Abstract
In this paper we studied the antioxidant and antiproliferative activities of the heated pepsin hydrolysate from a marine fish half-fin anchovy (HAHp-H). Furthermore, we compared the chemical profiles including the amino acid composition, the browning intensity, the IR and UV-visible spectra, and the molecular weight distribution between the half-fin anchovy pepsin hydrolysate (HAHp) and HAHp-H. Results showed that heat sterilization on HAHp improved the 1,1-diphenyl-2-picryl-hydrazil (DPPH) radical-scavenging activity and reducing power. In addition, the antiproliferative activities were all increased for HAHp-H on DU-145 human prostate cancer cell line, 1299 human lung cancer cell line and 109 human esophagus cancer cell line. The contents of free amino acid and reducing sugar of HAHp-H were decreased (P < 0.05). However, hydrophobic amino acid residues and the browning intensity of HAHp-H were increased. FT-IR spectroscopy indicated that amide I and amide III bands of HAHp-H were slightly modified, whereas band intensity of amide II was reduced dramatically. Thermal sterilization resulted in the increased fractions of HAHp-H with molecular weight of 3000–5000 Da and below 500 Da. The enhanced antioxidant and antiproliferative activities of HAHp-H might be attributed to the Maillard reaction.
Keywords: half-fin anchovy pepsin hydrolysate, heated sterilization, antioxidant activity, antiproliferative activity, chemical profile
1. Introduction
Heated sterilization is a common operation in food processing, and this process induces a variety of physical and biochemical changes that influence the nutritional and functional values of products, as well as the acceptable textures. During thermal processing of protein or hydrolysate-rich foods, the Maillard reaction occurs at high temperature [1,2]. In recent years, Maillard reaction products (MRPs) have been increasingly studied. MRPs have been reported to possess multiple-bioactivities, including antioxidant [3,4], antibacterial [5], antihypertensive [5], antimutagenic [6] and antiradical activities [7]. Moreover, MRPs have been recognized as an important flavoring enhancer for heat-processed food products [8].
Most MPRs were prepared through single pure amino acid, peptide or protein reacting with different reducing sugars [4,9,10]. Only a few studies have investigated the MPRs from peptides, hydrolysates, or proteins [1,2]. In our previous study we found that the pepsin hydrolysate of the marine fish half-fin anchovy (HAHp) was composed of small peptides, and demonstrated in vitro antioxidant and antiproliferative activities [11]. However, the activity changes for HAHp after thermal sterilization were not investigated. Thus, in this study we measured the antioxidant and antiproliferative activities of heated sterilized HAHp. Furthermore, the chemical profiles between HAHp and HAHp-H were compared, aiming at revealing the possible reasons for these enhanced bioactivities.
2. Results and Discussion
2.1. In Vitro Antioxidant Activity
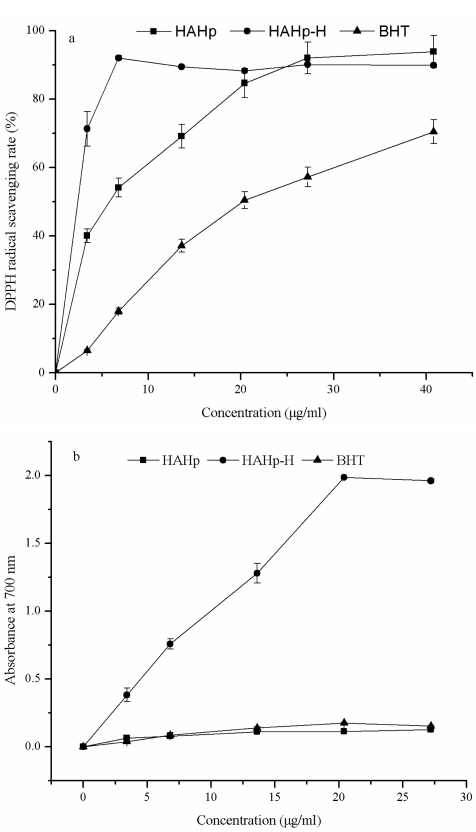
The antioxidant activities of HAHp, HAHp-H and butylated hydroxytoluene (BHT) were compared in Figure 1. HAHp-H exhibited a strong DPPH radical-scavenging activity at a concentration of 6.80 μg/mL and kept stable above that concentration (Figure 1a). By comparison, HAHp and BHT showed the increasing DPPH radical-scavenging activity in a concentration-dependent manner. The 50% scavenging concentrations (ED50 value) of HAHp, HAHp-H and BHT were 4.46 μg/mL, 1.19 μg/mL and 22.78 μg/mL, respectively. The lower the ED50 value, the higher the DPPH scavenging activity was. Obviously, HAHp-H had a stronger DPPH radical-scavenging activity than HAHp.
Figure 1.
Comparison of antioxidant activities between half-fin anchovy pepsin hydrolysate (HAHp) and heated pepsin hydrolysate from a marine fish half-fin anchovy (HAHp-H). (a) DPPH radical-scavenging activity; (b) Reducing power. The concentration of HAHp and HAHp-H was represented as protein concentration. Each value was expressed as mean ± standard deviation (n = 3).
