Abstract
The administration of tetradecylthioacetic acid (TTA), a hypolipidemic and anti-inflammatory modified bioactive fatty acid, has in several experiments based on high fat diets been shown to improve lipid transport and utilization. It was suggested that increased mitochondrial and peroxisomal fatty acid oxidation in the liver of Wistar rats results in reduced plasma triacylglycerol (TAG) levels. Here we assessed the potential of TTA to prevent tumor necrosis factor (TNF) α-induced lipid modifications in human TNFα (hTNFα) transgenic mice. These mice are characterized by reduced β-oxidation and changed fatty acid composition in the liver. The effect of dietary treatment with TTA on persistent, low-grade hTNFα overexpression in mice showed a beneficial effect through decreasing TAG plasma concentrations and positively affecting saturated and monounsaturated fatty acid proportions in the liver, leading to an increased anti-inflammatory fatty acid index in this group. We also observed an increase of mitochondrial β-oxidation in the livers of TTA treated mice. Concomitantly, there were enhanced plasma levels of carnitine, acetyl carnitine, propionyl carnitine, and octanoyl carnitine, no changed levels in trimethyllysine and palmitoyl carnitine, and a decreased level of the precursor for carnitine, called γ-butyrobetaine. Nevertheless, TTA administration led to increased hepatic TAG levels that warrant further investigations to ascertain that TTA may be a promising candidate for use in the amelioration of inflammatory disorders characterized by changed lipid metabolism due to raised TNFα levels.
Keywords: Tetradecylthioacetic acid, hTNFα transgenic mice, Low-grade inflammation, Dietary treatment, Plasma, Liver
Introduction
Chronic low-grade inflammation accompanies the development of metabolic syndrome features, like abdominal obesity, dyslipidemia, hypertension, and insulin resistance, known risk factors for cardiovascular disease affecting millions of people worldwide. The first indication that there is a connection between obesity, diabetes and chronic inflammation came from the observation that the pro-inflammatory cytokine tumor necrosis factor (TNF) α is overexpressed in adipose tissues of obese mice and humans [1, 2]. Moreover, its inactivation with anti-TNFα antibodies improved insulin resistance in obese mice [2], whereas TNFα null mice would not develop insulin resistance after diet-induced obesity [3]. The multifunctional cytokine TNFα was shown thereafter to perturb lipid metabolism by increasing free fatty acid production [4, 5], promoting lipolysis [6–9], affecting lipid-metabolism-related gene expression [10, 11], controlling cholesterol metabolism [12, 13], and influencing expression and secretion of other adipokines [14–17].
Used as a model for persistent low-grade TNFα exposure, we have found previously that hTNFα transgenic mice show a down-regulation of peroxisome proliferator-activated receptor (PPAR) α target genes [18]. The mitochondrial enzymes involved in hepatic lipid metabolism were influenced, leading to changes in fatty acid synthesis and oxidation [18]. In particular, not only carnitine palmitoyltransferase-I and -II (CPT-I and -II), proteins important for the β-oxidation of long-chain fatty acids in mitochondria, but also fatty acyl-CoA oxidase (FAO), which is important for peroxisomal β-oxidation, proved to have decreased hepatic activity in the hTNFα transgenic mice [18]. In addition, TNFα overexpression was associated with a significant reduction of hepatic mRNA levels of mitochondrial HMG-CoA synthase, the rate-limiting enzyme in ketogenesis [18]. Moreover, lipogenesis is affected by TNFα. Namely, the two important enzymes in lipogenesis, acetyl-CoA carboxylase (ACC), which produces malonyl-CoA for fatty acid synthesis, showed a tendency for lowered activity, whereas fatty acid synthase (FAS), an enzyme involved in long-term regulation of fatty acid synthesis, displayed a significantly decreased activity [18].
As lipids have the ability to modulate metabolic, inflammatory and innate immune processes [19], we investigated in the present study, whether the fatty acid analogue tetradecylthioacetic acid (TTA) could counteract the health risks as a consequence of TNFα-overexpression. To be able to investigate inflammation in relation to lipid accumulation as seen in the nutritional disorder of obesity, we chose to administer a high-fat diet. TTA is a saturated fatty acid containing 16 carbons and one sulfur atom at position three of the carbon chain from the alpha end, a characteristic that results in its increased metabolic stability [20]. TTA is known to act, at least partly, through the activation of PPAR [21–23], and influence plasma lipids by increasing hepatic β-oxidation [24, 25]. Moreover, we have reported that TTA has antioxidant and antiinflammatory properties [26].
In the present work, we show that the administration of TTA to hTNFα transgenic mice fed a high-fat diet showed beneficial effects on serum cholesterol and triacylglycerol (TAG) levels, hepatic fatty acid composition and cholesterol levels, with a concurrent increase in hepatic β-oxidation and fatty inflammatory index.
Experimental Procedure
Transgenic Mice
The study was performed on female transgenic mice expressing low levels of human TNFα (hTNFα) mice from Taconic (Germantown, USA). The transgenic mouse line was generated in strain C57Bl/6 [27]. The experiments were performed in accordance with, and under the approval of, the Norwegian State Board for Biological Experiments, the Guide for the Care and Use of Laboratory Animals, and the Guidelines of the Animal Welfare Act. The mice were between 6 and 8 weeks of age at the start of the experimental feeding and were divided into two experimental groups of five animals each with comparable mean body weight. They were housed in cages with constant temperature (22 ± 2 °C) and humidity (55 ± 5%), where they were exposed to a 12 h light-dark cycle (light from 07.00 to 19.00) and had unrestricted access to tap water and food. The mice were acclimatized to these conditions before the start of the experiment.
Protein and fat of the feeding diets were from casein sodium salt from bovine milk, 20% (Sigma-Aldrich Norway AS, Oslo, Norway) and lard, 23% (Ten Kate Vetten BV, Musselkanaal, Netherlands) plus soy oil, 2% (Dyets Inc., Bethlehem, PA, USA). In addition, in the TTA group 0.6% of the lard was substituted by TTA. The TTA was synthesized as described earlier [28]. There were no significant differences in fatty acid composition between control and TTA diets, except the TTA content (SFA 43.9 vs. 42.8 wt%, MUFA 38.8 vs. 37.8 wt%, PUFAn-3 1.51 vs. 1.48 wt%, PUFAn-6 15.6 vs. 15.3 wt%, TTA 0.0 vs 2.5 wt%).
The mice were anaesthetized under fasting conditions by inhalation of 2% isoflurane (Schering-Plough, Kent, UK) after two weeks of feeding. Blood was collected by aortic puncture with 7.5% EDTA and immediately chilled on ice. Plasma was prepared and stored at −80 °C prior to analysis. Parts of the liver were used for β-oxidation analysis and chilled on ice, and the rest was freeze-clamped and stored at −80 °C until the analysis of fatty acids, triacylglycerols, cholesterol, and enzyme activities.
