Abstract
Introduction
Although patients with end-stage organ failure are at high risk for vitamin D deficiency because of limited sunlight exposure and hepatic dysfunction, few studies have measured 25-hydroxy vitamin D (25OHD) at the time of transplantation.
Methods
We measured serum 25OHD immediately after transplantation in 69 heart and liver transplant recipients.
Results
Forty-six heart and 23 liver transplant recipients were evaluated (mean age 53 yr). Mean 25OHD was well below the lower limit of the normal range (43.2 ± 21.2 nmol/L). Ninety-one percent had levels below 75 nmol/L, the threshold commonly used to denote sufficiency, and 71% had levels below 50 nmol/L. Severe deficiency (25OHD <25 nmol/L) was found in 16%. Vitamin D levels did not differ by race, age, gender, or season. Mean 25OHD was lower among liver than heart transplant recipients (34.4 ± 17.5 vs. 47.7 ± 20.7 nmol/L; p < 0.03). Among liver transplant recipients, 22% had undetectable levels (<17 nmol/L).
Conclusions
Vitamin D deficiency is highly prevalent among heart and liver transplant recipients; those with liver failure are at greatest risk. As vitamin D deficiency has many serious skeletal and extra-skeletal sequelae, physicians who treat transplant patients should maintain a high degree of vigilance for this problem.
Keywords: heart transplant, liver transplant, vitamin D
Vitamin D deficiency is an increasingly recognized condition with many serious sequelae. In addition to secondary hyperparathyroidism, bone loss, and fracture, recent data support an indirect effect on fracture incidence through increased muscle weakness and falls (1, 2). Newly recognized non-skeletal effects of vitamin D deficiency include insulin resistance, diabetes mellitus, hypertension, and malignancy (3, 4). Moreover, evidence of the role of vitamin D in the regulation of immune cell proliferation, differentiation, and responsiveness (5) suggests that vitamin D deficiency may be of particular consequence in the transplant population.
Despite the potential for morbidity associated with vitamin D deficiency, few studies have evaluated vitamin D status in transplant recipients, and only one has examined vitamin D levels at the time of transplant, a point at which intervention may prevent further complications.
Insufficient vitamin D levels have been documented in organ transplant candidates with congestive heart failure (6), end-stage pulmonary disease (7), liver failure (8), and chronic kidney disease (9). Several factors place patients with end-stage organ failure at particular risk for vitamin D deficiency. These include limited sunlight exposure and low dietary intake of vitamin D containing foods. In addition, hepatic dysfunction, resulting from hepatic congestion in heart failure patients, or intrinsic liver disease may contribute. The majority of studies documenting vitamin D deficiency in transplant recipients studied patients many years after the transplant operation (10–13). The prevalence of this condition at the time of transplantation has not been commonly reported. To date, there is only one study evaluating patients at the time of kidney transplant (14).
The purpose of this cross-sectional study was to evaluate and directly compare the prevalence of vitamin D insufficiency in cardiac or liver transplant recipients at the time of organ transplantation. We hypothesized that vitamin D insufficiency (25OHD <75 nmol/L) would be prevalent among patients at the time of heart and liver transplantation, and that levels would be particularly low among liver transplant recipients, in whom hepatic 25-hydroxylation of vitamin D might be impaired.
Methods
Subjects were recruited immediately after heart or liver transplantation as part of a randomized trial comparing two bisphosphonates for prevention of bone loss after transplantation. This study was conducted at Columbia University Medical Center, which is located in New York City at a latitude 40°N. Serum 25-hydroxy vitamin D (25OHD) was measured shortly after the transplant operation, and before initiation of bisphosphonate therapy. The Columbia University Medical Center Institutional Review Board approved this protocol, and all subjects signed written informed consent.
As part of the double blind placebo controlled randomized trial, subjects received either oral alendronate for one yr or a single intravenous infusion of zoledronic acid; changes in bone mineral density and microarchitecture were assessed. Herein, we describe the baseline characteristics of the subjects enrolled.
Serum calcium, albumin and creatinine were measured using automated techniques. Serum 25OHD was measured using the DiaSorin Chemiluminescent assay (ARUP Laboratories, Salt Lake City, UT, USA). This is a commonly used clinical assay with a reference range of 75–200 nmol/L; the intra-assay coefficient of variation (CV) is 10%, and the interassay CV is 16%.
