 1096 ± 1.2 | 1197 ± 1.2 | 1998 ± 1.1 | 1207 ± 1.1 |
| 13 | 74.90 | 3769 ± 1.1 | 1075 ± 1.2 | 16571 ± 1.5 | 1998 ± 1.2 |
This is percent overall parasite growth inhibition.
Overall IC50 values were determined based upon the number of DNA positive erythrocytes.
Ring, trophozoite, and schizont IC50 values were calculated based upon the number of each life cycle stage present at each dose of drug.
The compounds 1, 2, and 5 all show potent (i.e., IC50 < 1 μM) overall growth inhibitory activity and the strongest activity against the ring stage (Table 2). Their level of ring stage growth inhibition is approximately equal to that determined for chloroquine (Table 1), but an order of magnitude less active when compared to artemisinin. However, it is important to emphasize that the three compounds 1, 2, and 5 show a different life cycle activity profile than the three known antimalarials: artemisinin, mefloquine, and chloroquine. Compounds 1, 2, and 5 are most active against the ring stage and less active against trophozoites whereas the known antimalarials artemisinin, mefloquine, and chloroquine are more active against trophozoites and less active against rings.
It is evident in the current malaria literature that the antimalarials in common use have a limited range of cellular targets. Furthermore, how these drugs act mechanistically is not well understood. Antimalarial compounds, such as chloroquine and mefloquine, act on a pathway that interrupts the parasite’s effective removal of free heme which is a byproduct of hemoglobin digestion. Hemoglobin degradation appears to be a cooperative process involving multiple proteases to include cysteine, aspartic, and metalloproteases.26–28 Because of the development of wide-spread resistance, there is growing trend to develop new antimalarials that inhibit parasite specific cellular targets that do not involve hemoglobin degradation. A few of these targets are the following: cyclin-dependent protein kinases;29 dihydrofolate reductase-thymidylate synthase;30 lactate dehydrogenase;31 DOXP-reductoisomerase; 32 and fatty acid synthesis.33
The cytometry based platform described herein has identified three new potent antimalarial compounds 1, 2, and 5 with strong growth inhibition against highly resistant ring stage P. falciparum. The mechanisms by which these compounds inhibit the growth of P. falciparum remain to be clearly determined. Empirically, if we consider the difference in structures of compounds 1, 2, and 5 to the structures of known antimalarials, and that they were specifically designed to inhibit particular mammalian or viral enzymes that have not been identified in malaria parasites, it is reasonable to assume that they are inhibiting malaria parasite growth by novel mechanisms.
In humans, it is known that Bay 43-9006 1 targets the Raf/MEK/ERK pathway, several tyrosine receptor kinases, and the serine–threonine kinase B-Raf.9,34 To date, tyrosine kinases have not been identified in the malaria parasite, and the parasite does not have a homologous MAP/ERK kinase cascade as found in other eukaryotes. 35 However, there are a few published studies of compounds that inhibit tyrosine kinases that have in vitro activity against P. falciparum species. For example, extracts of the Sudanese plant Combretum hartmannianum which demonstrate strong inhibition of p56(lck) tyrosine kinase have been reported to be significantly active against the chloroquine-sensitive P. falciparum NF54 strain.36 Nevertheless, activity in P. falciparum protein kinase research is expanding tremendously.37 The potent antimalarial activity determined for Bay 43-9006 1 should serve to initiate a search for similar kinase parasite growth inhibitors. At this time, the most productive direction of this search for similar kinase parasite growth inhibitors would be to focus on screening some derivatives of 1 and other compounds that have selectivity against tyrosine kinases that include: FGFR-1; wt BRAF and V599E mutant BRAF; as well as members of the split kinase family: VEGFR-2, VEGFR-3, PDGFR-β, c-KIT, and FIT3.
