Abstract
As one of the evolutionary oldest parts of the brain, the diencephalon evolved to harmonize changing environmental conditions with the internal state for survival of the individual and the species. The pioneering work of physiologists and psychologists around the middle of the last century clearly demonstrated that the hypothalamus is crucial for the display of motivated behaviors, culminating in the discovery of electrical self-stimulation behavior and providing the first neurological hint accounting for the concepts of reinforcement and reward. Here we review recent progress in understanding the role of the lateral hypothalamic area in the control of ingestive behavior and the regulation of energy balance. With its vast array of interoceptive and exteroceptive afferent inputs and its equally rich efferent connectivity, the lateral hypothalamic area is in an ideal position to integrate large amounts of information and orchestrate adaptive responses. Most important for energy homeostasis, it receives metabolic state information through both neural and humoral routes and can affect energy assimilation and energy expenditure through direct access to behavioral, autonomic, and endocrine effector pathways. The complex interplays of classical and peptide neurotransmitters such as orexin carrying out these integrative functions are just beginning to be understood. Exciting new techniques allowing selective stimulation or inhibition of specific neuronal phenotypes will greatly facilitate the functional mapping of both input and output pathways.
Keywords: Food intake, energy expenditure, leptin receptor, glucose sensing, orexin, MCH, reward seeking, obesity
Introduction and historical perspective
The diencephalon first gained attention in the mid 19th century, after the group around the Swiss neurologist, Walter Hess showed that electrical stimulation of different hypothalamic areas in cats elicited a variety of behaviors, including fight, flight, copulation, and voracious eating [1,2]. The influential discoveries of two hypothalamic areas with opposing effects on food intake and body weight in rats soon followed: a lateral area resulting in eating when electrically stimulated and in aphagia and weight loss when lesioned [3] was dubbed “feeding center” and a ventromedial area resulting in hyperphagia and obesity when destroyed [4] was called “satiety center”. In parallel to these studies focusing on food intake, Olds and Milner interested in reinforcement learning discovered the phenomenon of self-stimulation in the brain [5,6]. Soon thereafter, the first paper published by the young Bartley G. Hoebel under the mentorship of Phillip Teitelbaum put the two phenomena together in the journal Science entitled: “Hypothalamic control of feeding and self-stimulation” [7] (Fig. 1).
Fig. 1.
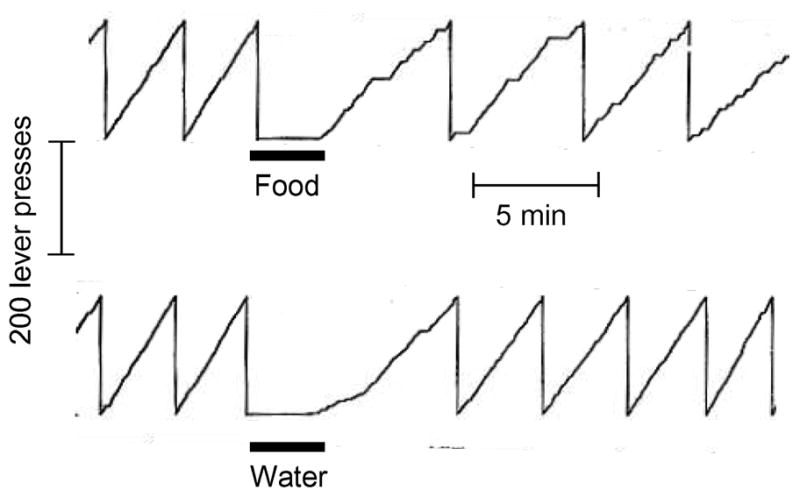
Sustained inhibition of self-stimulation by intragastric food but not water load in rats as demonstrated by Hoebel and Teitelbaum [7] in 1962. The authors concluded that: “Self-stimulation rate was slowed to about half the normal rate by a stomach load of 18 ml of liquid milk diet. The same amount of water had only a transient effect, suggesting that some consequence of food intake other than taste or stomach distension was responsible for prolonged inhibition [7].
This started a decade of intense investigation of the physiological determinants of these phenomena, culminating in an impressive number of highly visible publications. However, hypothalamic stimulation and lesion studies eventually tapered off, because little was known, at the time, about neural connectivity and neurochemistry both within and outside the hypothalamus. A first bout of anatomical studies was then fueled by the newly discovered neural tract tracing methods with tritiated amino acids in the seventies (see discussion by Swanson [8]). A second bout followed the discovery of leptin in the mid nineties and capitalized on the identification of the “feeding” neuropeptides. Most recently, revolutionary new ways have been developed to selectively stimulate specific types of neurons in restricted brain areas, which definitely relegated the non-selective electrical stimulation to the past. The new opto-genetic approach takes advantage of genetic methodology for insertion of light-sensitive excitatory or inhibitory ion channels into specific neurons and subsequent stimulation by light [9]. Thus, it is now possible to selectively activate or suppress orexin or any other neuron type in the lateral hypothalamus with maximal temporal control [10]. Similarly, “designer receptors” exclusively activated by “designer drugs” (DREADD) can be genetically inserted into specific populations of neurons and then selectively activated or suppressed by administration of the corresponding designer drug [11,12].
This review is a tribute to the seminal work of Bartley G. Hoebel, whose work was dedicated to a neurological understanding of ingestive behavior, specifically of food and drug reward mechanisms. We will argue that the lateral hypothalamic area by virtue of its connectivity and neurochemistry plays a key role in these behaviors. We believe that the newly developed tracing and stimulating techniques will be essential for a detailed understanding of how these complex pathways and circuits lead to the expression of adaptive behaviors and ultimately to the regulation of energy balance which is so important in health and disease. Given the large body of literature, we will not be able to cite all relevant studies, but several excellent reviews, mainly focusing on the role of orexin/hypocretin neurons, have recently been published [10,13–18].
Background of anatomy and chemistry of the lateral hypothalamus
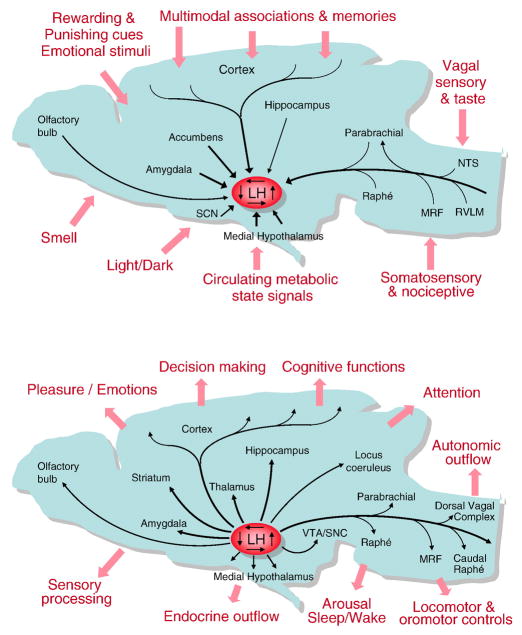
The lateral hypothalamic area or zone is a large and heterogeneous area with several distinct nuclear groups and is one of the most extensively interconnected area of the hypothalamus, allowing it to receive a vast array of interoceptive and exteroceptive information and to modulate cognitive, skeletal motor, autonomic, and endocrine functions (Fig. 2). The lateral hypothalamic area merges rostrally into the preoptic area and caudally into the ventral tegmental area. It borders medially to the dorsomedial, ventromedial, and arcuate nuclei and the anterior hypothalamic and medial preoptic areas, and laterally to the internal capsule, the optic tract, and more caudal to the subthalamic nucleus. There is no doubt that the LHA consists of numerous distinct nuclei [19,20], but the function and connectivity of most of these subnuclei has not been systematically studied. Generally, the lateral hypothalamic area can be divided into anterior, tuberal (roughly at the level of the ventromedial hypothalamus) and posterior portions based on its efferent connectivity as first described by Saper [20] (for a more detailed review see [21]). Another useful anatomic guide is the distribution pattern of two well studied neuronal populations that express either orexin/hypocretin or melanin-concentrating hormone (MCH) [19].