The reducing power of HAHp-H increased with increasing concentration (Figure 1b). At a concentration of 27.20 μg/mL, the absorbance at 700 nm reached 1.984, thereafter, the absorbance did not change significantly.
2.2. Antiproliferative Activity
It can be shown that both HAHp and HAHp-H were able to inhibit the proliferation of DU-145 human prostate cancer cell line, 1299 human lung cancer cell line and 109 human esophagus cancer cell line (Table 1). In addition, HAHp and HAHp-H displayed a dose-dependent manner on the antiproliferation of DU-145 and 1299 cell lines. The inhibitory rate of HAHp-H on the DU-145 cell line was significantly higher than that of HAHp (P < 0.05) at the same concentration, with an IC50 value of 13.67 mg/mL, about three-fold stronger than that of HAHp (IC50: 41.67 mg/mL). In contrast, there was no significant difference (P < 0.05) on the antiproliferation of 1299 human lung cancer cell line for HAHp and HAHp-H at concentrations ranging from 5 to 20 mg/mL. However, at a concentration of 40 mg/mL, the inhibitory rate of HAHp-H (95.68%) was significantly higher than that of HAHp (46.06%) (P < 0.05). The IC50 values of HAHp-H and HAHp on 1299 human lung cancer cells were 25.17 mg/mL and 40.28 mg/mL, respectively.
Table 1.
Comparison of antiproliferative activity between HAHp and HAHp-H a.
| Cell lines | Samples |
Concentration (mg/mL) |
|||
|---|---|---|---|---|---|
| 5 | 10 | 20 | 40 | ||
| DU-145 human prostate cancer cell | HAHp | 0.21 ± 0.11 aA | 8.39 ± 0.11 bA | 23.36 ± 0.69 cA | 44.40 ± 0.74 dB |
| HAHp-H | 17.31 ± 0.28 aC | 50.07 ± 0.76 bB | 71.28 ± 6.21 cB | 98.81 ± 0.34 dC | |
| 1299 human lung cancer cell | HAHp | – | 4.47 ± 2.05 aA | 20.62 ± 5.70 bA | 46.06 ± 0.95 cB |
| HAHp-H | 4.92 ± 1.10 aB | 9.27 ± 2.21 aA | 21.31 ± 7.93 bA | 95.68 ± 3.68 cC | |
| 109 human esophagus cancer cell | HAHp | – | – | – | 29.90 ± 7.18 A |
| HAHp-H | – | – | – | 55.99 ± 6.26 B | |
Data were shown as mean ± standard deviation of five replicates. Different letter superscripts (a–d) represented significant difference in the same row (P < 0.05). Different capital letter superscripts (A–C) represented significant difference in the same column (P < 0.05).
As for the antiproliferation of 109 human esophagus cancer cells, both HAHp and HAHp-H indicated inhibitory effects at a high concentration of 40 mg/mL. Moreover, HAHp-H showed stronger antiproliferative activity than HAHp (P < 0.05). Different types of cancer cells might have different cell membrane composition, fluidity and surface area [12], therefore, HAHp-H demonstrated variability in antiproliferative activities at the same concentration. The antiproliferative activity of fish hydrolysates or peptides on cancer cell lines was less studied compared with their other bioactivities. Picot et al. [13] reported fish protein hydrolysates from three blue whitings, three cods, three plaices and one salmon, showed significant antiproliferation on MCF-7/6 and MDA-MB-231 human breast cancer cell lines. Peptides were mainly responsible for these activities. Lee et al. [14] and Hsu et al. [15] isolated hydrophobic anticancer peptides from anchovy sauce and tuna dark muscle byproduct hydrolysates, respectively. To our knowledge, this is the first time that the antiproliferative activity of the pepsin hydrolysate of half-fin anchovy and its heated sterilization products (HAHp-H) have been reported.
2.3. Chemical Profiles
2.3.1. Changes in Free Amino Acid and Reducing Sugar Levels
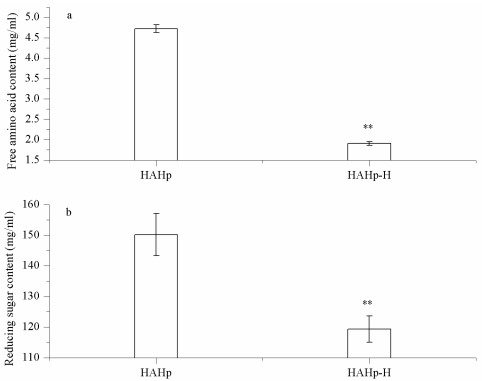
As shown in Figure 2a, the content of free amino acid of HAHp was decreased from 4.72 to 1.91 mg/mL (P < 0.05) after heat sterilization (121 °C, 30 min). In addition, the reducing sugar content of HAHp-H was reduced to 119.3 mg/mL, statistically lower than that of HAHp (150.2 mg/mL) (Figure 2b). During thermal processing, peptides/free amino acids in hydrolysates interacted directly with reducing sugar (cross-linking) to form larger molecules, resulted in the reduced free amino acid and reducing sugar contents. On the other hand, higher molecular weight of peptides could degrade to smaller peptides or free amino acids [2,16]. The balance between peptides/amino acids cross-linking and peptides degradation determined the free amino acids content in the heated products. The result of Figure 2 indicated that reducing sugar or its degraded products attached to free amino acids (cross-linking) played an important role in HAHp-H.
