Abstract
Marine macrophytes contain a variety of biologically active compounds, some reported to have antiprotozoal activity in vitro. As a part of a screening program to search for new natural antiprotozoals, we screened hydroalcoholic and ethyl acetate extracts of 20 species of seaweeds from three phyla (Rhodophyta, Heterokontophyta and Chlorophyta), sampled along the Normandy (France) coast. We tested them in vitro against the protozoa responsible for three major endemic parasitic diseases: Plasmodium falciparum, Leishmania donovani and Trypanosoma cruzi. The selectivity of the extracts was also evaluated by testing on a mammalian cell line (L6 cells). Ethyl acetate extracts were more active than hydroalcoholic ones. Activity against T. cruzi and L. donovani was non-existent to average, but almost half the extracts showed good activity against P. falciparum. The ethyl acetate extract of Mastocarpus stellatus showed the best antiplasmodial activity as well as the best selectivity index (IC50 = 2.8 μg/mL; SI > 30). Interestingly, a red algae species, which shares phylogenetic origins with P. falciparum, showed the best antiplasmodial activity. This study is the first to report comparative antiprotozoal activity of French marine algae. Some of the species studied here have not previously been biologically evaluated.
Keywords: seaweeds, Phaeophyceae, Rhodophyceae, Plasmodium, Trypanosoma, Leishmania
1. Introduction
Protozoal diseases, especially malaria, leishmaniasis, and Chagas disease, are major causes of mortality in various tropical and subtropical regions [1]. Moreover, treatment failure is increasingly common with current drugs. The resistance of Plasmodium falciparum, for example, to the former first-line antimalarials, chloroquine and sulfadoxine/pyrimethamine, has reached critical levels in many malaria-endemic regions and is producing deleterious effects on human health, wealth, and lifespans. Recent reports that malaria parasites have begun to develop tolerance to the artemisinin-based combination therapies threaten the last mainstay for treating uncomplicated malaria in endemic countries [2,3]. Furthermore, no new class of antimalarials has been introduced into clinical practice since 1996 [4]. Worse, chemotherapy of visceral leishmaniasis and Chagas disease still relies on first-line drugs that are old and far from satisfactory because of their major side effects, limited efficacy, and difficulty of administration. New more effective chemotherapeutic agents with novel modes of action are needed, and marine biodiversity might be a source of chemodiversity for that purpose.
Marine biodiversity contains resources composed of a variety of biologically active compounds [5–7]. Marine macrophytes produce an array of secondary compounds [8] still underexploited for their biomedical potential although they are a particularly available biomass. Studies of tropical marine macrophytes have shown their extensive biological activity, including antiprotozoal [9–12], but very little is known about Western seaweed species.
As a part of a screening program to search for new natural antiprotozoal products, we report here the in vitro screening of 35 polar (hydroalcoholic) and apolar (ethyl acetate) extracts from 20 species of seaweeds from the Normandy (France) coast against cultured protozoa responsible for human malaria, visceral leishmaniasis, and Chagas disease.
2. Results and Discussion
The sampling resulted in the selection of 20 species of seaweeds that were brown (8), red (9) and green algae (3). The species were collected from rocky habitats along the Normandy coast in northern France (Table 1).
Table 1.
Marine algal species selected for the study, and the sites and times of their collection (Normandy coast, France).