Fig. 2.
Schematic diagrams showing major inputs (top)and outputs (bottom) of the lateral hypothalamic area on an outline of the rat brain.
Two prominent fiber bundles traverse the lateral hypothalamic area, the medial forebrain bundle extending from the brainstem to the olfactory bulb and integrating neuronal processes from several brain areas including lateral hypothalamic neurons [22], and the fornix, connecting the hippocampal complex with the mammillary nuclei in the posterior ventral hypothalamus. This makes interpretation of electrical stimulation and lesion studies difficult, as involvement of nonspecific fibers of passage must be taken into consideration [22–24].
Connectivity of the lateral hypothalamic area
Afferents to the lateral hypothalamic area have been classically studied with retrograde tracing techniques, but because such tracers can be taken up not only by axon terminals but also by fibers of passage, some caution is necessary, and prospective afferent sites need to be verified with anterograde tracers. Based on such verification, afferents to the lateral hypothalamic area have been demonstrated to originate from various cortico-limbic structures such as the prefrontal/orbitofrontal, insular, and olfactory cortex, amygdala, hippocampal formation, the shell of the nucleus accumbens, and from brainstem structures including most aminergic cell groups such as the nucleus of the solitary tract [21,25]. Afferents from medial portions of the hypothalamus, although generally sparse, are functionally highly significant, as for example, projections from the arcuate nucleus POMC/CART and NPY/AgRP neurons [26–28] (Fig. 3). The perifornical area within the lateral hypothalamus receives substantial NPY-ergic input from the arcuate nucleus, and the strongest feeding response to NPY can be elicited by local injection into the perifornical area [29]. Furthermore, given its size and structural complexity, there is considerable connectivity within the lateral hypothalamic area itself, particularly projections from anterior to more posterior portions [26,30].
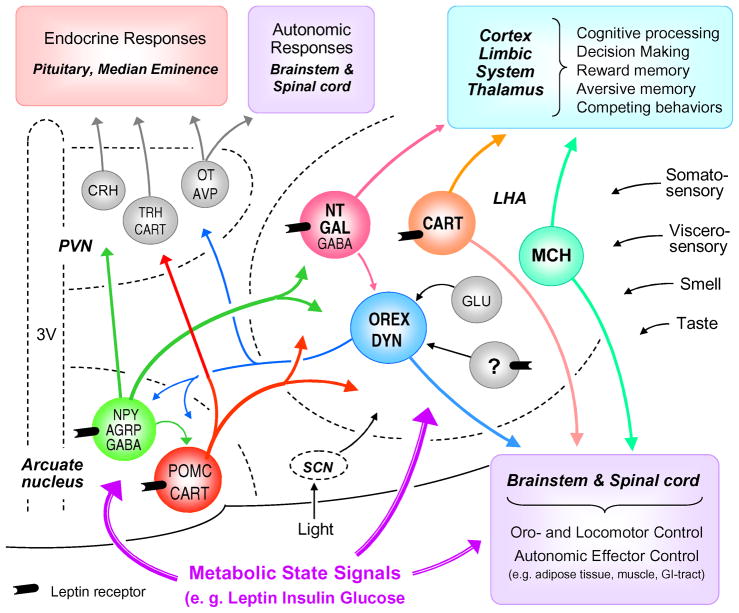
Fig. 3.
Interactions of lateral hypothalamic neurons with other hypothalamic areas and major behavioral, autonomic, and endocrine output pathways and functions. This highly simplified diagram does not show the relationship with other important hypothalamic nuclei such as the dorsomedial and ventral hypothalamic nuclei. Also not shown are the massive reciprocal connections from cortex and limbic structures to the lateral hypothalamic area. Arrows entering the three nuclei but not contacting individual neurons signifies potential input to all the different neuron types in that area. Abbreviations: AgRP, agouti-related protein; AVP, arginine-vasopressin; CART, cocaine and amphetamine-regulated transcript; CRH, corticotrophin-releasing hormone; DYN, dynorphin; GABA, gamma-aminobutyric acid; Gal, galanin, Glu, glutamate; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; NT, neurotensin; ORX, orexin/hypocretin; OT, oxytocin; POMC, proopio-melanocortin; TRH, Thyrotropin-releasing hormone; LHA, lateral hypothalamic area; PVN, paraventricular nucleus of the hypothalamus; SCN, suprachiasmatic nucleus; 3V, third ventricle.
More recently, Sakurai and colleagues used a transgenic method to map upstream neuronal populations that have synaptic connections to orexin neurons and confirmed most of the older findings with classical tracing techniques [31] (Fig. 2). In another recent study retrogradely transported neurotrophic viruses where used to map circuits including the lateral hypothalamic area [32]. They revealed projections from the arcuate nucleus, particularly the lateral POMC neuron containing portion, to insular and anterior cingulate cortex via synaptic relays in the lateral hypothalamic area (including orexin and MCH neurons) and midline thalamic nuclei. Similar multisynaptic projections relaying in the lateral hypothalamus to the nucleus accumbens shell originated in both arcuate POMC and NPY/AgRP neurons [32].
The LHA has vast efferent projections to the entire cortical mantle including the hippocampal formation, extended amygdala, basal ganglia and thalamus, the midbrain and pons, the brainstem and spinal cord, as well as most other nuclei of the hypothalamus [26,33,34] (and see [21] for a review) (Figs. 2 and 3). These projections have been established using mainly retrograde tracer injections into the various projection targets resulting in labeled perikarya in the lateral hypothalamic area, and erroneous co-labeling of fibers of passage is not a problem. More recently, many of these projections have been confirmed on the basis of immunohistochemical studies using antibodies to peptide neurotransmitters, which are almost exclusively produced in lateral hypothalamic neurons such as orexin and MCH (for a review see [35]). Within the hypothalamus the lateral zone has efferent projections to most medial zone nuclei such as the arcuate, paraventricular, dorsomedial, ventromedial, and anterior hypothalamic nuclei[21]. In particular, orexin neurons have been shown to project to the arcuate and paraventricular nuclei [36,37].
With respect to the theme of this review, Mogenson was the first to recognize that the nucleus accumbens, with its efferent projections to the lateral hypothalamus, may provide an interface between motivation and behavioral action [38], and his basic idea has been further developed in more recent review articles [39,40]. Specifically, Zahm has presented an integrative neuroanatomical perspective and proposed a convincing conceptual framework implicating this circuitry in general adaptive responding [39]. Particularly relevant, significant projections from the nucleus accumbens to the hypothalamus have been demonstrated. As shown with various tracing methods, these projections originate mainly from the shell and terminate predominantly in the lateral and perifornical hypothalamus [30,41–44]. In addition to these direct inputs, the nucleus accumbens may influence hypothalamic function via its very strong projections to the ventral pallidum, located ventrally to the nucleus accumbens [40,42], and via the pedunculopontine tegmental area [45]. The ventral pallidum projects directly to the far lateral hypothalamic area [46,47], and this pathway could also be involved in accumbens-induced food intake, as suggested by Stratford and colleagues [48,49].
Feeding peptides and neurotransmitters in lateral hypothalamic neurons
Several neuronal populations expressing neuropeptides categorized either as orexigenic such as MCH [50], orexin/hypocretin [51], galanin [52,53], or anorexigenic such as neurotensin [54] and CART [55] have been described.
MCH neurons project very broadly throughout the CNS [56,57], and similarly MCH receptors (SLC-1) are distributed equally broad in the brain, with particularly strong in situ hybridization signals throughout the cortex, including orbitofrontal, prelimbic, sensorimotor, motor and piriform cortex, as well as in olfactory pathways, nucleus accumbens shell, striatum, hippocampus, locus coeruleus and NTS[58–60]. Similar to other orexigenic peptides such as NPY and AgRP, MCH expression levels increase with fasting and are restored to fed levels with leptin injections [61]. Furthermore, intracerebroventricular MCH injections increase food intake [62], and MCH overexpression leads to obesity and insulin resistance [63], thus suggesting that MCH acts as a typical orexigenic neuropeptide that may regulate and integrate various aspects of feeding behavior [63].
