Abstract
In our ongoing search for new pharmacologically active leads from Solomon organisms, we have examined the sponge Theonella swinhoei. Herein we report the isolation and structure elucidation of swinholide A (1) and one new macrolide, swinholide J (2). Swinholide J is an unprecedented asymmetric 44-membered dilactone with an epoxide functionality in half of the molecule. The structural determination was based on extensive interpretation of high-field NMR spectra and HRESIMS data. Swinholide J displayed potent in vitro cytotoxicity against KB cells (human nasopharynx cancer) with an IC50 value of 6 nM.
Keywords: marine cytotoxin, swinholide J, Theonella swinhoei
1. Introduction
Theonella sponges represent an extraordinary source of bioactive secondary metabolites, particularly peptides and macrolides. Swinholide A (1) was the first symmetric 44-membered macrolide to be isolated from the Red Sea marine sponge Theonella swinhoei [1], and then demonstrated as product of biochemistry of symbiontic microorganisms [2]. The structure was first assigned as a monomer, revised later to a symmetric cyclic dimer [3], followed by determination of its stereochemistry [4–6]. Swinholide A (1) displays impressive biological properties including antifungal activity and potent cytotoxicity against a number of tumor cells. Its mechanism of action has been clarified in detail and its activity has been attributed to its ability to dimerize actin and disrupt the actin cytoskeleton [7–9]. Today, swinholide A is one of the better-characterized membrane permeable and specific inhibitors of actin filament networks and is actively used in cell biology studies [10].
Several derivatives of swinholide A were reported in the literature. They differ from the parent compound in the carbon backbone as in misakinolides [11–13] and hurghadolide [14] which feature a 40- and 42-membered dilactone structure, respectively, in the regiochemistry of the ring closure as in isoswinholide A [15], in the glycosidation, as in ankaraholides A and B [2], in the monomeric structure of preswinholide A [16], and in the different symmetric or asymmetric functionalization of the carbon backbone as in swinholides B (16′-demethyl) [15], C (29′-O-demethyl) [15], D (15′-O-demethyl) [17], E (6′-hydroxy) [17], F (2′-Z conformer) [17], G (20′-demethyl) [17], H (7,7′-O-dimethyl) [18] and I (26′-hydroxy) [14].
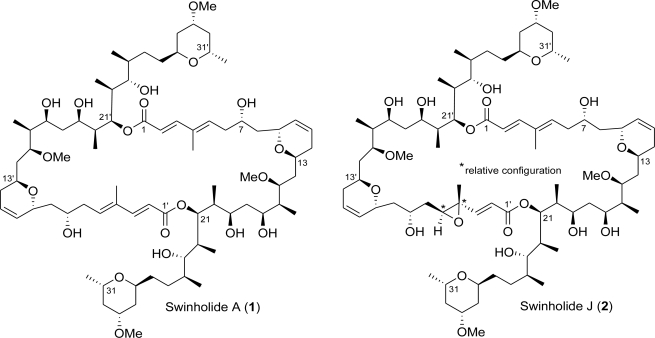
In our ongoing search for new pharmacologically active lead compounds from Solomon organisms [19–24], we have examined the sponge Theonella swinhoei. Separation of the cytotoxic fractions from the CHCl3 extract of a specimen of the marine sponge collected in Vangunu at the Solomon Islands, resulted in the identification of swinholide A (1) and of a new potently cytotoxic macrolide, swinholide J (2) (Figure 1).
Figure 1.
Swinholides A (1) and J (2) from Theonella swinhoei.
2. Results and Discussion
Swinholide J (2) showed an intense ion peak at m/z 1427.9181 [(M + Na)+] in the HR ESIMS, 16 mass units higher than that observed for 1, corresponding to one additional oxygen atom. As already reported for several swinholide derivatives [14,15,17], inspection of the 1H NMR spectrum clearly revealed an asymmetric dimeric nature for 2. Notably, the 1H NMR spectrum of 2 showed seven resonances in the region 7.46–5.67 ppm (H-2, H-2′, H-3, H-3′, H-5, H-10/H-10′, H-11/H-11′), instead of resonances for five pairs of equivalent protons as in 1 (Table 1). Furthermore, in contrast to 1, the 1H NMR spectrum of swinholide J (2) displayed two additional resonances at δH 3.22 [H-5′, dd (J = 4.3, 7.4 Hz)] and 1.42 (Me-4′, s). Also, the 13C NMR spectrum (Table 1), interpreted with the help of the HSQC and HMBC experiments, revealed the loss of symmetry in the dilactone skeleton of 2. In the 152.4–115.9 ppm region, the 13C NMR spectrum of 2 showed eight resonances for olefinic carbons (nine methines, two of which corresponding to two pairs of equivalent carbons (C-10/C-10′ and C-11/C-11′) and one quaternary carbon, C-4), instead of six signals for six pairs of equivalent olefinic carbons as seen in 1, and two resonances at δC 170.2 and 168.7 for the lactone carbons C-1 and C-1′, respectively. Moreover, in the region 55–80 ppm, two additional resonances respect to 1 were inferred from analysis of NMR data: one oxygen-bearing methine carbon (δC 64.6, δH 3.22, C-5′) and one oxygen-bearing quaternary carbon (δC 59.7, C-4′).