| Species | Family | Collection site | Collection time |
|---|---|---|---|
| Chlorophyta | |||
| Codium tomentosum Stackhouse | Codiaceae | Cap Lévy (Manche) | June 2007 |
| Ulva lactuca (Linnaeus) | Ulvaceae | Luc-sur-Mer (Calvados) | October 2006 |
| Ulva clathrata (Roth) C. Agardh | Ulvaceae | Anse St Martin (Manche) | June 2007 |
| Heterokontophyta | |||
| Bifurcaria bifurcata R. Ross | Sargassaceae | Cap Lévy (Manche) | June 2007 |
| Dictyopteris polypodioides (A.P. de Candolle) | Dictyotaceae | Barneville (Calvados) | October 2007 |
| J.V. Lamouroux | June 2007 | ||
| Dictyota dichotoma (Hudson) J.V. Lamouroux | Dictyotaceae | Anse St Martin (Manche) | June 2007 |
| Fucus serratus (Linnaeus) | Fucaceae | Luc-sur-mer (Calvados) | November 2005 |
| Himanthalia elongata (Linnaeus) | Himanthaliaceae | Cap Lévy (Manche) | June 2006 |
| Laminaria digitata (Linnaeus) J.V. Lamouroux | Laminariaceae | Langrunes-sur-Mer (Calvados) | January 2007 |
| Pelvetia canaliculata Decaisne & Thuret | Fucaceae | Cap Lévy (Manche) | June 2006 |
| Sargassum muticum (Yendo) Fensholt | Sargassaceae | Cap Lévy (Manche) | June 2006 |
| Rhodophyta | |||
| Calliblepharis jubata (Goodenough & woodward) Kützing | Cystocloniaceae | Cap Lévy (Manche) | June 2007 |
| Chondrus crispus Stackhouse | Gigartinaceae | Cap Lévy (Manche) | June 2007 |
| Dilsea carnosa (Schmidel) Kuntze | Dumontiaceae | Langrune-sur-Mer (Calvados) | January 2007 |
| Gelidium latifolium Bornet ex Hauck | Gelidiaceae | Cap Lévy (Manche) | June 2006 |
| Gracilaria gracilis (Stackhouse) Steentoft, L.M. Irvine & Farnham | Gracilariaceae | Anse St Martin (Manche) | June 2007 |
| Grateloupia turuturu Yamada | Halymeniaceae | St Vaast-la-Hougue (Manche) | September 2007 |
| Halurus flosculosus (J. Ellis) Maggs & Hommersand | Ceramiaceae | Anse St Martin (Manche) | June 2007 |
| Mastocarpus stellatus (Stackhouse) Guiry | Phyllophoraceae | Cap Lévy (Manche) | June 2006 |
| Palmaria palmata (Linnaeus) Kuntze | Palmariaceae | Luc-sur-Mer (Calvados) | November 2005 |
The potential antiplasmodial activity of the resultant ethyl acetate and hydroalcoholic extracts was evaluated in vitro against erythrocytes infected by a resistant K1 strain of P. falciparum, as well as against T. cruzi trypomastigotes and Leishmania donovani amastigotes (Table 2). Extracts were first screened at two concentrations (1.6 and 9.7 μg/mL), and parasite growth inhibition was measured. Extracts for which parasite growth inhibition was greater than 50% at the concentration of 9.7 μg/mL were subsequently assayed to determine their IC50. An extract was considered as active if the IC50 value was less than 5 μg/mL. Cytotoxicity to primary mammalian L6 cells was also evaluated to determine the selectivity of its activity. Table 3 presents the IC50 values and selectivity indexes (ratio of cytotoxic to antiprotozoal activity).
Table 2.
In vitro antiprotozoal medium throughput screening of extracts obtained from the selected species. P. falciparum: Multidrug-resistant K1 strain erythrocytic stages; T. cruzi: Talahuen strain trypomastigotes; L. donovani: MHOM/ET/67/L82 strain axenic amastigotes.