Orexin neurons are distinct from MCH neurons and co-express orexin-A and dynorphin [64]. Their projection pattern is equally widespread throughout the brain, including dense projections to areas in the brainstem and spinal cord, such as the locus coeruleus and dorsal vagal complex [36,64–68]. Orexin-A acts via two receptor isoforms (OxR-1 and OxR-2) that are also broadly expressed throughout the brain. Orexin-A injections into the lateral ventricle increase food intake [69] and systemic orexin receptor antagonist decreases food intake [70]. Orexin knockout mice exhibit hypophagia and narcolepsy [71]. However, in contrast to other orexigenic neuropeptides such as MCH, orexin gene expression does not increase by fasting but is strongly increased by leptin administration [61,72,73]. Thus orexin is not a typical orexigenic neuropeptide and based on its striking effect on sleep-wakefulness regulation it was suggested that the feeding related properties of orexin might be secondary to its regulation of arousal (a sleeping animal does not eat) [74,75] and regulation of arousal may well interact with other orexin modulated behaviors such as reward and anxiety.
Neurotensin expressing neurons are not restricted to, but are found abundantly in the lateral hypothalamic area [76], and centrally administered neurotensin may suppress food intake by modulation of the mesolimbic dopamine system [77,78]. Consistent with this interpretation are observations that anorexigenic leptin action induces neurotensin expression [79] and are suggested to involve leptin receptor expressing lateral hypothalamic neurotensin neurons [80] and personal communication with Dr. Martin G Myers).
Galanin expressing neurons are found throughout most of the brain, including the lateral hypothalamic area. When injected into the paraventricular nucleus, galanin stimulates consumption of food, particularly high-fat diets, and alcohol, and high-fat consumption stimulates galanin gene expression in a positive feedback manner [81,82]. Galanin expression is not changed by fasting or leptin administration [83], but galanin deficient mice show enhanced leptin sensitivity [84]. Several studies demonstrated that central galanin also modulates the mesolimbic DA system [85–88], likely via galanin actions in the ventral tegmental area [87], possibly involving galanin projections from the paraventricular nucleus of the hypothalamus or the locus coeruleus. However, given the intense co-localization of galanin and neurotensin specifically in the perifornical area of the lateral hypothalamus (Fig. 4, unpublished observations), galanin expressing neurons in the lateral hypothalamic area may very well contribute to the modulation of the mesolimbic DA system. More recently, the role of galanin in stress related behavior as well as drug addiction [89,90] has been intensely studied and is thought to involve dopaminergic transduction also (see review by Picciotto [91]).
Fig. 4.

Co-existence of orexigenic (galanin) and anorexigenic (neurotensin) neuropeptides in the LHA was demonstrated in colchicine-treated reporter mice with green fluorescent protein expression in galanin neurons (green) and co-staining for neurotensin (red).
CART expressing neurons are found scattered throughout the lateral hypothalamic area and other brain areas [92]. Intracerebroventricular CART inhibits food intake [93] and has effects on reward and anxiety (for a recent review see [94]). Leptin induces and fasting inhibits CART mRNA expression in the arcuate nucleus and more moderately in the dorsomedial nucleus and medial parts of the lateral hypothalamic area [93]. Research has been hindered by the absence of an identified CART-receptor and lack of antagonists [94].
Most neurons in the lateral hypothalamic area express more than one peptide and in addition may express either one of the classical neurotransmitters glutamate or GABA. The physiological significance of this co-expression of multiple neuotransmitters is in general not well understood and has not been investigated specifically for inputs to and downstream signaling of lateral hypothalamic neurons. Studies in sympathetic neurons expressing the classical neurotransmitters noradrenaline and acetylcholine together with the peptide NPY [95] demonstrate a degree of segregation of transmitters in different synapses [96] and preferential release of noradrenaline at low and NPY at high firing frequencies [97,98]. If such principles apply to lateral hypothalamic neurons it is conceivable that given neurons do not rigidly excite or inhibit downstream neurons, but can preferentially excite and inhibit downstream neurons in an activity-dependent and location-specific manner.
The new opto-genetic and designer drug tools are very promising to answer some of these questions. In a recent study, AgRP and POMC neurons were targeted with channelrhodopsin (ChR2) and light induced stimulation, resulting in increased or decreased food intake respectively, confirming earlier data (as reviewed by Schwartz [99]). However, these studies also showed that the firing frequency in AgRP neurons directly translated into feeding behavior (the higher the firing frequency, the more intense the hyperphagia observed). Furthermore, anorexia evoked by light stimulated POMC neurons required functional MC4R signaling, as expected from earlier findings, but orexigenic effects of optogenetically stimulated AgRP neurons were surprisingly independent of melacocortin receptor function [100]. These data are, however, consistent with other recent findings showing that GABAergic, but melanocortin independent brainstem inputs from AgRP neurons into the parabrachial nucleus are sufficient to explain orexigenic actions from AgRP neurons [101].
In summary, the lateral hypothalamic area with its rich inputs and outputs is in an ideal anatomical position to integrate both internal and external information and access all major output axes, behavioral, autonomic, and endocrine. However, much future research will be necessary to identify the details of input-output relationships of functionally specific sub-areas of the larger lateral hypothalamic area. These studies show clear evidence that arcuate feeding circuits are segregated into peptiderdic transmission and transmission via classic neurotransmitters and much has to be learned for their relative importance for feeding and other behaviors. Optogenetic tools will allow the study of other hypothalamic neurons in discrete brain sites and their functional (modulation of neuronal activity) and behavioral importance.
Role of the lateral hypothalamic area in sensing of the internal milieu
Sensing the internal milieu by the brain, including the availability of nutrients, is fundamental for the orchestration of optimal adaptive responses under given environmental conditions. Although the basomedial hypothalamus and caudal brainstem have been identified as key areas involved in nutrient sensing (as reviewed in [102,103]), there is accumulating evidence for a similar role of the lateral hypothalamus and other brain areas. There are two ways by which a brain area can sense availability of nutrients, through neural inputs from primary nutrient-sensing areas elsewhere in the brain (or periphery) and by direct action of nutrient availability signals on neurons and glial cells within a given area. In the case of the lateral hypothalamic area, neural inputs from both the arcuate nucleus and the caudal brainstem (as discussed above) are likely to convey information about the availability of nutrients, although the relevant experiments necessary to demonstrate such a function, namely selective elimination of these inputs, have not yet been carried out.
After food is ingested, a cascade of signals is generated along the alimentary canal and the metabolic pathways in various organs after absorption. Together, these hormonal, metabolite, and neural signals provide comprehensive information regarding availability of nutrients acutely and long-term. The gustatory system is at the interface between environment and internal milieu and will be discussed together with the other external sensory modalities below.
Glucose and insulin as signals for acute fuel availability
Glucose sensing was already a hot topic soon after the hypothalamic centers were discovered. Using in vivo extracellular recording in a number of species, the pioneering work of the Japanese researcher Yutaka Oomura identified and characterized glucose sensitive neurons throughout the central and peripheral nervous system, including the lateral hypothalamus [104–106]. These and other earlier studies from the pre-leptin era on food intake-related functional aspects of the lateral hypothalamus are discussed in an extensive review by Bernardis and Bellinger [107].
While many of the earlier in vitro studies used glucose concentrations well above the physiological range found in normal brain tissue [108,109], the general observation of glucose-inhibited and glucose-excited neurons in the lateral hypothalamus was confirmed in studies using more physiological glucose concentrations. Specifically, it was demonstrated that while physiologically relevant glucose concentrations decrease excitability and inhibit orexin neurons, they increase excitability of co-mingled MCH neurons [110,111] and that a distinct population of orexin neurons exhibits only a transient inhibitory response to sustained rises in glucose levels, allowing cell firing to maintain sensitivity to small fluctuations while simultaneously encoding a large range of basal glucose concentrations [112].