Table 1.
NMR data (700 MHz, CD3OD) for swinholides A (1) and J (2) (δ in ppm, J in Hz).
|
1a |
2a |
|||||
|---|---|---|---|---|---|---|
| Position | Type | δH/δH′ (J in Hz) | δC/δC′ | δH/δH′ (J in Hz) | δC/δC′ | HMBCb |
| 1/1′ | C | - | 170.6 | - | 170.2/168.7 | |
| 2/2′ | CH | 5.84 d (15.7) | 115.6 | 5.90 d (15.6)/6.14 d (15.7) | 115.9/122.2 | C1, C4 |
| C1′, C4′ | ||||||
| 3/3′ | CH | 7.43 d (15.7) | 152.3 | 7.46 d (15.6)/6.82 d (15.7) | 152.0/152.4 | C1, C5 |
| C1′ | ||||||
| 4/4′ | C | - | 135.5 | - | 135.5/59.7 | |
| 4/4′-Me | CH3 | 1.77 s | 12.4 | 1.85 s/1.42 s | 12.7/15.5 | C3, C4, C5 |
| C3′, C4′, C5′ | ||||||
| 5/5′ | CH | 6.14 t (7.3) | 140.5 | 6.16 t (7.2)/3.22 dd (4.3, 7.4) | 140.6/64.6 | C3, 4-Me |
| 6/6′ | CH2 | 2.40 t (6.9) | 38.8 | 2.44 m/1.81 m, 1.63 m | 38.7/37.9 | C4, C5, C7, C8 |
| 7/7′ | CH | 4.02 m | 68.1 | 3.99 ovl/4.12 m | 68.2/66.5 | |
| 8/8′ | CH2 | 1.28 m | 41.0 | 1.87 m, 1.37 m/ | 41.8/41.6 | |
| 1.76 m | 1.78 m, 1.37 m | |||||
| 9/9′ | CH | 4.47 br d (10.5) | 70.5 | 4.48 br d (10.2) | 70.4 | |
| 10/10′ | CH | 5.65 dd (1.8, 10.5) | 130.9 | 5.67 br d (10.3) | 130.8 | |
| 11/11′ | CH | 5.81 m | 124.9 | 5.83 m | 124.8 | |
| 12/12′ | CH2 | 1.94 m | 32.2 | 1.96 m | 32.2 | |
| 13/13′ | CH | 3.49 m | 65.3 | 3.54 m | 65.4 | |
| 14/14′ | CH2 | 1.58 m | 37.2 | 1.58 m | 36.9 | |
| 1.77 m | 1.82 m | |||||
| 15/15′ | CH | 3.76 m | 78.2 | 3.76 m/3.83 m | 78.4/77.9 | |
| 15/15′-OMe | CH3 | 3.32 s | 56.7 | 3.34 s/3.35 s | 56.9/57.2 | C15/C15′ |
| 16/16′ | CH | 1.52 m | 43.8 | 1.55 m | 43.3 | |
| 16/16′-Me | CH3 | 0.83 d (6.7) | 8.8 | 0.83 d (7.0)/0.84 d (7.0) | 9.2 | C15, C16, C17 |
| C15′, C16′, C17′ | ||||||
| 17/17′ | CH | 3.61 m | 73.2 | 3.60 m | 73.2 | |
| 18/18′ | CH2 | 1.63 m | 39.0 | 1.61 m | 39.2 | |
| 1.74 m | 1.75 m | |||||
| 19/19′ | CH | 3.97 ovl | 70.1 | 3.97 ovl/3.88 m | 70.0/70.1 | |
| 20/20′ | CH | 1.94 m | 39.4 | 1.94 m | 39.4 | |
| 20/20′-Me | CH3 | 0.91 d (7.0) | 8.9 | 0.90 d (7.1)/0.91 d (7.1) | 9.1 | |
| 21/21′ | CH | 5.46 d (10.5) | 75.6 | 5.45 t (10.3)/5.46 t (10.3) | 75.7/76.3 | |
| 22/22′ | CH | 1.98 m | 37.9 | 1.97 m | 38.0 | |
| 22/22′-Me | CH3 | 0.94 d (6.9) | 9.6 | 0.92 d (7.0)/0.93 d (7.0) | 9.7 | C21, C22, C23 |
| C21′, C22′, C23′ | ||||||
| 23/23′ | CH | 3.11 dd (1.8, 9.5) | 77.2 | 3.10 m | 77.3 | |
| 24/24′ | CH | 1.70 m | 34.4 | 1.70 m | 34.5 | |
| 24/24′-Me | CH3 | 0.98 d (6.7) | 17.7 | 0.97 d (6.7)/0.98 d (6.7) | 17.9 | C23, C24, C25 |
| C23′, C24′, C25′ | ||||||
| 25/25′ | CH2 | 1.24 m | 25.1 | 1.23 m, 1.41 m | 25.1 | |
| 1.42 m | ||||||
| 26/26′ | CH2 | 1.27 m | 29.7 | 1.28 m, 1.94 m | 29.8 | |
