Abstract
Chemical examination of the Taiwanese soft coral Sinularia triangular led to the isolation of five cembrane-based diterpenoids 1–5, including two new metabolites, triangulenes A (1) and B (2). The structures of the new metabolites were determined on the basis of extensive spectroscopic analysis, particularly mass spectroscopy and 2D NMR (1H–1H COSY, HMQC, HMBC, and NOESY) spectroscopy. Metabolites 3 and 5 exhibited moderate cytotoxicity to human tumor cell lines CCRF-CEM and DLD-1. Furthermore, 3–5 displayed significant in vitro anti-inflammatory activity in lipopolysaccharide-stimulated RAW264.7 macrophage cells by inhibiting the expression of the iNOS protein. Metabolites 4 and 5 also effectively reduced the expression of the COX-2 protein in the macrophages.
Keywords: soft coral, Sinularia triangular, cytotoxicity, anti-inflammatory
1. Introduction
Our previous chemical examination of soft corals of the genus Sinularia led to the isolation and identification of various oxygenated cembrane-type metabolites [1–5]. Some of these metabolites exhibit anti-inflammatory activity [2–4] and/or cytotoxicity to the growth of some cancer cell lines [1]. Our ongoing research to discover bioactive metabolites from the soft coral Sinularia triangular (Tixier-Durivault, 1970; family Alcyoniidae) (Figure 1) led to the isolation of two new cembrane-based diterpenoids, triangulenes A (1) and B (2), along with three known metabolites, sinularin (3) [6], dihydrosinularin (4) [6], and (−)14-deoxycrassin (5) [7]. The structures of 1 and 2 were established by extensive spectroscopic analysis, including 2D NMR spectroscopy. The cytotoxicity of 1–5 to the human tumor cell lines CCRF-CEM (T-cell acute lymphoblastic leukemia) and DLD-1 (colon adenocarcinoma) was studied, and the ability of 1–5 to inhibit the expression of the pro-inflammatory iNOS (inducible nitric oxide synthase) and COX-2 (cyclooxygenase-2) proteins in lipopolysaccharide (LPS)–stimulated RAW264.7 macrophage cells was also evaluated.
Figure 1.
Soft coral Sinularia triangular.
2. Results and Discussion
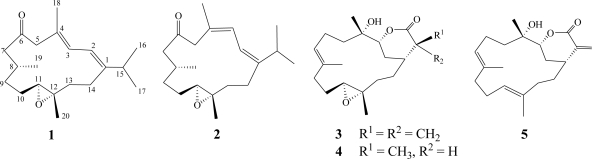
Frozen samples of S. triangular were extracted with EtOAc. The dry EtOAc extracts were fractionated by silica gel gravity column chromatography, and the eluted fractions were further purified by HPLC to yield cembranoids 1–5 (Figure 2).
Figure 2.
Structures of 1–5.