It was originally thought that neuronal metabolism of glucose via the GLUT2 glucose transporter, glucokinase and the ATP-sensitive potassium channel (KATP) was necessary for glucose to change neuronal excitability, but several alternative mechanisms of glucose sensing have recently been described. First, the sweet taste receptor T1R2 is expressed in lateral hypothalamus and may activate neurons in a metabolism-independent fashion [113]. Second, orexin neurons may function as lactate sensors, as lactate produced in astrocytes and taken up by neighboring neurons through the monocarboxylate transporter (MCT1/2) may sustain spontaneous activity of orexin neurons and keep them sensitized for excitation by other stimuli, independent of glucose [114].
With the availability of c-Fos immunohistochemistry as a neuronal activity stain, it was also found that hypoglycemia induced by acute insulin administration in rats stimulated neurons throughout the lateral hypothalamic area, many of them co-expressing orexin [115]. A similar activation of lateral hypothalamic orexin and other neurons was found after acute food deprivation in rats [116] and monkeys [117], as well as after food restriction and 2-deoxy-D-glucose administration in rats [118]. However, these studies do not rule out activation of distant glucose sensing mechanisms and mediation by neural inputs to the lateral hypothalamus.
Finally, in a recent study in mice, it was shown that the transcription factor Foxa2, a downstream target of insulin signaling, regulates the expression of orexin and MCH during fasting. Constitutive activation of Foxa2 in the brain resulted in increased neuronal orexin and MCH expression and increased food consumption, metabolism, insulin sensitivity, and increased physical activity in the fed state (reaching the level in fasted mice) [119].
Leptin as a signal for availability of stored nutrients
Within the hypothalamus, the arcuate nucleus, with its NPY and POMC neurons, had been originally thought to play an exclusive role in integrating metabolic signals such as leptin. But clearly, leptin receptors are located in other hypothalamic areas such as the ventromedial, dorsomedial, and premammillary nuclei, as well as the lateral and perifornical areas, where they likely contribute to leptin’s effects on food intake and energy expenditure. Indeed, with novel transgenic leptin receptor specific tracing methods it was shown that LHA leptin receptor neurons modulate the mesolimbic dopamine system in the ventral tegmental area. While some leptin receptor-bearing lateral hypothalamic neurons project directly to the ventral tegmental area [72], they also locally synapse onto orexin (but not MCH neurons), which in turn also project to dopamine neurons in the ventral tegmental area [73]. Furthermore, leptin action in these lateral hypothalamic neurons, some of which also co-express neurotensin (personal communication with Dr. Martin G. Myers), increases orexin gene expression and decreases food intake [73]. Thus, orexin neurons do not themselves express leptin receptors but receive input from neighboring leptin receptor-expressing neurons [73] (Fig. 3). In addition, leptin responsive POMC/CART and NPY/AgRP neurons in the arcuate nucleus project to the lateral hypothalamus [27] and some of them make close anatomical contacts with orexin and MCH neurons [27,120]. It will be interesting to determine whether these two leptin-sensitive inputs to orexin neurons play different roles.
Signals from the gut
Ghrelin, a hormone secreted mainly from the gastric mucosa and showing the highest circulating levels in the absence of digestible nutrients, increases c-Fos expression in orexin but not MCH neurons when administered intracerebroventricularly [121,122] and directly depolarizes and increases firing frequency of orexin neurons in vitro [123]. Local administration of ghrelin into the lateral hypothalamic area increases food intake and wakefulness [124] and central pretreatment with anti-orexin antibody attenuated peripheral ghrelin-induced increase in food intake [121]. These findings strongly suggest that at least one site of action for endogenous ghrelin to stimulate arousal, foraging, and appetitive behavior is orexin neurons in the lateral hypothalamus [123].
A potential role for the lateral hypothalamic area in the effects on food intake by other gut hormones is much less clear. Although, as expected from a putative satiety hormone, direct lateral hypothalamic injections of GLP-1 suppressed and its receptor antagonist Exendin-9 increased short-term food intake in rats [125], GLP-1 unexpectedly depolarized orexin neurons and increased their spike frequency in vitro [126]. It is thus not clear whether the two gut hormones ghrelin and GLP-1, which have clearly opposite effects on food intake, act on different populations of orexin neurons. Except for a report of no effect of intraperitoneal injection of PYY(3–36) on orexin gene expression in mice, there are no data available suggesting a role for the LHA in the satiating effects of the other lower gut hormone PYY.
In addition to a direct action via the circulation and blood barrier transport mechanisms, gut hormones and mechanical signals can potentially reach the lateral hypothalamic area via neural pathways including vagal afferents and medullary-hypothalamic projections including A2 catecholaminergic and GLP-1 expressing NTS neurons [127,128]. Functional input from vagal afferents to lateral hypothalamic neurons was demonstrated using extracellular recording techniques in intact rats [129]. This latter study further showed a remarkable degree of convergence on single lateral hypothalamic neurons of inputs from various sources. About half of all neurons tested responded to both vagal and cerebellar (somatic) input, and of all neurons doubly responsive, 60% were also glucose sensitive. Also, when the vagal and cerebellar inputs were stimulated simultaneously, a summation of the responses was observed [129].
In summary, glucose, insulin, ghrelin, and leptin have been quite convincingly demonstrated to directly act on various types of lateral hypothalamic neurons and to provide negative (insulin, leptin) and positive (ghrelin) feedback in the control of food intake. However, the specific circuitries and physiological roles of glucose-inhibited and glucose-stimulated neurons within the lateral hypothalamic area remain unclear.
Role of the lateral hypothalamic area in monitoring environmental stimuli and conditions
Olfactory, gustatory, somatosensory, and visual and information
Using single unit recording in intact animals, it was already shown during the height of the hypothalamic feeding center days that lateral hypothalamic neurons in the far-lateral hypothalamus receive olfactory and gustatory input [130–134]. Gustatory pathways from the parabrachial taste area to the lateral hypothalamus were confirmed with tracing techniques [135]. Extensive studies in Rhesus monkeys further identified both glucose excited and glucose inhibited LH neurons as recipients of olfactory and gustatory inputs [136–139]. The lateral hypothalamus also receives direct, monosynaptic input from nociceptive neurons in the spinal cord [140] and periaqueductul gray [141], and noxious stimuli increased Fos protein expression in orexin neurons [142].
Threat and stress
Besides input from nociceptive somatosensory afferents, orexin neurons are activated by immobilization and cold stress [143]. Because it was demonstrated that corticotrophin-releasing factor (CRF)-immunoreactive terminals make direct contact with orexin neurons and that CRF increases the firing rate of a subpopulation of orexin neurons in a CRF receptor-1 dependent fashion, it is likely that stress-induced arousal depends on a CRF-orexin pathway [143,144].
The LHA and behavioral effector pathways
Reward seeking
As mentioned in the introduction, one of the hallmarks of the lateral hypothalamus is its support of electrical self-stimulation, but that because of the indiscriminate activation of local neurons and fibers of passage with electrical stimulation, its underlying neurology is far from clear. Recent studies strongly implicate projections of lateral hypothalamic orexin neurons to the midbrain ventral tegmental area in this behavior. Orexin fibers innervate ventral tegmental dopamine neurons [145–147] which express orexin-1 receptors [148–150], and both dopaminergic and non-dopaminergic neurons in the ventral tegmental area are excited by orexins [151,152]. Ventral tegmental area orexin signaling is involved in cocaine and morphine-induced hyperlocomotion and place preference through the mesolimbic dopamine system, partly by potentiating NMDA-mediated excitatory currents in dopaminergic neurons [150,152]. Orexin-deficient mice are less susceptible to develop drug dependence [153], and orexin injection into the ventral tegmental area can reinstate an extinguished preference for drugs of abuse [154].
