Abstract
Nanoparticle technology is being incorporated into many areas of molecular science and biomedicine. Because nanoparticles are small enough to enter almost all areas of the body, including the circulatory system and cells, they have been and continue to be exploited for basic biomedical research as well as clinical diagnostic and therapeutic applications. For example, nanoparticles hold great promise for enabling gene therapy to reach its full potential by facilitating targeted delivery of DNA into tissues and cells. Substantial progress has been made in binding DNA to nanoparticles and controlling the behavior of these complexes. In this article, we review research on binding DNAs to nanoparticles as well as our latest study on non-viral gene delivery using polyethylenimine-coated magnetic nanoparticles.
Keywords: magnetic nanoparticles, Magnetofection, gene delivery, polyethylenimine
1. Introduction
Nanotechnology describes the creation and utilization of materials, devices, and systems through the control of nanometer-sized materials and their application to physics, chemistry, biology, engineering, materials science, medicine, and other endeavors. In particular, intensive efforts are in progress to develop nanomaterials for medical use as agents that can be targeted to specific organs, tissues, and cells. For example, magnetic nanoparticles (MNPs) are being used clinically as contrast agents for magnetic resonance imaging (MRI) (Table 1). MRI is a noninvasive technique that can provide real-time high-resolution soft tissue information [1,2]. MRI image quality can be further improved by utilizing contrast agents that alter proton relaxation rates [3–8]. MNP-based drug delivery systems (DDS) [9–11], and treatments of hyperthermia [12–21], using MNPs have been studied for over a decade. Furthermore, researchers have reported that MNPs have been useful in hyperthermic treatment for various cancers in vivo [22–31]. Nanotechnology-based anti-cancer agent DDS have already been approved, such as pegylated liposomal doxorubicin (DOXIL) for ovarian cancer [32–37]. MNPs have been used effectively as transfection reagents for introducing nucleic acids (plasmids or siRNAs) [38–53], or viruses (retrovirus, or adenovirus) [44,54–56] into cells. Our own research is focused on MNP-mediated gene delivery systems (called as “Magnetofection”).
Table 1.
Biomedical Applications of Magnetic Nanoparticles (MNPs).
2. Gene Delivery
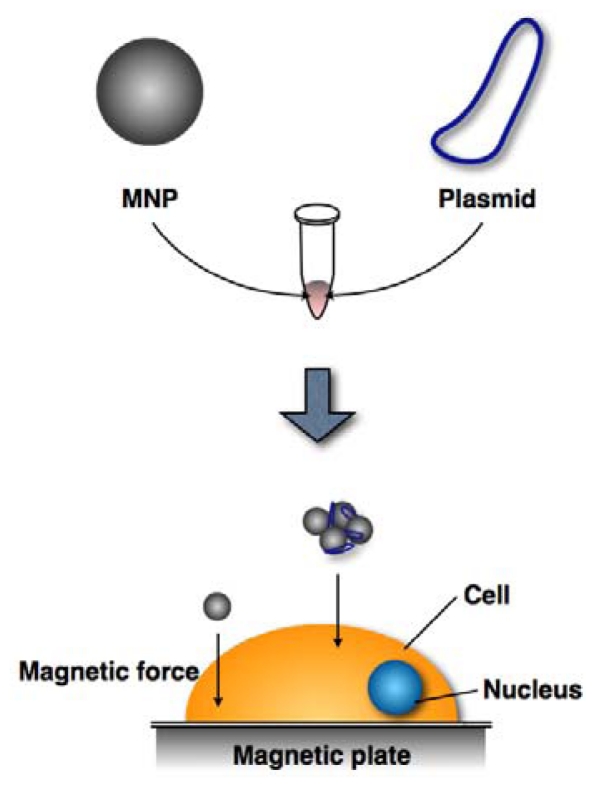
Gene delivery techniques efficiently introduce a gene of interest in order to express its encoded protein in a suitable host or host cell. Currently, there are three primary gene delivery systems that employ viral vectors (retroviruses and adenoviruses), nucleic acid electroporation, and nucleic acid transfection. These systems vary in efficacy (Table 2). Gene delivery by viral vectors can be highly efficient (80–90%) but may insert viral vector nucleic acid sequences into the host genome, potentially causing unwelcome effects, such as inappropriate expression of deleterious genes. Electroporation is also a highly efficient technique for introducing foreign genes into a host (50–70%); however, half of the recipient cells die due to the electrical stimulation. Transfection reagents do not efficiently deliver nucleic acids into cells (20–30%); however, cell viability is largely preserved and the method is safe enough for clinical use. Therefore, this method holds relatively more promise for medical applications, provided that its efficiency can be improved. MNPs are already in use by basic researchers to increase transfection efficiencies of cultured cells. Thus, MNP-nucleic acid complexes are added to cell culture media and then onto the cell surface by applying a magnetic force (Figure 1).
Table 2.
Gene delivery systems.
| Expression Type | Efficiency (%) | Cell Viability (%) | Safety | |
|---|---|---|---|---|
| Virus* | Stable, or Transient | 80–90% | 80–90% | Low |
| Electroporation | Transient | 50–70% | 40–50% | High |
| TF reagent ** | Transient | 20–30% | 80–90% | High |
Virus including adenovirus (transient), retrovirus (stable), and lentivirus (stable);
TF reagent, transfection reagents including PEI (Polysciences Inc.), FuGENE HD (Promega), and Lipofectamine 2000 (Invitrogen);
All values are ours (unpublished experiments).
Figure 1.
MNP gene delivery system (Magnetofection). Plasmids are bound to MNPs, which then move from the media to the cell surface by applying a magnetic force.
Oxide nanoparticles mixed with high magnetic moment compounds such as CoFe2O4, NiFe2O4, and MnFe2O4 exhibit superior performance compared to other magnetic materials [62,63]. However, these nanoparticles are highly toxic to cells, limiting their use for in vivo, and in vitro biomedical applications [64–67]. However, iron oxides such as magnetite (Fe3O4) and maghemite (γ-Fe2O3), in particular, possess high magnetic moments, are relatively safe, and currently in clinical use as MRI contrast agents [57–61]. These iron oxide based-magnetic materials are also suitable for biomedical applications. Fe3+ is widely dispersed in the human body so leaching of this metal ion from nanoparticles should not reach toxic concentrations [68,69]. As a result, maghemite is a popular choice for MNPs used biomedical applications. It is very important to modify the surface of MNPs so that they can be used for biomedical applications. Thus, MNPs are coated with compounds such as natural polymers (proteins and carbohydrates) [70–75], synthetic organic polymers (polyethylene glycol), polyvinyl alcohol, poly-l-lactic acid) [72,76–78], silica [79], and gold [80,81]. These surface coating agents prevent nanoparticle agglomeration, cytotoxicity, and add functionality. MNPs agglomerate readily in aqueous solutions around pH 7 [82], and it is difficult to control the properties and amounts of agglomerated MNPs. The greater toxicity of MNPs compared to those of microparticles can be attributed to their high surface to volume ratio [83]. Coating agents prevent the leaching of potentially toxic components from MNPs. In fact, the cytotoxicity of uncoated NiFeO4 MNPs is dramatically decreased by coating with cationic polymer, polyethylenimine (PEI) [84–86]. PEI, a cationic polymer, is widely used for nucleic acid transfection [87–89] and also serves as a nanoparticle dispersant [90]. PEI-coated MNPs enhance transfection efficiency [38,41,42,44–46,48,49,51,54,55].