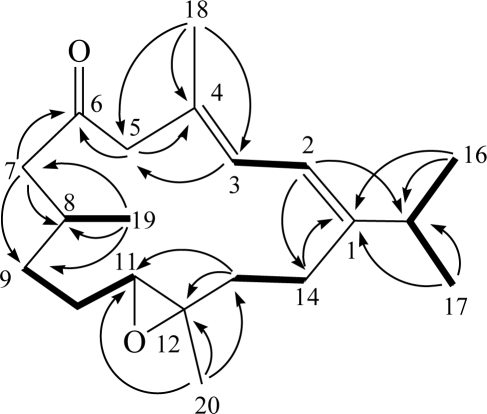
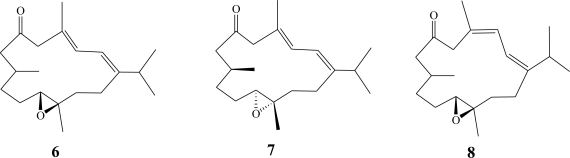
The HRESIMS spectrum of triangulene A (1) contained a molecular ion peak consistent with the molecular formula C20H32O2, indicating the molecule has five double-bond equivalent. A UV absorption maxima at 240 nm (logɛ = 4.0) was attributed to double bond conjugation. The IR spectrum of 1 revealed the presence of a carbonyl functionality (νmax = 1703 cm−1). The 13C NMR data of 1 showed the presence of 20 carbons (Table 1): five methyls, six sp3 methylenes, three sp3 methines (including an oxygenated carbon at δ 62.7), two sp2 methines, and four quaternary carbons (including an oxygenated carbon at δ 61.5, two olefinic carbons with resonances at δ 148.3 and δ 129.3, and a keto-carbonyl at δ 209.8). The 1H NMR data revealed the presence of two olefinic methine protons as doublets at δ 6.15 and δ 6.06. A proton signal at δ 2.71 (1H, dd, J = 8.4, 4.0 Hz) that correlated with a carbon signal at δ 62.7 in the HMQC spectrum of 1 was attributed to the proton of a trisubstituted epoxide. The gross planar structure of 1 was determined by detailed analysis of its 1D and 2D NMR spectra. From the 1H–1H COSY correlations (Figure 3), it was possible to establish five partial structures of consecutive proton spin systems extending from H-2 to H-3; H-8 to H3-19; H2-9 to H-11; H2-13 to H2-14; and H-15 to H3-16 and H3-17. The following key HMBC correlations permitted connection of the carbon skeleton: H-2 to C-1, C-14, and C-15; H-3 to C-5; H-5 to C-4 and C-6 (carbonyl carbon); H-7 to C-6, C-8, and C-9; H-13 to C-11 and C-12; H3-16 and H3-17 to C-1 and C-15; H3-18 to C-3, C-4, and C-5; H3-19 to C-7, C-8, and C-9; and H3-20 to C-11, C-12, and C-13. Thus, 1 was found to possess a tetrasubstituted diene at C-1/C-2 and C-3/C-4, a ketone group at C-6, and a trisubstituted epoxide at C-11/C-12. The above results indicate that 1 possessed the same molecular framework as known cembranoids 6 and 7 (Figure 4), which were isolated previously from octocorals Eunicea tourniforti [8] and Eunicea sp. [9], respectively.
Table 1.
1H and 13C NMR data for 1 and 2.
|
1 |
2 |
|||
|---|---|---|---|---|
| 1Ha | 13Cb | 1Ha | 13Cb | |
| 1 | 148.3 (C) | 146.7 (C) | ||
| 2 | 6.06 d (10.8) | 118.2 (CH) | 6.19 d (10.8) | 118.7 (CH) |
| 3 | 6.15 d (10.8) | 125.7 (CH) | 6.29 d (10.8) | 124.4 (CH) |
| 4 | 129.3 (C) | 130.5 (C) | ||
| 5 | 3.20 d (13.6); 3.06 d (13.6) | 54.7 (CH2) | 3.90 d (13.6); 2.70 d (13.6) | 48.9 (CH2) |
| 6 | 209.8 (C) | 208.0 (C) | ||
| 7 | 2.54 dd (13.2, 8.4); 2.17 m | 51.2 (CH2) | 2.52 m; 2.12 m | 52.4 (CH2) |
| 8 | 2.03 m | 31.2 (CH) | 1.83 m | 32.0 (CH) |
| 9 | 1.48 m; 1.18 m | 33.1 (CH2) | 1.31 m; 1.14 m | 33.3 (CH2) |
| 10 | 1.92 m; 1.14 m | 26.4 (CH2) | 1.88 m; 1.09 m | 26.4 (CH2) |
| 11 | 2.71 dd (8.4, 4.0) | 62.7 (CH) | 2.49 m | 64.4 (CH) |
| 12 | 61.5 (C) | 60.6 (C) | ||
| 13 | 2.15 m; 1.32 m | 36.7 (CH2) | 2.12 m; 1.36 m | 36.7 (CH2) |
| 14 | 2.37 m; 2.28 m | 25.9 (CH2) | 2.73 m; 2.12 m | 25.7 (CH2) |
| 15 | 2.35 m | 32.2 (CH) | 2.35 m | 30.8 (CH) |
| 16 | 1.06 d (7.2) | 22.6 (CH3) | 1.18 d (6.8) | 20.6 (CH3) |
| 17 | 1.08 d (7.6) | 22.1 (CH3) | 1.02 d (6.8) | 23.4 (CH3) |
| 18 | 1.89 s | 18.2 (CH3) | 1.93 s | 25.1 (CH3) |
| 19 | 0.93 d (6.8) | 19.5 (CH3) | 0.85 d (6.4) | 19.2 (CH3) |
| 20 | 1.20 s | 17.6 (CH3) | 1.23 s | 16.3 (CH3) |
Spectra were recorded at 400 MHz in CDCl3; J values (Hz) are given in parentheses;
spectra were recorded at 100 MHz in CDCl3; attached protons were deduced by DEPT experiments.