Our own observations implicate lateral hypothalamic orexin neurons in natural food reward. We used nucleus accumbens mu-opioid-induced intake of palatable food that was pioneered by the group of the late Anne Kelley as a model of reward-driven food intake in metabolically satiated rats [155–159], which is accompanied by activation of orexin neurons in the perifornical lateral hypothalamus [158,160], and can be blocked by inhibiting lateral hypothalamic activity with GABA receptor agonists [158] or glutamate receptor antagonist [48]. This suggests that glutamatergic neurons within the hypothalamus mediate the response, consistent with the finding that accumbens shell projections terminate in the anterior LH, rich in glutamatergic neurons that connect with orexin neurons in the more posterior lateral hypothalamus [30]. Because medium-spiny accumbens output neurons express GABA, the most parsimonious explanation is that stimulation of food intake is mediated by GABA-projections from the accumbens to the lateral hypothalamic area. These projection neurons seem to be normally (tonically) active and inhibit certain lateral hypothalamic neurons (e.g. orexin neurons) probably by presynaptically inhibiting glutamate release from local interneurons (Fig. 3). Inhibition of accumbens-lateral hypothalamus projection neurons leads to an arrest of GABA release from their terminals, disinhibition of these lateral hypothalamic neurons, and increased food intake. This model would fit the observation that activation of NMDA receptors in the lateral hypothalamus by glutamate is necessary for food-deprivation-induced food intake [161] and that injection of the GABA-antagonist bicuculline into the anterior lateral hypothalamic area increases ingestion of sweet milk [162].
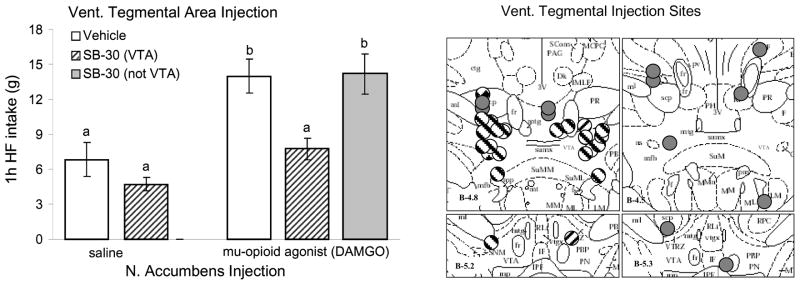
Using this nucleus accumbens-driven intake of high-fat food in satiated rats, we showed that local bilateral injection of orexin receptor-1 antagonist into the ventral tegmental area blocked accumbens-induced palatable food intake [163] (Fig. 4). Findings by Harris and Aston-Jones suggest that largely separate orexin neuron populations in the lateral (lateral to fornix) and medial portions of the lateral hypothalamic area mediate reward- and stress-guided behaviors, respectively [154,164,165]. In contrast, we found significant increases in Fos-activated orexin neurons after accumbens DAMGO only in the perifornical area, but not in the more lateral orexin neuron population [163]. There is considerable literature demonstrating that metabolic stress such as food deprivation and restriction, insulin-induced hypoglycemia, and 2DG-induced glucoprivation, activates orexin neurons [115–118], although such activated orexin neurons can be found in both the medial and lateral fields [118]. Given the importance of the hypothalamic orexin neurons in these diverse functional aspects, it will be important to further examine functional specificity of subpopulations [166].
In addition, orexin neurons feed back specifically to cholinergic striatal interneurons via the paraventricular nucleus of the thalamus [167]. The seminal work by the group of Berridge and colleagues identified a “liking” hotspot in the shell of the nucleus accumbens where mu-opioid activity enhances positive hedonic reactions to palatable foods in rats [168,169]. Together, these findings strongly suggest a role for an accumbens - LH orexin – VTA circuit in the expression of natural food reward.
While most of the above described experiments use pharmacological levels of drug applications, it remains elusive if endogenous orexin levels stimulate dopaminergic VTA neurons and if this would translate into DA release and behavioral changes. A recent study by Tsai et. al. [170] used an optogenetic approach to test the behavioral effects of different firing frequencies in dopaminergic DA neurons in the VTA. The study convincingly showed that light evoked high frequency phasic firing, but not low frequency tonic firing, caused a conditioned place preference and transient DA release in the nucleus accumbens [170]. Therefore, phasic dopaminergic activity is sufficient to evoke behavioral conditioning. Thus, future experiments using neuron specific stimulation/inhibition of LHA (e.g. orexin) neurons should reveal exiting new insights linking neuronal activity with appetitive behavior and reward function.
Food intake
Although reward seeking is an important component, a number of other neural systems are required for the orchestration of ingestive behavior. These include access to appropriate oro-motor and locomotor functions and its autonomic support, which are generally organized in the hindbrain and spinal cord. One approach we and others have used to address hindbrain participation in orexin-induced food intake is 4th ventricular administration of orexin in rats [68,171]. We demonstrated that sub-populations of about 20% and 10% of lateral hypothalamic orexin and MCH neurons, respectively, project to the nucleus of the solitary tract and dorsal motor nucleus with axon terminals in close contact to neurons expressing tyrosine hydroxylase and GLP-1, both allegedly involved in satiation and suppression of food intake. Similar contacts were frequently observed with neurons of the nucleus of the solitary tract, activation of which by gastrointestinal food stimuli was demonstrated by the expression of nuclear c-Fos immunoreactivity, and orexin-A administration to the fourth ventricle induced significant Fos-expression in many of the catecholaminergic neurons. Finally, fourth ventricular orexin injections significantly stimulated chow and water intake in nonfood-deprived rats, and direct bilateral injections of orexin into the dorsal vagal complex increased intake of palatable high-fat diet [68].
To further characterize the role of hindbrain orexin signaling in ingestive behavior, Baird and colleagues used sucrose licking microstructure analysis [171]. Fourth ventricular administration of orexin increased both meal size and meal frequency. Prolonging meals without affecting early ingestion rate or lick burst size suggested that orexin affected inhibitory postingestive feedback rather than taste evaluation [171]. This interpretation was supported by the observation that third ventricular orexin, while still able to increase meal frequency, was no longer able to increase meal size in rats with lesions of the area postrema and adjacent NTS [171]. Together, the findings suggest that areas in the hindbrain mediate the increase in consummatory (meal size) and the hypothalamus and other forebrain sites mediate the appetitive (meal frequency) components of orexin-induced hyperphagia.
The hypothalamic effect on meal frequency (meal initiation) could be mediated by orexin projections to the arcuate nucleus NPY/AgRP and POMC/CART neuron populations [172]. Specifically, POMC neurons are presynaptically inhibited by orexin in vivo [173]. This pathway may also play a permissive role in food intake induced by mu-opioid stimulation of the nucleus accumbens [160].
In summary, we have come a long way in better understanding what is happening in the classical “feeding center”. A circuitry that includes at least parts of the lateral hypothalamic area, the midbrain dopamine system with its numerous cortico-limbic targets, and the nucleus accumbens, appears to be important for reward seeking and the initiation of appetitive behavior. Equally important circuits including reciprocal connections with the medullary oromotor pattern generators and projections to the brainstem and spinal cord autonomic preganglionic neurons prepare the internal milieu for an ingestive bout and sustain ingestive behavior.
The LHA and autonomic effector pathways
Gut, pancreas, and hepatic functions
Again, electrical stimulation and lesions of the LHA were the first to show changes in gastrointestinal [174], pancreatic [175], hepatic [176,177], and adipose tissue functions [178], as mediated by the sympathetic and parasympathetic nervous system. However, only the discovery of neuropeptides and other technological advances made it possible to identify the specific pathways and confirm some of these earlier claims.
We demonstrated that local administration of minute amounts of orexin-A into the dorsal motor nucleus of the vagus nerve increased gastric motility and intragastric pressure [179]. Together with demonstrating orexin receptor-1 on gastric retrogradely identified vagal motor neurons [179,180] and our anatomical findings discussed above, these observations strongly suggest that lateral hypothalamic orexin neurons can directly influence gastrointestinal functions via vagal excitatory motor neurons in preparation for handling ingested nutrients. Similarly, central orexin administration appears to stimulate pancreatic exocrine secretion in a vagus-dependent but gastric acid secretion-independent fashion [181], and hypoglycemia-induced increases in vagal efferent signaling to the pancreas depends on orexin-signaling in the dorsal motor nucleus of the vagus [182].
Orexin projections to the spinal cord appear to specifically innervate sympathetic preganglionic neurons, which are activated and synchronized by orexin in an orexin receptor-1 dependent fashion [183].