Figure 2.
Comparison of free amino acid and reducing sugar levels between HAHp and HAHp-H. (a) Free amino acid content; (b) Reducing sugar content. Each value was expressed as mean ± standard deviation (n = 3).
2.3.2. Amino Acid Analysis
It was observed that the sterilization product of HAHp had slightly higher total amino acid content compared with HAHp, this result was similar to the observations of Oluwaniyi et al. [17] that the processes of boiling (100 °C, 10 min) and roasting (145 °C, 15 min) increased the total amino acid contents of four marine fishes including herring, Atlantic mackerel, horse mackerel and white hake. As seen in Table 2, cysteine was lost completely in HAHp-H. Additionally, the content of lysine was decreased from 0.961 to 0.814 mg/100 mg. Since cysteine has the ability to form a dehydroalanyl residue and bind with the ɛ-amino group of lysine in the Maillard reaction [18], result of Table 2 implied that cysteine and losses of lysine under the heat treatment probably contribute to form cysteine-derived crosslink compounds, e.g., lysinoalanine. The levels of amino acid, i.e., valine, leucine, phenylalanine, histidine and arginine, were all increased. In particular, the histidine level of HAHp-H was dramatically increased from 0.294 to 1.368 mg/100 mg. An interesting finding was that these amino acid levels, except for the losses of cysteine and lysine, were reported to be increased or damaged in different protein, peptide, or free amino acid/sugar heating systems [4,17]. We conjectured that differences in substrates and heating conditions determined amino acid composition in the thermal products.
Table 2.
Comparison of amino acid composition between HAHp and HAHp-H.
| Amino acid | HAHp (mg/100 mg) | Relative percent (%) | HAHp-H (mg/100 mg) | Relative percent (%) |
|---|---|---|---|---|
| Aspartic acid | 1.808 | 10.32 | 0.000 | 0.00 |
| Threonine | 0.755 | 4.31 | 1.413 | 7.70 |
| Serine | 0.859 | 4.90 | 0.780 | 4.25 |
| Glutamic acid | 2.393 | 13.66 | 2.256 | 12.29 |
| Glycine | 1.298 | 7.41 | 1.288 | 7.02 |
| Alanine | 2.327 | 13.28 | 2.535 | 13.81 |
| Cysteine | 0.157 | 0.90 | 0.000 | 0.00 |
| Valine | 1.480 | 8.45 | 1.812 | 9.87 |
| Methionine | 0.783 | 4.47 | 0.819 | 4.46 |
| Isoleucine | 0.584 | 3.33 | 0.818 | 4.46 |
| Leucine | 0.922 | 5.26 | 1.192 | 6.49 |
| Tyrosine | 0.170 | 0.97 | 0.589 | 3.21 |
| Phenylalanine | 0.303 | 1.73 | 0.326 | 1.78 |
| Lysine | 0.961 | 5.49 | 0.814 | 4.43 |
| Histidine | 0.294 | 1.68 | 1.368 | 7.45 |
| Arginine | 0.778 | 4.44 | 0.903 | 4.92 |
| Proline | 1.645 | 9.39 | 1.445 | 7.87 |
| Tryptophan | ND a | ND a | ND a | ND a |
| ΣAA b | 17.518 | 100 | 18.357 | 100 |
| ΣHAA c | 8.044 | 45.92 | 8.947 | 48.74 |
ND, not determined;
ΣAA represented the total content of amino acids;
ΣHAA represented the total content of hydrophobic amino acids.
Table 2, clearly shows that the contents of alanine, valine, leucine, arginine and histidine amino acids accounting for the presence of antioxidant activity [19], were slightly or dramatically increased in HAHp-H. A higher ratio of hydrophobic amino acids was also reported to improve the antioxidant activities of the Maillard reaction products (MPRs) [19,20]. Furthermore, the hydrophobic amino acid residues were important for the formation of hydrophobic tail in the COOH-terminal region, which exhibited a predominant role for anticancer peptides to mediate their cytotoxic effect [21]. The hydrophobic amino acids content of HAHp-H were increased to 8.947 mg/100 mg with a relative percent of 48.74%, indicating that HAHp-H should display higher antioxidant ability and antiproliferative activity than HAHp. Results shown in Figure 1 and Table 1 confirmed this viewpoint.