Figure 3.
Key 1H–1H COSY and HMBC correlations of 1.
Figure 4.
Structures of 6–8.
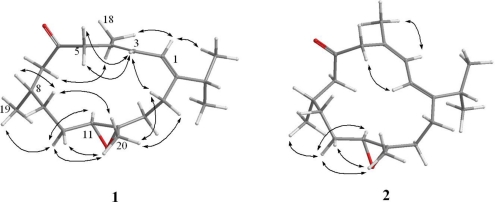
The relative configuration of 1 was determined from NOE correlations observed in the NOESY spectrum (Figure 5). The NOE correlations between H-2 and methyl protons H3-16 and H3-18 and between H-3 and H2-5 indicated E configurations for the double bonds at C-1/C-2 and C-3/C-4. In addition, one proton of C-10 methylene (δ 1.92) was found to exhibit correlations with H-11 (δ 2.71, dd, J = 8.4, 4.0 Hz) and H3-19 (δ 0.93, d, J = 6.8 Hz), indicating that these protons were situated on the same face; they were assigned as α protons, as C-20 methyl was β-oriented at C-12, which were verified by the absence of correlation between H-11 and H3-20. Furthermore, H3-20 correlated with protons of C-10 (δ 1.92 and 1.14) and C-14 (δ 2.37 and 2.28) methylenes, respectively. Consideration of molecular models found that H3-20 was reasonably close to H2-10 and H2-14 when H3-20 was β-oriented. Based on the above findings, the structure of 1, including its relative configuration was established, and the chiral centers for 1 were assigned as 8S*, 11S*, and 12S*. Furthermore, the chemical shifts of 1 were shifted downfield at C-7 (ΔδC +1.7 ppm) and C-8 (ΔδC +2.5 ppm) and upfield at C-19 (ΔδC −0.8 ppm) relative to the corresponding chemical shifts of 7. On the basis of the above findings, we determined the relative structure of 1, which was determined to be the C-8 epimer of 7.
Figure 5.
Selective NOESY correlations of 1 and 2.
Triangulene B (2) had the same molecular formula (C20H32O2) as 1, as indicated by HRESIMS and NMR spectra (Table 1). Comparison of the 1H and 13C NMR data of 2 with those of 1 revealed that the two compounds possessed similar structures. The trisubstituted double bonds at C-1/C-2 and C-3/C-4 of 2 had Z geometries, as indicated by NOE interactions (Figure 5) between H-3 (δ 6.29) and H3-18 (δ 1.93) and between H-2 (δ 6.19) and H-5 (δ 3.90). After determining the structure of 2, we discovered that its planar structure has been obtained previously as diterpenoid 8 from the octocoral Eunicea sp. [8]. Furthermore, we found that the NMR data for 2 were similar to those of 8, except that C-7 and C-8 of 2 were shifted markedly downfield (ΔδC +3.9 ppm and ΔδC +3.6 ppm, respectively) relative to the corresponding carbons of 8. Further analysis of other NOE interactions revealed that 1 and 2 possessed the same relative configurations at C-8, C-11, and C-12. Thus, the structure of 2 was established unambiguously.