Energy expenditure
We also examined in detail orexin-A innervation of the caudal raphé nuclei in the medulla, known to harbor sympathetic preganglionic motor neurons involved in thermal, cardiovascular, and gastrointestinal regulation. All three components of the caudal raphé nuclei, raphé pallidus, raphé obscurus, and parapyramidal nucleus, are innervated by orexin-A-immunoreactive fibers [184]. Using confocal microscopy, we demonstrate close anatomical appositions between varicose orexin-A immunoreactive axon profiles and sympathetic premotor neurons identified with either a transneuronal retrograde pseudorabies virus tracer injected into the interscapular brown fat pads, or with in situ hybridization of pro-TRH mRNA [184]. Furthermore, orexin-A injected into the fourth ventricle induced c-Fos expression in the raphé pallidus and parapyramidal nucleus [184]. These findings suggest that orexin neurons in the hypothalamus can modulate brown fat thermogenesis, cardiovascular, and gastrointestinal functions by acting directly on neurons in the caudal raphé nuclei, and support the idea that orexin’s simultaneous stimulation of food intake and sympathetic activity might have evolved as a mechanism to stay alert while foraging [184]
Fourth ventricular administration of melanin-concentrating hormone in freely moving rats decreased core body temperature but did not change locomotor activity and food and water intake[58]. We conclude that the rich hypothalamo-medullary melanin-concentrating hormone projections in the rat are mainly inhibitory to nucleus of the solitary tract neurons, but are not involved in the control of food intake. Projections to ventral medullary sites may play a role in the inhibitory effect of melanin-concentrating hormones on energy expenditure [58,185]
Conclusions and Perspective
An exciting new discovery more than 50 years ago showed that electrical stimulation of the lateral hypothalamic area induces feeding and self-stimulation behavior. However, only the continuous progress in neuroanatomical, neurochemical, and genetically-based techniques has allowed us to have at least a glimpse of understanding the neurology behind these phenomena. As could have been suspected 50 years ago, the lateral hypothalamus “does it not alone”; it is the rich connectivity with key downstream effector circuits and mechanisms and feedback from the metabolic periphery that underlies these phenomena. Despite these new insights, there are still more questions than answers. One issue is the connectivity and functional specificity of lateral hypothalamic sub-areas. Are all orexin or MCH neurons serving the same physiological functions, or are there different orexin or MCH-fields that serve different aspects of a unifying function or different functions altogether? Another unsolved issue is the physiological significance of co-expression of multiple classical and peptide neurotransmitters in a given neuron. We believe the new generation of methodological tools such as the ability to selectively stimulate specific neurons will greatly facilitate exciting future research.
Highlights
A historical perspective of lateral hypothalamic functions is provided.
The anatomy, connectivity, and chemistry of the lateral hypothalamic area is reviewed.
Lateral hypothalamic mechanisms involved in energy homeostasis are discussed.
The utility of cutting-edge modern methodology is highlighted.
Fig. 5.
Orexin-1R antagonist administration into the VTA blocks high-fat intake induced by accumbens administration of DAMGO. a: Vehicle or the orexin receptor antagonist SB334867 (15 nmol/side) was injected into the VTA and saline or DAMGO (250 ng) into the nucleus accumbens after overnight access to high-fat chow for pre-satiation. The robust DAMGO-induced feeding response over saline baseline (p<0.001) was almost completely abolished by VTA pretreatment with the orexin receptor antagonist. In animals with either one or both of the bilateral cannula tips not within the VTA, the orexin receptor antagonist was unable to block DAMGO-induced high-fat feeding. Bars that do not share the same letter are significantly different from each other (based on ANOVA, followed by Bonferroni-adjusted multiple comparisons test, p<0.05). b: Verification of orexin receptor antagonist injection sites aimed at the VTA. Striped circles depict animals with both sites within the VTA (n = 11), gray circles depict animals with one or both sites outside the VTA (n = 6), and diamond-filled circles depict animals with unilateral injections (n = 2). Injection sites are superimposed on images from the Paxinos and Watson stereotaxic atlas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bruegger M. Fresstrieb als hypothalamisches Syndrom. Helvetica Physiologica Acta. 1943;1:183–198. [Google Scholar]
- 2.Hess W. Das Zwischenhirn: Syndrome, Localisationen, Funktionen. Basel: Schwabe; 1949. [Google Scholar]
- 3.Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77(2):323–4. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- 4.Brobeck JR. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol Rev. 1946;26(4):541–59. doi: 10.1152/physrev.1946.26.4.541. [DOI] [PubMed] [Google Scholar]
- 5.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47(6):419–27. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 6.Olds J. Self-stimulation of the brain; its use to study local effects of hunger, sex, and drugs. Science. 1958;127(3294):315–24. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- 7.Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science. 1962;135:375–7. doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- 8.Swanson LW. The neuroanatomy revolution of the 1970s and the hypothalamus. Brain Res Bull. 1999;50(5–6):397. doi: 10.1016/s0361-9230(99)00163-x. [DOI] [PubMed] [Google Scholar]
- 9.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470(7333):221–6. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamanaka A, Tsunematsu T. New approaches for the study of orexin function. J Neuroendocrinol. 2010;22(7):818–24. doi: 10.1111/j.1365-2826.2010.02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nawaratne V, Leach K, Suratman N, Loiacono RE, Felder CC, Armbruster BN, Roth BL, Sexton PM, Christopoulos A. New insights into the function of M4 muscarinic acetylcholine receptors gained using a novel allosteric modulator and a DREADD (designer receptor exclusively activated by a designer drug) Mol Pharmacol. 2008;74(4):1119–31. doi: 10.1124/mol.108.049353. [DOI] [PubMed] [Google Scholar]
- 12.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011 doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–81. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 14.Boutrel B, Cannella N, de Lecea L. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 2009 doi: 10.1016/j.brainres.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao XB, Wang AH. Experience-dependent plasticity in hypocretin/orexin neurones: re-setting arousal threshold. Acta Physiol (Oxf) 2010;198(3):251–62. doi: 10.1111/j.1748-1716.2009.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadel J, Burk JA. Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention. Brain Res. 2010;1314:112–23. doi: 10.1016/j.brainres.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharf R, Sarhan M, Dileone RJ. Role of orexin/hypocretin in dependence and addiction. Brain Res. 2010;1314:130–8. doi: 10.1016/j.brainres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci Lett. 2005;387(2):80–4. doi: 10.1016/j.neulet.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 20.Saper CB, Swanson LW, Cowan WM. An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J Comp Neurol. 1979;183(4):689–706. doi: 10.1002/cne.901830402. [DOI] [PubMed] [Google Scholar]
- 21.Simerly RB. Anatomical Substrates of Hypothalamic Integration. In: Paxinos G, editor. The Rat Nervous System. 2. San Diego: Academic Press; 1995. pp. 353–376. [Google Scholar]
- 22.Nieuwenhuys R, Geeraedts LM, Veening JG. The medial forebrain bundle of the rat. I. General introduction. J Comp Neurol. 1982;206(1):49–81. doi: 10.1002/cne.902060106. [DOI] [PubMed] [Google Scholar]
- 23.Ungerstedt U. Is interruption of the nigro-striatal dopamine system producing the “lateral hypothalamus syndrome”? Acta Physiol Scand. 1970;80(4):35A–36A. doi: 10.1111/j.1748-1716.1970.tb04858.x. [DOI] [PubMed] [Google Scholar]