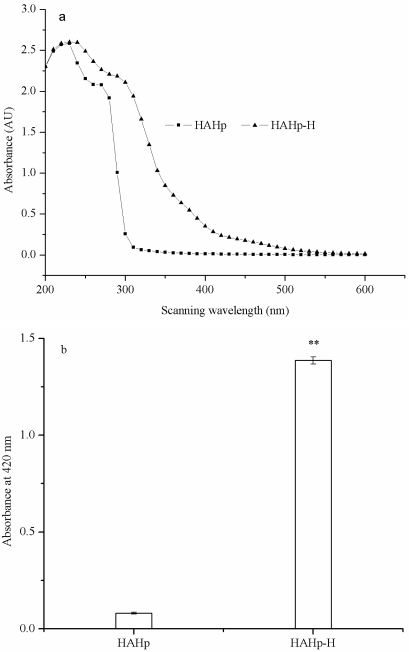
2.3.3. UV-Visible Spectra and Browning Intensity
The UV-visible spectra of HAHp and HAHp-H showed a similar contour. However, the absorption values of HAHp-H were generally higher than that of HAHp under same wavelength (Figure 3a). Sugar caramelization might occur in addition to the Maillard reaction when heated at temperatures above 120 °C or 9 < pH < 3, leading to a browning of the mixture [1,22]. However, provided amino compounds such as amino acids, peptides and proteins were present, the Maillard reaction usually took place [22]. In Figure 3a, HAHp-H had a maximum absorbance appeared in the range of 260–320 nm, which was characteristic of melanoidins [23,24]. The absorbance under 420 nm (browning intensity) was often used as an indicator for browning development in the Maillard reaction [25]. As can be seen in Figure 3b, the browning intensity of HAHp-H was increased to 1.386, significantly higher than that of HAHp (0.081) (P < 0.05), suggesting that the Maillard reaction might play a predominant role in HAHp-H. The results of Figure 3 were in accordance with the decreased free amino acid and reducing sugar contents in HAHp-H (seen in Figure 2).
Figure 3.
Comparison of UV-visible spectra and browning intensity between HAHp and HAHp-H. (a) UV-visible spectra; (b) Browning intensity, data in Figure 1b were expressed as mean ± standard deviation (n = 3).
2.3.4. FT-IR Measurement
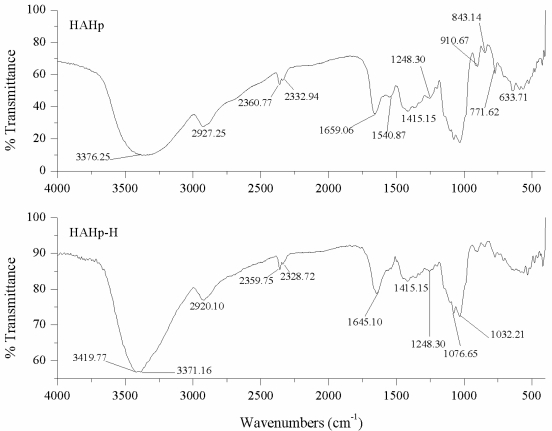
The vibration regions of the amide bonds of proteins and the chemical fingerprints of carbohydrates were readily identified in FT-IR spectroscopy [26,27]. The most distinctive spectral features for proteins were amide I band at 1600–1700 cm−1 (C═O stretching), amide II band at 1500–1550 cm−1 (N–H deformation) and amide III band at 1200–1300 cm−1 (C–N stretching and N–H deformation). Normally, the amide I band was strong, the amide II band was weak and the amide III band was moderate [26]. A series of overlapping peaks located in the region of 1180–953 cm−1, which were described as the “saccharide” bands resulting from vibration modes such as the stretchings of C–C and C–O and the bending mode of C–H bonds. These absorptions were weak in the spectra of most proteins [27].
As can be seen in Figure 4, the bands of amide I in HAHp and HAHp-H were located at 1659.06 cm−1 and 1645.10 cm−1, respectively. The result meant that the amide I band of HAHp-H was modified by thermal sterilization, meanwhile, the result of Figure 4 also indicated that both HAHp and HAHp-H might contain the second structure of alpha-helical, which has a peak maximum around 1660–1650 cm−1 [28]. The IR spectral features of HAHp and HAHp-H between 1500 cm−1 and 1550 cm−1 showed a clear change and the band intensity of HAHp-H at 1540.87 cm−1 was decreased dramatically, indicating that heat sterilization resulted in a big conformation change of amide II band (N–H deformation). As for amide III bands, which were very complex in proteins, there was slight variation from the range of 1200 cm−1 to 1300 cm−1 region (Figure 4). Nevertheless, the FT-IR spectroscopy would not be sufficiently sensitive to distinguish such small changes.
Figure 4.
Comparison of FT-IR between HAHp and HAHp-H.
In the IR region of 1180–953 cm−1, which associated with “saccharide” bands, the absorbances of HAHp and HAHp-H were not weak, implying that saccharides were contained in HAHp and HAHp-H. The result of FT-IR could contribute to explain the reason for MRPs formation in HAHp-H even without addition of sugars. In the mid-infrared spectrum of MRPs, several chemical structures were changed. For example, the group of NH2, especially from lysine, was lost. While the amount of groups, including the Amadori compound (C═O), Schiff base (C═N) and pyrazines (C═N) associated with Maillard products might be increased [4].