| 1.94 m | ||||||
| 27/27′ | CH | 3.99 ovl | 72.7 | 3.98 ovl | 72.8 | |
| 28/28′ | CH2 | 1.52 m | 35.8 | 1.52 m | 35.8 | |
| 1.87 br d (12.8) | 1.87 br d (12.5) | |||||
| 29/29′ | CH | 3.61 m | 74.2 | 3.61 m | 74.3 | C29-OMe |
| C29′-OMe | ||||||
| 29/29′-OMe | CH3 | 3.34 s | 55.3 | 3.34 s | 55.3 | C29 |
| C29′ | ||||||
| 30/30′ | CH2 | 1.09 dd (10.4, 12.6) | 39.7 | 1.09 dd (10.4, 12.6) | 39.7 | |
| 2.01 br d (12.6) | 2.02 br d (12.6) | |||||
| 31/31′ | CH | 3.74 m | 65.7 | 3.74 m | 65.8 | |
| 31/31′-Me | CH3 | 1.19 d (6.2) | 21.8 | 1.19 d (6.2) | 21.8 | |
Data from COSY, HSQC, and HMBC experiments;
HMBC correlations, optimized for 6 Hz, are from proton(s) stated to the indicated carbon.
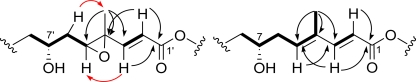
All these data clearly suggested a perturbation in the diene moiety of one half of the molecule with the introduction of an epoxide functionality. Extensive study of COSY, HSQC, and HMBC spectra allowed us to establish the presence in the molecule of one half identical to that of symmetric swinholide A (Figure 2). A conjugated double bond in the other half (C-1′–C-3′) was inferred by COSY correlation between proton signals at δH 6.14 (d, J = 15.7 Hz) and 6.82 (d, J = 15.7 Hz) and HMBC cross-peaks H-2′/C-1′ and H-3′/C-1′. The two additional oxygen-bearing carbons were placed at C-4′ and C-5′, respectively, on the basis of HMBC correlations from methyl protons at δH 1.42 (3H, s, Me-4′) to C-3′, C-4′ and C-5′. Definitive confirmation of the proposed structure for swinholide J (2), derived from 2D-HOHAHA analysis, showed correlations starting from H-5′ (δH 3.22) to H-9′ (δH 4.48).
Figure 2.
COSY/TOCSY connectivities (bold bonds), HMBC (black arrows) and ROE (red arrows) correlations for C-1/C-7 and C-1′/C-7′ partial structures of swinholide J (2).
As shown in Table 1, the presence of an epoxy-functionality at C-4′/C-5′ positions at one side of the molecule caused twinning of most of the 1H and 13C NMR resonances of the nuclei belonging to dilactone ring skeleton, without any effect on the signals of the side chain.
The large coupling constant (15.7 Hz) between two vinyl protons H-2′ and H-3′ revealed the E-configuration of Δ2′-double bond whereas ROE correlations Me-4′/H-6′ and H-5′/H-3′ allowed us to established a relative configuration around the epoxide moiety as depicted in Figure 2. The stereochemistry of all the remaining stereocenters of swinholide J (2) is suggested to be the same as the parent swinholide A (1) on the basis of the similarity in their chemical shift and in the coupling constant values.