SU 11274 2 was originally developed as an anti-cancer drug that acts as a human ATP competitive inhibitor of the catalytic activity of MET.10 Recently, it has been reported that hepatocyte growth factor/MET kinase (HGF/MET) signaling protects P. falciparum infected host cells from apoptosis.38 It has been shown that inhibition of HGF/MET signaling induces a specific increase in apoptosis of infected cells leading to great reduction of infection. This may suggest that there is a possibility that 2 demonstrates antimalarial activity via its inhibition of the catalytic activity of MET. The design and synthesis of c-MET inhibitors are a very active area of anti-cancer research, and potent and selective inhibitors have been developed.39 This finding provides initiative to screen other ATP competitive inhibitors as well as allosteric inhibitors of MET particularly because apoptosis in malarial parasites and unicellular organisms is currently thought to be significantly different from the respective processes in the human host.40
Compounds TMC 125 5 and R278474 6 were designed as nonnucleoside reverse transcriptase inhibitors, and have been shown to be highly active against wild type and mutant HIV.11 Our screening results have demonstrated potent antimalarial activity for TMC 125 5 and moderate activity for R278474 6. It has been reported that other anti-HIV drugs (protease inhibitors) have shown potent activity against P. falciparum.41 Recently, it has been disclosed that P. falciparum contain a gene that encodes a catalytic reverse transcriptase component of telomerase (PfTERT).42 PfTERT is unique in being three times larger than TERTs characterized in other species. It is also expressed in asexual blood stage parasites that have begun DNA synthesis and localizes into a discrete nuclear compartment associated with the nucleolus. Our results suggest screening other reverse transcriptase inhibitors for antimalarial activity with the hope of discovering other compounds with similar convergent activity. This takes on added significance given that 5 is active against wild type and mutant HIV and a highly resistant form of P. falciparum, and that malaria and HIV infection co-exist in sub-Saharan Africa.
The flow cytometry application used here represents a new opportunity for effectively determining the drug susceptibility of P. falciparum in vitro cultures. The method is a rapid, relatively inexpensive, accurate, and safe strategy. Technically, this methodology is less cumbersome, can be used in high throughput screening, and efficiently uses asynchronous cultures to obtain antimalarial stage specific data. In addition, we have determined that the method enables preliminary screening for possible toxic effects of compounds on uninfected red blood cells. It was established during the course of our drug analyses that all the compounds tested did not effect changes in red blood cell shape43 and internal complexity.44 Greater than 98.5% of uninfected erythrocytes remained intact with no evidence of cell membrane toxicity. 45 No other method allows for simultaneous determinations of compound effects on red blood cells and drug susceptibility of P. falciparum in vitro cultures. Other methods that are used to assess life cycle stage in vitro drug susceptibility require parasite culture synchronization and thus independent stage assessment. Future applications of this new technique will pursue continued antimalarial drug and drug combination screening as well as adaptation for high throughput screening.
In regard to the utility of this flow cytometry technique for determining life cycle stage drug susceptibility, it is important to mention the future goals of antimalarial drug strategies.46 Artemisinin and its derivatives, recommended by the World Health Organization to treat malaria, have been found to cause ring stage parasites to enter a dormant phase for a period of 10 days. It has been proposed that partner drugs to an artemisinin derivative must have activity against dormant forms or a long enough half life to remain active in the blood when dormant ring stage parasites begin to emerge and grow. Our studies have enabled the discovery of compounds that have potent ring stage antimalarial activity, and certainly provide a possible avenue for addressing this immediate concern. Further evaluation of the possible mechanisms and pharmacology of antimalarial action of the most potent compounds 1, 2, and 5 and new compound high throughput screening are ongoing.
Acknowledgments
The authors thank Kerry O. Grimberg and William F. Hickey for helpful discussions and critical evaluation of the methods and manuscript. The authors thank R. Michael Sramkoski and James W. Jacobberger from the Flow Cytometry Core Facility of the Comprehensive Cancer Center of Case Western Reserve University and University Hospitals of Cleveland (P30CA43703) for their generous technical support.