- 24.Geeraedts LM, Nieuwenhuys R, Veening JG. Medial forebrain bundle of the rat: IV. Cytoarchitecture of the caudal (lateral hypothalamic) part of the medial forebrain bundle bed nucleus. J Comp Neurol. 1990;294(4):537–68. doi: 10.1002/cne.902940404. [DOI] [PubMed] [Google Scholar]
- 25.Ter Horst GJ, de Boer P, Luiten PG, van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31(3):785–97. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- 26.Ter Horst GJ, Luiten PG. Phaseolus vulgaris leuco-agglutinin tracing of intrahypothalamic connections of the lateral, ventromedial, dorsomedial and paraventricular hypothalamic nuclei in the rat. Brain Res Bull. 1987;18(2):191–203. doi: 10.1016/0361-9230(87)90190-0. [DOI] [PubMed] [Google Scholar]
- 27.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23(4):775–86. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 28.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402(4):442–59. [PubMed] [Google Scholar]
- 29.Stanley BG, Magdalin W, Seirafi A, Thomas WJ, Leibowitz SF. The perifornical area: the major focus of (a) patchily distributed hypothalamic neuropeptide Y-sensitive feeding system(s) Brain Res. 1993;604(1–2):304–17. doi: 10.1016/0006-8993(93)90382-w. [DOI] [PubMed] [Google Scholar]
- 30.Sano H, Yokoi M. Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin- or melanin-concentrating hormone-containing neurons. J Neurosci. 2007;27(26):6948–55. doi: 10.1523/JNEUROSCI.0514-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46(2):297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Kampe J, Tschop MH, Hollis JH, Oldfield BJ. An anatomic basis for the communication of hypothalamic, cortical and mesolimbic circuitry in the regulation of energy balance. Eur J Neurosci. 2009;30(3):415–30. doi: 10.1111/j.1460-9568.2009.06818.x. [DOI] [PubMed] [Google Scholar]
- 33.Berk ML, Finkelstein JA. Efferent connections of the lateral hypothalamic area of the rat: an autoradiographic investigation. Brain Res Bull. 1982;8(5):511–26. doi: 10.1016/0361-9230(82)90009-0. [DOI] [PubMed] [Google Scholar]
- 34.Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153(1):1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- 35.Saper CB. Hypothalamic connections with the cerebral cortex. Prog Brain Res. 2000;126:39–48. doi: 10.1016/S0079-6123(00)26005-6. [DOI] [PubMed] [Google Scholar]
- 36.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96(2):748–53. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(2–3):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 39.Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24(1):85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 40.Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- 41.Conrad LC, Pfaff DW. Autoradiographic tracing of nucleus accumbens efferents in the rat. Brain Res. 1976;113(3):589–96. doi: 10.1016/0006-8993(76)90060-3. [DOI] [PubMed] [Google Scholar]
- 42.Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223(3):347–67. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- 43.Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797(1):73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- 44.Otake K, Nakamura Y. Possible pathways through which neurons of the shell of the nucleus accumbens influence the outflow of the core of the nucleus accumbens. Brain Dev. 2000;22 (Suppl 1):S17–26. doi: 10.1016/s0387-7604(00)00142-x. [DOI] [PubMed] [Google Scholar]
- 45.Inglis WL, Winn P. The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol. 1995;47(1):1–29. doi: 10.1016/0301-0082(95)00013-l. [DOI] [PubMed] [Google Scholar]
- 46.Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57(1):113–42. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- 47.Zahm DS, Williams E, Wohltmann C. Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain. J Comp Neurol. 1996;364(2):340–62. doi: 10.1002/(SICI)1096-9861(19960108)364:2<340::AID-CNE11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 48.Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19(24):11040–8. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stratford TR, Kelley AE, Simansky KJ. Blockade of GABAA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res. 1999;825(1–2):199–203. doi: 10.1016/s0006-8993(99)01239-1. [DOI] [PubMed] [Google Scholar]
- 50.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380(6571):243–7. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 51.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 52.Skofitsch G, Jacobowitz DM, Zamir N. Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res Bull. 1985;15(6):635–49. doi: 10.1016/0361-9230(85)90213-8. [DOI] [PubMed] [Google Scholar]
- 53.Melander T, Hokfelt T, Rokaeus A. Distribution of galaninlike immunoreactivity in the rat central nervous system. J Comp Neurol. 1986;248(4):475–517. doi: 10.1002/cne.902480404. [DOI] [PubMed] [Google Scholar]
- 54.Luttinger D, King RA, Sheppard D, Strupp J, Nemeroff CB, Prange AJ., Jr The effect of neurotensin on food consumption in the rat. Eur J Pharmacol. 1982;81(3):499–503. doi: 10.1016/0014-2999(82)90116-9. [DOI] [PubMed] [Google Scholar]
- 55.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393(6680):72–6. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 56.Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319(2):218–45. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 57.Bittencourt JC, Frigo L, Rissman RA, Casatti CA, Nahon JL, Bauer JA. The distribution of melanin-concentrating hormone in the monkey brain (Cebus apella) Brain Res. 1998;804(1):140–3. doi: 10.1016/s0006-8993(98)00662-3. [DOI] [PubMed] [Google Scholar]
- 58.Zheng H, Patterson LM, Morrison C, Banfield BW, Randall JA, Browning KN, Travagli RA, Berthoud HR. Melanin concentrating hormone innervation of caudal brainstem areas involved in gastrointestinal functions and energy balance. Neuroscience. 2005;135(2):611–25. doi: 10.1016/j.neuroscience.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 59.Kokkotou EG, Tritos NA, Mastaitis JW, Slieker L, Maratos-Flier E. Melanin-concentrating hormone receptor is a target of leptin action in the mouse brain. Endocrinology. 2001;142(2):680–6. doi: 10.1210/endo.142.2.7981. [DOI] [PubMed] [Google Scholar]
- 60.Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435(1):26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- 61.Tritos NA, Mastaitis JW, Kokkotou E, Maratos-Flier E. Characterization of melanin concentrating hormone and preproorexin expression in the murine hypothalamus. Brain Res. 2001;895(1–2):160–6. doi: 10.1016/s0006-8993(01)02066-2. [DOI] [PubMed] [Google Scholar]
- 62.Tritos NA, Vicent D, Gillette J, Ludwig DS, Flier ES, Maratos-Flier E. Functional interactions between melanin-concentrating hormone, neuropeptide Y, and anorectic neuropeptides in the rat hypothalamus. Diabetes. 1998;47(11):1687–92. doi: 10.2337/diabetes.47.11.1687. [DOI] [PubMed] [Google Scholar]
- 63.Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107(3):379–86. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, et al. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21(19):RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrison TA, Chen CT, Dun NJ, Chang JK. Hypothalamic orexin A-immunoreactive neurons project to the rat dorsal medulla. Neurosci Lett. 1999;273(1):17–20. doi: 10.1016/s0304-3940(99)00611-4. [DOI] [PubMed] [Google Scholar]
- 66.Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van Den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415(2):145–59. [PubMed] [Google Scholar]
- 67.van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19(8):3171–82. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng H, Patterson LM, Berthoud HR. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol. 2005;485(2):127–42. doi: 10.1002/cne.20515. [DOI] [PubMed] [Google Scholar]
- 69.Sakurai T. Orexins and orexin receptors: implication in feeding behavior. Regul Pept. 1999;85(1):25–30. doi: 10.1016/s0167-0115(99)00076-2. [DOI] [PubMed] [Google Scholar]
- 70.Haynes AC, Chapman H, Taylor C, Moore GB, Cawthorne MA, Tadayyon M, Clapham JC, Arch JR. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regul Pept. 2002;104(1–3):153–9. doi: 10.1016/s0167-0115(01)00358-5. [DOI] [PubMed] [Google Scholar]