Swinholide J (2) was isolated as a very minor component with respect to the parent compound swinholide A (1) (relative composition of 1/2 in the sponge extract being 15:1 whereby the % yield was calculated based on the wet weight of the CHCl3 extract, swinholide A 0.55% and swinholide J 0.038%). On this basis, swinholide J (2) could be considered to be artifact arising from the extraction and isolation procedures utilized. However, careful HPLC and 1H NMR (700 MHz) analyses of all swinholide-containing fractions did not show the presence of other isomeric mono-epoxide or diepoxide derivatives that could be obtained through an abiotic radical oxidation of swinholide A (1). As such, swinholide J (2) is considered to be a new natural derivative of swinholide A (1) arising from a regio- and stereo-selective enzyme mediated oxidation of one of the two dienoate moieties in the parent compound.
Swinholide J (2) showed potent in vitro antiproliferative activity against KB cells, with an IC50 value of 6.7 nM, comparable to that of the parent compound (IC50 1.2 nM against KB cells) [25].
3. Experimental Section
3.1. General Procedures
Specific rotations were measured on a Perkin-Elmer 243 B polarimeter. High-resolution ESI-MS spectra were performed with a Micromass QTOF Micromass spectrometer. ESI-MS experiments were performed on an Applied Biosystem API 2000 triple-quadrupole mass spectrometer. NMR spectra were obtained on Varian Inova 700 NMR spectrometer (1H at 700 MHz, 13C at 175 MHz, respectively) equipped with a Sun hardware, δ (ppm), J in Hz, spectra referred to CD3OH as internal standard (δH 3.31, δC 49.0). HPLC was performed using a Waters Model 510 pump equipped with Waters Rheodine injector and a differential refractometer, model 401.
Through-space 1H connectivities were evidenced using a ROESY experiment with mixing times of 200 ms.
Silica gel (200–400 mesh) from Macherey-Nagel Company was used for flash chromatography.
3.2. Sponge Material and Separation of Individual Macrolides
Theonella swinhoei (order Lithistida, family Theonellidae) was collected on the barrier reef of Vangunu Island, Solomon Islands, in July 2004. The samples were frozen immediately after collection and lyophilized to yield 207 g of dry mass. Taxonomic identification was performed by Dr. John Hooper of Queensland Museum, Brisbane, Australia, where specimen is deposited under the accession number G3122662.
The lyophilized material (207 g) was extracted with methanol (3 × 1.5 L) at room temperature and the crude methanolic extract was subjected to a modified Kupchan’s partitioning procedure as follows. The methanol extract was dissolved in a mixture of MeOH/H2O containing 10% H2O and partitioned against n-hexane (15.2 g). The water content (% v/v) of the MeOH extract was adjusted to 30% and partitioned against CHCl3 (5.8 g). The aqueous phase was concentrated to remove MeOH and then extracted with n-BuOH (6.0 g).
The CHCl3 extract (5.8 g) was chromatographed by silica gel MPLC using a solvent gradient system from CH2Cl2 to CH2Cl2:MeOH 1:1.
Fractions eluted with CH2Cl2:MeOH 95:5 (239 mg) were further purified by HPLC on a Nucleodur 100-5 C18 (5 μm; 7.8 mm i.d. × 250 mm) with MeOH:H2O (9:1) as eluent (flow rate 1 mL/min) to give 2.2 mg of swinholide J (2) (tR = 7.0 min) and 31.7 mg of swinholide A (1) (tR = 7.4 min).
Biological evaluation. The antiproliferative activity of swinholide A and J was determined on KB (nasopharyngeal epidermoid carcinoma) as previously reported [26].
3.3. Characteristic Data for Each Compound
Swinholide A (1): light yellow solid; [α]D23 +16.1 (c 0.12, MeOH); 1H and 13C NMR data in CD3OD given in Table 1; ESIMS: m/z 1411.9 [M + Na]+. HRMS (ESI): calcd for C78H132NaO20: 1411.9210; found 1411.9257 [M + Na]+.
Swinholide J (2): light yellow solid; [α]D23 −11.7 (c 0.14, MeOH); 1H and 13C NMR data in CD3OD given in Table 1; ESIMS: m/z 1427.9 [M + Na]+. HRMS (ESI): calcd for C78H132NaO21: 1427.9159; found 1427.9181 [M + Na]+.