The assay used in this study was developed and validated using funds from the NIH (AI52312). The screening of experimental drugs was supported by departmental funds from the CWRU Center for Global Health and Diseases. LBH was supported by the National Institute on Drug Abuse (DA-03816).
References and notes
- 1.Aregawi M, Williams R, Dye C, Cibulskis R, Otten M. World Malaria Report 2008. World Health Organization (WHO); Geneva: 2008. [Google Scholar]
- 2.(a) White NJ. J Clin Invest. 2004;113:1084. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Knobler S. The Resistance Phenomenon In Microbes And Infectious Disease Vectors. National Academy; Washington, DC: 2003. [PubMed] [Google Scholar]
- 3.(a) Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Antimicrob Agents Chemother. 1979;16:710. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Scheibel LW, Adler A, Trager W. Proc Natl Acad Sci USA. 1979;76:5303. doi: 10.1073/pnas.76.10.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noedl H, Wernsdorfer WH, Miller RS, Wongsrichanalai C. Antimicrob Agents Chemother. 2002;46:1658. doi: 10.1128/AAC.46.6.1658-1664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. Antimicrob Agents Chemother. 2007;51:1926. doi: 10.1128/AAC.01607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baniecki ML, Wirth DF, Clardy J. Antimicrob Agents Chemother. 2007;51:716. doi: 10.1128/AAC.01144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimberg BT, Erickson JJ, Sramkoski RM, Jacobberger JW, Zimmerman PA. Cytometry Part A. 2008;73:546. doi: 10.1002/cyto.a.20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compounds 1–27 in Figure 1 were all prepared at Curragh Chemistries Inc. according to procedures described in the patent and journal literature. The compounds were fully characterized. 1H NMR (300 MHz) spectra of the compounds were obtained through the use of instrumentation in the department of chemistry at Case Western Reserve University.
- 9.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. Cancer Res. 2004;64:7099. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 10.Berthou S, Aebersold DM, Schmidt LS, Stroka D, Heigl C, Streit B, Stalder D, Gruber G, Liang C, Howlett AR, Candinas D, Greiner RH, Linson KE, Zimmer Y. Oncogenes. 2004;23:5387. doi: 10.1038/sj.onc.1207691. [DOI] [PubMed] [Google Scholar]
- 11.Janssen PAJ, Lewi PJ, Arnold E, Daeyaert F, de Jong M, Heeres J, Koymans L, Vinkers M, Guillemont J, Pasquier E, Kukla M, Ludovici D, Andries K, de Bethune MP, Pauwels R, Das K, Clark AD, Jr, Frenkel YV, Hughes SH, Medaer B, De Knaep F, Bohets H, De Clerck F, Lampo A, Williams P, Stoeffels P. J Med Chem. 2005;48:1901. [Google Scholar]
- 12.Shuto S, Takada H, Mochizuki D, Tsujita R, Hase Y, Ono S, Shibuya N, Matsuda A. J Med Chem. 1995;38:2964. doi: 10.1021/jm00015a019. [DOI] [PubMed] [Google Scholar]
- 13.(a) Hough LB, Nalwalk JW, Phillips JG, Kern B, Shan Z, Wentland MP, de Esch IJ, Janssen E, Barr T, Stadel R. Neuropharmacology. 2007;52:1244. doi: 10.1016/j.neuropharm.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hough LB, de Esch IJ, Janssen E, Phillips J, Svokos K, Kern B, Trachler J, Abood ME, Leurs R, Nalwalk JW. Neuropharmacology. 2006;51:447. doi: 10.1016/j.neuropharm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 14.(a) Angibaud PR, Venet MG, Filliers W, Broeckx R, Ligny YA, Muller P, Poncelet VS, End DW. Eur J Org Chem. 2004:479. [Google Scholar]; (b) Ninkovic S, Bennett MJ, Rui Y, Wang F, Benedict SP, Teng M. WO 2004063198 (A1) ; (c) Price DA, Gayton S, Selby MD, Ahman J, Haycock-Lewandowski S, Stammen BL, Warren A. Tetrahedron Lett. 2005;46:5005. [Google Scholar]; (d) Borzilleri RM, Cai Z, Ellis C, Fargnoli J, Fura A, Gerhardt T, Goyal B, Hunt JT, Mortillo S, Qian L, Tokarski J, Vyas V, Wautlet B, Zheng X, Bhide RS. Bioorg Med Chem Lett. 2005;15:1429. doi: 10.1016/j.bmcl.2004.12.079. [DOI] [PubMed] [Google Scholar]; (e) Wallace EM, Lyssikatos JP, Hurley BT, Marlow AL. WO2003077914 (A1) ; (f) Chachoyan AA, Garibdzhanyan BT, Markaryan EZ. Biol Zhurnal Armenii. 1972;25:102. [Google Scholar]