|
Parasite growth inhibition (%) |
|||||||
|---|---|---|---|---|---|---|---|
|
P. falciparum Erythrocytic stages |
T. cruzi Trypomastigotes |
L. donovani Axenic amastigotes |
|||||
| Species | Extract | 1.6 μg/mL | 9.7μg/mL | 1.6 μg/mL | 9.7 μg/mL | 1.6 μg/mL | 9.7 μg/mL |
| B. bifurcata | E | 0 | 25 | 18 | 0 | 16 | 40 |
| A | 36 | 100 | 0 | 78 | 31 | 100 | |
| C. jubata | E | 0 | 0 | 11 | 0 | 13 | 34 |
| A | 3 | 71 | 19 | 0 | 20 | 40 | |
| C. tomentosum | E | 0 | 2 | 0 | 0 | 15 | 29 |
| A | 0 | 0 | 0 | 0 | 0 | 0 | |
| C. crispus | E | 0 | 0 | 0 | 0 | 13 | 95 |
| A | 28 | 92 | 1 | 0 | 0 | 12 | |
| D. polypodioides | E | nd | |||||
| A | 8 | 81 | 6 | 7 | 14 | 41 | |
| D. dichotoma | E | nd | |||||
| A | 19 | 98 | 21 | 17 | 27 | 83 | |
| D. carnosa | E | 0 | 6 | 0 | 0 | 0 | 15 |
| A | 9 | 71 | 10 | 14 | 9 | 31 | |
| F. serratus | E | 0 | 0 | 0 | 0 | 0 | 15 |
| A | 0 | 42 | 0 | 3 | 0 | 14 | |
| G. latifolium | E | nd | |||||
| A | 1 | 93 | 0 | 0 | 18 | 49 | |
| G. gracilis | E | 0 | 0 | 0 | 9 | 21 | 29 |
| A | 5 | 92 | 0 | 0 | 19 | 36 | |
| G. turuturu | E | nd | |||||
| A | 42 | 97 | 15 | 14 | 9 | 33 | |
| H. flosculosus | E | nd | |||||
| A | 19 | 94 | 17 | 21 | 22 | 49 | |
| H. elongata | E | 0 | 5 | 17 | 13 | 20 | 40 |
| A | 14 | 81 | 0 | 18 | 13 | 43 | |
| L. digitata | E | 0 | 0 | 0 | 9 | 0 | 11 |
| A | 0 | 64 | 0 | 3 | 0 | 8 | |
| M. stellatus | E | 0 | 0 | 0 | 4 | 11 | 20 |
| A | 42 | 94 | 8 | 0 | 22 | 39 | |
| P. palmata | E | 0 | 0 | 0 | 0 | 0 | 0 |
| A | 0 | 1 | 17 | 36 | 0 | 10 | |
| P. canaliculata | E | 15 | 30 | 0 | 0 | 7 | 32 |
| A | 0 | 41 | 0 | 13 | 14 | 37 | |
| S. muticum | E | 0 | 0 | 0 | 1 | 8 | 37 |
| A | 50 | 96 | 0 | 9 | 4 | 48 | |
| U. lactuca | E | 0 | 0 | 0 | 3 | 0 | 26 |
| A | 0 | 28 | 0 | 0 | 0 | 11 | |
| U. clathrata | E | 0 | 0 | 0 | 0 | 0 | 0 |
| A | 0 | 13 | 0 | 0 | 0 | 7 | |
| Standard drugs | 0.003 μg/mL | 0.018 μg/mL | 0.5 μg/mL | 2.4 μg/mL | 0.2 μg/mL | 1.2 μg/mL | |
| Artemisinin | 68 | 100 | |||||
| Benznidazole | 45 | 91 | |||||
| Miltefosine | 59 | 85 | |||||
(E): EtOH 60% extract, (A): ethyl acetate extract, nd: not determined. Compounds for which parasite growth inhibition was greater than 50% at 9.7 μg/mL were assayed to determine IC50 and evaluate cytotoxicity.
Table 3.
In vitro antiprotozoal and cytotoxic activities of the active ethyl acetate extracts. Data shown are means of two independent assays.
| IC50 (μg/mL) | Selectivity index (SI) | |||
|---|---|---|---|---|
|
Antiprotozoal activity |
Cytotoxic activity |
|||
| Species | P. falciparum | L. donovani | L6 cells | |
| B. bifurcata | >5 | 3.8 | 6 | 1.6 b |
| C. jubata | 5 | nd | 71 | 14 a |
| C. crispus | 2.9 | nd | 84 | 29 a |
| D. polypodioides | nd | 10.8 | 87 | 8 b |
| D. dichotoma | 3.1 | 8.8 | 27 | 9 a |
| D. carnosa | 3.9 | 9.5 | 74 | 19 a |
| G. latifolium | 3.4 | nd | 62 | 18 a |
| G. gracilis | 3.3 | nd | 71 | 21 a |
| G. turuturu | 3.1 | nd | 71 | 23 a |
| H. flosculosus | 4.6 | nd | 58 | 12 a |
| H. elongata | 3.5 | nd | 88 | 25 a |
| M. stellatus | 2.8 | nd | >90 | >30 a |
| P. canaliculata | nd | nd | 87 | 11 a |
| S. muticum | 2.9 | nd | 63 | 11 a |
|
Standards |
||||
| Chloroquine | 0.069 | - | - | - |
| Miltefosine | - | 0.181 | - | - |
| Podophyllotoxin | - | - | 0.007 | - |
SI: selectivity index, ratio of cytotoxic activity on L6 cells to the best antiprotozoal activity measured, that is, to antiplasmodial (SIa) or leishmanicidal (SIb) activity; nd: not determined.