Plasma and Hepatic Lipids
Liver lipids were extracted according to Bligh and Dyer [29], evaporated under nitrogen, and redissolved in isopropanol before analysis. Lipids were subsequently measured enzymatically on a Hitachi 917 system (Roche Diagnostics GmbH, Mannheim, Germany) using the triacylglycerol (GPO-PAP) and cholesterol kit (CHOD-PAP) from Roche Diagnostics (Mannheim, Germany) and the phospholipid kit from bioMérieux SA (Marcy l’Etoile, France).
Hepatic Fatty Acid and Plasma Carnitine Compositions
Total hepatic fatty acid composition was analyzed as described previously [18]. The anti-inflammatory fatty acid index was calculated as (docosapentaenoic acid + docosahexaenoic acid + dihomo-γ-linolenic acid + eicosapentaenoic acid)x100/arachidonic acid. Slightly different indexes have been used by [30, 31]. Free carnitine, short-, medium-, and long-chain acylcarnitines, and the precursors for carnitine, trimethyllysine and γ-butyrobetaine, respectively, were analysed in plasma using HPLC/MS/MS as described previously [32] with some modifications (Svardal et al., in preparation).
Hepatic Enzyme Activities
The livers were homogenized and fractionated as described earlier [33]. Palmitoyl-CoA oxidation was measured in a mitochondria-enriched extract from liver as acid-soluble products [34]. The activities of carnitine palmitoyltransferase (CPT)-I [35] and acyl-CoA synthetase (ACS) [35] were measured in the mitochondrial fraction. Fatty acid synthase (FAS) was measured in the post-nuclear fraction as described by Skorve et al. [36].
Statistical Analysis
Data sets were analyzed using Prism Software (Graph-Pad Software, San Diego, CA) to generate the figures and determine statistical significance. The results are shown as means with their standard deviations (S.D.). A t-test was used to determine significant differences between the control and the TTA treatment group. P-values < 0.05 were considered significant.
Results
Body/Liver Weights and Feed Intake
We found that TTA supplementation for two weeks to hTNFα-overexpressing mice promoted a significant decrease in body weights (−0.8 ± 0.8 g) as compared to high-fat-fed control animals (1.4 ± 0.6 g) (Table 1). Whereas liver weights and liver index were significantly increased in the TTA supplemented group (1.4 ± 0.09 and 7.2 ± 0.43) in comparison to the control group (0.9 ± 0.08 and 4.3 ± 0.3). The TTA group displayed a reduced total feed intake, 30 g versus 34 g of diet per TTA- or control-fed mouse, respectively. Thus, the TTA group seems more efficient in converting feed into increased body mass, as shown by a lower total feed efficiency (−0.03) in comparison to the control group (0.04).
Table 1.
Body and liver weights, liver index [100 × (liver weight in g/body weight in g)], total feed intake, and feed efficiency (weight gain in g/food intake in g)
| Control | TTA | |
|---|---|---|
| Initial body weight (g) | 19.2 ± 1.1 | 20.2 ± 1.3 |
| Final body weight (g) | 20.6 ± 1.5 | 19.4 ± 1.3 |
| Body weight gain (g) | 1.4 ± 0.6 | −0.8 ± 0.8 |
| Liver weight (g) | 0.9 ± 0.08 | 1.4 ± 0.09 |
| Liver index | 4.3 ± 0.3 | 7.2 ± 0.43 |
| Total feed intake (g) | 34.2 | 30 |
| Total feed efficiency | 0.04 | −0.03 |
Values are mean ± S.D. (n = 5)
Serum and Hepatic Lipids
In previous studies, comparing transgenic hTNFα-overexpressing with wildtype mice, TNFα interfered with lipid metabolism, leading to increased hepatic TAG and total cholesterol levels. Serum cholesterol concentrations were decreased, whereas serum TAG levels were unchanged [18].
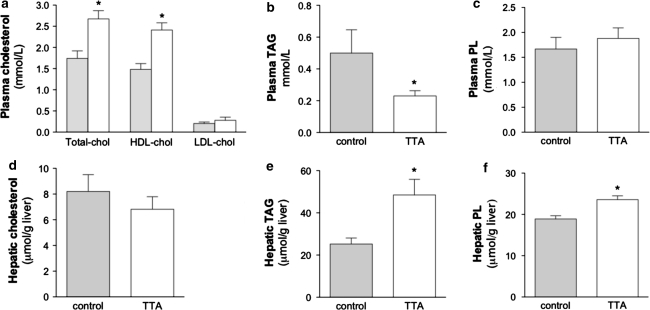
In this study, two weeks of 0.6% TTA administration promoted positive effects on plasma parameters in hTNFα-overexpressing mice fed a high fat diet. The transgenic mice showed increased levels of total cholesterol due to a 39% increase in HDL-cholesterol in the TTA treated group (Fig. 1a) as compared to the high fat-fed controls. With regard to plasma TAG, a drastic reduction of 54% was observed after TTA treatment (Fig. 1b) as compared to the control. Plasma phospholipids (Fig. 1c) and free fatty acids (data not shown) were not significantly changed. The hepatic levels of total cholesterol showed a non-significant tendency to decrease in the TTA-treated group (Fig. 1d), and the already high TAG amounts in transgenic mice further increased after TTA treatment (Fig. 1e). The hepatic phospholipid level was also elevated after TTA administration in comparison to control mice (Fig. 1f).
Fig. 1.
TTA treatment induces a significant increase in plasma HDL-cholesterol levels in hTNFα transgenic mice (a) and a decrease in plasma TAG levels (b), whereas phospholipids showed no change (c). Hepatic cholesterol showed no significant change (d), but a TAG and phospholipid-increasing effect in the liver was found after treatment with TTA (e, f). Data are means ± S.D. (n = 5). * denotes statistical significant differences by Student’s t-test between control (grey bars) and TTA (white bars) (P < 0.05). chol, cholesterol; HDL, high-density lipoprotein; LDL, low density lipoprotein; PL, phospholipid; AG, triacylglycerol; TTA, tetradecylthioacetic acid
Fatty Acid Composition in Liver
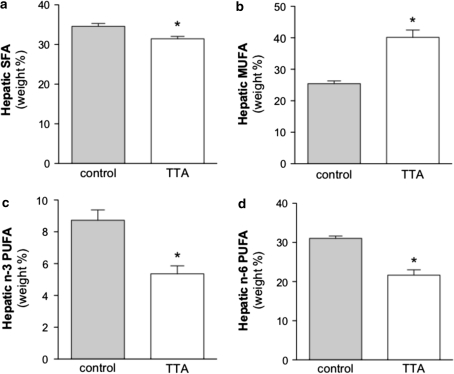
In order to investigate if the results obtained for hepatic lipids were specific for cholesterol and TAG or if fatty acids were also affected significantly, we performed an analysis of the total hepatic fatty acid composition. We have previously demonstrated an increased weight % of saturated fatty acids (SFAs) in TNFα-overexpressing mice on a chow diet [18].