- 15.P. falciparum in vitro drug assay: Asynchronous cultures of P. falciparum strainW2-Mef (MRA-615 deposited to ATCC/MR4 by AF Cowman) were maintained at a 5% hematocrit of human O+ blood. For the initial drug screening parasites were exposed to 10 μM of each of the 27 drugs, in duplicate, and allowed to grow for 48 h under standard conditions. Cells were then stained with Hoescht 33342 and thiazole orange for 40 min as previously described.7 Dead cells were detected using 74.8 nM propidium iodide membrane integrity stain which is excluded by intact cells. Dead parasites within live RBC were detected using 25 nM DiIC1-5 membrane potential stain. Erythrocytes with live parasites within show an increase in the levels of membrane potential because of the presence of live parasite membranes. Dead parasites would not increase the membrane potential of its host erythrocyte but still would show remnants of DNA. Test drugs which showed a >50% inhibition of overall parasite growth were further tested to determine their IC50 value. Parasites were again exposed to test drugs (1, 2, 5, 6, and 13) which were diluted in 1/2 log10 steps from 10 μM down to 0.33 nM, or currently available antimalarial drugs (Artemisinin, Mefloquine, Chloroquine) which were diluted in log2 from 1024 nM down to 2 nM. Cultures were grown in triplicate and tested as above. The number of parasites of each life cycle stage and overall was recorded. Artemisinin and Chloroquine diphosphate salt were obtained from Sigma (St. Louis, MO) and mefloquine (formulated as found in the drug, Lariam®) was kindly provided by the Walter Reed Army Institute of Research. Currently the throughput of this assay is approximately 100 compounds per day. Recently we have added a High Throughput Sampler to our existing LSR2 from Becton-Dickinson allowing for 300–900 compounds (or drug dilutions) to be tested per day. The rate limiting step is dilution of samples to the appropriate concentration.
- 16.Calculation of IC50 values: The use of optimized nucleic acid staining allowed us to perform quantitative assessment of nuclei (N) and gene expression through detection of DNA and RNA, respectively. In addition to observing the overall change in parasitemia (DNA+), this method also enabled separate gating on rings (DNA+/RNA−), trophozoites (DNA < 4 N/RNA+), and schizonts (DNA ≥ 4 N/RNA+). The number of parasites or parasite lifecycles stages found in each concentration of drug was averaged across replicates and divided by the average number of corresponding parasites in control wells grown in the absence of any drug. To calculate IC50 values, a non-linear sigmoid dose–response curve for variable slope was fitted to the data to determine the overall and lifecycle stage specific IC50 values using GraphPad Prism version 5.00 for Windows (San Diego, CA). In the future, collaborations with Verity Software House will allow the authors access to Gemstone automated FACS analysis software (Beta version) to deal with the vast amounts of data generated with our high content analysis. FACS plots can be found in Grimberg et al. (2008) (Ref. 7). We would like to emphasize to the malarial research community and to those not familiar with flow cytometry techniques that the essence of this Letter describes the first application of this new flow cytometry method to measure the stage in vitro drug susceptibility of the 3 known antimalarials and to discover new antimalarials. Additional details as to how the flow cytometry method enables these measurements and analysis and its preliminary validation and optimization in comparison to hypoxanthine uptake measurements can be found in Ref. 7.