The active extracts were almost entirely (97%) ethyl acetate extracts, while the hydroalcoholic extracts were mainly inactive. This finding suggests that the active antiprotozoal compounds were relatively apolar, except for the hydroalcoholic extract of C. crispus, which was quite active against L. donovani (95% inhibition of parasite growth at 9.7 μg/mL).
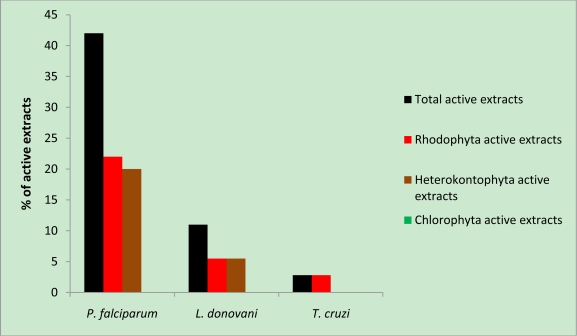
P. falciparum was the pathogen most responsive to these extracts: 40% of the extracts showed activity against this protozoon. L. donovani was less sensitive (11% of the extracts were active against it) and T. cruzi quite insensitive (3%). Red and brown seaweeds were almost equally active against P. falciparum and L. donovani, but green seaweeds were inactive (Figure 1).
Figure 1.
Percentage of extracts active against the protozoan parasites tested.
Few studies have reported antiprotozoal screening of marine algae, and those that did looked at tropical and Asian species [13,14], most often individually [5,6]. Trypanocidal and leishmanicidal activity was recently assessed in British and Irish species [15,16]. Those studies found that organic extracts from several British green algae species were active, although weakly, against the same strains of T. cruzi and L. donovani that we used here [16]. U. lactuca, the only green algae common to their study and ours, was inactive in ours at 9.7 μg/mL, despite similar experimental conditions. This discrepancy may stem either from the different extraction solvents or from their different phytochemical compositions, probably due to the different habitats of the two samples.
Brown tropical algae have previously been shown to be moderately active against L. mexicana promastigotes, while red and green algal organic extracts were almost inactive, as in our study [14]. Here, the most active extract against L. donovani axenic amastigotes was the ethyl acetate extract of B. bifurcata, which had an IC50 value of 3.9 μg/mL. Nevertheless, the selectivity index of 1.6 found for this extract seems to indicate general toxicity. Moreover, crude organic extracts of Irish B. bifurcata were recently shown to be active against both L. donovani and T. brucei rhodesiense, the protozoan parasite responsible for sleeping sickness [15]. B. bifurcata contains arrays of terpenoids [17–19] with cytotoxic activity [19,20]. The ethyl acetate that we used here efficiently extracts this type of compound. Their presence could explain the absence of selectivity.
Our results provide further evidence that marine seaweeds may be active against protozoa, especially Plasmodium species. Investigations of red algae species are rare, still more so against P. falciparum. We showed here, for the first time, in vitro antiplasmodial activity by eight French species of red algae: C. jubata, C. crispus, D. carnosa, G. latifolium, G. gracilis, G. turuturu, H. flosculosus, and M. stellatus. Moreover, the IC50 values measured for these extracts reflect activity similar to that reported for such reference extracts as ethanolic crude extracts of Artemisia annua (Quinguao) and of Azadirachta indica (Neem) in the same in vitro microdilution test under similar experimental conditions [21,22].