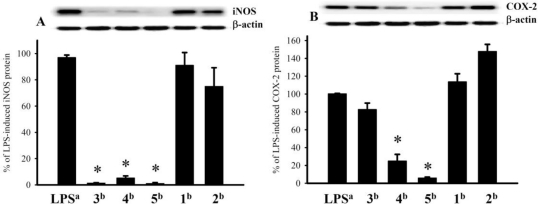
Study of the cytotoxicity of diterpenoids 1–5 to human tumor cell lines CCRF-CEM and DLD-1 showed that 3 and 5 moderately inhibited the growth of the tested cell lines (the ED50 values were 26.0 and 37.1 μM for 3 and 29.8 and 32.2 μM for 5 for CCRF-CEM and DLD-1, respectively). The in vitro anti-inflammatory effects of 1–5 were also tested. The inhibition of LPS-stimulated upregulation of the pro-inflammatory proteins iNOS and COX-2 in RAW264.7 macrophage cells was measured by immunoblot analysis. At a concentration of 10 μm, 3–5 reduced the levels of the iNOS protein to 1.2 ± 0.3%, 5.1 ± 1.6%, and 0.9 ± 0.7%, respectively, of the levels in control cells stimulated with LPS alone (set at 100%). At the same concentration, 4 and 5 markedly reduced the levels of COX-2 to 24.9 ± 7.4% and 5.9 ± 1.0%, respectively, relative to controls (Figure 6).
Figure 6.
Immunoblot analysis of the effects of 1–5 (10 μM) on the expression of the iNOS and COX-2 proteins of RAW264.7 macrophage cells: (A) Immunoblots of iNOS and β-actin and (B) immunoblots of COX-2 and β-actin. The relative intensity for the cells stimulated with LPS alone was set at 100%. Band intensities were quantified by densitometry and are indicated as percentages relative to the intensities for the LPS-stimulated cells. Western blotting with β-actin was performed to verify that equivalent amounts of protein were loaded in each lane. Values represent mean ± SEM (n = 6). *Significantly different from the values for cells stimulated with LPS alone (*P < 0.05). aStimulated with LPS alone; bstimulated with LPS in the presence of 1–5.
3. Experimental Section
3.1. General Experimental Procedures
Melting points were measured on Fargo apparatus and are uncorrected. Optical rotation values were measured with a Jasco P-1010 digital polarimeter. Ultraviolet spectra were recorded on a Jasco V-650 spectrophotometer. IR spectra were obtained with a Varian Digilab FTS 1000 FT-IR spectrophotometer. NMR spectra were recorded with a Varian Mercury Plus 400 FT-NMR, at 400 MHz for 1H NMR and 100 MHz for 13C NMR, in CDCl3. ESIMS and HRESIMS data were recorded with a Bruker APEX II mass spectrometer. Silica gel 60 (230–400 mesh; Merck, Darmstadt, Germany) was used for column chromatography. Gravity column chromatography was performed on silica gel (230–400 mesh; Merck). TLC was carried out on precoated Kieselgel 60 F254 (0.2 mm; Merck), and spots were visualized by spraying with 10% H2SO4 solution followed by heating. HPLC was performed on a system comprising a Hitachi L-7100 pump, a Hitachi photodiode array detector L-7455, and a Rheodyne 7725 injection port. A semi-preparative reverse-phase column (Hibar 250 × 10 mm, LiChrospher 100 RP-18e, 5 μm, Merck) and a preparative normal-phase column (Hibar 250 × 21 mm, Si-60 column, 7 μm, Merck) were used for HPLC.