4. Conclusions
In this paper we report the isolation and the structural characterization of a new swinholide congener, swinholide J endowed with potent cytotoxic activity from the marine sponge Theonella swinhoei. The structure was determined by extensive application of 2D NMR techniques. The discovery of swinholide J reaffirms the utility of examining marine sponge for identifying novel potential antitumor lead compounds.
Acknowledgments
This work is part of the CRISP (Coral Reef Initiative in the South Pacific) project and granted by the Agence Française de Développement. We thank the Solomon and the Fiji Islands governments for allowing us to collect there, their Fisheries departments for their help and assistance. We thank the IRD diving team for the collection of the sponges and John Hooper for the identification of the sponges. NMR spectra were provided by the CSIAS, Centro Interdipartimentale di Analisi Strumentale, Faculty of Pharmacy, University of Naples.
Footnotes
Samples Availability: Available from the authors.
References and Notes
- 1.Carmely S, Kashman Y. Structure of swinholide A, a new macrolide from the marine sponge. Theonella swinhoei Tetrahedron Lett. 1985;26:511–514. [Google Scholar]
- 2.Andrianasolo EH, Gross H, Goeger D, Musafija-Girt M, McPhail K, Leal RM, Mooberry SL, Gerwick WH. Isolation of swinholide A and related glycosylated derivatives from two field collections of marine cyanobacteria. Org Lett. 2005;7:1375–1378. doi: 10.1021/ol050188x. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi M, Tanaka J, Katori T, Matsuura M, Kitagawa I. Structure of swinholide A, a potent cytotoxic macrolide from the Okinawan marine sponge. Theonella swinhoei Tetrahedron Lett. 1989;30:2963–2966. [Google Scholar]
- 4.Kobayashi M, Tanaka J, Katori T, Matsuura M, Yamashita M, Kitagawa I. Marine natural products. XXII. The absolute stereostructure of swinholide A, a potent cytotoxic dimeric macrolide from the Okinawan marine sponge. Theonella swinhoei Chem Pharm Bull. 1990;38:2409–2418. doi: 10.1248/cpb.38.2960. [DOI] [PubMed] [Google Scholar]
- 5.Kitagawa I, Kobayashi M, Katori T, Yamashita M, Tanaka J, Doi M, Ishida T. Absolute stereostructure of swinholide A, a potent cytotoxic macrolide from the Okinawan marine sponge. Theonella swinhoei J Am Chem Soc. 1990;112:3710–3712. [Google Scholar]
- 6.Doi M, Ishida T, Kobayashi M, Kitagawa I. Molecular conformation of swinholide A, a potent cytotoxic dimeric macrolide from the Okinawan marine sponge Theonella swinhoei: X-ray crystal structure of its diketone derivative. J Org Chem. 1991;56:3629–3632. [Google Scholar]
- 7.Klenchin VA, King R, Tanaka J, Marriott G, Rayment I. Structural basis of swinholide A binding to actin. Chem Biol. 2005;12:287–291. doi: 10.1016/j.chembiol.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Allingham JS, Zampella A, D’Auria MV, Rayment I. Structures of microfilament destabilizing toxins bound to actin provide insight into toxin design and activity. PNAS. 2005;102:14527–14532. doi: 10.1073/pnas.0502089102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanley FM. Insulin-Increased prolactin gene expression requires actin treadmilling: Potential pole for P21 activated kinase. Endocrinology. 2007;148:5874–5883. doi: 10.1210/en.2007-0127. [DOI] [PubMed] [Google Scholar]
- 10.Braet F, Soon L, Vekemans K, Thordarson P, Spector I. Actin-binding drugs: An elegant tool to dissect subcellular processes in endothelial and cancer cells. Protein Rev. 2008;8:37–49. [Google Scholar]
- 11.Sakai R, Higa T, Kashman Y. Misakinolide-A, an antitumor macrolide from the marine sponge Theonella sp. Chem Lett. 1986;9:1499–1502. [Google Scholar]