Here we observed a comparable level of SFAs in TNFα-overexpressing mice (in spite of higher dietary SFA content in the high-fat diet used- see Discussion). TTA treatment lead to decreased weight % in saturated fatty acid levels (Fig. 2a). The most significant individual decreases could be found in the fatty acids C15:0, C17:0, C18:0, C22:0, C23:0, and C24:0 (Table 2). The most impact on decreasing total SFAs had C18:0.
Fig. 2.
Dietary treatment for 2 weeks with TTA affects hepatic fatty acid composition and changes saturated fatty acids (a), monounsaturated fatty acids (b), n-3 polyunsaturated fatty acids (c), and n-6 polyunsaturated fatty acids (d) levels significantly. The data is given as weight % of total fatty acid ± S.D. (n = 5). * denotes statistical significant differences to the control by Student’s t test (P < 0.05). SFA saturated fatty acids, MUFA monounsaturated fatty acids, PUFA polyunsaturated fatty acids, TTA tetradecylthioacetic acid
Table 2.
Comparison of hepatic fatty acid composition in control and TTA treated hTNFα transgenic mice
| Fatty acid | control | TTA | Level of significance |
|---|---|---|---|
| SFA | |||
| C15:0 | 0.07 ± 0.002 | 0.03 ± 0.001 | P < 0.0001 |
| C16:0 | 21.42 ± 0.35 | 24.3 ± 0.204 | P < 0.0001 |
| C17:0 | 0.19 ± 0.012 | 0.10 ± 0.009 | P < 0.0001 |
| C18:0 | 12.02 ± 0.554 | 6.42 ± 0.585 | P < 0.0001 |
| C22:0 | 0.15 ± 0.017 | 0.07 ± 0.016 | P < 0.0001 |
| C23:0 | 0.08 ± 0.004 | 0.03 ± 0.004 | P < 0.0001 |
| C24:0 | 0.11 ± 0.009 | 0.05 ± 0.006 | P < 0.0001 |
| MUFA | |||
| C16:1 | 0.02 ± 0.003 | 0.01 ± 0.001 | P < 0.002 |
| C16:1n-7 | 1.03 ± 0.127 | 1.44 ± 0.291 | P < 0.03 |
| C18:1n-7 | 1.6 ± 0.079 | 1.72 ± 0.23 | NS |
| C18:1n-9 | 21.77 ± 0.748 | 35.22 ± 1.796 | P < 0.0001 |
| C20:1n-9 | 0.26 ± 0.022 | 0.49 ± 0.064 | P < 0.0001 |
| n-6 PUFA | |||
| C18:2n-6 | 16 ± 0.540 | 9.90 ± 0.743 | P < 0.0001 |
| C20:4n-6 | 12.96 ± 0.788 | 8.61 ± 0.628 | P < 0.0001 |
| C20:3n-6 | 0.9 ± 0.059 | 2.51 ± 0.291 | P < 0.0001 |
| n-3 PUFA | |||
| C18:3n-3 | 0.42 ± 0.027 | 0.13 ± 0.010 | P < 0.0001 |
| C18:4n-3 | 0.04 ± 0.005 | 0.01 ± 0.002 | P < 0.0001 |
| C22:6n-3 | 7.60 ± 0.646 | 4.49 ± 0.431 | P < 0.0001 |
| C20:5n-3 | 0.29 ± 0.028 | 0.27 ± 0.043 | NS |
| Anti-inflammatory index | 70.12 ± 2.883 | 88.94 ± 4.551 | P < 0.0002 |
| C20:4n-6/C20:3n-6 | 14.41 ± 1.429 | 3.46 ± 0.380 | P < 0.0001 |
| C18:1n-9/C18:0 | 1.81 ± 0.117 | 5.54 ± 0.752 | P < 0.0001 |
The anti-inflammatory index is calculated as described in Experimental Procedure. The ratio of C20:4n-6 to C20:3n-6 gives an indirect index of the n-6 ∆5 desaturase activity. The data are given as weight % ± S.D. (n = 5). A two sample, two-tailed t-test assuming equal variance was calculated. NS means not significant
The relative amounts of monounsaturated fatty acids (MUFA) that were shown to be decreased in hTNFα transgenic in comparison to wildtype mice [18], were significantly increased by TTA in the diet (Fig. 2b). This was mainly due to the increased amount of oleic acid (C18:1n-9) that was to some extent induced by increased Δ9 desaturase activity as the index Δ9 desaturase activity suggests (Table 2).
A significant decrease in weight % of n-3 and n-6 polyunsaturated fatty acids (PUFA) was observed in the transgenic mice treated with TTA (Fig. 2c and d) when compared to control animals. This was reflected in decreased amounts of the n-3 PUFA alpha-linolenic acid (18:3n-3), stearidonic acid (C18:4n-3) and docosahexaenoic acid (C22:6n-3), along with linoleic acid (C18:2n-6) and arachidonic acid (C20:4n-6) that can mainly be attributed to lowered levels of n-6 PUFA (Table 1). The sums result in a decreased ratio of n-3 to n-6 PUFA (Table 1). TTA seems to counteract the increased PUFA levels seen in transgenic mice in contrast to wildtype mice that were due to increased weight % of n-3, with unchanged n-6 levels, giving an increased n-3 to n-6 ratio [18].
In addition, after TTA administration, there was a strong decrease in the C20:4n-6/C20:3n-6 ratio as an indirect measure of the n-6 ∆5 desaturase activity, an enzyme important for the production of arachidonic acid (Table 2). This indicates that TTA might decrease the hepatic desaturation of dihomo-γ-linolenic acid (20:3n-6) to arachidonic acid (20:4n-6) in hTNFα transgenic mice. Whereas beforehand, we observed increased ratios pointing towards increased activities of ∆6 and ∆5 desaturases in transgenic mice in comparison to control animals [18].
The anti-inflammatory fatty acid index, calculated as (docosapentaenoic acid + docosahexaenoic acid + dihomo-γ-linolenic acid + eicosapentaenoic acid) × 100/arachidonic acid [30], increased after TTA treatment.