3. Cell Transplantation Therapy Using MNPs
Autologous cell transplantation has been widely used in the clinic for decades. Delivering therapeutic genes to patients using their own cells avoids using immunosuppressive drugs. We reasoned, therefore, that a non-viral gene delivery system using iron oxide-based MNPs could provide a powerful tool for next-generation therapies. Gene delivery using MNPs has been successful for delivering nucleic acids into living cells with high efficiency and low cytotoxicity [38,41,42,44–46,48,49,51,54,55]. Currently, there are several methods for inducing cellular differentiation.
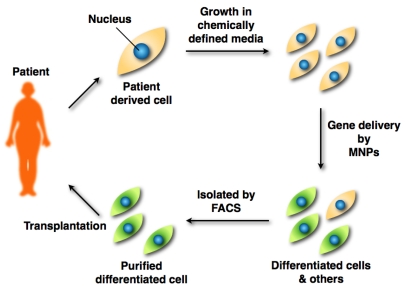
One of these methods, termed direct reprogramming, or direct conversion, has successfully yielded induced cardiomyocytes, induced neurons, reprogrammed pancreatic β cells, and induced pluripotent stem cells (iPSCs) [91–95]. Direct reprogramming represents a more straightforward strategy to treat diseases involving loss of function by specific cell populations compared to approaches requiring an intermediate embryonic stem cell. Thus, patient-derived differentiated cells by gene transfer are suitable for autologous cell transplantation, potentially resulting in faster patient recoveries. The scheme is classified into ex vivo gene therapy. The steps involved in this technique are as follows: (1) Patient-derived cells (such as fibroblasts) are cultured in chemically defined media in vitro; (2) These cells are transfected by MNPs, and differentiated into functional cells; (3) Differentiated cells are isolated by fluorescence-activated cell sorting (FACS); (4) FACS-purified differentiated cells are transplanted into the patient’s target tissue (Figure 2).
Figure 2.
Strategy for cell transplantation therapy. A patient’s cells are cultured in chemically defined media. MNP-transfected cells by the introduced gene are isolated by FACS. FACS-purified differentiated cells are transplanted into the patient.
Here we briefly describe the magnetofection [96], and our latest study concerning non-viral gene delivery using deacylated polyethylenimine coated MNPs.
4. Gene Delivery Using MNPs and Magnetic Force
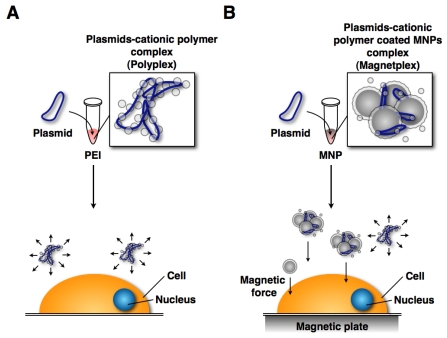
The mechanism of magnetofection is similar to using transfection reagents (Lipofectamine 2000, FuGENE HD, and PEI). The only difference is that the plasmids form complexes with cationic polymer-coated MNPs (called as “Magnetoplex”) [42,48,97–99] (Figure 3). Figure 3 shows the two difference techniques. The behavior of magnetoplex is readily controlled by magnetic force. Upon binding to the cell surface they are taken up by endocytosis [51,100,101]. Thus, the transfection efficiency was increased.
Figure 3.
Gene delivery systems using a transfection reagent (cationic polymer) and MNPs: (A) Gene delivery system using transfection reagent. The polyplex moves randomly in culture medium; (B) Magnetofection system. The magnetoplex only moves to the cell surface.
Many researchers have described magnetofection methods (Table 3). They modified the surface of iron oxide-based MNPs to increase transfection efficiency and reduce cytotoxicity. To achieve this, some investigators selected coating agents such as anionic surfactants (oleic acid, lauroyl sarcosinate) [42,50,102], a non-ionic water-soluble surfactant (Pluronic F-127) [42], fluorinated surfactant (lithium 3-[2-(perfluoroalkyl) ethylthio]propionate) [54], a polymer (polyethylene glycol, poly-l-lysine, poly(propyleneimine) dendrimers) [40,103,104], carbohydrates (Chitosan, Heparan sulfate) [41,47], silica particles (MCM48) [49], proteins (serum albumin, streptavidin) [40,55], hydroxyapatite [105], phospholipids [49,50], a cationic cell penetrating peptide (TAT peptide) [43], non-activated virus envelope (HVJ-E) [47], a transfection reagent (Lipofectamine 2000) [53], and viruses (adenovirus, retrovirus) [44,54–56]. These coating agents are often used in conjunction with PEI. PEI is a well-known cationic gene carrier with high transfection efficiency. However, the high toxicity, depended on its molecular weight, has limited its use as a potential gene carrier. Thus, the PEI was modified to increase transfection efficiency, and decrease cytotoxicity [88,106]. To enhance transfection efficiency, most researchers used the PEI, or the modified PEI to coat the nanoparticle surface [38,41,42,44–46,48,49,51,54,55,102,107]. PEI-coated MNPs are stable in water, bind nucleic acids, and control MNP behavior by magnetic force. In addition, linear PEI possesses low cytotoxicity compared with branched PEI in vivo and in vitro [108,109] The highest transfection efficiencies have been achieved using 25,000 molecular weight linear PEI [89]. However, PEI cytotoxicity due to its acyl groups has been described [88]. Therefore, our group focused on commercial deacylated PEI (Polyethylenimine “Max” (PEI “Max”), Polysciences Inc.) as an MNP (γ-Fe2O3, d = 70 nm, CIK NanoTek) coating agent.
Table 3.
Summary of magnetofection literature.
