Abstract
Aims/hypothesis
Traditional blood glucose lowering agents do not prevent the progressive loss of beta cell function in patients with type 2 diabetes. The dipeptidylpeptidase (DPP)-4 inhibitor vildagliptin improves beta cell function both acutely and chronically (up to 2 years). Whether this effect persists after cessation of treatment remains unknown. Here, we assessed the insulin secretory capacity in drug-naive patients with type 2 diabetes after a 52 week treatment period with vildagliptin or placebo, and again after a 12 week washout period.
Methods
This study was conducted at a single university medical centre, and was a double-blind, randomised clinical trial in 59 drug-naive patients with type 2 diabetes and mild hyperglycaemia to either vildagliptin 100 mg (n = 29) or placebo (n = 30). Randomisation was performed by a validated 1:1 system. Neither patient, nor caregiver, was informed about the assigned treatment. Inclusion criteria were drug-naive patients ≥30 years, with HbA1c ≤7.5% and BMI of 22–45 kg/m2. The mildly hyperglycaemic patient population was chosen to minimise glucose toxicity as a confounding variable. Beta-cell function was measured during an arginine-stimulated hyperglycaemic clamp at week 0, week 52 and after a 12 week washout period. All patients with at least one post-randomisation measure were analysed (intent-to-treat).
Results
Fifty-two week vildagliptin 100 mg (n = 26) treatment increased the primary efficacy variable, combined hyperglycaemia and arginine-stimulated C-peptide secretion (AIRarg), by 5.0 ± 1.8 nmol/l × min, while it decreased by 0.8 ± 1.8 nmol/l × min with placebo (n = 25) (between-group difference p = 0.030). No significant between-group difference in AIRarg was seen after the 12 week washout period. The between-group difference adjusted mean 52 week changes from baseline was −0.19 ± 0.11, p = 0.098 and −0.22 ± 0.23%, p = 0.343 for HbA1c and fasting plasma glucose, respectively. There were no suspected drug treatment-related serious adverse events.
Conclusions/interpretation
One year treatment with vildagliptin significantly increased beta cell secretory capacity. This effect was not maintained after the washout, indicating that this increased capacity was not a disease modifying effect on beta cell mass and/or function.
Trial registration:
ClinicalTrials.gov NCT00260156
Funding:
This study was sponsored by the Novartis Pharmaceutical Cooperation.
Keywords: Beta cell function, Hyperglycaemic clamp, Randomised clinical trial, Type 2 diabetes, Vildagliptin
Introduction
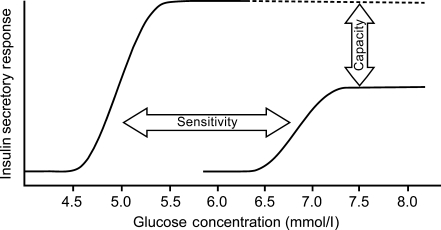
The relationship between the insulin secretion rate and increasing glucose concentration is the most basic reflection of beta cell function. This relationship includes components of sensitivity and capacity [1] as outlined in Fig. 1.
Fig. 1.
Relationship between the insulin secretion rate and increasing glucose concentration
The sensitivity of insulin secretion to glucose is an early defect in beta cell function [2]. The capacity to secrete insulin is thought to be related to beta cell mass, which is determined by the balance of the rate of beta cell neogenesis and apoptosis. Insulin secretory capacity [2] and beta cell mass [3, 4] are known to be reduced in patients with type 2 diabetes. Glucagon-like peptide (GLP)-1 increases beta cell mass via increased beta cell neogenesis and decreased beta cell apoptosis in animal models [5] and the levels of GLP-1 required for these effects are in the range seen with dipeptidylpeptidase (DPP)-4 inhibitors [6].
The DPP-4 inhibitor vildagliptin has been shown to increase beta cell function in humans as a result of improved sensitivity of beta cells to glucose [7]. Furthermore, after 12 weeks of vildagliptin treatment, a small increase in the capacity for insulin secretion has been shown [8]. The present study was designed to test the hypothesis that this capacity would increase further after 1 year of vildagliptin treatment and that, if the increased capacity was due to a disease modifying effect on beta cell mass, it would still be present after a 3 month washout.