2.3.5. Molecular Weight (MW) Distribution
The MW profiles of HAHp and HAHp-H were displayed in Table 3. HAHp was a mixture of small peptides and the percent of peptides below 3000 Da was above 95%, while in HAHp-H it decreased to 60.77%. The decreased fractions were within the ranges of 1000–3000 Da and 500–1000 Da. However, the higher MW of 3000–5000 Da and lower MW below 500 Da in HAHp-H were increased to 38.06% and 19.00%, respectively (P < 0.05). The result was similar to the study of Liu et al. [3]. Because of the presence of peptides/amino acids cross-linking in the Maillard reaction, larger molecules tended to form, while peptides/proteins degradation resulted in the increased smaller peptides or free amino acids. High MW and low MW components in MRPs were accountable for the enhanced antioxidant activities [29], and hydroxyl groups of MRPs were effective electron donors to increase reducing power [30].
Table 3.
Comparison of molecular weight distribution between HAHp and HAHp-H a.
| MW (Da) | >5000 | 3000–5000 | 3000–1000 | 1000–500 | <500 |
|---|---|---|---|---|---|
| HAHp | 4.29 ± 0.35 a | 0.50 ± 0.16 a | 53.57 ± 1.82 a | 31.67 ± 0.25 a | 9.99 ± 1.22 a |
| HAHp-H | 1.63 ± 0.67 b | 38.06 ± 0.15 b | 24.74 ± 1.49 b | 17.03 ± 0.11 b | 19.00 ± 0.06 b |
Data in the table were mean of relative areas ± standard deviation of duplicate analyses. Different superscripts in the same column indicated significant difference (P < 005).
3. Experimental Section
3.1. Preparation of Heated Products from Half-Fin Anchovy Pepsin Hydrolysate
Pepsin hydrolysate of half-fin anchovy (HAHp) was prepared as the optimized method described previously [31]. The soluble peptides content and degree of hydrolysis of HAHp were (81.78 ± 0.04)% and (18.12 ± 0.39)%, respectively, determined by the following methods [32]. Briefly, HAHp was added to 15% of trichloroacetic acid (TCA) at a ratio of 1:1. The mixture was maintained for 30 min and centrifuged (8000 rpm/min, 15 min). The total soluble peptides content was computed as the proportion (%) of total nitrogen content in the supernatant with respect to the total nitrogen content in HAHp. The degree of hydrolysis was evaluated as the proportion (%) of total free amino nitrogen in the supernatant with respect to the total nitrogen content in fish muscle. The contents of total nitrogen and free amino nitrogen were assayed through micro Kjeldahl and ninhydrin reaction methods, respectively.
HAHp was heated for 30 min at 121 °C to imitate food steaming sterilization procedure. The thermal products of HAHp were designated as HAHp-H. Some portions of HAHp-H were also freeze-dried to obtain powder for further amino acid composition analysis and FT-IR spectroscopy. Protein concentrations of HAHp and HAHp-H were determined using Bradford’s method [33].
3.2. In Vitro Antioxidant Activity
3.2.1. DPPH Radical-Scavenging Assay
The free radical scavenging activity was determined by the 1,1-diphenyl-2-picryl-hydrazil (DPPH) method with few modifications [34]. Briefly, 375 μL of 0.02% DPPH solution in 99.5% ethanol was added to 1.5 mL of HAHp and 1.5 mL of 99.5% ethanol, then mixed thoroughly and kept in the dark at room temperature for 60 min. The absorbance of the mixtures was measured at 517 nm. HAHp and butylated hydroxytoluene (BHT) were tested in the same way for comparison. DPPH radical scavenging activity was calculated as follows:
where control, replaced 1.5 mL of sample with 1.5 mL of distilled; blank, replaced 375 μL of 0.02% DPPH with 375 μL of 99.5% ethanol.
3.2.2. Reducing Power Assay
The presence of reductants of samples could result in reducing Fe3+/ferricyanide complex to the ferrous form (Fe2+), and the Fe2+ could therefore be monitored by measuring the formation of Perl’s Prussian blue at 700 nm. A higher absorbance at 700 nm meant a better reducing power [30]. The reducing power of HAHp-H was measured according to the method of Oyaizu [35] with slight modifications. 1.0 mL of HAHp-H was mixed with 1.0 mL of 0.2 M sodium phosphate buffer (pH 6.6) and 1.0 mL of 1% potassium ferricyanide (K3Fe(CN6)). The mixture was incubated at 50 °C for 20 min, followed by addition of 1.0 mL of 10% trichloroacetic acid. Then, the mixture was centrifuged at 3000 g for 10 min and 2.0 mL of supernatant was blended with 2.0 mL of deionized water and 0.8 mL of 0.1% ferric chloride (FeCl3). The absorbance of the mixture was measured at 700 nm. The reducing power of HAHp and BHT were also determined for comparison.