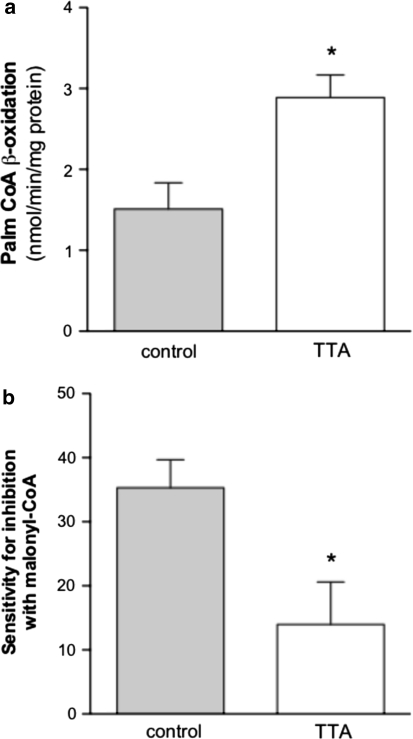
Hepatic β-Oxidation of Fatty Acids
The TAG-lowering effect observed in plasma of hTNAα transgenic mice after TTA treatment, could be due to an enhanced mitochondrial and peroxisomal β-oxidation that is responsible for the shortening of long-chain fatty acyl-CoAs. Indeed, β-oxidation increased by 48% in the TTA treated mice, when using 14C-palmitoyl-CoA as substrate (Fig. 3a), counterbalancing the decreased β-oxidation values from transgenic mice [18]. In the previous study, the sensitivity towards malonyl-CoA, an inhibitor of CPT-I, was unaffected by TNFα [18]. However, in the TTA treated group we detected a lowering of the inhibition capacity of malonyl-CoA from 35% to 14% inhibition (Fig. 3b) and the transport of fatty acids across the mitochondrial outer membrane is facilitated even in the presence of malonyl-CoA.
Fig. 3.
Palmitoyl-CoA β-oxidation is increased in liver mitochondria of TTA-treated mice (a) and the sensitivity for inhibition of oxidation of palmitoyl-CoA with malonyl-CoA (given in % inhibition) is lower in the TTA group compared to high fat-fed controls (b). Oxidation of palmitoyl-CoA was measured in purified mitochondria as acid-soluble products. Bars indicate ± S.D. (n = 5). * P < 0.05 different from the control. Palm-CoA, palmitoyl-CoA; TTA, tetradecylthioacetic acid
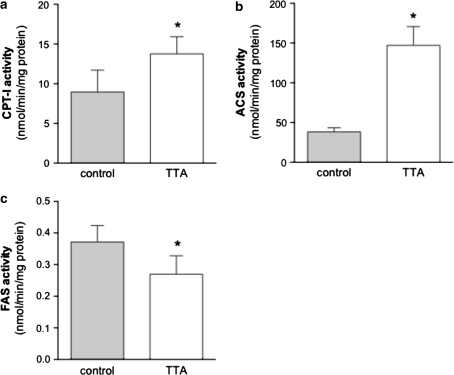
Activities of Enzymes Involved in Degradation and Biosynthesis of Fatty Acids
In order to explain the mechanisms of TTA in more detail, enzyme activities were measured in the post-nuclear fraction of the liver. The enzymatic activity of CPT-I, the rate-limiting enzyme of mitochondrial β-oxidation, involved in acyl group transport into the matrix, was increased after TTA treatment (Fig. 4a). Likewise, the mitochondrial enzyme acyl-CoA synthetase (ACS) that activates fatty acids prior to oxidation, showed increased activity (Fig. 4b). On the other hand, a major cytosolic, multi-functional enzyme involved in the biosynthesis of fatty acids, called fatty acid synthase (FAS), showed reduced activity (Fig. 4c).
Fig. 4.
Enzyme activities of the mitochondrial enzymes CPT-I (a) and ACS (b) involved in β-oxidation are increased in the liver of hTNFα transgenic mice treated with TTA, whereas the cytosolic FAS activity involved in biosynthesis of fatty acids shows reduced activity after two weeks of treatment (c). Values are means ± S.D. (n = 5) and * shows a P < 0.05 difference from the control. CPT-I, carnitine palmitoyl transferase I; ACS, acyl-CoA synthetase; FAS, fatty acid synthase; TTA, tetradecylthioacetic acid
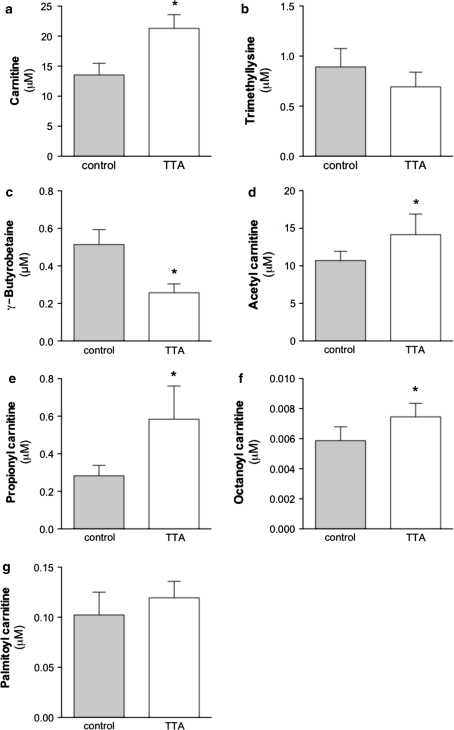
Oxidation of fatty acids can be facilitated not only by the up-regulation of CPT-I, but also by an increased concentration of its substrate carnitine, to ensure efficient transport of fatty acids into mitochondria. Carnitine can be taken up from dietary sources or is endogenously synthesized primarily in the liver and kidney [37, 38]. All other tissues have to take carnitine actively up from the blood. We found that in plasma, free carnitine concentrations have increased during TTA treatment by 36% in comparison to control animals (Fig. 5a). The precursors for carnitine, were either unchanged (trimethyllysine, Fig. 5b) or reduced (γ-butyrobetaine, Fig. 5c). The two quantitatively most important acyl-esters, acetyl carnitine (Fig. 5d) and propionyl carnitine (Fig. 5e) were significantly increased by TTA compared to high fat-fed controls. The plasma medium-chain acylcarnitine, octanoyl carnitine, was significantly increased (Fig. 5f) and the long-chain acylcarnitine, palmitoyl carnitine, remained unchanged by TTA treatment (Fig. 5g).
Fig. 5.
A change in plasma levels of free carnitine (a), trimethyllysine (b), γ-butyrobetaine (c), acetyl carnitine (d), propionyl carnitine (e), octanoyl carnitine (f), and palmitoyl carnitine (g) could be detected after TTA treatment. * denotes statistical significant differences by Student’s t-test between control (grey bars) and TTA (white bars) (P < 0.05) and bars indicate ± S.D. (n = 5)
Discussion
Obesity-related metabolic disorders are associated with a state of chronic low-intensity inflammation. Inflammation induces a wide array of metabolic changes in the body and affects expression of many proteins involved in lipid metabolism. The different subtypes of the nuclear ligand-dependent transcription factors, PPAR, play key roles in lipid homeostasis. They are involved in the regulation of lipoprotein metabolism, fatty acid oxidation, glucose and carnitine homeostasis and also seem to be involved in inflammatory processes [39]. TNFα is one of the factors that mediate alterations in lipid metabolism, including decreased PPARα mRNA and protein levels [40]. With the knowledge in mind that previous studies have shown that the biological response to TTA treatment is at least partly a result of PPAR activation, and that this bioactive fatty acid analogue has the ability to activate all three isoforms of PPAR [21, 23, 41], as well as increase the mRNA level of PPARα [42], we wanted to test if TTA was able to counteract TNFα-induced metabolic aberrations.