Methods
Subjects and study design
This was a single centre, double-blind, randomised, parallel-group study that was designed to investigate the mechanisms by which vildagliptin improves beta cell function. The study was not designed to investigate the therapeutic potential of vildagliptin on glycaemic efficacy in patients with type 2 diabetes. In total, 59 drug-naive patients with type 2 diabetes and mild hyperglycaemia were randomly assigned to receive vildagliptin 100 mg once daily or placebo (1:1 ratio). No treatment with oral glucose lowering agents for at least 12 weeks prior to study entry, or for >3 consecutive months at any time in the past, was allowed.
The mildly hyperglycaemic patient population was chosen to minimise glucose toxicity as a confounding variable. All patients received lifestyle counselling. Inclusion criteria were age ≥30 years, HbA1c ≤7.5%, and BMI of 22–45 kg/m2. The study protocol was approved by the ethics review committee and was in accordance with the principles described in the declaration of Helsinki. All participating patients gave their written informed consent before screening.
Study endpoints
Insulin secretion and sensitivity were measured during a combined euglycaemic–hyperinsulinaemic and hyperglycaemic clamp procedure, as previously described [9]. First and second phase C-peptide secretion were calculated as the incremental (i)AUC180-190min and iAUC240-260min. Arginine-stimulated C-peptide secretion (AIRarg) was calculated as the iAUC260–270min. All incremental AUCs were calculated above the C-peptide concentration just prior to the start of the hyperglycaemic clamp procedure (average of 170 and 175 min). Arginine was administered during the hyperglycaemic clamp to measure insulin secretory capacity at a steady-state glucose concentration of 15 mmol/l. Clamps were performed prior to randomisation, following 52 weeks of treatment, and after a 12 week off-drug period (week 64). Following an overnight fast, an indwelling cannula was inserted into an antecubital vein for infusion of glucose and insulin. To obtain arterialised venous blood samples, an indwelling cannula was inserted in a retrograde fashion into a dorsal hand or wrist vein and maintained in a heated box at 50°C. During the clamp at week 52, patients received their study medication at the protocol specified time, i.e. 15 min prior to the start of the euglycaemic–hyperinsulinaemic and hyperglycaemic clamp procedure.
HbA1c (using a DCCT standardised HA 8160 analyser, Menarini, Florence, Italy; normal range: 4.3–6.1%), fasting plasma glucose (FPG), and safety variables were measured prior to randomisation and during each follow-up visit until the end of the 12 week off-drug period, by a central laboratory (Covance, Geneva, Switzerland). Plasma glucose concentrations during the clamp were measured at the bedside using a YSI 2300 STAT Plus (Yellow Springs Instruments, Yellow Springs, OH, USA). C-peptide samples were analysed at the VU University Medical Centre using an immunoradiometric assay (Centaur; Bayer Diagnostics, Mijdrecht, the Netherlands).
Statistical analysis
A sample size of 30 patients per group was required to provide 90% power to detect a significant between-group difference in AIRarg. All patients randomised with at least one post-randomisation measure were analysed, i.e. intent-to-treat. All outcome measures are compared between the two treatment groups using an analysis of covariance (ANCOVA) model. The dependent variable used in the model is the change from pre-treatment for the beta cell function variables (AIRarg, first phase, second phase). For all other endpoints the dependent value used is the mean at the corresponding visit. The model included a factor for treatment group (vildagliptin/placebo), and the pre-treatment variable of the corresponding dependent variable as a covariate. Statistical analysis was done using SAS software (SAS Institute, Cary, NC, USA). All inferential statistical tests were conducted at a significance level of 0.05 (two sided). Data are presented as mean ± SEM.