| Author | Year | Vector | Magnetic Nanoparticles | Modifying Agent | Targeting Cell, or Tissue | TF Efficiency | Cell Viability (% of Control) | Reference |
|---|---|---|---|---|---|---|---|---|
| Kami D | 2011 | Plasmid | Iron oxide (γ-Fe2O3) | PEI max (MW: 25 k) | P19CL6 | * 82% | 100% | [107] |
| Pickard MR | 2011 | Plasmid | NeuroMag | - | Neural precursor cell | * 30% | 70% | [39] |
| Hashimoto M | 2011 | Adenovirus, Biotin | SPION | PEI, Streptoavidin | HeLa | ** 4-fold | - | [55] |
| Adenovirus, Biotin | SPION | PEI, Streptoavidin | NIH3T3 | ** 10-fold | - | |||
| Adenovirus, Biotin | SPION | PEI, Streptoavidin | Mouse embryonic brain | - | - | |||
| Biswas S | 2011 | Plasmid | Iron oxide (Fe3O4) | Aminooxy, Oxime ether | MCF-7 | ** 1425-fold | 89% | [110] |
| B González | 2011 | Plasmid | SPION | Poly(propyleneimine) dendrimers | Saos-2 osteoblasts | * 12% | 75% | [104] |
| Zhang H | 2010 | Plasmid | SPION | Branch PEI (MW: 25 k) | NIT3T3 | * 64% | 100% | [38] |
| siRNA | SPION | Branch PEI (MW: 25 k) | NIT3T3 | * 77% | 100% | |||
| Song HP | 2010 | Plasmid | PolyMag | Tat peptide | U251 | * 60% | 80% | [43] |
| Plasmid | PolyMag | Tat peptide | Rat spinal cord | ** 2-fold | - | |||
| Arsianti M | 2010 | Plasmid | Iron oxide | Branch PEI (MW: 25 k) | BHK-21 | - | 60–90% | [51] |
| Shi Y | 2010 | Plasmid | Magnetite | Hyperbranch PEI (MW: 10 k) | COS-7 | ** 13-fold | - | [45] |
| Ang D | 2010 | Plasmid | Magnetite | Branch PEI (MW: 25 k) | COS-7 | ** 6-fold | 70% | [46] |
| Tresilwised N | 2010 | Adenovirus | Iron oxide (Fe2O3, Fe3O4) | Branch PEI (MW: 25 k), Zonyl FSA fluorosurfactant | EPP85-181RDB | ** 10-fold | - | [54] |
| Namgung R | 2010 | Plasmid | SPION | PEG, Branch PEI (MW: 25 k) | HUVEC | ** 12-fold | 80% | [48] |
| Yiu HH | 2010 | Plasmid | Iron oxide (Fe3O4) | PEI (MW: 25 k), MCM48 (Silica particle) | NCI-H292 | ** 4-fold | - | [49] |
| HC Wu | 2010 | Plasmid | Magnetite | Hydroxyapatite | Rat marrow stromal cells | * 60–70% | 100% | [105] |
| Namiki Y | 2009 | Plasmid | Magnetite | Oleic acid, Phospholipid | HSC45 | ** 8-fold | - | [50] |
| siRNA | Magnetite | Oleic acid, Phospholipid | Tissue sample from gastric cancer | - | - | |||
| Kim TS | 2009 | Plasmid | PolyMag | - | Boar spermatozoa | - | - | [52] |
| Kievit FM | 2009 | Plasmid | SPION | PEI (MW: 25 k) | C6 | * 90% | 10% | [41] |
| Plasmid | SPION | PEI (MW: 25 k), Chitosan | C6 | * 45% | 100% | |||
| Plasmid | PolyMag | - | C6 | * 32% | 66% | |||
| Lee JH | 2009 | siRNA | MnMEIO | Serum albumin, PEG-RGD | MDA-MB-435-GFP | * 30% | - | [40] |
| Li Z | 2009 | Plasmid | Iron oxide | Poly-l-lysine | Lung tissue | *** 60% | - | [103] |
| Yang SY | 2008 | Plasmid | Iron oxide (Fe3O4) | Lipofectamine 2000 | He99 | - | - | [53] |
| Plasmid | Iron oxide (Fe3O4) | DOTAP:DOPE | He99 | - | - | |||
| Pan X | 2008 | Plasmid | Magnetite | Oleic acid, Branch PEI (MW: 25 k), Transferrin | KB | ** 300-fold | 92% | [102] |
| Mykhaylyk O | 2007 | Plasmid | Iron oxide (Fe2O3, Fe3O4) | Branch PEI (MW: 25 k) | H441 | * 49% | - | [42] |
| Plasmid | Iron oxide (Fe2O3, Fe3O4) | Pluronic F-127 | H441 | * 37% | - | |||
| Plasmid | Iron oxide (Fe2O3, Fe3O4) | Lauroyl sarcosinate | H441 | - | - | |||
| Plasmid | Iron oxide (Fe2O3, Fe3O4) | Branch PEI (MW: 25 k), Lauroyl sarcosinate | H441 | - | - | |||
| Morishita N | 2005 | Plasmid | Iron oxide (γFe2O3) | HVJ-E, protamine sulfate | BHK-21 | ** 4-fold | - | [47] |
| Plasmid | Iron oxide (γ-Fe2O3) | HVJ-E, heparin sulfate | Liver, BALB/c mice (8 weeks age) | ** 3-fold | - | |||
| Scherer F | 2002 | Plasmid | SPION | PEI (MW: 800 k) | NIH3T3 | ** 5-fold | - | [44] |
| Adenovirus | SPION | PEI (MW: 800 k) | K562 | ** 100-fold | - | |||
| Retrovirus | SPION | PEI (MW: 800 k) | NIH3T3 | * 20% | - | |||
| Mah C | 2002 | Adenovirus | Avidinylated magnetite | Biotunylated heparan sulfate | C12S | * 75% | - | [56] |
| Adenovirus | Avidinylated magnetite | Biotunylated heparan sulfate | Adult 129/SvJ mice | - | - | |||
indicates % of fluorescent positive cells analyzed by flow cytometric analysis.
indicates analysis by luciferase activity assay compared with control. Transfection efficiency was indicated optimal transfection condition.
indicates transfection without magnetic force.
PEI: Polyethylenimine; PEI max: Deacaylated PEI; MNP: Magnetic nanoparticle; SPION: Superparamagnetic iron oxide nanoparticle; MW: Molecular weight; TF: transfection; PolyMag: Commercial Magnetofection reagent), NeuroMag (Commercial Magnetofection reagent); HVJ-E: hemagglutinating virus of Japan-envelope; DOTAP: 1,2-dioleoyl- 3-trimethylammonium-propane; DOPE: 1,2-dioleoyl-3-sn- phosphatidyl-ethanolamine; Tat peptide: cationic cell penetrating peptide; MeMEIO: Manganese-doped magnetism-engineered iron oxide; PEG: polyethylene glycol, Zonyl FSA fluorosurfactant: Lithium 3-[2-(perfluoroalkyl)ethylthio]propionate).
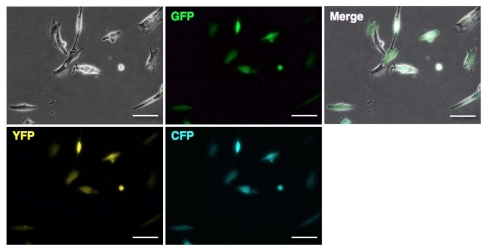
Deacylated polyethylenimine (linear, 25,000 molecular weight) is built from the same polymer backbone as the popular linear polyethylenimine, and possesses high cationic reactivity. PEI “Max”-coated MNPs (PEI max-MNPs) are stable in deionized water, and positively charged. Thus, PEI max-MNPs electrostatically bind to plasmids. We attempted to introduce the green fluorescent protein (GFP) gene into a mouse embryonic carcinoma cell line, P19CL6 using PEI max-MNPs, and succeeded in establishing a highly efficient and low cytotoxic gene delivery system [107]. Furthermore, we applied this system to human fetal lung-derived fibroblasts (TIG-1 cells) using sixwell plates. Using MNPs, the transfected gene’s expression level increased 2-to 4-fold under optimum conditions (Figure 4, unpublished data). Furthermore, to assess whether the multiple plasmids were expressed in a single cell, we attempt to induce the expression of three fluorescent proteins GFP, cyan fluorescent protein (CFP), and yellow fluorescent protein (YFP). Most cells expressed these three proteins (Figure 5, unpublished data) indicating that gene delivery using MNPs could introduce and allow expression of multiple genes in a single cell.