Interestingly, Plasmodium parasites contain a cellular peculiarity, called the apicoplast, a rudimentary plastid that was acquired long ago by secondary endosymbiosis between a free-living ancestor of Apicomplexans and a red algal species [23]. Accordingly, this organelle probably shares several metabolic pathways and housekeeping processes with red algae. We can hypothesize that some algal compounds contained in these extracts kill malaria parasites by interfering with these common pathways or processes.
The most active and interesting extract was the ethyl acetate extract of M. stellatus, which had the best IC50 and SI values (2.8 μg/mL and >30, respectively). Its selectivity towards P. falciparum was also good, compared with the other protozoan parasites we tested. To our knowledge, no antiplasmodial activity has previously been shown for Mastocarpus species. Moreover, phytochemical data for these genera are sparse. Nevertheless, some tropical species of red algae have been shown to contain such antiplasmodial compounds as halogenated diterpenes [6] or bromophycolides [5,24]. Bioguided fractionation associated with dereplication techniques should allow us to identify new antiplasmodial compounds from this species and thus help us to determine its mode of action.
3. Experimental Section
3.1. Algae Collection and Identification
The 20 algae species were collected between November 2005 and September 2007 at several locations on the coast of Basse-Normandie. Table 1 reports the collection dates and sites.
Taxonomic determination was performed by Dr. A.-M. Rusig and voucher specimens of the algae are deposited in the Herbarium of the University of Caen.
3.2. Preparation of Crude Extracts
Freeze-dried algal material of each species (200 g) was powdered and stirred overnight at room temperature for complete extraction, with 70% EtOH or ethyl acetate (10%, w/v). The filtrates were dried under vacuum at 35 °C and the residues were stored at 4 °C until testing. Tannins were removed from the crude hydroalcoholic extracts with Sephadex LH-20 exclusion chromatography, according to the method described by Houghton and Raman [25].
3.3. In Vitro Antiprotozoal Assays
The extracts were dissolved in dimethylsulfoxide (DMSO) to obtain a concentration of 10 mg/mL and screened for antiprotozoal activity against P. falciparum, T. cruzi and L. donovani and cytotoxicity against rat skeletal muscle myoblasts (L-6 cells). The in vitro assays were conducted as described by Scala et al. [26]. A brief description is given below.
3.3.1. Activity against P. Falciparum
In vitro activity against erythrocytic stages of P. falciparum was determined by a modified [3H]-hypoxanthine incorporation assay with the chloroquine- and pyrimethamine-resistant K1 strain [27]. Briefly, parasite cultures incubated in RPMI 1640 medium with 5% Albumax (without hypoxanthine) were exposed to serial drug dilutions in microtiter plates. After 48 h of incubation at 37 °C in a reduced oxygen atmosphere, 0.5 μCi [3H]-hypoxanthine was added to each well. Cultures were incubated for a further 24 h before they were harvested onto glass-fiber filters and washed with distilled water. The radioactivity was counted with a BetaplateTM liquid scintillation counter (Wallac, Zurich, Switzerland). The results were recorded as counts per minute (CPM) per well at each drug concentration and expressed as the percentage of untreated controls. IC50 values were calculated from graphically plotted dose-response curves by linear interpolation. Chloroquine (Sigma C6628) and artemisinin (Sigma 36,159-3) were used as positive references.
3.3.2. Activity against Trypanosoma cruzi
Rat skeletal myoblasts (L6 cells) were seeded in 96-well microtiter plates at 2000 cells/well in 100 μL RPMI 1640 medium with 10% FBS and 2 mM l-glutamine. After 24 h the medium was removed and replaced by 100 μL per well containing 5000 trypomastigote forms of T. cruzi Tulahuen strain C2C4 with the β-galactosidase (Lac Z) gene [28]. After 48 h, the medium was removed from the wells and replaced by 100 μL fresh medium with or without a serial drug dilution of seven 3-fold dilution steps covering a range from 90 to 0.123 μg/mL. After 96 h of incubation the plates were inspected under an inverted microscope to assure growth of the controls and sterility. Then the substrate CPRG/Nonidet (50 μL) was added to all wells. A color reaction developed within 2–6 h and could be read photometrically at 540 nm. The IC50 values were calculated from the sigmoidal inhibition curves with SoftmaxPro software. Benznidazole (Roche) was used as a positive reference.