Results
Characteristics of the study subjects
Sixty-nine heart and liver transplant recipients (mean age 53 yr; range 22–72) were evaluated. Forty-six (67%) subjects were heart transplant recipients; 23 (33%) were liver transplant recipients. The group included 56 men (81%) and 13 women (19%). Forty-four (64%) were Caucasian, nine (13%) were Hispanic, eight (12%) were African American, two (3%) were Asian, and six (9%) were of another racial background. Characteristics of the study population, summarized in Table 1, are similar to the larger population of patients receiving organ transplants at our institution (15). The majority of subjects was evaluated, and had serum 25OHD measurements, within two wk of transplantation (mean 10 ± 7 d). The subjects had normal serum calcium concentrations according to inclusion criteria for the randomized clinical trial. Mean estimated glomerular filtration rate (eGFR), calculated using the Modification of Diet in Renal Disease (MDRD) formula, was 91 ± 38 mL/min. The majority of subjects (78%) had normal renal function eGFR >60 mL/min. Fourteen (20%) had stage 3 chronic kidney disease (CKD) or an eGFR between 30 and 60 mL/min, and one subject had stage 4 CKD (eGFR was 29 mL/min) at the time the study measurements were obtained. The immunosuppression regimen utilized included glucocorticoids, calcineurin inhibitors, predominantly cyclosporine, and mycophenolate mofetil. As part of the routine post-transplant regimen, all subjects received calcium supplements containing 400–600 IU of vitamin D and a multivitamin containing 400 IU of vitamin D. These supplements were started after the transplant operation as soon as patients were stable and able to tolerate oral medications.
Table 1.
Characteristics of the sample (mean ± SD except where otherwise noted)
| Organ transplanted – n (%) | |
| Heart | 46 (67) |
| Liver | 23 (33) |
| Race – n (%) | |
| Caucasian | 44 (64) |
| African American | 8 (12) |
| Hispanic | 9 (13) |
| Asian | 2 (3) |
| Other | 6 (9) |
| Gender – n (%) | |
| Male | 56 (81) |
| Female | 13 (19) |
| Age (yr) | 53 ± 11 |
| Calcium (2.1–2.5 mmol/L) | 2.3 ± 0.1 |
| Creatinine (44–106 µmol/L) | 88 ± 35 |
| Albumin (40–50 g/L) | 32 ± 5 |
| 25OHD (75–200 nmol/L) | 43 ± 21 |
Vitamin D status
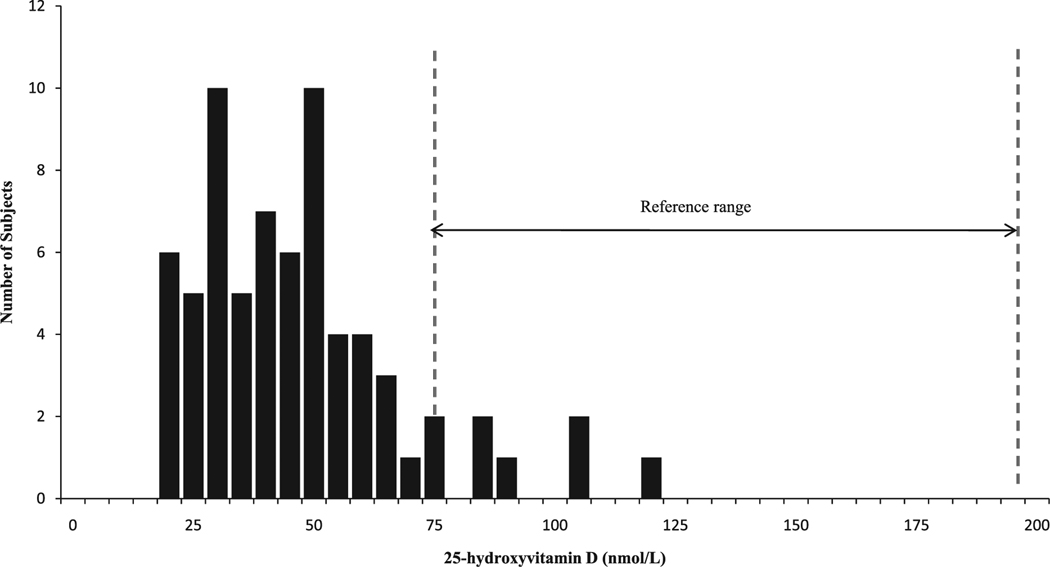
Low 25OHD levels were virtually ubiquitous; 91% of subjects had levels below 75 nmol/L, the threshold commonly used to denote sufficiency. Even using a less stringent threshold of 50 nmol/L, 71% of subjects were insufficient. Severe deficiency (25OHD <25 nmol/L) was found in 16%, and 9% of subjects had levels below 17 nmol/L, the lower limit of detection of the 25OHD assay (Fig. 1). Mean concentration of 25OHD was well below the normal range for the assay (43.2 ± 21.2 nmol/L; normal 75–200 nmol/L). Vitamin D concentrations did not differ by ethnicity, age, or gender. There was no evidence of seasonal variation in 25OHD; specifically, levels in summer months were not higher than those observed at other times of the year (data not shown). Higher serum 25OHD was associated with higher serum albumin (r = 0.32; p < 0.01). There was no evidence of an association between 25OHD and kidney function.