Results
Patient disposition and pre-treatment clinical characteristics
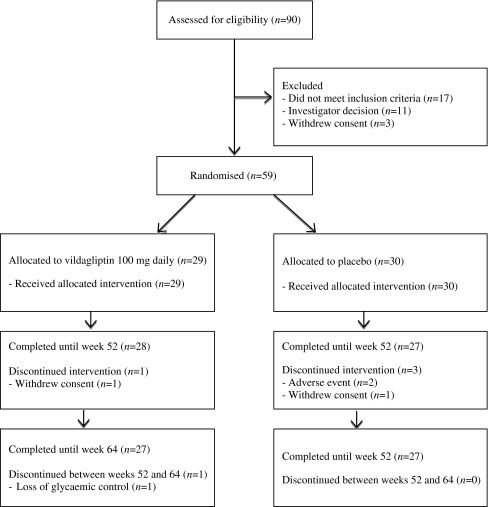
Patient disposition and pre-treatment clinical characteristics are depicted in Fig. 2 and Table 1, respectively. Ninety patients entered the screening phase of our study, during which 17 did not meet the inclusion criteria. Of the patients found eligible to participate in the current study, 11 patients were excluded because of failure to agree to participate after screening in a potential placebo arm or in the clamp, or because of the investigators’ opinion that the participant could not meet the demands of this 15 month study, which included three hyperinsulinaemic–euglycaemic and hyperglycaemic clamps. Finally, three patients withdrew their consent prior to randomisation. In the vildagliptin group, 29 patients were randomised and 27 patients completed the study; one patient withdrew consent because of an unsatisfactory therapeutic effect and another was excluded because of a protocol violation. In the placebo group 30 patients were randomised and 27 patients completed the study; two patients discontinued because of an adverse effect; a third withdrew consent. Baseline clinical characteristics of the randomised population were comparable in both treatment groups: mean age 57.4 ± 9.4 years vs 57.0 ± 6.7 years, 58.6% vs 60.0% male, 96.6% vs 90.0% Europid, BMI 29.9 ± 4.9 kg/m2 vs 29.2 ± 4.4 kg/m2, HbA1c 6.0 ± 0.7% vs 6.0 ± 0.7%, FPG 7.0 ± 1.2 mmol/l vs 7.1 ± 1.0 mmol/l and duration of diabetes 1.4 ± 2.8 years vs 0.6 ± 1.1 years, for vildagliptin and placebo groups, respectively.
Fig. 2.
Protocol flow-chart and patient disposition
Table 1.
Demographics and baseline characteristics
| Variable | Vildagliptin 100 mg once daily | Placebo |
|---|---|---|
| n | 29 | 30 |
| Age (years) | 57.4 ± 9.4 | 57.0 ± 6.7 |
| Men | 17 (58.6) | 18 (60.0) |
| Europid | 28 (96.6) | 27 (90.0) |
| Body weight (kg) | 90.2 ± 15.9 | 87.6 ± 19.1 |
| BMI (kg/m2) | 29.9 ± 4.9 | 29.2 ± 4.4 |
| Waist circumference (cm) | 100.7 ± 10.9 | 99.5 ± 12.8 |
| HbA1c (%) | 6.0 ± 0.7 | 6.0 ± 0.7 |
| FPG (mmol/l) | 7.0 ± 1.2 | 7.1 ± 1.0 |
| Disease duration (y) | 1.4 ± 2.8 | 0.6 ± 1.1 |
Data are presented as mean ± SD or n (%)
Haemoglobin A1c and fasting plasma glucose
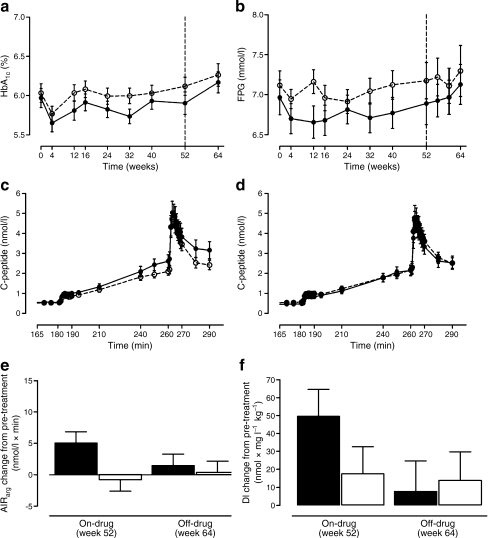
Figure 3a,b depicts the HbA1c and FPG concentrations during the duration of the study. After 52 weeks of treatment, in the vildagliptin group the mean change from baseline in HbA1c (6.0 ± 0.7%) was −0.1% and in FPG (7.0 ± 1.2 mmol/l) was −0.1 mmol/l, and in the placebo group the mean change from baseline in HbA1c (6.0 ± 0.7%) was +0.1% and in FPG (7.1 ± 1.0 mmol/l) was +0.1 mmol/l (Fig. 2a,b), resulting in a non-significant between-group difference of −0.19 ± 0.11, p = 0.098 and −0.22 ± 0.23%, p = 0.343 for HbA1c and FPG, respectively.