In the present study we demonstrated that TTA, in addition to its health-promoting effects in previous in vivo [43] and in vitro experiments [26, 44], could also ameliorate several parameters in a mouse model of chronic inflammation. TNFα might induce hypertriglyceridemia, characterized by the accumulation of very low density lipoprotein (VLDL) in the plasma, due to impaired removal of VLDL [45] and increased hepatic lipogenesis [46]. TAG-rich lipoproteins were shown to be important for the acute phase response by binding to endotoxins to reduce their harmful action [47]. However, systemic low-grade inflammation with elevated circulating levels of TNFα seen in obese subjects is typically associated with high plasma TAG levels that will increase the risk for metabolic and cardiovascular complications [48]. It is therefore of importance to note that TTA has the capability to strongly reduce plasma TAG levels in a chronic inflammatory state with persistently high TNFα levels.
The observation of decreased TAG in the plasma is compatible with the hypothesis that there is a higher flux of fatty acids from the plasma to the liver, with a simultaneous reduction in fatty acid biosynthesis. But even though TTA up-regulates hepatic mitochondrial CPT-I and ACS activities, accompanied by an increase in hepatic β-oxidation and plasma carnitine levels, it is not sufficient to deal with the already existing high TAG levels in the transgenic mice. It is noteworthy that TTA has the capability to downregulate the hepatic biosynthesis of fatty acids, since the FAS activity is reduced by 27% in the TTA treated animals. Reduced lipogenesis and increased fatty acid oxidation after TTA treatment seem to be the reason for decreased plasma TAG levels by 54% after TTA treatment. Yet, the exact reasons why hepatic TAG were not affected similarly by TTA are not yet elucidated at the molecular level and will require further detailed studies. It might be that a higher dose of TTA is required to get a more pronounced effect on lipogenesis, thereby influencing hepatic TAG levels. Previously, we have found indications that the effect on hepatic TAG metabolism may be dose dependent and that the amount of hepatic TAG is decreased with increasing doses of TTA [49]. It could also be that a longer feeding period than the two weeks applied is needed to overcome hepatic TAG accumulation. Noteworthy is that we have never detected a TTA-induced liver steatosis in any of the mouse or rat models used previously. It is however of importance to verify in future studies if increasing amounts of TTA could counteract this effect in the mouse model used and if this finding is of significance for humans.
The increased hepatic phospholipid level that we measure in the TTA treated group, is probably a consequence of increased membrane production due to mitochondrial and peroxisomal proliferation as seen after TTA treatment previously [50–52]. Moreover, administration of PPARα ligands has been described to lead to hepatic hypertrophy and hyperplasia leading to increased liver weights as seen in our study. The liver enlargement is however restricted to rodents and has never been described in humans [53–58].
By measuring plasma free carnitine and its acyl-esters, in particular the two most abundant species, acetyl carnitine and propionyl carnitine, we looked at overall changes in the β-oxidation process. We found that plasma free carnitine levels were increased due to TTA administration, as well as the short- and medium-chain carnitine esters, but not long-chain fatty acyl carnitines. It is important to note that in addition to being essential for transport of fatty acids into mitochondria, carnitine is crucial to increase acyl and acetyl group export out of mitochondria into the blood [59]. By increasing carnitine levels through TTA administration, lipotoxicity of β-oxidation metabolites is reduced and mitochondrial capacity is improved. The excess short-chain acyl-esters, that cannot be used by the TCA cycle, will be secreted in urine. The formation of trimethyllysine and its conversion to γ-butyrobetaine is reported to occur in most tissues, but the last step in carnitine biosynthesis, the hydroxylation of γ-butyrobetaine to carnitine, occurs only in liver and kidney in mice. Therefore interorgan transport of γ-butyrobetaine and carnitine are of importance. It was of interest that plasma γ-butyrobetaine levels were decreased, whereas carnitine levels were increased. This may be due to increased consumption of γ-butyrobetaine for carnitine biosynthesis and increased mitochondrial oxidation of long-chain fatty acids.
TTA affects the capacity for insertion of double bonds and counteracts the abnormal hepatic fatty acid composition levels under the influence of TNFα. Correct proportions of fatty acids are crucial to maintain cellular functions, a balance that was disturbed in the TNFα transgenic mice. hTNFα overexpression was accompanied by increased saturated and polyunsaturated fatty acids, but decreased levels of monounsaturated fatty acids in liver [18]. The basic values of these fatty acid families in hTNFα control group in our high fat diet experiment compared to the previous study were less pronounced, which could be partially attributed to a different diet in [18] (low fat standard mouse chow with 26 % SFA, 25 % MUFA, 4 % PUFAn-3 and 45 % PUFAn-6). Nevertheless, it did not affect the trends of fatty acid changes inside the dietary groups (high fat diet and standard chow diet, respectively). Moreover, TTA reduced the n-6 ∆5 desaturase activity index in transgenic mice as fatty acid saturation was increased. This was accompanied by decreases of n-3 and n-6 polyunsaturated fatty acids including stearidonic acid (C18:4n-3) and docosahexaenoic acid (C22:6n-3), as well as linoleic acid (C18:2n-6) and arachidonic acid (C20:4n-6). The amount of arachidonic acid is of importance for the calculation of the anti-inflammatory fatty acid index, and decreased amounts after TTA treatment increased the fatty anti-inflammatory index in this group. Saturated fatty acids, that have pro-inflammatory and insulin-antagonizing properties [60], were decreased by TTA, thereby lowering the risk for the development of metabolic syndrome. On the other hand, monounsaturated fatty acids were increased after TTA treatment. This is potentially beneficial, as high levels were associated with low rates of cardiovascular disease [61]. Another risk factor for cardiovascular disease in humans is low HDL-cholesterol due to its important function in reverse cholesterol transport to the liver [62]. We found elevated plasma HDL-cholesterol levels with an increase of 63% in TTA treated TNFα transgenic mice. However, in a study including a small group of HIV-infected patients, we found that TTA in combination with dietary intervention can reduce total cholesterol, LDL-cholesterol, and LDL/HDL cholesterol, but no increase in HDL-cholesterol could be observed [63]. It has been shown previously that apolipoprotein compositions and responses to PPARα activation are different between humans and rodents [64, 65]. The result of increased HDL-cholesterol in hTNFα overexpressed mice in our study might therefore be rodent-specific. However, effects of PPARα activation on other pathways of lipid metabolism including FA uptake and activation, β-oxidation, and lipogenesis support similarities between mice and humans [39]. This suggests similar responses upon TTA treatment in humans as seen in our mice study.
In summary, given the ability of TTA to increase β-oxidation, reduce plasma TAG, and positively affect hepatic saturated and monounsaturated fatty acid compositions leading to an increase in the anti-inflammatory fatty acid index, indicates that TTA has a high potential to ameliorate chronic inflammation such as with obesity, arthritis or atherosclerosis.