Fig. 1.
Distribution of serum 25-hydroxy vitamin D in 69 heart and liver transplant recipients. The reference range for the assay is denoted by the area between the two dashed lines.
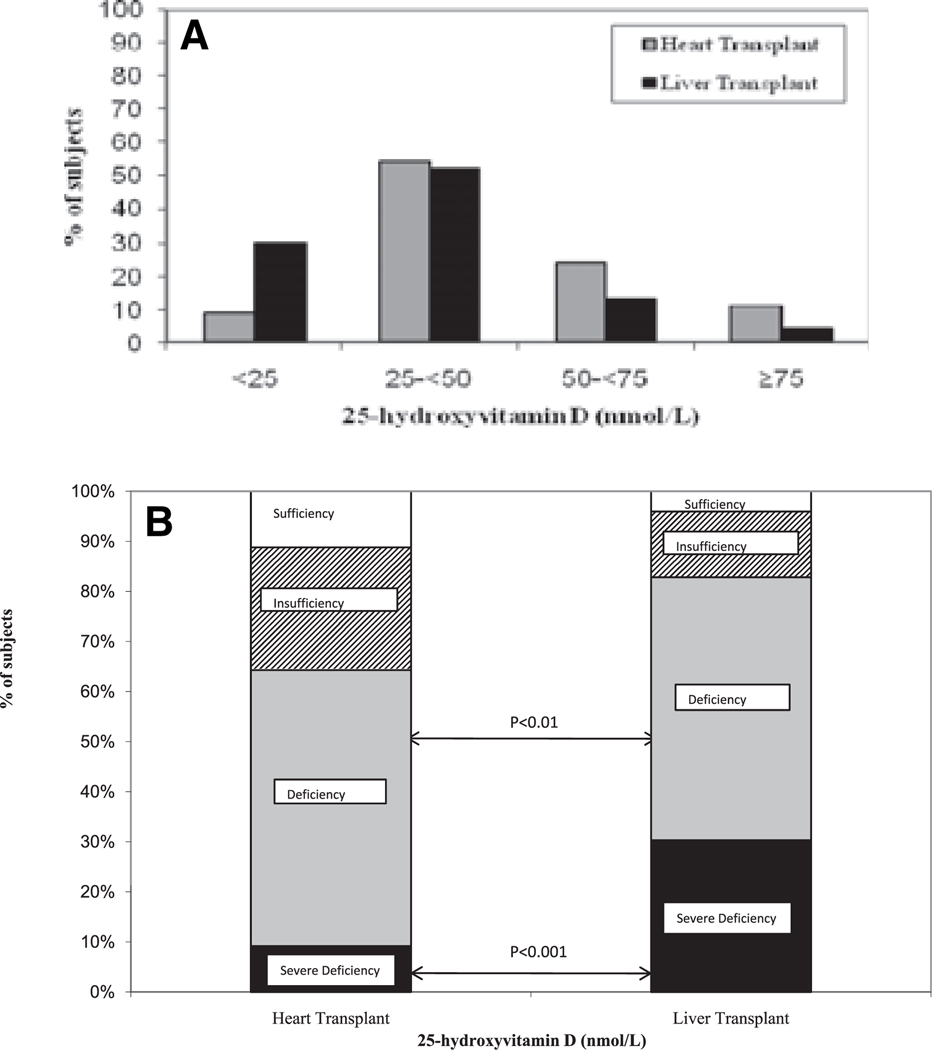
Liver transplant recipients had significantly lower mean 25OHD levels than heart transplant recipients (34.4 ± 17.5 nmol/L vs. 47.7 ± 20.7 nmol/L; p < 0.03). Severe vitamin D deficiency (25OHD <25 nmol/L) was found in 30% of liver transplant recipients and was more common among liver than heart transplant recipients (χ2 = 13.8, p < 0.001). Vitamin D deficiency (25OHD <50 nmol/L) was also more common among liver transplant recipients (χ2 = 8.72, p < 0.01; Fig. 2). Five liver transplant recipients (22%) had undetectable levels (25OHD <17 nmol/L). Serum albumin levels were significantly lower among liver transplant recipients (29 ± 1 g/L vs. heart: 34 ± 1 g/L; p < 0.01), reflecting poorer general health and diminished hepatic synthetic capacity in this group. Liver and heart transplant recipients did not significantly differ on the basis of race, gender, age, calcium, or creatinine. In a stepwise regression model of all potential predictors of vitamin D status, and their first order interaction terms, the only variable that was found to be a significant predictor of 25OHD level was type of organ transplanted (r2 = 0.09; p < 0.02).
Fig. 2.
Comparison of percentage of subjects with severe vitamin D deficiency (25OHD <25 nmol/L or 10 ng/mL), deficiency (25OHD 25–<50 nmol/L or 10–20 ng/mL), insufficiency (50–<75 nmol/L or 20–30 ng/mL), and sufficiency (25OHD ≥75 nmol/L or 30 ng/mL) among heart and liver transplant recipients. Severe vitamin D deficiency was more common among liver than heart transplant recipients (χ2 = 13.8; p < 0.001). Vitamin D deficiency (25OHD <50 nmol/L) was also more common among liver transplant recipients (χ2 = 8.72; p < 0.01).
Discussion