- 12.Kato Y, Fusetani N, Matsunaga S, Hashimoto K, Sakai R, Higa T, Kashman Y. Bioactive marine metabolites. Part XXIII. Antitumor macrodiolides isolated from a marine sponge Theonella sp.: Structure revision of misakinolide A. Tetrahedron Lett. 1987;28:6225–6228. [Google Scholar]
- 13.Kobayashi J, Tsukamoto S, Tanabe A, Sasaki T, Ishibashi M. New congeners of bistheonellides from Okinawan marine sponges of the Genus. Theonella J Chem Soc Perkin Trans 1. 1991:2379–2383. doi: 10.1039/P19910002379. [DOI] [Google Scholar]
- 14.Youssef DTA, Mooberry LS. Hurghadolide A and swinholide I, potent actin-microfilament disrupters from the red sea sponge. Theonella swinhoei J Nat Prod. 2006;69:154–157. doi: 10.1021/np050404a. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Tanaka J, Katori T, Kitagawa I. Marine natural products. XXIII. Three new cytotoxic dimeric macrolides, swinholides B and C and isoswinholide A, congeners of swinholide A, from the Okinawan marine sponge. Theonella swinhoei Chem Pharm Bull. 1990;38:2960–2966. doi: 10.1248/cpb.38.2960. [DOI] [PubMed] [Google Scholar]
- 16.Todd JS, Alvi KA, Crews P. The isolation of a monomeric carboxylic acid of swinholide a from the indo-pacific sponge. Theonella swinhoei Tetrahedron Lett. 1992;33:441–442. [Google Scholar]
- 17.Tsukamoto S, Ishibashi M, Sasaki T, Kobayashi J. New congeners of swinholides from the Okinawan marine sponge Theonella sp. J Chem Soc Perkin Trans 1. 1991:3185–3188. doi: 10.1039/P19910003185. [DOI] [Google Scholar]
- 18.Dumdei EJ, Blunt JW, Munro MHG, Pannell LK. Isolation of Calyculins, Calyculinamides, and Swinholide H from the New Zealand Deep-Water Marine Sponge Lamellomorpha strongylata. J Org Chem. 1997;62:2635–2639. doi: 10.1021/jo961745j. [DOI] [PubMed] [Google Scholar]
- 19.Festa C, De Marino S, Sepe V, Monti MC, Luciano P, D’Auria MV, Debitus C, Bucci M, Vellecco V, Zampella A. Perthamides C and D, two new potent anti-inflammatory cyclopeptides from a Solomon Lithistid sponge. Theonella swinhoei Tetrahedron. 2009;65:10424–10429. [Google Scholar]
- 20.Sepe V, D’Auria MV, Bifulco G, Ummarino R, Zampella A. Concise synthesis of AHMHA unit in perthamide C. Structural and stereochemical revision of perthamide C. Tetrahedron. 2010;66:7520–7526. [Google Scholar]
- 21.Festa C, De Marino S, D’Auria MV, Bifulco G, Renga B, Fiorucci S, Petek S, Zampella A. Solomonsterols A and B from Theonella swinhoei. The First Example of C-24 and C-23 Sulfated Sterols from a Marine Source Endowed with a PXR Agonistic Activity. J Med Chem. 2011;54:401–405. doi: 10.1021/jm100968b. [DOI] [PubMed] [Google Scholar]
- 22.Festa C, De Marino S, Sepe V, D’Auria MV, Bifulco G, Debitus C, Bucci M, Vellecco V, Zampella A. Solomonamides A and B, new anti-inflammatory peptides from Theonella swinhoei. Org Lett. 2011;13:1532–1535. doi: 10.1021/ol200221n. [DOI] [PubMed] [Google Scholar]
- 23.De Marino S, Ummarino R, D’Auria MV, Chini MG, Bifulco G, Renga B, D’Amore C, Fiorucci S, Debitus C, Zampella A. Theonellasterols and conicasterols from Theonella swinhoei. Novel marine natural ligands for human nuclear receptors. J Med Chem. 2011;54:3065–3075. doi: 10.1021/jm200169t. [DOI] [PubMed] [Google Scholar]
- 24.De Marino S, Sepe V, D’Auria MV, Bifulco G, Renga B, Petek S, Fiorucci S, Zampella A. Towards new ligands of nuclear receptors. Discovery of malaitasterol A, an unique bis-secosterol from marine sponge. Theonella swinhoei Org Biomol Chem. 2011;9:4856–4862. doi: 10.1039/c1ob05378g. [DOI] [PubMed] [Google Scholar]
- 25.The scarcity of isolated material hampered further pharmacological investigation.
- 26.Zampella A, Sepe V, Bellotta F, Luciano P, D’Auria MV, Cresteil T, Debitus C, Petek S, Poupat C, Ahon A. Homophymines B–E and A1–E1a family of bioactive cyclodepsipeptides from the sponge Homophymia sp. Org Biomol Chem. 2009;7:4037–4044. doi: 10.1039/b910015f. [DOI] [PubMed] [Google Scholar]