3.3.3. Activity against Leishmania Donovani
Amastigotes of L. donovani strain MHOM/ET/67/L82 were grown in axenic culture at 37 °C in SM medium at pH 5.4 supplemented with 10% heat-inactivated fetal bovine serum under an atmosphere of 5% CO2 in air. Culture medium (100 L) was seeded in 96-well microtiter plates with 105 amastigotes from axenic culture with or without a serial drug dilution. Serial drug dilutions covering a range from 90 to 0.123 μg/mL were prepared. After 72 h of incubation the plates were inspected under an inverted microscope to assure growth of the controls and sterile conditions. Then 10 μL of a resazurin solution (12.5 mg resazurin dissolved in 100 mL double-distilled water) was added to each well, and the plates incubated for another 2 h. They were then read in a Spectramax Gemini XS microplate fluorometer at an excitation wavelength of 536 nm and an emission wavelength of 588 nm. The IC50 values were calculated from the sigmoidal inhibition curves with SoftmaxPro software. Miltefosin (Zentaris GmbH, Germany) was used as a positive reference.
3.4. Cytotoxicity against L6 cells
Assays were performed in 96-well microtiter plates, each well containing 100 L of RPMI 1640 medium supplemented with 1% l-glutamine (200 mM) and 10% fetal bovine serum, and 4 × 104 l-6 cells (rat skeletal myoblasts). Serial drug dilutions of seven 3-fold dilution steps covering a range from 90 to 0.123 μg/mL were prepared. After 72 h of incubation the plates were inspected under an inverted microscope to assure growth of the controls and sterile conditions. Then 10 μL of a resazurin solution (12.5 mg resazurin dissolved in 100 mL distilled water) was added to each well and the plates incubated for another 2 h. They were then read with a Spectramax Gemini XS microplate fluorometer at an excitation wavelength of 536 nm and an emission wavelength of 588 nm. The IC50 values were calculated from the sigmoidal inhibition curves with SoftmaxPro software. Podophyllotoxin (Sigma, P4405) was used as a positive reference.
3.5. Calculation of IC50
To measure antiplasmodial activity, the concentration of extract at which the parasite growth (=[3H]hypoxanthine uptake) was inhibited by 50% (IC50) was calculated by linear interpolation between the two concentrations above and below 50% [29]. To assess leishmanicidal, antitrypanosomal, and cytotoxic activity we transferred data into the graphic Softmax Pro program (Molecular Devices), which calculated IC50 values from the sigmoidal inhibition curve. The values given in Table 3 are the means of two independent assays.
3.6. Selectivity Index Determination
The selectivity index (SI) corresponds to the ratio of the IC50 value of the cytotoxic activity to the IC50 value of antiprotozoal activity. An SI value >10 is generally considered to indicate antiprotozoal activity not due to general cytotoxicity.
4. Conclusions
Almost all the marine macrophytes species tested in the current study showed activity against at least one protozoan. Some of them may be promising sources for further bio-guided isolation of new active components. Most of these components are probably apolar compounds. The most interesting species, in terms of antiplasmodial activity and selectivity, is the red algae M. stellatus. Bioguided fractionation is underway.
Acknowledgments
We are grateful to Sandra Sritharan for her excellent technical assistance in the treatment of the seaweed material. We thank Monica Cal, Sibylle Sax and Christoph Stalder of the Swiss TPH for their assistance with the protozoan assays.
References
- 1.World Health Organization. Working to Overcome the Global Impact of Neglected Tropical Diseases. First WHO report on neglected tropical diseases, 12 October 2010. Available online: http://www.who.int/neglected_diseases/2010report/NTD_2010report_web.pdf (accessed on 14 January 2011).