3.3. Antiproliferative Activity
3.3.1. Cell Lines and Cell Culture
Three human cancer cell lines, DU-145 prostate cancer cell line, 1299 lung cancer cell line and 109 esophagus cancer cell, were provided by College of Food Science and Pharmacy, Zhejiang Ocean University and cultured in RPMI 1640 medium at 37 °C in a humidified atmosphere with 5% CO2 incubator (ThermoForma, USA), supplemented with 10% heat-inactivated fetal calf serum, 200 mM l-glutamine, 100 units/mL of penicillin, and 100 units/mL streptomycin.
3.3.2. MTT Assay
The inhibition rate of cancer cell lines after treatment with HAHp and HAHp-H was determined by the MTT method [36]. Cancer cell lines were transferred to fresh media every 2–3 days to maintain in an exponential growth and digested by 0.25% trypsin to obtain cell suspension. Then, 200 μL of cell suspension (1 × 104 cells/mL) was transferred into a 96 wells plate and incubated for 24 h. After this period, the suspension was removed and 200 μL of the sample (HAHp and HAHp-H, concentration ranging from 5 mg/mL to 40 mg/mL) was added, followed by incubation for 48 h at 37 °C. Then, 200 μL of MTT (1 mg/mL) was added to these cells respectively and incubated for 4 h at 37 °C. The medium was removed and 150 μL of DMSO was added to dissolve the formazan crystals formed and precipitated. Finally, the absorbance was read at 490 nm with an ELISA reader (BR680, USA). The inhibition rate was calculated using the following equation:
where AC, absorbance of control, using 200 μL of RPMI 1640 complete medium instead of 200 μL of HAHp-H; AS, absorbance of sample; AB, absorbance of RPMI 1640 complete medium.
3.4. Measurement of Chemical Profiles
3.4.1. Free Amino Acid Content
The content of free amino acids was determined by the method of ninhydrin reaction [37]. 0.2 mL of sample was mixed with 3.8 mL of distilled water, 1.0 mL of ninhydrin and 1.0 mL of 0.2125 mol/L sodium phosphate buffer (pH 8.2) in a 25 mL of cylinder. The solution was heated at 100 °C for 15 min and cooled down to room temperature quickly. Then, the volume of the mixture was adjusted to 25 mL by addition of distilled water. After being kept at room temperature for 15 min, the absorbance of the mixture was measured at 570 nm. A calibration curve was obtained by using l-alanine as a standard. The content of free amino acids was expressed as mg per mL of sample.
3.4.2. Reducing Sugar Level
Reducing sugar levels of HAHp and HAHp-H were determined by the Fehling method with some modifications [38]. Briefly, HAHp and HAHp were diluted to 100 mL after removal of proteins by addition of zinc acetate and potassium ferrocyanide solution. 5 mL of Fehling solution A (15 g copper sulfate and 0.05 g methylene blue dissolved in distilled water), 5 mL of Fehling solution B (50 g sodium potassium tartrate tetrahydrate, 75 g sodium hydroxide and 4 g potassium ferrocyanide dissolved in distilled water) and 10 mL of distilled water were added into a 250 mL of conical flask. The mixture was heated to boilling in 2 min, followed by titration with HAHp and HAHp to reach bright yellow colour. The consumed volumes of HAHp and HAHp-H were recorded, respectively. HAHp and HAHp-H were replaced by glucose, which was used as the standard of reducing sugar, to standardize the mixture of Fehling solution A and B under the same condition. The content of reducing sugar was expressed as mg per mL of sample.
3.4.3. Amino Acid Analysis
HAHp and HAHp-H powders were respectively dissloved in 6 mol/L HCl and hydrolyzed at 110 °C for 24 h. After removal of HCl under vacuum, the dried samples were then redissolved in 0.1 M sodium citrate buffer (pH 2.2). Subsequent amino acid analysis was carried out in an automatic amino acid analyzer (Hitachi 835-50, Tokyo, Japan). Results were determined as mg per 100 mg of sample.
3.4.4. UV-Visible Spectra and Browning Intensity
UV-visible spectra of HAHp and HAHp-H were recorded by a Shimadzu 1200UV-vis spectrophotometer (Tokyo, Japan) with the wavelength ranging from 200 to 600 nm. The browning intensity was measured at 420 nm [39].
3.4.5. Fourier Transform Infrared Spectroscopy (FT-IR) Measurement
All IR spectra of HAHp and HAHp-H were detected using an FT-IR spectrometer (NICOLET NEXUS670, DTGS). HAHp and HAHp-H powders were mixed with potassium bromide (KBr) powder separately. Scanning was carried out in the range of 4000–400 cm−1 with a resolution of 4 cm−1 and 32 scans were calculated to obtain an average for each sample.