To our knowledge, this is the first study to directly compare the prevalence of vitamin D deficiency among patients immediately after heart and liver transplantation. We found evidence of marked vitamin D insufficiency (91%) and deficiency (16%) in patients at the time of heart and liver transplantation. Liver transplant recipients had significantly lower vitamin D levels than heart transplant recipients.
Of the small number of studies evaluating vitamin D status in patients after organ transplantation, the majority have been performed in kidney transplant recipients, and have focused on patients several years after the transplant operation. The reported prevalence of vitamin D insufficiency (25OHD <75 nmol/L) ranges from 51% to 97% and of severe deficiency (25OHD <25 nmol/L) from 26% to 33% (10–13). The prevalence of insufficiency in our cohort was among the highest reported. Variability in these estimates may relate to the patient population, type of organ transplanted, and assay utilized for measurement of 25OHD. Significant variability in serum 25OHD results that have been reported with several commonly used commercial assays (16–18). We used the DiaSorin chemiluminescent assay, a commercial assay, as opposed to a more precise research method, such as high performance liquid chromatography or mass spectroscopy, because it is the assay used by New York-Presbyterian Hospital clinical laboratories and is in wide clinical use.
Factors associated with worse vitamin D status in transplant recipients include African American race (10), avoidance of sun, low dietary intake (13), and transplant during winter months (14). A recent study of African American kidney transplant recipients, approximately 23 months after transplant, found that 95% of subjects had levels below 75 nmol/L and 58% had levels below 40 nmol/L (10). In one study that evaluated vitamin D status in patients at the time of renal transplant, vitamin D insufficiency was prevalent (25OHD <75 nmol/L: 51%; 25OHD <25 nmol/L: 29%) and exhibited seasonal variation (14).