3.2. Animal Material
The marine soft coral S. triangular (specimen No. 200807-15) was collected by scuba divers at a depth of around 10 m off the coast of Taitung County, Taiwan, in July 2008, and the samples were frozen immediately after collection. A voucher sample was deposited at the Department of Marine Biotechnology and Resources, National Sun Yat-sen University, Taiwan.
3.3. Extraction and Separation
The frozen bodies of S. triangular (1.2 kg, wet weight) were minced and exhaustively extracted with EtOAc (1 L × 5). The combined EtOAc extracts (15.5 g) were subjected to silica gel column chromatography with elution by EtOAc in n-hexane (0–100%, stepwise) followed by 100% acetone; and the fractions were pooled on the basis of TLC analysis to yield 17 fractions. Fraction 8 (265 mg), which eluted with n-hexane–EtOAc (10:1), was subjected to silica gel column chromatography with gradient elution (n-hexane–acetone, 12:1 to 6:1) to afford five subfractions (A1–A5). Subfraction A2 (20 mg) was subjected to reverse-phase HPLC with MeOH–H2O (5:1) elution to afford 1 (2.5 mg) and 2 (2.0 mg). Subfraction A3 (90 mg) was subjected to normal-phase HPLC using n-hexane–acetone (10:1) to afford 5 (50.3 mg). Fraction 11 (160 mg), which eluted with n-hexane–EtOAc (5:1), was subjected to silica gel column chromatography with gradient elution (n-hexane–acetone, 8:1 to 5:1) to yield six subfractions (B1–B6). Subfraction B3 was subjected to normal-phase HPLC with n-hexane–acetone (7:1) elution to afford 3 (20.5 mg) and 4 (10.8 mg).
Triangulene A (1): colorless oil; [α]25D +70.8 (c 0.5, CHCl3); IR (neat) vmax 2961, 2928, 1703, 1456, 1385, and 1261 cm−1; UV (MeOH) λmax 240 (log ɛ = 4.0); 13C and 1H NMR data, see Table 1; ESIMS m/z 327 [M + Na]+; HRESIMS m/z 327.2302 [M + Na]+ (calcd for C20H32O2Na, 327.2300).
Triangulene B (2): colorless oil; [α]25D +50.6 (c 0.5, CHCl3); IR (neat) vmax 2959, 2928, 1709, 1460, and 1385 cm−1; UV (MeOH) λmax 239 (log ɛ = 3.8); 13C and 1H NMR data, see Table 1; ESIMS m/z 327 [M + Na]+; HRESIMS m/z 327.2301 [M + Na]+ (calcd for C20H32O2Na, 327.2300).
Sinularin (3): white powder; mp 151–153 °C; [α]25D −120 (c 0.5, CHCl3); ESIMS m/z 357 [M + Na]+ [6].
Dihydrosinularin (4): white powder; mp 116–118 °C; [α]25D –42 (c 0.3, CHCl3); ESIMS m/z 359 [M + Na]+ [6].
(−)14-Deoxycrassin (5): colorless oil; [α]25D −15 (c 1.0, CHCl3); ESIMS m/z 341 [M + Na]+ [7].
3.4. Cytotoxicity Testing
The cytotoxicity of 1–5 to CCRF-CEM and DLD-1 tumor cells was evaluated by means of the tetrazolium-based colorimetric assay [10,11]. As a positive control, we employed doxorubicin, which exhibited cytotoxicity to CCRF-CEM and DLD-1 cells with ED50 values of 0.57 and 0.25 μm, respectively.
3.5. In Vitro Anti-Inflammatory Assay
A macrophage (RAW264.7) cell line was purchased from ATCC. We measured the in vitro anti-inflammatory activities of 1–5 by examining the inhibition of LPS-simulated upregulation of the iNOS (inducible nitric oxide synthetase) and COX-2 (cyclooxygenase-2) proteins in macrophages using western blotting analysis [12,13].