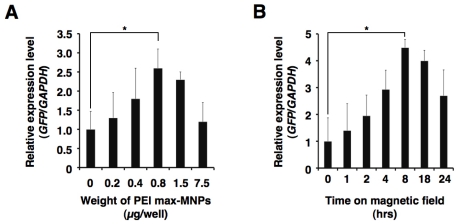
Figure 4.
Optimum conditions for PEI max-MNPs magnetofection. To optimize conditions, we varied volume (A) and time on the magnetic plate (B). These results were evaluated by quantitative real-time RT-PCR. The relative expression level (GFP/GAPDH) in the human fetal lung-derived fibroblasts (TIG-1 cells) treated with PEI max alone (A), and in the absence of magnetic force (0 h) (B) was defined as 1. Optimal transfection conditions were established when TIG-1 cells were treated with 0.8 μg PEI max-MNPs and 2.0 μg pCAG-GFP for 8 h on the magnetic plate in either a six-well plate or a 35 mm dish. The asterisk (*) indicates a significant difference (P < 0.05).
Figure 5.
Transfection of TIG-1 cells with multiple genes using PEI max-MNPs. TIG-1 cells were simultaneously transfected with GFP, CFP, and YFP expression vector plasmids. TIG-1 cells were treated with 0.8 μg of PEI max-MNPs and 0.7 μg each of pCAG-GFP (GFP, provided by Dr. Nishino), pPhi-Yellow-N (YFP, Evrogen), and pAmCyan1-C1 (CFP, Clonetech) for 8 h on the magnetic plate in a six-well plate or a 35 mm dish. White bar indicates 200 μm.
5. Conclusions
The great promise of gene therapy for treating devastating, incurable diseases has yet to be realized. Less toxic and more efficient systems will be required, and robust research efforts in this regard are currently underway. Rapid advances have been made in adapting nanoparticle technology for basic biomedical and clinical research. Nanoparticles are already being used clinically to enhance MRI imaging, and drug delivery for cancer patients. Our own research has focused on gene delivery systems for autologous cell transplantation therapy, in which the patient’s own cells are transfected with the gene required to correct their condition. In particular, our laboratory and those of others have aimed to optimize magnetofection by developing better nanoparticle coating agents [38,40–51,53–55]. Nanoparticle size is another important parameter but there were few reports addressing this subject [111]. Since cells endocytose MNPs [51,100,101], MNP size has significant implications for transfection efficiency. PEI-MNPs forms magnetoplex, which increased its influence on the magnetic force. Furthermore, MNP size influences cytotoxicity [112], and more studies on this aspect of MNP technology will be crucial for enhancing transfection efficiencies.
The two research groups reported the important developments in the field of magnetofection. The first is the influence of the oscillating magnetic force on transfection [113,114]. The second is the use of MNP-heating, and -transfection [15,16]. The purpose of these studies have increased the efficiency of transfection, and/or induced a fever by oscillating MNPs for hyperthermia. The latter, a combination of MNP-heating and -transfection, was expected to research the efficacy of both hyperthermia and gene delivery. In the future, the studies of magnetofection using the oscillating MNPs could be developed as a novel methodology.
We found that PEI is an excellent cationic polymer for dispersing MNPs and that its water solubility, stability, and low toxicity contribute to enhancing transfection efficiency [95,115–119]. Derivation of iPSCs with the use of non-viral gene delivery using PEI max MNPs should provide a powerful tool for treating diseases such as Alzheimer’s, Huntington’s, and Parkinson’s by autologous cell transplantation. Reprogramming cells requires the action of multiple transcription factors. Our studies demonstrate that MNP-mediated transfection efficiently introduces at least three genes in a single cell. This indicates the feasibility of our system for one-step reprogramming.
References
- 1.Chouly C, Pouliquen D, Lucet I, Jeune JJ, Jallet P. Development of superparamagnetic nanoparticles for MRI: Effect of particle size, charge and surface nature on biodistribution. J. Microencapsul. 1996;13:245–255. doi: 10.3109/02652049609026013. [DOI] [PubMed] [Google Scholar]
- 2.Schlorf T, Meincke M, Kossel E, Gluer CC, Jansen O, Mentlein R. Biological properties of iron oxide nanoparticles for cellular and molecular magnetic resonance imaging. Int. J. Mol. Sci. 2010;12:12–23. doi: 10.3390/ijms12010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo B, Pagel MD. An overview of responsive MRI contrast agents for molecular imaging. Front. Biosci. 2008;13:1733–1752. doi: 10.2741/2796. [DOI] [PubMed] [Google Scholar]
- 4.Sun C, Fang C, Stephen Z, Veiseh O, Hansen S, Lee D, Ellenbogen RG, Olson J, Zhang M. Tumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticles. Nanomedicine (Lond. UK) 2008;3:495–505. doi: 10.2217/17435889.3.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medarova Z, Rashkovetsky L, Pantazopoulos P, Moore A. Multiparametric monitoring of tumor response to chemotherapy by noninvasive imaging. Cancer Res. 2009;69:1182–1189. doi: 10.1158/0008-5472.CAN-08-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu YL, Ye Q, Sato K, Foley LM, Hitchens TK, Ho C. Noninvasive evaluation of cardiac allograft rejection by cellular and functional cardiac magnetic resonance. JACC Cardiovasc. Imaging. 2009;2:731–741. doi: 10.1016/j.jcmg.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu YL, Ye Q, Foley LM, Hitchens TK, Sato K, Williams JB, Ho C. In situ labeling of immune cells with iron oxide particles: An approach to detect organ rejection by cellular MRI. Proc. Natl. Acad. Sci. USA. 2006;103:1852–1857. doi: 10.1073/pnas.0507198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CL, Zhang H, Ye Q, Hsieh WY, Hitchens TK, Shen HH, Liu L, Wu YJ, Foley LM, Wang SJ, et al. A New Nano-sized Iron Oxide Particle with High Sensitivity for Cellular Magnetic Resonance Imaging. Mol Imaging Biol. 2010 doi: 10.1007/s11307-010-0430-x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 10.Maeda H, Matsumura Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit. Rev. Ther. Drug Carrier Syst. 1989;6:193–210. [PubMed] [Google Scholar]