- 2.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maude RJ, Pontavornpinyo W, Saralamba S, Aguas R, Yeung S, Dondorp AM, Day NP, White NJ, White LJ. The last man standing is the most resistant: Eliminating artemisinin-resistant malaria in Cambodia. Malar J. 2009;8:31. doi: 10.1186/1475-2875-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekland EH, Fidock DA. In vitro evaluations of antimalarial drugs and their relevance to clinical outcomes. Int J Parasitol. 2008;38:743–747. doi: 10.1016/j.ijpara.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin AS, Stout EP, Prudhomme J, Le Roch K, Fairchild CR, Franzblau SG, Aalbersberg W, Hay ME, Kubanek J. Bioactive bromophycolides R–U from the Fijian red alga. Callophycus serratus J Nat Prod. 2010;73:275–278. doi: 10.1021/np900686w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afolayan AF, Mann M, Lategan CA, Smith PJ, Bolton JJ, Beukes DR. Antiplasmodial halogenated monoterpenes from the marine red alga. Plocamium cornutum Phytochemistry. 2009;70:597–600. doi: 10.1016/j.phytochem.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Assreuy AMS, Gomes DM, da Silva MSJ, Torres VM, Siqueira RCL, Pires A, Criddle DN, de Alencar NMN, Cavada BS, Sampaio AH, Farias WRL. Biological effects of a sulfated polysaccharide isolated from the marine red algae. Champia feldmannii Biol Pharm Bull. 2008;31:691–695. doi: 10.1248/bpb.31.691. [DOI] [PubMed] [Google Scholar]
- 8.Mayer AM, Rodríguez AD, Berlinck RG, Hamann MT. Marine pharmacology in 2005–2006: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, antiinflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochem Biophys Acta. 2009;1790:283–308. doi: 10.1016/j.bbagen.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty K, Lipton AP, Paulraj R, Chakraborty RD. Guaiane sesquiterpenes from seaweed Ulva fasciata Delile and their antibacterial properties. Eur J Med Chem. 2010;45:2237–2244. doi: 10.1016/j.ejmech.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 10.Moo-Puc R, Robledo D, Freile-Pelegrin Y. Evaluation of selected tropical seaweeds for in vitro anti-trichomonal activity. J Ethnopharmacol. 2008;120:92–97. doi: 10.1016/j.jep.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Orhan I, Sener B, Atici T, Brun R, Perozzo R, Tasdemir D. Turkish freshwater and marine macrophyte extracts show in vitro antiprotozoal activity and inhibit FabI, a key enzyme of Plasmodium falciparum fatty acid biosynthesis. Phytomedicine. 2006;13:388–393. doi: 10.1016/j.phymed.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Kubanek J, Jensen PR, Keifer PA, Sullards MC, Collins DO, Fenical W. Seaweed resistance to microbial attack: A targeted chemical defense against marine fungi. Proc Natl Acad Sci USA. 2003;100:6916–6921. doi: 10.1073/pnas.1131855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nara T, Kamei Y, Tsubouchi A, Annoura T, Hirota K, Iizumi K, Dohmoto Y, Ono T, Aoki T. Inhibitory action of marine algae extracts on the Trypanosoma cruzi dihydroorotate dehydrogenase activity and on the protozoan growth in mammalian cells. Parasitol Int. 2005;54:59–64. doi: 10.1016/j.parint.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Freile-Pelegrin Y, Robledo D, Chan-Bacab MJ, Ortega-Morales BO. Antileishmanial properties of tropical marine algae extracts. Fitoterapia. 2008;79:374–377. doi: 10.1016/j.fitote.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Spavieri J, Allmendinger A, Kaiser M, Casey R, Hingley-Wilson S, Lalvani A, Guiry MD, Blunden G, Tasdemir D. Antimycobacterial, antiprotozoal and cytotoxic potential of twenty-one brown algae (Phaeophyceae) from British and Irish waters. Phytother Res. 2010;24:1724–1729. doi: 10.1002/ptr.3208. [DOI] [PubMed] [Google Scholar]