Fig. 3.
HbA1c (a) and fasting plasma glucose (b) profiles during the treatment period; C-peptide concentrations during hyperglycaemic clamp at week 52, on-drug (c) and week 64, off-drug (d); change from pre-treatment in combined hyperglycaemic and arginine-stimulated C-peptide secretion (e), and disposition index (f), in the vildagliptin and placebo treated group. Data represent mean (SEM) in a–d and adjusted LS mean (SEM) in e–f. AIRarg, C-peptide response to arginine at 15 mmol/l glucose concentration; DI, disposition index. See the Methods section for calculations of beta cell function measures. Black circles and black bars, vildagliptin 100 mg daily; white circles and white bars, placebo
Insulin sensitivity and hyperglycaemic clamp derived measures of beta cell function
Prior to randomisation, no between-group differences were present in beta cell function measures (Table 2). One year treatment with vildagliptin significantly increased arginine-stimulated C-peptide secretion during hyperglycaemia, the primary endpoint of the study, compared with placebo (adjusted mean ± SEM change from pre-treatment: +5.0 ± 1.8 nmol/l × min and −0.8 ± 1.8 nmol/l × min respectively; between-group adjusted mean ± SEM difference: 5.8 ± 2.6 nmol/l × min p = 0.030) (Fig. 3c,e). First and sustained phase glucose stimulated C-peptide secretion also improved after 1 year of vildagliptin treatment (between-group adjusted mean ± SEM difference: +0.77 ± 0.38, p = 0.047 and +9.89 ± 3.19, p = 0.003, respectively). In our study no statistically significant effect on insulin sensitivity, measured using the euglycaemic–hyperinsulinaemic clamp, was observed. Treatment-induced change in M value was +0.77 ± 1.9 mg kg−1 min−1 and +0.55 ± 1.72 mg kg−1 min−1, for vildagliptin and placebo, respectively.
Table 2.