Acknowledgments
We thank Kari Williams, Liv Kristine Øysæd, Randi Sandvik, Svein Krüger, and Torunn Eide for excellent technical assistance. This work was supported by grants from NordForsk, grant no. 070010, MitoHealth; EEA Polish-Norwegian Research Fund, grant no. PNRF-104-Al-1/07; the Research Council of Norway, grant no. 190287/110; and the European Community’s Seventh Framework Programme (FP7/2007-2013), grant no. 201668, AtheroRemo.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- ACC
Acetyl-CoA carboxylase
- ACS
Acyl-CoA synthetase
- CPT-I and -II
Carnitine palmitoyltransferase-I and -II
- FAO
Fatty acyl-CoA oxidase
- FAS
Fatty acid synthase
- HDL
High-density lipoprotein
- HMG-CoA synthase
3-Hydroxy-3-methylglutaryl-coenzyme A synthase
- HPLC
High-performance liquid chromatography
- MUFA
Monounsaturated fatty acid(s)
- PPAR
Peroxisome proliferator-activated receptor(s)
- PUFA
Polyunsaturated fatty acid(s)
- SFA
Saturated fatty acid(s)
- TAG
Triacylglycerol
- TCA
Tricarboxylic acid cycle (Krebs cycle)
- hTNFα
Human tumor necrosis factor α
- TTA
Tetradecylthioacetic acid
- VLDL
Very low density lipoprotein
References
- 1.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 3.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 4.Beier K, Volkl A, Fahimi HD. Suppression of peroxisomal lipid beta-oxidation enzymes of TNF-alpha. FEBS Lett. 1992;310:273–276. doi: 10.1016/0014-5793(92)81347-O. [DOI] [PubMed] [Google Scholar]
- 5.Grunfeld C, Verdier JA, Neese R, Moser AH, Feingold KR. Mechanisms by which tumor necrosis factor stimulates hepatic fatty acid synthesis in vivo. J Lipid Res. 1988;29:1327–1335. [PubMed] [Google Scholar]
- 6.Gasic S, Tian B, Green A. Tumor necrosis factor alpha stimulates lipolysis in adipocytes by decreasing Gi protein concentrations. J Biol Chem. 1999;274:6770–6775. doi: 10.1074/jbc.274.10.6770. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa H, Nielsen S, Kawakami M. Cachectin/tumor necrosis factor and interleukin-1 show different modes of combined effect on lipoprotein lipase activity and intracellular lipolysis in 3T3–L1 cells. Biochim Biophys Acta. 1989;1003:131–135. doi: 10.1016/0005-2760(89)90246-4. [DOI] [PubMed] [Google Scholar]
- 8.Plomgaard P, Fischer CP, Ibfelt T, Pedersen BK, van Hall G. Tumor necrosis factor-alpha modulates human in vivo lipolysis. J Clin Endocrinol Metab. 2008;93:543–549. doi: 10.1210/jc.2007-1761. [DOI] [PubMed] [Google Scholar]
- 9.Ryden M, Dicker A, van Harmelen V, Hauner H, Brunnberg M, Perbeck L, Lonnqvist F, Arner P. Mapping of early signaling events in tumor necrosis factor-alpha-mediated lipolysis in human fat cells. J Biol Chem. 2002;277:1085–1091. doi: 10.1074/jbc.M109498200. [DOI] [PubMed] [Google Scholar]
- 10.Pape ME, Kim KH. Effect of tumor necrosis factor on acetyl-coenzyme A carboxylase gene expression and preadipocyte differentiation. Mol Endocrinol. 1988;2:395–403. doi: 10.1210/mend-2-5-395. [DOI] [PubMed] [Google Scholar]
- 11.Sumida M, Sekiya K, Okuda H, Tanaka Y, Shiosaka T. Inhibitory effect of tumor necrosis factor on gene expression of hormone sensitive lipase in 3T3–L1 adipocytes. J Biochem. 1990;107:1–2. doi: 10.1093/oxfordjournals.jbchem.a122990. [DOI] [PubMed] [Google Scholar]
- 12.Memon RA, Grunfeld C, Moser AH, Feingold KR. Tumor necrosis factor mediates the effects of endotoxin on cholesterol and triglyceride metabolism in mice. Endocrinology. 1993;132:2246–2253. doi: 10.1210/en.132.5.2246. [DOI] [PubMed] [Google Scholar]
- 13.Memon RA, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. In vivo and in vitro regulation of sterol 27-hydroxylase in the liver during the acute phase response. potential role of hepatocyte nuclear factor-1. J Biol Chem. 2001;276:30118–30126. doi: 10.1074/jbc.M102516200. [DOI] [PubMed] [Google Scholar]
- 14.Hector J, Schwarzloh B, Goehring J, Strate TG, Hess UF, Deuretzbacher G, Hansen-Algenstaedt N, Beil FU, Algenstaedt P. TNF-alpha alters visfatin and adiponectin levels in human fat. Horm Metab Res. 2007;39:250–255. doi: 10.1055/s-2007-973075. [DOI] [PubMed] [Google Scholar]
- 15.Komai N, Morita Y, Sakuta T, Kuwabara A, Kashihara N. Anti-tumor necrosis factor therapy increases serum adiponectin levels with the improvement of endothelial dysfunction in patients with rheumatoid arthritis. Mod Rheumatol. 2007;17:385–390. doi: 10.1007/s10165-007-0605-8. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Yang G, Shi S, Yang M, Liu H, Boden G. The adipose triglyceride lipase, adiponectin and visfatin are downregulated by tumor necrosis factor-alpha (TNF-alpha) in vivo. Cytokine. 2009;45:12–19. doi: 10.1016/j.cyto.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Xun K, Chen L, Wang Y. TNF-alpha, a potent lipid metabolism regulator. Cell Biochem Funct. 2009;27:407–416. doi: 10.1002/cbf.1596. [DOI] [PubMed] [Google Scholar]
- 18.Glosli H, Gudbrandsen OA, Mullen AJ, Halvorsen B, Rost TH, Wergedahl H, Prydz H, Aukrust P, Berge RK. Down-regulated expression of PPARalpha target genes, reduced fatty acid oxidation and altered fatty acid composition in the liver of mice transgenic for hTNFalpha. Biochim Biophys Acta. 2005;1734:235–246. doi: 10.1016/j.bbalip.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 20.Lau SM, Brantley RK, Thorpe C. The reductive half-reaction in Acyl-CoA dehydrogenase from pig kidney: studies with thiaoctanoyl-CoA and oxaoctanoyl-CoA analogues. Biochemistry. 1988;27:5089–5095. doi: 10.1021/bi00414a021. [DOI] [PubMed] [Google Scholar]
- 21.Berge K, Tronstad KJ, Flindt EN, Rasmussen TH, Madsen L, Kristiansen K, Berge RK. Tetradecylthioacetic acid inhibits growth of rat glioma cells ex vivo and in vivo via PPAR-dependent and PPAR-independent pathways. Carcinogenesis. 2001;22:1747–1755. doi: 10.1093/carcin/22.11.1747. [DOI] [PubMed] [Google Scholar]