- 11.Oh KT, Baik HJ, Lee AH, Oh YT, Youn YS, Lee ES. The reversal of drug-resistance in tumors using a drug-carrying nanoparticular system. Int. J. Mol. Sci. 2009;10:3776–3792. doi: 10.3390/ijms10093776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan A, Scholz R, Wust P, Fahling H, Roland F. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999;201:413–419. [Google Scholar]
- 13.Mornet S, Vasseur S, Grasset F, Veverka P, Goglio G, Demourgues A, Portier J, Pollert E, Duguet E. Magnetic nanoparticle design for medical applications. Prog. Solid State Chem. 2006;34:237–247. [Google Scholar]
- 14.Kim DH, Kim KN, Kim KM, Lee YK. Targeting to carcinoma cells with chitosan- and starch-coated magnetic nanoparticles for magnetic hyperthermia. J. Biomed. Mater. Res. , Part A. 2009;88:1–11. doi: 10.1002/jbm.a.31775. [DOI] [PubMed] [Google Scholar]
- 15.Ito A, Shinkai M, Honda H, Kobayashi T. Heat-inducible TNF-alpha gene therapy combined with hyperthermia using magnetic nanoparticles as a novel tumor-targeted therapy. Cancer Gene Ther. 2001;8:649–654. doi: 10.1038/sj.cgt.7700357. [DOI] [PubMed] [Google Scholar]
- 16.Tang QS, Zhang DS, Cong XM, Wan ML, Jin LQ. Using thermal energy produced by irradiation of Mn-Zn ferrite magnetic nanoparticles (MZF-NPs) for heat-inducible gene expression. Biomaterials. 2008;29:2673–2679. doi: 10.1016/j.biomaterials.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Salloum M, Ma RH, Weeks D, Zhu L. Controlling nanoparticle delivery in magnetic nanoparticle hyperthermia for cancer treatment: Experimental study in agarose gel. Int. J. Hyperthermia. 2008;24:337–345. doi: 10.1080/02656730801907937. [DOI] [PubMed] [Google Scholar]
- 18.Wust P, Gneveckow U, Johannsen M, Bohmer D, Henkel T, Kahmann F, Sehouli J, Felix R, Ricke J, Jordan A. Magnetic nanoparticles for interstitial thermotherapy—feasibility, tolerance and achieved temperatures. Int. J. Hyperth. 2006;22:673–685. doi: 10.1080/02656730601106037. [DOI] [PubMed] [Google Scholar]
- 19.Ito A, Honda H, Kobayashi T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: A novel concept of “heat-controlled necrosis” with heat shock protein expression. Cancer Immunol. Immunother. 2006;55:320–328. doi: 10.1007/s00262-005-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka K, Ito A, Kobayashi T, Kawamura T, Shimada S, Matsumoto K, Saida T, Honda H. Intratumoral injection of immature dendritic cells enhances antitumor effect of hyperthermia using magnetic nanoparticles. Int. J. Cancer. 2005;116:624–633. doi: 10.1002/ijc.21061. [DOI] [PubMed] [Google Scholar]
- 21.Ito A, Tanaka K, Honda H, Abe S, Yamaguchi H, Kobayashi T. Complete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticles. J. Biosci. Bioeng. 2003;96:364–369. doi: 10.1016/S1389-1723(03)90138-1. [DOI] [PubMed] [Google Scholar]
- 22.Muggia FM. Doxorubicin-polymer conjugates: Further demonstration of the concept of enhanced permeability and retention. Clin. Cancer Res. 1999;5:7–8. [PubMed] [Google Scholar]
- 23.Gabizon A, Chemla M, Tzemach D, Horowitz AT, Goren D. Liposome longevity and stability in circulation: Effects on the in vivo delivery to tumors and therapeutic efficacy of encapsulated anthracyclines. J. Drug Target. 1996;3:391–398. doi: 10.3109/10611869608996830. [DOI] [PubMed] [Google Scholar]
- 24.Sakakibara T, Chen FA, Kida H, Kunieda K, Cuenca RE, Martin FJ, Bankert RB. Doxorubicin encapsulated in sterically stabilized liposomes is superior to free drug or drug-containing conventional liposomes at suppressing growth and metastases of human lung tumor xenografts. Cancer Res. 1996;56:3743–3746. [PubMed] [Google Scholar]
- 25.Harrington KJ, Mohammadtaghi S, Uster PS, Glass D, Peters AM, Vile RG, Stewart JS. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin. Cancer Res. 2001;7:243–254. [PubMed] [Google Scholar]
- 26.Noe LL, Becker RV, III, Gradishar WJ, Gore M, Trotter JP. The cost effectiveness of tamoxifen in the prevention of breast cancer. Am J Manag Care. 1999;5(Suppl 6):S389–406. [PubMed] [Google Scholar]
- 27.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, Esmaeli B, Ring SE, Bedikian A, Hortobagyi GN, et al. Phase I and pharmacokinetic study of ABI- 007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin. Cancer Res. 2002;8:1038–1044. [PubMed] [Google Scholar]
- 28.Ibrahim NK, Samuels B, Page R, Doval D, Patel KM, Rao SC, Nair MK, Bhar P, Desai N, Hortobagyi GN. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J. Clin. Oncol. 2005;23:6019–6026. doi: 10.1200/JCO.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Pinder MC, Ibrahim NK. Nanoparticle albumin-bound paclitaxel for treatment of metastatic breast cancer. Drugs Today. 2006;42:599–604. doi: 10.1358/dot.2006.42.9.1009902. [DOI] [PubMed] [Google Scholar]
- 30.Hamaguchi T, Kato K, Yasui H, Morizane C, Ikeda M, Ueno H, Muro K, Yamada Y, Okusaka T, Shirao K, et al. A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation. Br. J. Cancer. 2007;97:170–176. doi: 10.1038/sj.bjc.6603855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, Nakatomi I, Yokoyama M, Kataoka K, Kakizoe T. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer. 2005;92:1240–1246. doi: 10.1038/sj.bjc.6602479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muggia FM, Hainsworth JD, Jeffers S, Miller P, Groshen S, Tan M, Roman L, Uziely B, Muderspach L, Garcia A, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: Antitumor activity and toxicity modification by liposomal encapsulation. J. Clin. Oncol. 1997;15:987–993. doi: 10.1200/JCO.1997.15.3.987. [DOI] [PubMed] [Google Scholar]
- 33.Kikumori T, Kobayashi T, Sawaki M, Imai T. Anti-cancer effect of hyperthermia on breast cancer by magnetite nanoparticle-loaded anti-HER2 immunoliposomes. Breast Cancer Res. Treat. 2009;113:435–441. doi: 10.1007/s10549-008-9948-x. [DOI] [PubMed] [Google Scholar]
- 34.Johannsen M, Thiesen B, Wust P, Jordan A. Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperth. 2010;26:790–795. doi: 10.3109/02656731003745740. [DOI] [PubMed] [Google Scholar]
- 35.Rao W, Deng ZS, Liu J. A review of hyperthermia combined with radiotherapy/chemotherapy on malignant tumors. Crit. Rev. Biomed. Eng. 2010;38:101–116. doi: 10.1615/critrevbiomedeng.v38.i1.80. [DOI] [PubMed] [Google Scholar]