- 16.Spavieri J, Kaiser M, Casey R, Hingley-Wilson S, Lalvani A, Blunden G, Tasdemir D. Antiprotozoal, antimycobacterial and cytotoxic potential of some british green algae. Phytother Res. 2010;24:1095–1098. doi: 10.1002/ptr.3072. [DOI] [PubMed] [Google Scholar]
- 17.El Hattab M, Ben Mesaoud M, Daoudi M, Ortalo-Magne A, Culioli G, Valls R, Piovetti L. Trihydroxylated linear diterpenes from the brown alga Bifurcaria bifurcata (Fucales, Phaeophyta) Biochem Syst Ecol. 2008;36:484–489. [Google Scholar]
- 18.Ortalo-Magné A, Culioli G, Valls R, Pucci B, Piovetti L. Polar acyclic diterpenoids from Bifurcaria bifurcata (Fucales, Phaeophyta) Phytochemistry. 2005;66:2316–2323. doi: 10.1016/j.phytochem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Culioli G, Ortalo-Magné A, Daoudi M, Thomas-Guyon H, Valls R, Piovetti L. Trihydroxylated linear diterpenes from the brown alga. Bifurcaria bifurcata Phytochemistry. 2004;65:2063–2069. doi: 10.1016/j.phytochem.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Valls R, Banaigs B, Piovetti L, Archavlis A, Artaud J. Linear diterpene with antimitotic activity from the brown alga. Bifurcaria bifurcata Phytochemistry. 1993;34:1585–1588. [Google Scholar]
- 21.O’Neill MJ, Bray DH, Boardman P, Phillipson JD, Warhurst DC. Plants as sources of antimalarian drugs. In vitro test method for the evaluation of crude extracts from plants. Part. Planta Med. 1985;151:394–398. doi: 10.1055/s-2007-969529. [DOI] [PubMed] [Google Scholar]
- 22.Benoit F, Valentin A, Pelissier Y, Diafouka F, Marion C, Kone-Bamba D, Kone M, Mallie M, Yapo A, Bastide JM. In vitro antimalarial activity of vegetal extracts used in west african traditional medicine. Am J Trop Med Hyg. 1996;54:67–71. doi: 10.4269/ajtmh.1996.54.67. [DOI] [PubMed] [Google Scholar]
- 23.Baumeister S, Winterberg M, Przyborski JM, Lingelbach K. The malaria parasite Plasmodium falciparum: Cell biological peculiarities and nutritional consequences. Protoplasma. 2010;240:3–12. doi: 10.1007/s00709-009-0090-3. [DOI] [PubMed] [Google Scholar]
- 24.Lane AL, Stout EP, Lin AS, Prudhomme J, Le Roch K, Fairchild CR, Franzblau SG, Hay ME, Aalbersberg W, Kubanek J. Antimalarial bromophycolides J–Q from the Fijian red alga Callophycus serratus. J Org Chem. 2009;74:2736–2742. doi: 10.1021/jo900008w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houghton PJ, Raman A. Laboratory Handbook for the Fractionation of Natural Extracts. 1st ed. Chapman & Hall; London, UK: 1998. p. 49. [Google Scholar]
- 26.Scala F, Fattorusso E, Menna M, Taglialatela-Scafati O, Tierney M, Kaiser M, Tasdemir D. Bromopyrrole alkaloids as lead compounds against protozoan parasites. Mar Drugs. 2010;8:2162–2174. doi: 10.3390/md8072162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thaithong S, Beale GH. Resistance of ten Thai isolates of Plasmodium falciparum to chloroquine and pyrimethamine by in vitro tests. Trans Roy Soc Trop Med Hyg. 1981;75:271–273. doi: 10.1016/0035-9203(81)90333-3. [DOI] [PubMed] [Google Scholar]
- 28.Buckner FS, Verlinde CL, La Flamme AC, Van Voorhis WC. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob Agents Chemother. 1996;40:2592–2597. doi: 10.1128/aac.40.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber W, Koella JC. A comparison of the three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 1993;55:257–261. doi: 10.1016/0001-706x(93)90083-n. [DOI] [PubMed] [Google Scholar]