Measures of beta cell secretory function during hyperglycaemic clamp and change from pre-treatment in the vildagliptin (n = 29) and placebo (n = 30) groups
| Measurements | Pre-treatment (week 0) | On-drug (week 52) | Off-drug (week 64) | On-drug change to pre-treatment (week 52) | Off-drug change to pre-treatment (week 64) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted LS mean | Between-group difference | p value | Adjusted LS mean | Between-group difference | p value | ||||
| 1st phase | |||||||||
| Placebo | 2.95 ± 0.58 | 2.92 ± 0.52 | 4.14 ± 0.89 | −0.02 ± 0.27 | 0.95 ± 0.58 | ||||
| Vildagliptin | 2.94 ± 0.61 | 3.55 ± 0.65 | 3.44 ± 0.82 | 0.75 ± 0.26 | 0.77 ± 0.38 | 0.047 | 0.81 ± 0.60 | −0.14 ± 0.84 | 0.870 |
| 2nd phase | |||||||||
| Placebo | 34.05 ± 4.10 | 32.92 ± 4.05 | 32.83 ± 3.78 | −1.07 ± 2.21 | −2.16 ± 2.53 | ||||
| Vildagliptin | 35.06 ± 3.60 | 43.94 ± 5.36 | 38.06 ± 5.76 | 8.82 ± 2.21 | 9.89 ± 3.19 | 0.003 | 2.99 ± 2.59 | 5.15 ± 3.62 | 0.162 |
| AIRarg | |||||||||
| Placebo | 39.05 ± 4.56 | 38.29 ± 4.65 | 38.24 ± 4.59 | −0.77 ± 1.84 | 0.40 ± 1.73 | ||||
| Vildagliptin | 39.04 ± 3.58 | 42.81 ± 4.72 | 39.32 ± 5.28 | 5.00 ± 1.81 | 5.77 ± 2.58 | 0.030 | 1.44 ± 1.81 | 1.04 ± 2.51 | 0.680 |
Data are presented as mean ± SEM. Changes from pre-treatment are presented as adjusted LS mean ± SEM
1st phase, first phase C-peptide response to glucose (nmol/l × min); 2nd phase, second phase C-peptide response to glucose (nmol/l × min); AIRarg, C-peptide response to arginine at 15 mmol/l glucose concentration (nmol/l × min); See the Methods for calculations of beta cell function measures
Vildagliptin increased the disposition index (AIRarg × M value) after 52 weeks of treatment by 49.6 ± 15.0 nmol × mg l−1 kg−1 (Fig. 3f). However, the placebo-adjusted mean change from pre-treatment in the disposition index (AIRarg × M value) 32.0 nmol × mg l−1 kg−1 did not reach statistical significance (p = 0.142). The above-mentioned effects were not sustained following the 12 week washout period; no statistically significant difference between the two groups was observed at week 64 (p = 0.791; Fig. 3d–f).
Adverse effects and tolerability
Vildagliptin treatment was generally well-tolerated. Adverse events occurred at similar rates across treatment groups (89.7% in the vildagliptin group, 90.0% in the placebo group). Large or consistent differences between active treatments and the corresponding placebo groups for these types of adverse events were not observed. The most frequently observed adverse events were common infections such as nasopharyngitis and bronchitis. Most adverse events were isolated and occurred in just one patient in any treatment group, and no clustering of any specific event was noted. Two serious adverse events were observed during the study in the vildagliptin group (nephrolithiasis and prostate cancer); these events were not believed to be due to drug treatment and did not result in discontinuation from the study. One serious adverse event occurred in the placebo group (hyperkalaemia); this event led to discontinuation from the study.
Discussion
The combined glucose and arginine-stimulated C-peptide secretion rate is the established measure of beta cell capacity to secrete insulin [2, 9]. In the current study, 1 year treatment with vildagliptin 100 mg once daily significantly increased beta cell capacity to secrete insulin as reflected by a ~15% increase relative to placebo in the combined glucose and arginine-stimulated C-peptide secretion rate. By design this was measured after an overnight fast; thus, any increased meal-related acute stimulation of insulin secretion due to increased GLP-1 and glucose-dependent insulinotropic peptide (GIP) was not obscuring any potential increase in insulin secretion capacity. The glucose toxicity associated with increasing FPG is known to reduce beta cell function and any agent that reduces FPG is predicted to improve beta cell function [10]. In order to avoid this confounding variable, the design of the current study attempted to minimise glucose toxicity. The lack of important differences in glycaemic control as measured by either FPG or HbA1c, or of a significant effect on insulin resistance, indicates that glucose toxicity was in fact minimised in the study. It was also planned that the capacity would be corrected for any residual insulin resistance by calculating the disposition index (AIRarg × M value). The mean between-group difference in the disposition index tended to increase to a similar degree to the difference seen with