Abstract
Background
Gaucher's disease is an autosomal recessive, lysosomal storage disease caused by mutations of the β-glucocerebrosidase gene (GBA). There is increasing evidence that GBA mutations are a genetic risk factor for the development of Parkinson's disease (PD). We report herein a family of Koreans exhibiting parkinsonism-associated GBA mutations.
Case Report
A 44-year-old woman suffering from slowness and paresthesia of the left arm for the previous 1.5years, visited our hospital to manage known invasive ductal carcinoma. During a preoperative evaluation, she was diagnosed with Gaucher's disease and double mutations of S271G and R359X in GBA. Parkinsonian features including low amplitude postural tremors, rigidity, bradykinesia and shuffling gait were observed. Genetic analysis also revealed that her older sister, who had also been diagnosed with PD and had been taking dopaminergic drugs for 8-years, also possessed a heterozygote R359X mutation in GBA. 18F-fluoropropylcarbomethoxyiodophenylnortropane positron-emission tomography in these patients revealed decreased uptake of dopamine transporter in the posterior portion of the bilateral putamen.
Conclusions
This case study demonstrates Korean familial cases of PD with heterozygote mutation of GBA, further supporting the association between PD and GBA mutation.
Keywords: Gaucher's disease, glucocerebroside, Parkinson's diseases
Introduction
Gaucher's disease (GD) is an autosomal recessive, lysosomal storage disease that causes abnormal accumulation of a glucocerebroside in multiple organs, as a result of mutations in the β-glucocerebrosidase gene (GBA), which is located at chromosome 1q21.1 Clinically, GD has a wide spectrum of clinical manifestations, and the spectrum of the disease tends to be correlated with residual enzyme activity. The most severe form of infantile-onset GD presents with widespread accumulation of glucosylceramide in a various cell types, leading rapidly to fatal neurodegenerative disease, whereas in the late-onset form, residual GBA enzyme activity results in highly variable clinical manifestations including hepatosplenomegaly, anemia, thrombocytopenia, and skeletal symptoms.1-3
There is increasing evidence that GBA mutations represent a genetic risk factor for the development of Parkinson's disease (PD); at autopsy, GD produces α-synuclein-immunoreactive Lewy bodies, and parkinsonism appears to be more prevalent in relatives of GD patients.3,4 Furthermore, subsequent GBA genotyping studies on various PD cohorts have revealed that the frequency of GBA mutations is elevated in PD patients. Here we present Korean familial cases of PD with heterozygous mu-tations of GBA, which further supporting the association between these two disorders.
Case Reports
Patient 1
A 44-year-old woman visited the breast cancer clinic in our hospital to manage known invasive ductal carcinoma. Abdomen magnetic resonance imaging during a preoperative evaluation revealed thrombocytopenia (81,000 platelet/µl) and hepatosplenomegaly with four round hypointense nodules. In addition, whole body positron emission tomography-computer tomography (PET-CT) revealed bone marrow activation. At mastectomy, simultaneous bone marrow biopsy of the iliac bone was performed to further evaluate the thrombocytopenia and marrow abnormality. Immunohistochemical analysis revealed numerous lipid-containing CD-68-positive cells infiltrating the bone marrow (Fig. 1). The patient was then referred to our neurological department due to slowness and paresthesia of the left arm, from which she had been suffering for 1.5 years. A family history revealed that she had two brothers and six sisters, and her elder sister had been suffering from PD for 8 years. On neurological examination, the patient's higher cortical functions were not remarkable, but her facial expression was markedly decreased. She had low amplitude postural tremor with a frequency of 4-5 Hz and cogwheel rigidities in all 4 limbs. Bradykinesia with fatigability was observed in the bilateral limbs on finger and foot tapping. Moreover, she had a stooped posture and shuffling gait, with decre-ased bilateral arm swing. These parkinsonian features were dominant in the left extremities. Her score on the Unified Parkinson's Disease Rating Scale (UPDRS)-III was 22.
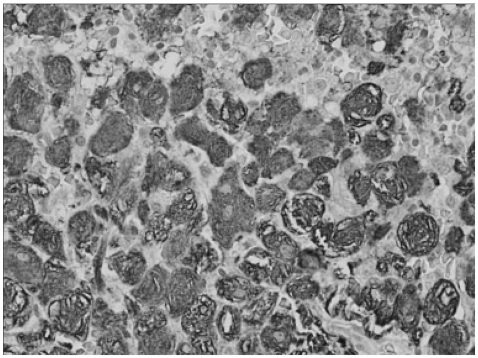
Fig. 1.