- 17.Saliba KJ, Folb PI, Smith P. J Biochem Pharmacol. 1998;56:313. doi: 10.1016/s0006-2952(98)00140-3. [DOI] [PubMed] [Google Scholar]
- 18.Cowman AF, Galatis FD, Thompson JK. Proc Natl Acad Sci USA. 1994;91:1143. doi: 10.1073/pnas.91.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peel SA, Bright P, Yount B, Handy J, Baric AS. Am J Trop Med Hyg. 1994;51:648. doi: 10.4269/ajtmh.1994.51.648. [DOI] [PubMed] [Google Scholar]
- 20.Yayon A, Vande Waa JA, Yayon M, Geary TG, Jensen JB. J Protozool. 1983;30:642. doi: 10.1111/j.1550-7408.1983.tb05336.x. [DOI] [PubMed] [Google Scholar]
- 21.ter Kuile F, White NJ, Holloway P, Pasvol G, Krishna S. Exp Parasitol. 1993;76:85. doi: 10.1006/expr.1993.1010. [DOI] [PubMed] [Google Scholar]
- 22.Gligorijevic B, Purdy K, Elliott DA, Cooper RA, Roepe PD. Mol Biochem Parasitol. 2008;159:7. doi: 10.1016/j.molbiopara.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demattei J, Shaky AS, Piscopio AD, Hache BP, Evans MC, Ford JG, Pointon SM, Peeters K, Lilley TJ, Leonard J. WO2007002157 (A2)
- 24.Andrews CW, III, Cheung M, Davis-Ward RG, Drewry DH, Emmitte KA, Hubbard RD, Kuntz KW, Linn JA, Mook RA, Smith GK, Veal JM. WO2004014899 (A1)
- 25.The data generated for compound #13 demonstrate an order of magnitude less growth inhibition for the trophozoite stage when compared to the ring and schizont stages. This may suggest that this compound is preventing maturation of the trophozoite stage to the schizont stage. Presently, we do not have additional supporting data. However, it points to the fact that our methodology even with the use of asynchronous cultures enables the detection of compounds that have unique stage growth inhibition profiles.
- 26.Lanteri CA, Johnson JD, Waters NC. Recent Pat Anti-Infect Drug Discovery. 2007;2:95. doi: 10.2174/157489107780832640. [DOI] [PubMed] [Google Scholar]; Liu J, Istvan ES, Gluzman IY, Gross J, Goldberg DE. Proc Natl Acad Sci USA. 2006;103:8840. doi: 10.1073/pnas.0601876103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg DE, Slater AF, Beavis R, Chait B, Cerami A, Henderson GB. J Exp Med. 1991;173:961. doi: 10.1084/jem.173.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenthal PJ, McKerrow JH, Aikawa M, Nagasawa H, Leech JH. J Clin Invest. 1988;82:1560. doi: 10.1172/JCI113766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geyer JA, Prigge ST, Waters NC. Biochim Biophys Acta. 2005;1754:160. doi: 10.1016/j.bbapap.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Bunyarataphan S, Leartsakulpanich U, Taweechai S, Tarnchompoo B, Kamchonwongpaisan S, Yuthavong Y. Antimicrob Agents Chemother. 2006;50:3631. doi: 10.1128/AAC.00448-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cameron A, Read J, Tranter R, Winter VJ, Sessions RB, Brady RL, Vivas L, Easton A, Kendrick H, Croft SL, Barros D, Lavandera JL, Martin JJ, Risco F, Garcia-Ochoa S, Garno FJ, Sanz L, Leon L, Ruiz JR, Gabarro R, Mallo A, Gomez de las Heras F. J Biol Chem. 2004;279:31429. doi: 10.1074/jbc.M402433200. [DOI] [PubMed] [Google Scholar]