In healthy populations, greater skin pigmentation or seasonal reductions in UVB radiation can lead to decreased cutaneous production of vitamin D3 from 7-dehydrocholesterol (4). Two recent studies of 25OHD levels in transplant recipients found that levels were lowest in African Americans (14) and subjects with decreased sun exposure (13). In contrast with those findings, we did not observe a variation in 25OHD levels in subjects by race or by season. Our subjects may have been more severely ill, and therefore had even less sun exposure than kidney transplant candidates and long-term transplant recipients. The association of serum 25OHD and albumin suggests that vitamin D insufficiency may be a marker of poor health in these subjects. Patients with low levels of albumin may have also had decreased synthesis of other hepatic proteins, including vitamin D binding protein (VDBP). Low levels of VBDP would result in low total 25OHD levels, even if free circulating 25OHD was normal. Whether low levels of VDBP contribute to the decreased total 25OHD levels in these patients remains to be answered by studies that directly measure this protein.
Bisphosphonates, which prevent bone loss following organ transplantation (15, 19), are increasingly administered to transplant recipients. While these medications can effectively reduce bone loss, they may not be optimally effective in the setting of severe vitamin D deficiency. Moreover, intravenous bisphosphonate treatment has been reported to precipitate symptomatic hypocalcemia in patients with severe, unrecognized vitamin D deficiency (20), making diagnosis of this condition at the time of transplantation even more crucial. Caution should also be used when administering intravenous bisphosphonates to transplant recipients, as these medications, particularly in patients with pre-existing renal insufficiency, have been associated with nephrotoxicity (21), and may compound effects of other medications or pre-renal azotemia.
Recent evidence from animal studies demonstrates that administration of 1,25-dihydroxyvitamin D can prevent acute allograft rejection following liver (22), kidney (23), and heart (24) transplantation. Patients treated with calcitriol following heart transplantation had a reduction in their requirement of cyclosporine (25). Further studies are needed to explore the role of 1,25(OH)2D and of parent vitamin D in prevention of graft rejection. Vitamin D has also been shown to potentiate the innate immune system, and be protective against bacterial infections and tuberculosis (26).
Our findings underscore the need for longitudinal studies to evaluate the efficacy of different repletion regimens to restore 25OHD levels after transplantation, and to examine whether restoring 25OHD at the time of transplant reduces the development of infectious complications and immunosuppressant requirements.
In conclusion, severe vitamin D deficiency is extremely prevalent among heart and liver transplant recipients. Patients with end-stage liver disease are at exceptionally high risk, likely because of disease-related factors, such as malabsorption, and impaired hepatic 25-hydroxylation of vitamin D. It is important that physicians who care for transplant patients maintain a high degree of vigilance for this condition. Although further work is required to explore the complex relationship between vitamin D and immune function in patients after transplant, vitamin D insufficiency is easy to treat, and improvement in vitamin D status may provide both skeletal and extra-skeletal benefits to these patients.
References
- 1.Dhesi JK, Bearne LM, Moniz C, et al. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002;17:891. doi: 10.1359/jbmr.2002.17.5.891. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of Vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 3.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Lemire JM. Immunomodulatory actions of 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 1995;53:599. doi: 10.1016/0960-0760(95)00106-a. [DOI] [PubMed] [Google Scholar]
- 6.Shane E, Mancini D, Aaronson K, et al. Bone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failure. Am J Med. 1997;103:197. doi: 10.1016/s0002-9343(97)00142-3. [DOI] [PubMed] [Google Scholar]
- 7.Shane E, Silverberg SJ, Donovan D, et al. Osteoporosis in lung transplantation candidates with end-stage pulmonary disease. Am J Med. 1996;101:262. doi: 10.1016/S0002-9343(96)00155-6. [DOI] [PubMed] [Google Scholar]