Immunohistochemical analysis with CD-68 antibodies. Numerous lipid-containing Gaucher cells, which were positive for CD-68 staining, have infiltrated the bone marrow.
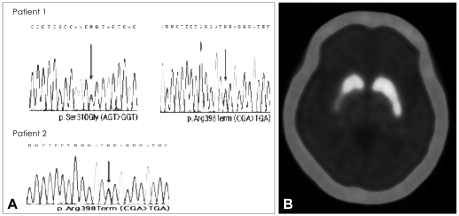
With hematological information, we performed genetic analysis of GBA mutations and 18F-fluoropropylcarbomethoxyiodophenylnortropane (18F-FP-CIT) PET. This revealed compound heterozygous mutations (S271G and R359X) in GBA (Fig. 2A) and decreased uptake of dopamine transporter in the posterior portion of the bilateral putamen, which was more severe on the right side (Fig. 2B). The patient's parkinso-nism was considerably improved by the introduction of levodopa medication. (UPDRS-III score 12).
Fig. 2.
Genetic analysis of GBA and dopamine-transporter imaging. Direct polymerase chain reaction sequencing revealed double mutations of S271G (p.Ser310Gly) and R359X (p.Arg398Term) in GBA in patient 1, and the R359X mutation in patient 2 (A). 18F-FP-CIT PET revealed decreased uptake of dopamine transporter in the posterior portion of bilateral putamen, which was more severe on the right side (B). GBA: β-glucocerebrosidase, 18F-FP-CIT: 18F-fluoropropy-lcarbomethoxyiodophenylnortropane, PET: positron-emission tomography.
Patient 2
The older sister of previous patient 1, a 55-year-old female, had been diagnosed with PD and taking dopaminergic drugs for 8-years. On neurological examination, her UPDRS-III scores during her best on-period was 25, and she exhibited motor complications of wearing off and peak-dose dyskinesia. She had no objective cognitive impairments, as evaluated by a detailed neuropsychological evaluation with the Seoul Neuropsychological Screening Battery. 18F-FP-CIT PET revealed markedly decreased uptake of dopamine transporter in the posterior portion of the bilateral putamen. Genetic analysis revealed a heterozygous mutation (R359X) in GBA. (Fig. 2A).
Discussion
The neurological symptoms encountered in adult-type GD include seizures, psychosis, and dementia. However, many case reports have suggested indirect evidence of an association between GD and PD.2,3 McKeran et al.5 reported a 55-year-old patient with GD who later developed parkinsonism. Neudorfer et al.6 described six patients in whom GD was accompanied by parkinsonism, and Machaczka et al.7 reported another patient with parkinsonism that preceded the clinical manifestations of GD by 12-years. Regarding the phenotypical analysis based on large case series, parkinsonism in GD shows classical symptoms of PD with a tendency toward the akinetic form, an early-onset form, and a relatively good response to levodopa.4,8 Furthermore, dementia and visual hallucinations are frequently observed in parkinsonism with GD, which may be associated with pathological findings, with Lewy bodies being observed more frequently in parkinsonism with GD compared to PD on its own.8
Our patients had also exhibited classic parkinsonism with a young age at onset and an akinetic form, and had an excellent response to levodopa. Even though one patient (a younger sister) revealed laboratory abnormalities and pathological infiltrations of Gaucher cells, which may be attribute to the effects of the S271G/R359X double mutation, these two sisters had no overt clinically systemic manifestations of GD except for parkinsonism, as in the case series of heterozygote mutation reported previously.8 In addition, the enormous clinical variation between genotype and phenotype presentations in GD may explain the sole clinical manifestation of parkinsonism in our cases.9,10
The association between parkinsonism and the S271G mutation in GBA has also been reported in both Greek and a Chinese cohort.11,12 In addition, the R359X mutation in GBA found in this study was identified by Beutler and Gelbart in non-Jewish patients with GD type 2,13 and its prevalence is reported very low in among Spanish patients with GD.14 However, the R359X mutation in those reports did not accompany a parkinsonian phenotype. Although it is difficult to determine which mutation may have impacted the development of a parkinsonian phenotype in our patients, since the R359X mutation was found in both patients, the R359X mutation may act as a pathogen in the PD phenotype. To our knowledge, this is the first report demonstrating the association between parkinsonism and the R359X mutation in GBA. The different phenotype of the same R359X mutation in GBA may be ascribed to racial differences as well as environmental factors influencing expression of the risk gene. Nevertheless, the pathogenic or functional effects of the novel R359X mutation found in our PD patients needs to be further clarified. Nevertheless, the pathogenic or functional effects of the novel R359X mutation found in our PD patients needs to be further clarified.