- 32.Silber K, Heidler P, Kurz T, Klebe G. J Med Chem. 2005;48:3547. doi: 10.1021/jm0491501. [DOI] [PubMed] [Google Scholar]
- 33.Lu JZ, Lee PJ, Waters NC, Priggs ST. Comb Chem High Throughput Screen. 2005;8:15. doi: 10.2174/1386207053328192. [DOI] [PubMed] [Google Scholar]
- 34.Plastaras JP, Kim SH, Liu YY, Dicker DT, Dorsey JF, McDonough J, Cerniglia G, Rajendran RR, Gupta A, Rustgi AK, Diehl JA, Smith CD, Flaherty KT, El-Deiry WS. Cancer Res. 2007;67:9443. doi: 10.1158/0008-5472.CAN-07-1473. [DOI] [PubMed] [Google Scholar]
- 35.Ward P, Equinet L, Packer J, Doerig C. BMC Genom. 2004;5:79. doi: 10.1186/1471-2164-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali H, Konig GM, Khalid SA, Wright AD, Kaminsky R. J Ethnopharmacol. 2002;83:219. doi: 10.1016/s0378-8741(02)00245-3. [DOI] [PubMed] [Google Scholar]
- 37.Doerig C, Billker O, Haystead T, Sharma P, Tobin AB, Waters NC. Trends Parasitol. 2008;24:570. doi: 10.1016/j.pt.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Leiriao P, Albuquerque SS, Corso S, van Gemert G, Sauerwein RW, Rodriguez A, Giordano S, Mota MM. Cell Microbiol. 2005;7:603. doi: 10.1111/j.1462-5822.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 39.D’Angelo ND, Bellon SF, Booker SK, Cheng Y, Coxon A, Dominguez C, Fellows I, Hoffman D, Hungate R, Kaplan-Lefko P, Lee MR, Li C, Liu L, Rainbeau E, Reider PJ, Rex K, Siegmund A, Sun Y, Tasker AS, Xi N, Xu S, Yang Y, Zhang Y, Burgess TL, Dussault I, Kim T. J Med Chem. 2008;51:5766. doi: 10.1021/jm8006189. [DOI] [PubMed] [Google Scholar]
- 40.Picot S, Burnod J, Bracchi V, Chumpitazi BFF, Ambroise-Thomas P. Trans R Soc Trop Med Hyg. 1997;91:590. doi: 10.1016/s0035-9203(97)90039-0. [DOI] [PubMed] [Google Scholar]
- 41.Andrews KT, Fairlie DP, Madala PK, Ray J, Wyatt DM, Hilton PM, Melville LA, Beattie L, Gardiner DL, Reid RC, Stoermer MJ, Skinner-Adams T, Berry C, McCarthy JS. Antimicrob Agents Chemother. 2006;50:639. doi: 10.1128/AAC.50.2.639-648.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Figueiredo LM, Rocha EPC, Mancio-Silva L, Prevost C, Hernandez-Verdun D, Scherf A. Nucleic Acids Res. 2005;33:1111. doi: 10.1093/nar/gki260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cell size is indicated by the forward scatter (FSC) on a flow cytometer. This parameter is proportional to the amount of laser light passing unobstructed around a cell.
- 44.Internal complexity is indicated by the side scatter (SSC) on a flow cytometer. This parameter is proportional to the amount of laser light which is reflected by cell membranes and organelles.
- 45.In addition to changes in cell shape, drug toxicity to cells can be detected as damage to the membrane and is indicated by the presence of propidium iodide stain within a cell.
- 46.White NJ. Science. 2008:330. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]