AIRarg,, but unlike AIRarg this difference was not significant. Presumably any discrimination between the treatment groups arising from this ratio appears to be offset by the increased variation. A previous study with 50 mg vildagliptin twice daily [8] demonstrated that after 3 months there was a ~9% increase in the capacity for insulin secretion. A 9% increase after 3 months in the previous study and a 15% increase after 12 months in the current study suggests, but does not provide adequate evidence, that the beta cell capacity is increasing over time. In any case, if beta cell capacity is increasing it is doing so at a very slow rate. The 0.2% decrease in HbA1c from a baseline of 6.0% in the current study is consistent with previous vildagliptin (50 mg daily) studies where there was a 0.15% decrease from a baseline of 5.9% in a study with participants with IGT [11] and a 0.3% decrease from a baseline of 6.7% in a study with patients with type 2 diabetes [12]. Although the changes in HbA1c in these previous IGT and diabetes studies were not large, the increase in beta cell function following treatment was much greater than that seen in the current study. Both of these previous studies measured overall beta cell function during meals and in the diabetes study it was determined that increase was due to an improvement in the sensitivity of insulin secretion to glucose [12]. In contrast, in the current study, as indicated above, by design any meal-related stimulation of insulin secretion due to increased GLP-1 and GIP was not obscuring any potential increase in insulin secretion capacity. Thus in the present study, the beta cell capacity component (after an overnight fast) of the improved insulin secretion seen with vildagliptin treatment is small relative to the glucose-sensing component observed during meals.
Twelve weeks of washout was chosen to test whether any increase in capacity was due to a disease modifying effect. This length of time was chosen so as eliminate any post-dosing effects of vildagliptin to maintain higher GLP-1 and GIP levels as well as any reversible effects due to an improved metabolic state. It was assumed that any increase in beta cell mass would not be reversible within a 3 month period of time. The ~15% effect on beta cell capacity in the current study was not maintained after a 3 month washout period, indicating that this increased beta cell capacity after 1 year of vildagliptin treatment was not a disease modifying effect on beta cell functional mass. This suggests that vildagliptin increased beta cell capacity after 1 year by a more reversible mechanism. It has recently been reported that, following vildagliptin treatment for 12 days, insulin secretion was increased during a morning intravenous glucose challenge after an overnight fast [13]; the effects on capacity seen in the current study could thus be due to increased basal GLP-1 and GIP.
The data from the current study are consistent with a previous 1 year study where the GLP-1 mimetic, exenatide, was shown to significantly improve beta cell capacity, which, however, returned to the pre-treatment values after a 4 week washout [9]. The two studies differ in that patients had higher levels of hyperglycaemia in the exenatide study; thus there was an important change from baseline in glucose toxicity that was controlled for by equivalent glycaemic control with insulin therapy in the control group. Furthermore, the acute effect of exenatide to increase insulin secretion above non-treated values is much greater than that seen with vildagliptin after an overnight fast.
Interestingly, there was improved beta cell function 4 weeks after washout from 2 years vildagliptin treatment [12] and after 3 years exenatide treatment [14]. However, in these longer-term studies, the contribution of increased capacity vs improved sensitivity of the beta cells to glucose, to the overall effect on beta cell function, was not assessed. It is entirely possible that, after several years of treatment, it is the improved glucose sensing that is persisting after 4 weeks washout, and not the capacity. Whether longer treatments with DPP-4 inhibitors or GLP-1 receptor activators have disease modifying effects on beta cell mass in humans remains to be determined.
Acknowledgements
We thank the participants for taking part in the study. The authors acknowledge L.W. van Golen and R.E. van Genugten, Diabetes Centre, Department of Internal Medicine, VU University Medical Centre, Amsterdam, the Netherlands, for their assistance during the study.