- 36.Yallapu MM, Othman SF, Curtis ET, Gupta BK, Jaggi M, Chauhan SC. Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials. 2011;32:1890–1905. doi: 10.1016/j.biomaterials.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen B, Wu W, Wang X. Magnetic iron oxide nanoparticles for tumor-targeted therapy. Curr. Cancer Drug Targets. 2011;11:184–189. doi: 10.2174/156800911794328475. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Lee MY, Hogg MG, Dordick JS, Sharfstein ST. Gene delivery in three-dimensional cell cultures by superparamagnetic nanoparticles. ACS Nano. 2010;4:4733–4743. doi: 10.1021/nn9018812. [DOI] [PubMed] [Google Scholar]
- 39.Pickard MR, Barraud P, Chari DM. The transfection of multipotent neural precursor/stem cell transplant populations with magnetic nanoparticles. Biomaterials. 2011;32:2274–2284. doi: 10.1016/j.biomaterials.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Lee JH, Lee K, Moon SH, Lee Y, Park TG, Cheon J. All-in-one target-cell-specific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew. Chem., Int. Ed. Engl. 2009;48:4174–4179. doi: 10.1002/anie.200805998. [DOI] [PubMed] [Google Scholar]
- 41.Kievit FM, Veiseh O, Bhattarai N, Fang C, Gunn JW, Lee D, Ellenbogen RG, Olson JM, Zhang M. PEI-PEG-Chitosan Copolymer Coated Iron Oxide Nanoparticles for Safe Gene Delivery: Synthesis, complexation, and transfection. Adv. Funct. Mater. 2009;19:2244–2251. doi: 10.1002/adfm.200801844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mykhaylyk O, Antequera YS, Vlaskou D, Plank C. Generation of magnetic nonviral gene transfer agents and magnetofection in vitro. Nat. Protoc. 2007;2:2391–2411. doi: 10.1038/nprot.2007.352. [DOI] [PubMed] [Google Scholar]
- 43.Song HP, Yang JY, Lo SL, Wang Y, Fan WM, Tang XS, Xue JM, Wang S. Gene transfer using self-assembled ternary complexes of cationic magnetic nanoparticles, plasmid DNA and cell-penetrating Tat peptide. Biomaterials. 2010;31:769–778. doi: 10.1016/j.biomaterials.2009.09.085. [DOI] [PubMed] [Google Scholar]
- 44.Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Kruger A, Gansbacher B, Plank C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 45.Shi Y, Zhou L, Wang R, Pang Y, Xiao W, Li H, Su Y, Wang X, Zhu B, Zhu X, Yan D, Gu H. In situ preparation of magnetic nonviral gene vectors and magnetofection in vitro. Nanotechnology. 2010;21:115103. doi: 10.1088/0957-4484/21/11/115103. [DOI] [PubMed] [Google Scholar]
- 46.Ang D, Nguyen QV, Kayal S, Preiser PR, Rawat RS, Ramanujan RV. Insights into the mechanism of magnetic particle assisted gene delivery. Acta Biomater. 2011;7:1319–1326. doi: 10.1016/j.actbio.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 47.Morishita N, Nakagami H, Morishita R, Takeda S, Mishima F, Terazono B, Nishijima S, Kaneda Y, Tanaka N. Magnetic nanoparticles with surface modification enhanced gene delivery of HVJ-E vector. Biochem. Biophys. Res. Commun. 2005;334:1121–1126. doi: 10.1016/j.bbrc.2005.06.204. [DOI] [PubMed] [Google Scholar]
- 48.Namgung R, Singha K, Yu MK, Jon S, Kim YS, Ahn Y, Park IK, Kim WJ. Hybrid superparamagnetic iron oxide nanoparticle-branched polyethylenimine magnetoplexes for gene transfection of vascular endothelial cells. Biomaterials. 2010;31:4204–4213. doi: 10.1016/j.biomaterials.2010.01.123. [DOI] [PubMed] [Google Scholar]
- 49.Yiu HH, McBain SC, Lethbridge ZA, Lees MR, Dobson J. Preparation and characterization of polyethylenimine-coated Fe3O4-MCM-48 nanocomposite particles as a novel agent for magnet-assisted transfection. J. Biomed. Mater. Res. , Part A. 2010;92:386–392. doi: 10.1002/jbm.a.32363. [DOI] [PubMed] [Google Scholar]
- 50.Namiki Y, Namiki T, Yoshida H, Ishii Y, Tsubota A, Koido S, Nariai K, Mitsunaga M, Yanagisawa S, Kashiwagi H, et al. A novel magnetic crystal-lipid nanostructure for magnetically guided in vivo gene delivery. Nat. Nanotechnol. 2009;4:598–606. doi: 10.1038/nnano.2009.202. [DOI] [PubMed] [Google Scholar]
- 51.Arsianti M, Lim M, Marquis CP, Amal R. Polyethylenimine based magnetic iron-oxide vector: The effect of vector component assembly on cellular entry mechanism, intracellular localization, and cellular viability. Biomacromolecules. 2010;11:2521–3251. doi: 10.1021/bm100748p. [DOI] [PubMed] [Google Scholar]
- 52.Kim TS, Lee SH, Gang GT, Lee YS, Kim SU, Koo DB, Shin MY, Park CK, Lee DS. Exogenous DNA uptake of boar spermatozoa by a magnetic nanoparticle vector system. Reprod. Domest. Anim. 2009;45:e201–e206. doi: 10.1111/j.1439-0531.2009.01516.x. [DOI] [PubMed] [Google Scholar]
- 53.Yang SY, Sun JS, Liu CH, Tsuang YH, Chen LT, Hong CY, Yang HC, Horng HE. Ex vivo magnetofection with magnetic nanoparticles: A novel platform for nonviral tissue engineering. Artif. Organs. 2008;32:195–204. doi: 10.1111/j.1525-1594.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 54.Tresilwised N, Pithayanukul P, Mykhaylyk O, Holm PS, Holzmuller R, Anton M, Thalhammer S, Adiguzel D, Doblinger M, Plank C. Boosting oncolytic adenovirus potency with magnetic nanoparticles and magnetic force. Mol. Pharmaceutics. 2010;7:1069–1089. doi: 10.1021/mp100123t. [DOI] [PubMed] [Google Scholar]
- 55.Hashimoto M, Hisano Y. Directional gene-transfer into the brain by an adenoviral vector tagged with magnetic nanoparticles. J. Neurosci. Methods. 2011;194:316–320. doi: 10.1016/j.jneumeth.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 56.Mah C, Fraites TJ, Jr, Zolotukhin I, Song S, Flotte TR, Dobson J, Batich C, Byrne BJ. Improved method of recombinant AAV2 delivery for systemic targeted gene therapy. Mol. Ther. 2002;6:106–112. doi: 10.1006/mthe.2001.0636. [DOI] [PubMed] [Google Scholar]
- 57.Basti H, Ben Tahar L, Smiri LS, Herbst F, Vaulay MJ, Chau F, Ammar S, Benderbous S. Catechol derivatives-coated Fe3O4 and gamma-Fe2O3 nanoparticles as potential MRI contrast agents. J. Colloid Interface Sci. 2010;341:248–254. doi: 10.1016/j.jcis.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 58.Gamarra LF, Amaro E, Jr, Alves S, Soga D, Pontuschka WM, Mamani JB, Carneiro SM, Brito GE, Figueiredo Neto AM. Characterization of the biocompatible magnetic colloid on the basis of Fe3O4 nanoparticles coated with dextran used as contrast agent in magnetic resonance imaging. J. Nanosci. Nanotechnol. 2010;10:4145–4153. doi: 10.1166/jnn.2010.2200. [DOI] [PubMed] [Google Scholar]