- 22.Raspe E, Madsen L, Lefebvre AM, Leitersdorf I, Gelman L, Peinado-Onsurbe J, Dallongeville J, Fruchart JC, Berge R, Staels B. Modulation of rat liver apolipoprotein gene expression and serum lipid levels by tetradecylthioacetic acid (TTA) via PPARalpha activation. J Lipid Res. 1999;40:2099–2110. [PubMed] [Google Scholar]
- 23.Westergaard M, Henningsen J, Svendsen ML, Johansen C, Jensen UB, Schroder HD, Kratchmarova I, Berge RK, Iversen L, Bolund L, Kragballe K, Kristiansen K. Modulation of keratinocyte gene expression and differentiation by PPAR-selective ligands and tetradecylthioacetic acid. J Invest Dermatol. 2001;116:702–712. doi: 10.1046/j.1523-1747.2001.01329.x. [DOI] [PubMed] [Google Scholar]
- 24.Berge RK, Tronstad KJ, Berge K, Rost TH, Wergedahl H, Gudbrandsen OA, Skorve J. The metabolic syndrome and the hepatic fatty acid drainage hypothesis. Biochimie. 2005;87:15–20. doi: 10.1016/j.biochi.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Lovas K, Rost TH, Skorve J, Ulvik RJ, Gudbrandsen OA, Bohov P, Wensaas AJ, Rustan AC, Berge RK, Husebye ES. Tetradecylthioacetic acid attenuates dyslipidaemia in male patients with type 2 diabetes mellitus, possibly by dual PPAR-alpha/delta activation and increased mitochondrial fatty acid oxidation. Diabetes Obes Metab. 2009;11:304–314. doi: 10.1111/j.1463-1326.2008.00958.x. [DOI] [PubMed] [Google Scholar]
- 26.Dyroy E, Yndestad A, Ueland T, Halvorsen B, Damas JK, Aukrust P, Berge RK. Antiinflammatory effects of tetradecylthioacetic acid involve both peroxisome proliferator-activated receptor alpha-dependent and -independent pathways. Arterioscler Thromb Vasc Biol. 2005;25:1364–1369. doi: 10.1161/01.ATV.0000171982.57713.96. [DOI] [PubMed] [Google Scholar]
- 27.Hayward MD, Jones BK, Saparov A, Hain HS, Trillat AC, Bunzel MM, Corona A, Li-Wang B, Strenkowski B, Giordano C, Shen H, Arcamone E, Weidlick J, Vilensky M, Tugusheva M, Felkner RH, Campbell W, Rao Y, Grass DS, Buiakova O. An extensive phenotypic characterization of the hTNFalpha transgenic mice. BMC Physiol. 2007;7:13. doi: 10.1186/1472-6793-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spydevold O, Bremer J. Induction of peroxisomal beta-oxidation in 7800 C1 Morris hepatoma cells in steady state by fatty acids and fatty acid analogues. Biochim Biophys Acta. 1989;1003:72–79. doi: 10.1016/0005-2760(89)90101-x. [DOI] [PubMed] [Google Scholar]
- 29.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 30.Chavali SR, Zhong WW, Utsunomiya T, Forse RA. Decreased production of interleukin-1-beta, prostaglandin-E2 and thromboxane-B2, and elevated levels of interleukin-6 and -10 are associated with increased survival during endotoxic shock in mice consuming diets enriched with sesame seed oil supplemented with Quil-A saponin. Int Arch Allergy Immunol. 1997;114:153–160. doi: 10.1159/000237661. [DOI] [PubMed] [Google Scholar]
- 31.Utsunomiya T, Chavali SR, Zhong WW, Forse RA. Effects of sesamin-supplemented dietary fat emulsions on the ex vivo production of lipopolysaccharide-induced prostanoids and tumor necrosis factor alpha in rats. Am J Clin Nutr. 2000;72:804–808. doi: 10.1093/ajcn/72.3.804. [DOI] [PubMed] [Google Scholar]
- 32.Vernez L, Wenk M, Krahenbuhl S. Determination of carnitine and acylcarnitines in plasma by high-performance liquid chromatography/electrospray ionization ion trap tandem mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:1233–1238. doi: 10.1002/rcm.1470. [DOI] [PubMed] [Google Scholar]
- 33.Berge RK, Flatmark T, Osmundsen H. Enhancement of long-chain acyl-CoA hydrolase activity in peroxisomes and mitochondria of rat liver by peroxisomal proliferators. Eur J Biochem. 1984;141:637–644. doi: 10.1111/j.1432-1033.1984.tb08239.x. [DOI] [PubMed] [Google Scholar]
- 34.Madsen L, Garras A, Asins G, Serra D, Hegardt FG, Berge RK. Mitochondrial 3-hydroxy-3-methylglutaryl coenzyme A synthase and carnitine palmitoyltransferase II as potential control sites for ketogenesis during mitochondrion and peroxisome proliferation. Biochem Pharmacol. 1999;57:1011–1019. doi: 10.1016/S0006-2952(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 35.Madsen L, Froyland L, Dyroy E, Helland K, Berge RK. Docosahexaenoic and eicosapentaenoic acids are differently metabolized in rat liver during mitochondria and peroxisome proliferation. J Lipid Res. 1998;39:583–593. [PubMed] [Google Scholar]
- 36.Skorve J, al-Shurbaji A, Asiedu D, Bjorkhem I, Berglund L, Berge RK. On the mechanism of the hypolipidemic effect of sulfur-substituted hexadecanedioic acid (3-thiadicarboxylic acid) in normolipidemic rats. J Lipid Res. 1993;34:1177–1185. [PubMed] [Google Scholar]
- 37.Rebouche CJ, Seim H. Carnitine metabolism and its regulation in microorganisms and mammals. Annu Rev Nutr. 1998;18:39–61. doi: 10.1146/annurev.nutr.18.1.39. [DOI] [PubMed] [Google Scholar]
- 38.Vaz FM, Wanders RJ. Carnitine biosynthesis in mammals. Biochem J. 2002;361:417–429. doi: 10.1042/0264-6021:3610417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burri L, Thoresen GH, Berge RK (2010) The role of PPARalpha activation in liver and muscle. PPAR Res 2010 pii: 542359 [DOI] [PMC free article] [PubMed]
- 40.Beier K, Volkl A, Fahimi HD. TNF-alpha downregulates the peroxisome proliferator activated receptor-alpha and the mRNAs encoding peroxisomal proteins in rat liver. FEBS Lett. 1997;412:385–387. doi: 10.1016/S0014-5793(97)00805-3. [DOI] [PubMed] [Google Scholar]