Duality of interest statement
J. E. Foley is an employee and stockholder of the Novartis Pharmaceutical Cooperation. M. C. Bunck has declared no dualities of interest. D. L. Möller-Goede has declared no dualities of interest. M. Poelma has declared no dualities of interest. G. Nijpels has declared no dualities of interest. E. M. Eekhoff has declared no dualities of interest. A. Schweizer is an employee and stockholder of Novartis Pharma AG. R. J. Heine is an employee and stockholder of Eli Lilly and Company. During the study, R. J. Heine was still employed at the VU University Medical Centre. M. Diamant is a consultant and speaker for the Novartis Pharmaceutical Cooperation. Through M. Diamant, the VU University Medical Centre has received research grants from the Novartis Pharmaceutical Cooperation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- AIRarg
Acute insulin response to arginine
- ANCOVA
Analysis of covariance
- DPP-4
Dipeptidylpeptidase-4
- FPG
Fasting plasma glucose
- GIP
Glucose-dependent insulinotropic peptide
- GLP-1
Glucagon-like peptide-1
- iAUC
Incremental AUC
- LS mean
Least squares mean
References
- 1.Ahrén B, Pratley RE, Soubt M, Dunning BE, Foley JE. Clinical measures of islet function: usefulness to characterize defects in diabetes. Curr Diab Rev. 2008;4:129–145. doi: 10.2174/157339908784220714. [DOI] [PubMed] [Google Scholar]
- 2.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D. Diminished beta-cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984;74:1318–1328. doi: 10.1172/JCI111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(Suppl 3):S16–S21. doi: 10.2337/diabetes.53.suppl_3.S16. [DOI] [PubMed] [Google Scholar]
- 4.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza R, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 5.Salehi M, Aulinger BA, D'Alessio DA. Targeting beta-cell mass in type 2 diabetes: promise and limitations of new drugs based on incretins. Endocr Rev. 2008;29:367–379. doi: 10.1210/er.2007-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duttaroy A, Voelker F, Merriam K, et al. The DPP-4 inhibitor vildagliptin increases pancreatic beta cell mass in neonatal rats. Eur J Pharmacol. 2011;650:703–707. doi: 10.1016/j.ejphar.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 7.Mari A, Scherbaum WA, Nilsson PM, et al. Characterization of the influence of vildagliptin on model-assessed -cell function in patients with type 2 diabetes and mild hyperglycaemia. J Clin Endocrinol Metab. 2008;93:103–109. doi: 10.1210/jc.2007-1639. [DOI] [PubMed] [Google Scholar]
- 8.D'Alessio DA, Denney AM, Hermiller LM, et al. Treatment with the dipeptidyl peptidase-4 inhibitor vildagliptin improves fasting islet-cell function in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:81–88. doi: 10.1210/jc.2008-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared to insulin glargine, in metformin treated type 2 diabetes patients: a randomized, controlled trial. Diabetes Care. 2009;32:762–768. doi: 10.2337/dc08-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yki-Järvinen H. Glucose toxicity. Endocr Rev. 1992;13:415–431. doi: 10.1210/edrv-13-3-415. [DOI] [PubMed] [Google Scholar]
- 11.Rosenstock J, Foley JE, Rendell M, et al. Effects of the dipeptidyl peptidase-IV inhibitor vildagliptin on incretin hormones, islet function, and postprandial glycemia in subjects with impaired glucose tolerance. Diabetes Care. 2008;31:30–35. doi: 10.2337/dc07-1616. [DOI] [PubMed] [Google Scholar]
- 12.Scherbaum WA, Schweizer A, Mari A, et al. Evidence that vildagliptin attenuates deterioration of glycaemic control during 2-year treatment of patients with type 2 diabetes and mild hyperglycaemia. Diab Obes Metab. 2008;10:1114–1124. doi: 10.1111/j.1463-1326.2008.00875.x. [DOI] [PubMed] [Google Scholar]
- 13.Vardarli I, Nauck MA, Köthe LD, et al. Inhibition of DPP-4 with vildagliptin improved insulin secretion in response to oral as well as "isoglycemic" intravenous glucose without numerically changing the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:945–954. doi: 10.1210/jc.2010-2178. [DOI] [PubMed] [Google Scholar]
- 14.Bunck MC, Corner A, Eliasson B, et al. Three-year exenatide therapy, followed by a 4-week off-drug period, had a sustainable effect on beta-cell disposition index in metformin treated patients with type 2 diabetes. Diabetes. 2010;59:A198–A199. [Google Scholar]