- 59.Jung CW, Jacobs P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: Ferumoxides, ferumoxtran, ferumoxsil. Magn. Reson. Imaging. 1995;13:661–674. doi: 10.1016/0730-725x(95)00024-b. [DOI] [PubMed] [Google Scholar]
- 60.Martina MS, Fortin JP, Menager C, Clement O, Barratt G, Grabielle-Madelmont C, Gazeau F, Cabuil V, Lesieur S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J. Am. Chem. Soc. 2005;127:10676–10685. doi: 10.1021/ja0516460. [DOI] [PubMed] [Google Scholar]
- 61.Widder DJ, Greif WL, Widder KJ, Edelman RR, Brady TJ. Magnetite albumin microspheres: A new MR contrast material. Am. J. Roentgenol. 1987;148:399–404. doi: 10.2214/ajr.148.2.399. [DOI] [PubMed] [Google Scholar]
- 62.Sun X, Gutierrez A, Yacaman MJ, Dong X, Jin S. Investigations on magnetic properties and structure for carbon encapsulated nanoparticles of Fe, Co, Ni. Mater. Sci. Eng. A. 2000;286:157–160. [Google Scholar]
- 63.Tomitaka A, Kobayashi H, Yamada T, Jeun M, Bae S, Takemura Y. Magnetization and self-heating temperature of NiFe2O4 nanoparticles measured by applying ac magnetic field. J. Phys.: Conf. Ser. 2010;200:122010. [Google Scholar]
- 64.Cho WS, Duffin R, Poland CA, Duschl A, Oostingh GJ, Macnee W, Bradley M, Megson IL, Donaldson K. Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology. 2011 doi: 10.3109/17435390.2011.552810. in press. [DOI] [PubMed] [Google Scholar]
- 65.George S, Xia T, Rallo R, Zhao Y, Ji Z, Lin S, Wang X, Zhang H, France B, Schoenfeld D, et al. Use of a high-throughput screening approach coupled with in vivo zebrafish embryo screening to develop hazard ranking for engineered nanomaterials. ACS Nano. 2011;5:1805–1817. doi: 10.1021/nn102734s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giri J, Pradhan P, Somani V, Chelawat H, Chhatre S, Banerjee R, Bahadur D. Synthesis and characterizations of water-based ferrofluids of substituted ferrites [Fe1-xBxFe2O4, B = Mn, Co (x = 0−1)] for biomedical applications. J. Magn. Magn. Mater. 2008;320:724–730. [Google Scholar]
- 67.Karlsson HL, Cronholm P, Gustafsson J, Moller L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008;21:1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- 68.McBain SC, Yiu HH, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008;3:169–180. doi: 10.2147/ijn.s1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buyukhatipoglu K, Clyne AM. Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J. Biomed. Mater. Res. , Part A. 2011;96:186–195. doi: 10.1002/jbm.a.32972. [DOI] [PubMed] [Google Scholar]
- 70.Schroder U, Segren S, Gemmefors C, Hedlund G, Jansson B, Sjogren HO, Borrebaeck CA. Magnetic carbohydrate nanoparticles for affinity cell separation. J. Immunol. Methods. 1986;93:45–53. doi: 10.1016/0022-1759(86)90431-x. [DOI] [PubMed] [Google Scholar]
- 71.Berry CC, Wells S, Charles S, Curtis AS. Dextran and albumin derivatised iron oxide nanoparticles: Influence on fibroblasts in vitro. Biomaterials. 2003;24:4551–7455. doi: 10.1016/s0142-9612(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 72.Nitin N, LaConte LE, Zurkiya O, Hu X, Bao G. Functionalization and peptide-based delivery of magnetic nanoparticles as an intracellular MRI contrast agent. J. Biol. Inorg. Chem. 2004;9:706–712. doi: 10.1007/s00775-004-0560-1. [DOI] [PubMed] [Google Scholar]
- 73.Ito A, Ino K, Kobayashi T, Honda H. The effect of RGD peptide-conjugated magnetite cationic liposomes on cell growth and cell sheet harvesting. Biomaterials. 2005;26:6185–6193. doi: 10.1016/j.biomaterials.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 74.de la Fuente JM, Penades S. Glyconanoparticles: Types, synthesis and applications in glycoscience, biomedicine and material science. Biochim. Biophys. Acta. 2006;1760:636–651. doi: 10.1016/j.bbagen.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 75.McDonald MA, Watkin KL. Investigations into the physicochemical properties of dextran small particulate gadolinium oxide nanoparticles. Acad. Radiol. 2006;13:421–427. doi: 10.1016/j.acra.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 76.Mertz CJ, Kaminski MD, Xie Y, Finck MR, Guy S, Rosengart AJ. In vitro studies of functionalized magnetic nanospheres for selective removal of a simulant biotoxin. J. Magn. Magn. Mater. 2005;293:572–577. [Google Scholar]
- 77.Mikhaylova M, Jo Y, Kim D, Bobrysheva N, Andersson Y, Eriksson T, Osmolowsky M, Semenov V, Muhammed M. The Effect of Biocompatible Coating Layers on Magnetic Properties of Superparamagnetic Iron Oxide Nanoparticles. Hyperfine Interact. 2004;156–157:257–263. [Google Scholar]
- 78.Qiu XP, Winnik F. Preparation and characterization of PVA coated magnetic nanoparticles. Chin. J. Polym. Sci. 2000;18:535–539. [Google Scholar]
- 79.Yiu HHP, Wright PA, Botting NP. Enzyme immobilisation using SBA-15 mesoporous molecular sieves with functionalised surfaces. J. Mol. Catal. B: Enzym. 2001;15:81–92. [Google Scholar]
- 80.Ameur S, Martelet C, Jaffrezic-Renault N, Chovelon J-M. Sensitive immunodetection through impedance measurements onto gold functionalized electrodes. Appl. Biochem. Biotechnol. 2000;89:161–170. doi: 10.1385/abab:89:2-3:161. [DOI] [PubMed] [Google Scholar]
- 81.Arsianti M, Lim M, Lou SN, Goon IY, Marquis CP, Amal R. Bi-functional gold-coated magnetite composites with improved biocompatibility. J. Colloid Interface Sci. 2011;354:536–545. doi: 10.1016/j.jcis.2010.10.061. [DOI] [PubMed] [Google Scholar]
- 82.Williams D, Gold K, Holoman T, Ehrman S, Wilson O. Surface modification of magnetic nanoparticles using gum arabic. J. Nanopart. Res. 2006;8:749–753. [Google Scholar]
- 83.Klabunde KJ, Stark J, Koper O, Mohs C, Park DG, Decker S, Jiang Y, Lagadic I, Zhang D. Nanocrystals as stoichiometric reagents with unique surface chemistry. J. Phys. Chem. 1996;100:12142–12153. [Google Scholar]