- 41.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gudbrandsen OA, Rost TH, Berge RK. Causes and prevention of tamoxifen-induced accumulation of triacylglycerol in rat liver. J Lipid Res. 2006;47:2223–2232. doi: 10.1194/jlr.M600148-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Berge RK, Skorve J, Tronstad KJ, Berge K, Gudbrandsen OA, Grav H. Metabolic effects of thia fatty acids. Curr Opin Lipidol. 2002;13:295–304. doi: 10.1097/00041433-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Aukrust P, Wergedahl H, Muller F, Ueland T, Dyroy E, Damas JK, Froland SS, Berge RK. Immunomodulating effects of 3-thia fatty acids in activated peripheral blood mononuclear cells. Eur J Clin Invest. 2003;33:426–433. doi: 10.1046/j.1365-2362.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- 45.Kaufmann RL, Matson CF, Beisel WR. Hypertriglyceridemia produced by endotoxin: role of impaired triglyceride disposal mechanisms. J Infect Dis. 1976;133:548–555. doi: 10.1093/infdis/133.5.548. [DOI] [PubMed] [Google Scholar]
- 46.Feingold KR, Grunfeld C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987;80:184–190. doi: 10.1172/JCI113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris HW, Grunfeld C, Feingold KR, Read TE, Kane JP, Jones AL, Eichbaum EB, Bland GF, Rapp JH. Chylomicrons alter the fate of endotoxin, decreasing tumor necrosis factor release and preventing death. J Clin Invest. 1993;91:1028–1034. doi: 10.1172/JCI116259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardman AE. Physical activity, obesity and blood lipids. Int J Obes Relat Metab Disord. 1999;23(Suppl 3):S64–S71. doi: 10.1038/sj.ijo.0800886. [DOI] [PubMed] [Google Scholar]
- 49.Skorve J, Berge RK. The hypocholesterolemic effect of sulfur-substituted fatty acid analogues in rats fed a high carbohydrate diet. Biochim Biophys Acta. 1993;1167:175–181. doi: 10.1016/0005-2760(93)90159-7. [DOI] [PubMed] [Google Scholar]
- 50.Berge RK, Aarsland A, Kryvi H, Bremer J, Aarsaether N. Alkylthio acetic acids (3-thia fatty acids)–a new group of non-beta-oxidizable peroxisome-inducing fatty acid analogues–II. Dose-response studies on hepatic peroxisomal- and mitochondrial changes and long-chain fatty acid metabolizing enzymes in rats. Biochem Pharmacol. 1989;38:3969–3979. doi: 10.1016/0006-2952(89)90676-X. [DOI] [PubMed] [Google Scholar]
- 51.Froyland L, Helland K, Totland GK, Kryvi H, Berge RK. A hypolipidemic peroxisome proliferating fatty acid induces polydispersity of rat liver mitochondria. Biol Cell. 1996;87:105–112. doi: 10.1016/S0248-4900(97)89842-5. [DOI] [PubMed] [Google Scholar]
- 52.Totland GK, Madsen L, Klementsen B, Vaagenes H, Kryvi H, Froyland L, Hexeberg S, Berge RK. Proliferation of mitochondria and gene expression of carnitine palmitoyltransferase and fatty acyl-CoA oxidase in rat skeletal muscle, heart and liver by hypolipidemic fatty acids. Biol Cell. 2000;92:317–329. doi: 10.1016/S0248-4900(00)01077-7. [DOI] [PubMed] [Google Scholar]
- 53.Ashby J, Brady A, Elcombe CR, Elliott BM, Ishmael J, Odum J, Tugwood JD, Kettle S, Purchase IF. Mechanistically-based human hazard assessment of peroxisome proliferator-induced hepatocarcinogenesis. Hum Exp Toxicol. 1994;13(Suppl 2):S1–S117. doi: 10.1177/096032719401300201. [DOI] [PubMed] [Google Scholar]
- 54.Bentley P, Calder I, Elcombe C, Grasso P, Stringer D, Wiegand HJ. Hepatic peroxisome proliferation in rodents and its significance for humans. Food Chem Toxicol. 1993;31:857–907. doi: 10.1016/0278-6915(93)90225-N. [DOI] [PubMed] [Google Scholar]
- 55.Hays T, Rusyn I, Burns AM, Kennett MJ, Ward JM, Gonzalez FJ, Peters JM. Role of peroxisome proliferator-activated receptor-alpha (PPARalpha) in bezafibrate-induced hepatocarcinogenesis and cholestasis. Carcinogenesis. 2005;26:219–227. doi: 10.1093/carcin/bgh285. [DOI] [PubMed] [Google Scholar]
- 56.Peters JM, Cheung C, Gonzalez FJ. Peroxisome proliferator-activated receptor-alpha and liver cancer: where do we stand? J Mol Med. 2005;83:774–785. doi: 10.1007/s00109-005-0678-9. [DOI] [PubMed] [Google Scholar]
- 57.Rao MS, Reddy JK. An overview of peroxisome proliferator-induced hepatocarcinogenesis. Environ Health Perspect. 1991;93:205–209. doi: 10.1289/ehp.9193205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reddy JK, Rao MS. Malignant tumors in rats fed nafenopin, a hepatic peroxisome proliferator. J Natl Cancer Inst. 1977;59:1645–1650. doi: 10.1093/jnci/59.6.1645. [DOI] [PubMed] [Google Scholar]
- 59.Mynatt RL. Carnitine and type 2 diabetes. Diabetes Metab Res Rev. 2009;25(Suppl 1):S45–S49. doi: 10.1002/dmrr.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79:969–973. doi: 10.1093/ajcn/79.6.969. [DOI] [PubMed] [Google Scholar]
- 61.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61:1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 62.Yaari S, Goldbourt, Even-Zohar S, Neufeld HN (1981) Associations of serum high density lipoprotein and total cholesterol with total, cardiovascular, and cancer mortality in a 7-year prospective study of 10 000 men. Lancet 1:1011-1015 [DOI] [PubMed]
- 63.Fredriksen J, Ueland T, Dyroy E, Halvorsen B, Melby K, Melbye L, Skalhegg BS, Bohov P, Skorve J, Berge RK, Aukrust P, Froland SS. Lipid-lowering and anti-inflammatory effects of tetradecylthioacetic acid in HIV-infected patients on highly active antiretroviral therapy. Eur J Clin Invest. 2004;34:709–715. doi: 10.1111/j.1365-2362.2004.01410.x. [DOI] [PubMed] [Google Scholar]
- 64.Rakhshandehroo M, Hooiveld G, Muller M, Kersten S. Comparative analysis of gene regulation by the transcription factor PPARalpha between mouse and human. PLoS One. 2009;4:e6796. doi: 10.1371/journal.pone.0006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramaswamy M, Wallace TL, Cossum PA, Wasan KM. Species differences in the proportion of plasma lipoprotein lipid carried by high-density lipoproteins influence the distribution of free and liposomal nystatin in human, dog, and rat plasma. Antimicrob Agents Chemother. 1999;43:1424–1428. doi: 10.1128/aac.43.6.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]