The prevalence of GBA mutations being considered as susceptibility genes in PD differs with ethnicitys; the carrier frequency of GBA mutations is reportedly 10-31% in the Ashkenazi Jewish population and 2.9-12% among the non-Ashkenazi-Jewish.15 A recent study in a Japanese population found that GBA mutations were strikingly associated with PD, exhibiting a markedly high relative risk of 28.0 compared to controls.16 Currently, data regarding the association between GBA mutations and PD are not available for the Korean population, and future studies of Korean PD patients should compare the prevalence of GBA mutations among Asian PD populations.
Acknowledgements
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2028 (6-2008-0097).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Butters TD. Gaucher disease. Curr Opin Chem Biol. 2007;11:412–418. doi: 10.1016/j.cbpa.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 2.Sidransky E. Gaucher disease and parkinsonism. Mol Genet Metab. 2005;84:302–304. doi: 10.1016/j.ymgme.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Goker-Alpan O, Lopez G, Vithayathil J, Davis J, Hallett M, Sidransky E. The spectrum of parkinsonian manifestations associated with gluco-cerebrosidase mutations. Arch Neurol. 2008;65:1353–1357. doi: 10.1001/archneur.65.10.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogaeva E, Hardy J. Gaucher and Parkinson diseases: unexpectedly related. Neurology. 2008;70:2272–2273. doi: 10.1212/01.wnl.0000314657.92762.0f. [DOI] [PubMed] [Google Scholar]
- 5.McKeran RO, Bradbury P, Taylor D, Stern G. Neurological involvement in type 1 (adult) Gaucher's disease. J Neurol Neurosurg Psychiatry. 1985;48:172–175. doi: 10.1136/jnnp.48.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neudorfer O, Giladi N, Elstein D, Abrahamov A, Turezkite T, Aghai E, et al. Occurrence of Parkinson's syndrome in type I Gaucher disease. QJM. 1996;89:691–694. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- 7.Machaczka M, Rucinska M, Skotnicki AB, Jurczak W. Parkinson's syndrome preceding clinical manifestation of Gaucher's disease. Am J Hematol. 1999;61:216–217. doi: 10.1002/(sici)1096-8652(199907)61:3<216::aid-ajh12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Neumann J, Bras J, Deas E, O'Sullivan SS, Parkkinen L, Lachmann RH, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain. 2009;132:1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lachmann RH, Grant IR, Halsall D, Cox TM. Twin pairs showing discordance of phenotype in adult Gaucher's disease. QJM. 2004;97:199–204. doi: 10.1093/qjmed/hch036. [DOI] [PubMed] [Google Scholar]
- 10.Sidransky E. Gaucher disease: complexity in a "simple" disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Kalinderi K, Bostantjopoulou S, Paisan-Ruiz C, Katsarou Z, Hardy J, Fi-dani L. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Greece. Neurosci Lett. 2009;452:87–89. doi: 10.1016/j.neulet.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Gutti U, Fung HC, Hruska KS, Lamarca ME, Chen CM, Wu YR, et al. The need for appropriate genotyping strategies for glucocerebrosidase mutations in cohorts with Parkinson disease. Arch Neurol. 2008;65:850–851. doi: 10.1001/archneur.65.6.850. author reply 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beutler E, Gelbart T. Two new Gaucher disease mutations. Hum Genet. 1994;93:209–210. doi: 10.1007/BF00210614. [DOI] [PubMed] [Google Scholar]
- 14.Alfonso P, Aznarez S, Giralt M, Pocovi M, Giraldo P Spanish Gaucher's Disease Registry. Mutation analysis and genotype/phenotype relationships of Gaucher disease patients in Spain. J Hum Genet. 2007;52:391–396. doi: 10.1007/s10038-007-0135-4. [DOI] [PubMed] [Google Scholar]
- 15.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsui J, Mizuta I, Toyoda A, Ashida R, Takahashi Y, Goto J, et al. Mu-tations for Gaucher disease confer high susceptibility to Parkinson dise-ase. Arch Neurol. 2009;66:571–576. doi: 10.1001/archneurol.2009.72. [DOI] [PubMed] [Google Scholar]