- 84.Zhang H, Xia T, Meng H, Xue M, George S, Ji Z, Wang X, Liu R, Wang M, France B, et al. Differential expression of syndecan-1 mediates cationic nanoparticle toxicity in undifferentiated versus differentiated normal human bronchial epithelial cells. ACS Nano. 2011;5:2756–2769. doi: 10.1021/nn200328m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sunoqrot S, Bae JW, Jin SE, Ryan MP, Liu Y, Hong S. Kinetically controlled cellular interactions of polymer-polymer and polymer-liposome nanohybrid systems. Bioconjugate Chem. 2011;22:466–474. doi: 10.1021/bc100484t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schweiger C, Pietzonka C, Heverhagen J, Kissel T. Novel magnetic iron oxide nanoparticles coated with poly(ethylene imine)-g-poly(ethylene glycol) for potential biomedical application: Synthesis, stability, cytotoxicity and MR imaging. Int. J. Pharm. 2011;408:130–137. doi: 10.1016/j.ijpharm.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 87.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc. Natl. Acad. Sci. USA. 2005;102:5679–5684. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: Polyethylenimine. Hum. Gene Ther. 1996;7:1947–1954. doi: 10.1089/hum.1996.7.16-1947. [DOI] [PubMed] [Google Scholar]
- 90.Zuo KH, Jiang DL, Zhang JX, Lin QL. Forming nanometer TiO2 sheets by nonaqueous tape casting. Ceram. Int. 2007;33:477–481. [Google Scholar]
- 91.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–711. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 96.Laurent N, Sapet CD, Le Gourrierec L, Bertosio E, Zelphati O. Nucleic acid delivery using magnetic nanoparticles: The Magnetofection™ technology. Ther. Deliv. 2011;2:471–482. doi: 10.4155/tde.11.12. [DOI] [PubMed] [Google Scholar]
- 97.Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J. Biol. Chem. 2003;278:44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 98.Brunner S, Sauer T, Carotta S, Cotten M, Saltik M, Wagner E. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Ther. 2000;7:401–407. doi: 10.1038/sj.gt.3301102. [DOI] [PubMed] [Google Scholar]
- 99.Nishiyama N, Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006;112:630–648. doi: 10.1016/j.pharmthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 100.Yu JH, Quan JS, Huang J, Nah JW, Cho CS. Degradable poly(amino ester) based on poly(ethylene glycol) dimethacrylate and polyethylenimine as a gene carrier: Molecular weight of PEI affects transfection efficiency. J. Mater. Sci.: Mater. Med. 2009;20:2501–2510. doi: 10.1007/s10856-009-3816-z. [DOI] [PubMed] [Google Scholar]
- 101.Veiseh O, Kievit FM, Gunn JW, Ratner BD, Zhang M. A ligand-mediated nanovector for targeted gene delivery and transfection in cancer cells. Biomaterials. 2009;30:649–657. doi: 10.1016/j.biomaterials.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pan X, Guan J, Yoo JW, Epstein AJ, Lee LJ, Lee RJ. Cationic lipid-coated magnetic nanoparticles associated with transferrin for gene delivery. Int. J. Pharm. 2008;358:263–270. doi: 10.1016/j.ijpharm.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Z, Xiang J, Zhang W, Fan S, Wu M, Li X, Li G. Nanoparticle delivery of anti-metastatic NM23-H1 gene improves chemotherapy in a mouse tumor model. Cancer Gene Ther. 2009;16:423–429. doi: 10.1038/cgt.2008.97. [DOI] [PubMed] [Google Scholar]
- 104.Gonzalez B, Ruiz-Hernandez E, Feito MJ, Lopez de Laorden C, Arcos D, Ramirez-Santillan C, Matesanz C, Portoles MT, Vallet-Regi M. Covalently bonded dendrimer-maghemite nanosystems: Nonviral vectors for in vitro gene magnetofection. J. Mater. Chem. 2011;21:4598–4604. [Google Scholar]
- 105.Wu H-C, Wang T-W, Bohn MC, Lin F-H, Spector M. Novel magnetic hydroxyapatite nanoparticles as non-viral vectors for the glial cell line-derived neurotrophic factor Gene. Adv. Funct. Mater. 2010;20:67–77. [Google Scholar]
- 106.Zhao D, Gong T, Zhu D, Zhang Z, Sun X. Comprehensive comparison of two new biodegradable gene carriers. Int J Pharm. 2011 doi: 10.1016/j.ijpharm.2011.04.040. in press. [DOI] [PubMed] [Google Scholar]
- 107.Kami D, Takeda S, Makino H, Toyoda M, Itakura Y, Gojo S, Kyo S, Umezawa A, Watanabe M. Efficient transfection method using deacylated polyethylenimine-coated magnetic nanoparticles. J Artif Organs. 2011 doi: 10.1007/s10047-011-0568-6. in press. [DOI] [PubMed] [Google Scholar]
- 108.Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. A twostage poly(ethylenimine)-mediated cytotoxicity: Implications for gene transfer/therapy. Mol. Ther. 2005;11:990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 109.Zou SM, Erbacher P, Remy JS, Behr JP. Systemic linear polyethylenimine (L-PEI)-mediated gene delivery in the mouse. J. Gene Med. 2000;2:128–134. doi: 10.1002/(SICI)1521-2254(200003/04)2:2<128::AID-JGM95>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 110.Biswas S, Gordon LE, Clark GJ, Nantz MH. Click assembly of magnetic nanovectors for gene delivery. Biomaterials. 2011;32:2683–2688. doi: 10.1016/j.biomaterials.2010.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahmed M, Deng Z, Narain R. Study of transfection efficiencies of cationic glyconanoparticles of different sizes in human cell line. ACS Appl. Mater. Interfaces. 2009;1:1980–1987. doi: 10.1021/am900357x. [DOI] [PubMed] [Google Scholar]
- 112.Frohlich E, Kueznik T, Samberger C, Roblegg E, Wrighton C, Pieber TR. Sizedependent effects of nanoparticles on the activity of cytochrome P450 isoenzymes. Toxicol. Appl. Pharmacol. 2010;242:326–332. doi: 10.1016/j.taap.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 113.McBain SC, Griesenbach U, Xenariou S, Keramane A, Batich CD, Alton EWFW, Dobson J. Magnetic nanoparticles as Gene delivery agents: Enhanced transfection in the presence of oscillating magnet arrays. Nanotechnology. 2008;19:405102. doi: 10.1088/0957-4484/19/40/405102. [DOI] [PubMed] [Google Scholar]
- 114.Kamau SW, Hassa PO, Steitz B, Petri-Fink A, Hofmann H, Hofmann-Amtenbrink M, von Rechenberg B, Hottiger MO. Enhancement of the efficiency of non-viral gene delivery by application of pulsed magnetic field. Nucleic Acids Res. 2006;34:e40. doi: 10.1093/nar/gkl035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 116.Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 118.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 119.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–782. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]