Acknowledgments
This research was supported by grants from the National Museum of Marine Biology & Aquarium and the National Science Council (NSC 99-2320-B-291-001), Taiwan, awarded to J.-H. Su.
Footnotes
Samples Availability: Not available.
References
- 1.Su J-H, Ahmed AF, Sung P-J, Chao C-H, Kuo Y-H, Sheu J-H. Manaarenolides A–I, new diterpenoids from the soft coral. Sinularia manaarensis J Nat Prod. 2006;69:1134–1139. doi: 10.1021/np050483q. [DOI] [PubMed] [Google Scholar]
- 2.Lu Y, Huang C-Y, Lin Y-F, Wen Z-H, Su J-H, Kuo Y-H, Chiang MY, Sheu J-H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and. Sinularia granosa J Nat Prod. 2008;71:1754–1759. doi: 10.1021/np8003563. [DOI] [PubMed] [Google Scholar]
- 3.Chen B-W, Chao C-H, Su J-H, Huang C-Y, Dai C-F, Wen Z-H, Sheu J-H. A novel symmetric sulfur-containing biscembranoid from the Formosan soft coral. Sinularia flexibilis Tetrahedron Lett. 2010;44:5764–5766. [Google Scholar]
- 4.Lu Y, Su J-H, Huang C-Y, Liu Y-C, Kuo Y-H, Wen ZH, Hsu C-H, Sheu J-H. Cembranoids from the Soft Corals Sinularia granosa and. Sinularia querciformis Chem Pharm Bull. 2010;58:464–466. doi: 10.1248/cpb.58.464. [DOI] [PubMed] [Google Scholar]
- 5.Su J-H, Lin Y-F, Lu Y, Yeh H-C, Wang W-H, Fan T-Y, Sheu JH. Oxygenated cembranoids from the cultured and wild-type soft corals. Sinularia flexibilis Chem Pharm Bull. 2009;57:1189–1192. doi: 10.1248/cpb.57.1189. [DOI] [PubMed] [Google Scholar]
- 6.Weinheimer AJ, Matson JA, Hossain MB, van der Helm D. Marine anticancer agents: Sinularin and dihydrosinularin, new cembranolides from the soft coral. Sinularia flexibilis Tetrahedron Lett. 1977;34:2923–2926. [Google Scholar]
- 7.Wen T, Ding Y, Deng Z, van Ofwegen L, Proksch P, Lin W. Sinulaflexiolides A–K, cembrane-type diterpenoids from the Chinese soft coral. Sinularia flexibilis J Nat Prod. 2008;71:1133–1140. doi: 10.1021/np070640g. [DOI] [PubMed] [Google Scholar]
- 8.Marville KI, McLean S, Reynolds WF, Tinto WF. New cembrane diterpenes of the marine octocoral Eunicea tourniforti from the eastern Caribbean. J Nat Prod. 2003;66:1284–1287. doi: 10.1021/np030091o. [DOI] [PubMed] [Google Scholar]
- 9.Wei X, Rodríguez AD, Baran P, Raptis RG, Sánchez JA, Ortega-Barria E, González J. Antiplasmodial cembradiene diterpenoids from a Southwestern Caribbean gorgonian octocoral of the genus. Eunicea Tetrahedron. 2004;60:11813–11819. [Google Scholar]
- 10.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 11.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 12.Jean Y-H, Chen W-F, Sung C-S, Duh C-Y, Huang S-Y, Lin C-S, Tai M-H, Tzeng S-F, Wen Z-H. Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats. Br J Pharmacol. 2009;158:713–725. doi: 10.1111/j.1476-5381.2009.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jean Y-H, Chen W-F, Duh C-Y, Huang S-Y, Hsu C-H, Lin C-S, Sung C-S, Chen I-M, Wen Z-H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral. Lemnalia cervicorni Eur J Pharmacol. 2008;578:323–331. doi: 10.1016/j.ejphar.2007.08.048. [DOI] [PubMed] [Google Scholar]