3.4.6. Molecular Weight (MW) Distribution
The MW distribution profiles of HAHp and HAHp-H were estimated by gel permeation chromatography (GPC) using an ÄKTA purifier system, equipped with a Superdex-75 HR 10/300 column from Amersham Pharmacia, Uppsala, Sweden. The elution consisting of 50 mM sodium phosphate buffer (pH 5.8 containing 0.15 mol/L NaCl) was delivered at a flow rate of 0.4 mL/min. 100 μL of sample (filtered by a 0.22 μm mircofiltre) was injected onto the column. Chromatograms were recorded by UV detection at 280 nm. Four MW standards, including bovine serum albumin (68,000 Da), aprotinin (6512 Da), vitamin B12 (1355 Da) and oxidized glutathinone (613 Da), were used to obtain a MW calibration curve. The data analysis was performed using gel permeation chromatography software.
3.4.7. Statistical Analysis
Data were represented as mean ± standard deviation. The significant differences (P < 0.05) between samples were evaluated through the student’s t-test.
4. Conclusions
In this study, the process of sterilization on HAHp had desirable effect on the antioxidant and antiproliferative activities. The results of chemical profiles revealed that it is likely that the Maillard reaction occurred in HAHp-H. Moreover, the higher content of hydrophobic amino acids and the formation of specific higher and lower molecular weight components in HAHp-H might be accountable for the enhanced antioxidant and antiproliferative activities. To summarize, heated sterilization treatment should be encouraged as a convenient approach for HAHp to improve the bioactivity in consumption.
Acknowledgments
This study was supported by grants from Educational project of Zhejiang Province (Y200909275) and Natural Science Foundation of Zhejiang Province (Y3110153).
Footnotes
Samples Availability: Available from the authors.
References
- 1.Benjakul S, Lertittikul W, Bauer F. Antioxidant activity of Maillard reaction products from a porcine plasma protein-sugar model system. Food Chem. 2005;93:189–196. [Google Scholar]
- 2.Lan XH, Liu P, Xia SQ, Jia CS, Mukunzi D, Zhang XM, Xia WS, Tian HX, Xiao ZB. Temperature effect on the nonvolatile compounds of Maillard reaction products derived from xylose–soybean peptide system: further insights into thermal degradation and crosslinking. Food Chem. 2010;120:967–972. [Google Scholar]
- 3.Liu P, Huang MG, Song SQ, Khizar H, Zhang XM, Xia SQ, Jia CS. Sensory characteristics and antioxidant activities of Maillard reaction products from soy protein hydrolysates with different molecular weight distribution. Food Bioprocess Technol. 2010 doi: 10.1007/s11947-010-0440-3. [DOI] [Google Scholar]
- 4.Gu FL, Kim JM, Abbas S, Zhang XM, Xia SQ, Chen ZX. Structure and antioxidant activity of high molecular weight Maillard reaction products from casein–glucose. Food Chem. 2010;120:505–511. [Google Scholar]
- 5.Rufián-Henares JA, Morales FJ. Functional properties of melanoidins: In vitro antioxidant, antimicrobial and antihypertensive activities. Food Res Int. 2007;40:995–1002. [Google Scholar]
- 6.Yen GC, Tsai LC. Antimutagenecity of partially fractionated Maillard reaction product. Food Chem. 1993;47:11–15. [Google Scholar]
- 7.Morales FJ, Babbel MB. Antiradical efficiency of Maillard reaction mixtures in a hydrophilic media. J Agric Food Chem. 2002;50:2788–2792. doi: 10.1021/jf011449u. [DOI] [PubMed] [Google Scholar]
- 8.Ogasawara M, Katsumata T, Egi M. Taste properties of Maillard-reaction products preparedfrom 1000 to 5000 Da peptide. Food Chem. 2006;99:600–604. [Google Scholar]
- 9.Chawla SP, Chander R, Sharma A. Antioxidant formation by γ-irradiation of glucose–amino acid model systems. Food Chem. 2007;103:1297–1304. [Google Scholar]
- 10.Martins SIFS, Van Boekel MAJS. A kinetic model for the glucose/glycine Maillard reaction pathway. Food Chem. 2005;90:257–269. [Google Scholar]
- 11.Song R. Zhejiang Ocean University, Zhoushan, China. Unpublished work, 2011.