- 8.Monegal A, Navasa M, Guanabens N, et al. Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporos Int. 2001;12:484. doi: 10.1007/s001980170094. [DOI] [PubMed] [Google Scholar]
- 9.Stavroulopoulos A, Porter CJ, Roe SD, Hosking DJ, Cassidy MJ. Relationship between vitamin D status, parathyroid hormone levels and bone mineral density in patients with chronic kidney disease stages 3 and 4. Nephrology (Carlton) 2008;13:63. doi: 10.1111/j.1440-1797.2007.00860.x. [DOI] [PubMed] [Google Scholar]
- 10.Tripathi SS, Gibney EM, Gehr TW, King AL, Beckman MJ. High prevalence of vitamin D deficiency in African American kidney transplant recipients. Transplantation. 2008;85:767. doi: 10.1097/TP.0b013e3181613fb5. [DOI] [PubMed] [Google Scholar]
- 11.Segal E, Baruch Y, Kramsky R, Raz B, Ish-Shalom S. Vitamin D deficiency in liver transplant patients in Israel. Transplant Proc. 2001;33:2955. doi: 10.1016/s0041-1345(01)02269-2. [DOI] [PubMed] [Google Scholar]
- 12.Querings K, Girndt M, Geisel J, Georg T, Tilgen W, Reichrath J. 25-hydroxyvitamin D deficiency in renal transplant recipients. J Clin Endocrinol Metab. 2006;91:526. doi: 10.1210/jc.2005-0547. [DOI] [PubMed] [Google Scholar]
- 13.Ewers B, Gasbjerg A, Moelgaard C, Frederiksen AM, Marckmann P. Vitamin D status in kidney transplant patients: need for intensified routine supplementation. Am J Clin Nutr. 2008;87:431. doi: 10.1093/ajcn/87.2.431. [DOI] [PubMed] [Google Scholar]
- 14.Sadlier DM, Magee CC. Prevalence of 25(OH) vitamin D (calcidiol) deficiency at time of renal transplantation: a prospective study. Clin Transplant. 2007;21:683. doi: 10.1111/j.1399-0012.2007.00696.x. [DOI] [PubMed] [Google Scholar]
- 15.Shane E, Addesso V, Namerow PB, et al. Alendronate versus calcitriol for the prevention of bone loss after cardiac transplantation. N Engl J Med. 2004;350:767. doi: 10.1056/NEJMoa035617. [DOI] [PubMed] [Google Scholar]
- 16.Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int. 1999;9:394. doi: 10.1007/s001980050162. [DOI] [PubMed] [Google Scholar]
- 17.Binkley N, Krueger D, Cowgill CS, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89:3152. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]
- 18.Glendenning P, Noble JM, Taranto M, et al. Issues of methodology, standardization and metabolite recognition for 25-hydroxyvitamin D when comparing the DiaSorin radioimmunoassay and the Nichols Advantage automated chemiluminescence protein-binding assay in hip fracture cases. Ann Clin Biochem. 2003;40(Pt 5):546. doi: 10.1258/000456303322326470. [DOI] [PubMed] [Google Scholar]
- 19.Crawford BA, Kam C, Pavlovic J, et al. Zoledronic acid prevents bone loss after liver transplantation: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144:239. doi: 10.7326/0003-4819-144-4-200602210-00005. [DOI] [PubMed] [Google Scholar]
- 20.Breen TL, Shane E. Prolonged hypocalcemia after treatment with zoledronic acid in a patient with prostate cancer and vitamin D deficiency. J Clin Oncol. 2004;22:1531. doi: 10.1200/JCO.2004.99.013. [DOI] [PubMed] [Google Scholar]
- 21.Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int. 2008;74:1385. doi: 10.1038/ki.2008.356. [DOI] [PubMed] [Google Scholar]
- 22.Zhang AB, Zheng SS, Jia CK, Wang Y. Effect of 1,25-dihydroxyvitamin D3 on preventing allograft from acute rejection following rat orthotopic liver transplantation. World J Gastroenterol. 2003;9:1067. doi: 10.3748/wjg.v9.i5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker BN, Hullett DA, O’Herrin JK, Malin G, Sollinger HW, DeLuca H. Vitamin D as immunomodulatory therapy for kidney transplantation. Transplantation. 2002;74:1204. doi: 10.1097/00007890-200210270-00030. [DOI] [PubMed] [Google Scholar]
- 24.Hullett DA, Cantorna MT, Redaelli C, et al. Prolongation of allograft survival by 1,25-dihydroxyvitamin D3. Transplantation. 1998;66:824. doi: 10.1097/00007890-199810150-00002. [DOI] [PubMed] [Google Scholar]
- 25.Briffa NK, Keogh AM, Sambrook PN, Eisman JA. Reduction of immunosuppressant therapy requirement in heart transplantation by calcitriol. Transplantation. 2003;75:2133. doi: 10.1097/01.TP.0000065179.06731.99. [DOI] [PubMed] [Google Scholar]
- 26.Bikle DD. Vitamin D and the immune system: role in protection against bacterial infection. Curr Opin Nephrol Hypertens. 2008;17:348. doi: 10.1097/MNH.0b013e3282ff64a3. [DOI] [PubMed] [Google Scholar]