- 12.Leuschner C, Hansel W. Membrane disrupting lytic peptides for cancer treatments. Curr Pharm Des. 2004;10:2299–2310. doi: 10.2174/1381612043383971. [DOI] [PubMed] [Google Scholar]
- 13.Picot L, Bordenave S, Didelot S, Fruitier-Arnaudin I, Sannier F, Thorkelsson G, Bergé JP, Guérard F, Chabeaud A, Piot JM. Antiproliferative activity of fish protein hydrolysates on human breast cancer cell lines. Process Biochem. 2006;41:1217–1222. [Google Scholar]
- 14.Lee YG, Lee KW, Kim JY, Kim KH, Lee HJ. Induction of apoptosis in a human lymphoma cell line by hydrophobic peptide fraction separated from anchovy sauce. Biofactors. 2004;21:63–67. doi: 10.1002/biof.552210112. [DOI] [PubMed] [Google Scholar]
- 15.Hsu KC, Li-Chan ECY, Jao CL. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2011;126:617–622. [Google Scholar]
- 16.Lu CY, Hao ZG, Payne R, Ho CT. Effects of water content on volatile generation and peptide degradation in the Maillard reaction of glycine, diglycine, and triglycine. J Agric Food Chem. 2005;53:6443–6447. doi: 10.1021/jf050534p. [DOI] [PubMed] [Google Scholar]
- 17.Oluwaniyi OO, Dosumu OO, Awolola GV. Effect of local processing methods (boiling, frying and roasting) on the amino acid composition of four marine fishes commonly consumed in Nigeria. Food Chem. 2010;123:1000–1006. [Google Scholar]
- 18.Ajandouz EH, Desseaux V, Tazi S, Puigserver A. Effects of temperature and pH on the kinetics of caramelisation, protein cross-linking and Maillard reactions in aqueous model systems. Food Chem. 2008;107:1244–1252. [Google Scholar]
- 19.Chen HM, Muramoto K, Yamauchi F, Nokihara K. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J Agric Food Chem. 1996;44:2619–2623. doi: 10.1021/jf970649w. [DOI] [PubMed] [Google Scholar]
- 20.Rajapakse N, Mendis E, Byun HG, Kim SK. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J Nutr Biochem. 2005;16:562–569. doi: 10.1016/j.jnutbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Hoskin DW, Ramamoorthy A. Studies on anticancer activites of antimicrobial peptides. Biochim Biophys Acta. 2008;1778:357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroh LW. Caramelisation in food and beverages. Food Chem. 1994;51:373–379. [Google Scholar]
- 23.Rafik M, Mas A, Elharfi A, Schue F. Decoloration de solutions sucrees par ultrafiltration sur une membrane a base de poly (organocyclophosphazene) Eur Polym J. 1997;33:679–690. [Google Scholar]
- 24.Kim JS, Lee YS. Study of Maillard reaction products derived from aqueous model systems with different peptide chain lengths. Food Chem. 2009;116:846–853. [Google Scholar]
- 25.Ajandouz EH, Tchiakpe LS, DalleOre F, Benajiba A, Puigserver A. Effect of pH on caramelization and Maillard reaction kinetics in fructose–lysine model systems. J Food Sci. 2001;66:926–931. [Google Scholar]
- 26.Chang MC, Tanaka JZ. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials. 2002;23:4811–4818. doi: 10.1016/s0142-9612(02)00232-6. [DOI] [PubMed] [Google Scholar]
- 27.Turner JA, Sivasundaram LR, Ottenhof MA, Farhat IA, Linforth RST, Taylor AJ. Monitoring chemical and physical changes during thermal flavor generation. J Agric Food Chem. 2002;50:5406–5411. doi: 10.1021/jf0203803. [DOI] [PubMed] [Google Scholar]
- 28.Vonhoff S, Condliffe J, Schiffter H. Implementation of an FTIR calibration curve for fast and objective determination of changes in protein secondary structure during formulation development. J Pharm Biomed Anal. 2010;51:39–45. doi: 10.1016/j.jpba.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 29.Jing H, Yap M, Wong PYY, Kitts DD. Comparison of physicochemical and antioxidant properties of egg-white proteins and fructose and inulin Maillard reaction products. Food Bioprocess Technol. 2010. [DOI]
- 30.Yoshimura Y, Iijima T, Watanabe T, Nakazawa H. Antioxidative effect of Maillard reaction products using glucose–glycine model system. J Agric Food Chem. 1997;45:4106–4109. [Google Scholar]
- 31.Song R, Wei RB, Zhang B, Wang DF. Optimization of the antibacterial activity of half-fin anchovy (Setipinna taty) hydrolysates. Food Bioprocess Technol. 2011. [DOI]
- 32.Song R, Wang DF, Xie C, Wang X. In vitro antioxidant and antibacterial activities of pepsin hydrolysate of half-fin anchovy (Setipinna taty) Food Sci. 2010;31:127–131. [Google Scholar]
- 33.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 34.Bersuder P, Hole M, Smith G. Antioxidants from a heated histidine-glucose model system. I. Investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J Am Oil Chem Soc. 1998;75:181–187. [Google Scholar]
- 35.Oyaizu M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. [Google Scholar]
- 36.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 37.Doi E, Shibata D, Matoba T. Modified colorimetric ninhydrin methods for peptidase assay. Anal Biochem. 1981;118:173–184. doi: 10.1016/0003-2697(81)90175-5. [DOI] [PubMed] [Google Scholar]
- 38.Wu MC. Food Analysis and Organoleptic Investigaation. China Agriculture Press; Beijing, China: 2002. pp. 85–87. [Google Scholar]
- 39.Samaras TS, Camburn PA, Chandra SX, Gordon MH. Antioxidant properties of kilned and roasted malts. J Agric Food Chem. 2005;53:8068–8074. doi: 10.1021/jf051410f. [DOI] [PubMed] [Google Scholar]