- 71.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 72.Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10(2):89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Louis GW, Leinninger GM, Rhodes CJ, Myers MG., Jr Direct innervation and modulation of orexin neurons by lateral hypothalamic LepRb neurons. J Neurosci. 2010;30(34):11278–87. doi: 10.1523/JNEUROSCI.1340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kodadek T, Cai D. Chemistry and biology of orexin signaling. Mol Biosyst. 2010;6(8):1366–75. doi: 10.1039/c003468a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–58. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 76.Beitz AJ. The sites of origin brain stem neurotensin and serotonin projections to the rodent nucleus raphe magnus. J Neurosci. 1982;2(7):829–42. doi: 10.1523/JNEUROSCI.02-07-00829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hawkins MF, Barkemeyer CA, Tulley RT. Synergistic effects of dopamine agonists and centrally administered neurotensin on feeding. Pharmacol Biochem Behav. 1986;24(5):1195–201. doi: 10.1016/0091-3057(86)90170-x. [DOI] [PubMed] [Google Scholar]
- 78.Cador M, Kelley AE, Le Moal M, Stinus L. Ventral tegmental area infusion of substance P, neurotensin and enkephalin: differential effects on feeding behavior. Neuroscience. 1986;18(3):659–69. doi: 10.1016/0306-4522(86)90061-8. [DOI] [PubMed] [Google Scholar]
- 79.Sahu A. Evidence suggesting that galanin (GAL), melanin-concentrating hormone (MCH), neurotensin (NT), proopiomelanocortin (POMC) and neuropeptide Y (NPY) are targets of leptin signaling in the hypothalamus. Endocrinology. 1998;139(2):795–8. doi: 10.1210/endo.139.2.5909. [DOI] [PubMed] [Google Scholar]
- 80.Leinninger GM, Myers MG., Jr LRb signals act within a distributed network of leptin-responsive neurones to mediate leptin action. Acta Physiol (Oxf) 2008;192(1):49–59. doi: 10.1111/j.1748-1716.2007.01784.x. [DOI] [PubMed] [Google Scholar]
- 81.Tempel DL, Leibowitz KJ, Leibowitz SF. Effects of PVN galanin on macronutrient selection. Peptides. 1988;9(2):309–14. doi: 10.1016/0196-9781(88)90265-3. [DOI] [PubMed] [Google Scholar]
- 82.Barson JR, Morganstern I, Leibowitz SF. Galanin and consummatory behavior: special relationship with dietary fat, alcohol and circulating lipids. EXS. 2010;102:87–111. doi: 10.1007/978-3-0346-0228-0_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sahu A. Leptin decreases food intake induced by melanin-concentrating hormone (MCH), galanin (GAL) and neuropeptide Y (NPY) in the rat. Endocrinology. 1998;139(11):4739–42. doi: 10.1210/endo.139.11.6432. [DOI] [PubMed] [Google Scholar]
- 84.Hohmann JG, Krasnow SM, Teklemichael DN, Clifton DK, Wynick D, Steiner RA. Neuroendocrine profiles in galanin-overexpressing and knockout mice. Neuroendocrinology. 2003;77(6):354–66. doi: 10.1159/000071308. [DOI] [PubMed] [Google Scholar]
- 85.Tsuda K, Tsuda S, Nishio I, Masuyama Y, Goldstein M. Effects of galanin on dopamine release in the central nervous system of normotensive and spontaneously hypertensive rats. Am J Hypertens. 1998;11(12):1475–9. doi: 10.1016/s0895-7061(98)00168-x. [DOI] [PubMed] [Google Scholar]
- 86.Rada P, Mark GP, Hoebel BG. Galanin in the hypothalamus raises dopamine and lowers acetylcholine release in the nucleus accumbens: a possible mechanism for hypothalamic initiation of feeding behavior. Brain Res. 1998;798(1–2):1–6. doi: 10.1016/s0006-8993(98)00315-1. [DOI] [PubMed] [Google Scholar]
- 87.Ericson E, Ahlenius S. Suggestive evidence for inhibitory effects of galanin on mesolimbic dopaminergic neurotransmission. Brain Res. 1999;822(1–2):200–9. doi: 10.1016/s0006-8993(99)01144-0. [DOI] [PubMed] [Google Scholar]
- 88.Robinson JK, Brewer A. Galanin: a potential role in mesolimbic dopamine-mediated instrumental behavior. Neurosci Biobehav Rev. 2008;32(8):1485–93. doi: 10.1016/j.neubiorev.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zachariou V, Parikh K, Picciotto MR. Centrally administered galanin blocks morphine place preference in the mouse. Brain Res. 1999;831(1–2):33–42. doi: 10.1016/s0006-8993(99)01476-6. [DOI] [PubMed] [Google Scholar]
- 90.Hawes JJ, Brunzell DH, Narasimhaiah R, Langel U, Wynick D, Picciotto MR. Galanin protects against behavioral and neurochemical correlates of opiate reward. Neuropsychopharmacology. 2008;33(8):1864–73. doi: 10.1038/sj.npp.1301579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Picciotto MR, Brabant C, Einstein EB, Kamens HM, Neugebauer NM. Effects of galanin on monoaminergic systems and HPA axis: Potential mechanisms underlying the effects of galanin on addiction- and stress-related behaviors. Brain Res. 2010;1314:206–18. doi: 10.1016/j.brainres.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol. 1997;9(11):823–33. doi: 10.1046/j.1365-2826.1997.00651.x. [DOI] [PubMed] [Google Scholar]
- 93.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393(6680):72–6. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 94.Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9(10):747–58. doi: 10.1038/nrn2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lundberg JM, Terenius L, Hokfelt T, Martling CR, Tatemoto K, Mutt V, Polak J, Bloom S, Goldstein M. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand. 1982;116(4):477–80. doi: 10.1111/j.1748-1716.1982.tb07171.x. [DOI] [PubMed] [Google Scholar]
- 96.Vega A, Luther JA, Birren SJ, Morales MA. Segregation of the classical transmitters norepinephrine and acetylcholine and the neuropeptide Y in sympathetic neurons: modulation by ciliary neurotrophic factor or prolonged growth in culture. Dev Neurobiol. 2010;70(14):913–28. doi: 10.1002/dneu.20834. [DOI] [PubMed] [Google Scholar]
- 97.Lundberg JM, Rudehill A, Sollevi A, Theodorsson-Norheim E, Hamberger B. Frequency- and reserpine-dependent chemical coding of sympathetic transmission: differential release of noradrenaline and neuropeptide Y from pig spleen. Neurosci Lett. 1986;63(1):96–100. doi: 10.1016/0304-3940(86)90020-0. [DOI] [PubMed] [Google Scholar]
- 98.Bartfai T, Iverfeldt K, Fisone G, Serfozo P. Regulation of the release of coexisting neurotransmitters. Annu Rev Pharmacol Toxicol. 1988;28:285–310. doi: 10.1146/annurev.pa.28.040188.001441. [DOI] [PubMed] [Google Scholar]
- 99.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 100.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351–5. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu Q, Palmiter RD. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. Eur J Pharmacol. 2010 doi: 10.1016/j.ejphar.2010.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jordan SD, Konner AC, Bruning JC. Sensing the fuels: glucose and lipid signaling in the CNS controlling energy homeostasis. Cell Mol Life Sci. 67(19):3255–73. doi: 10.1007/s00018-010-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav. 2004;81(5):781–93. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 104.Oomura Y, Kimura K, Ooyama H, Maeno T, Iki M, Kuniyoshi M. Reciprocal Activities of the Ventromedial and Lateral Hypothalamic Areas of Cats. Science. 1964;143:484–5. doi: 10.1126/science.143.3605.484. [DOI] [PubMed] [Google Scholar]
- 105.Oomura Y, Ooyama H, Sugimori M, Nakamura T, Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature. 1974;247(439):284–6. doi: 10.1038/247284a0. [DOI] [PubMed] [Google Scholar]
- 106.Shiraishi T. Feeding related lateral hypothalamic neuron responses to odors depend on food deprivation in rats. Physiol Behav. 1988;44(4–5):591–7. doi: 10.1016/0031-9384(88)90323-x. [DOI] [PubMed] [Google Scholar]
- 107.Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: ingestive behavior. Neurosci Biobehav Rev. 1996;20(2):189–287. doi: 10.1016/0149-7634(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 108.Silver MA, Stryker MP. A method for measuring colocalization of presynaptic markers with anatomically labeled axons using double label immunofluorescence and confocal microscopy. J Neurosci Methods. 2000;94(2):205–15. doi: 10.1016/s0165-0270(99)00145-4. [DOI] [PubMed] [Google Scholar]
- 109.Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki J, Zhang BB, Levin BE. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J Neurosci. 2009;29(21):7015–22. doi: 10.1523/JNEUROSCI.0334-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25(9):2429–33. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50(5):711–22. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 112.Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci U S A. 2008;105(33):11975–80. doi: 10.1073/pnas.0802687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci. 2009;3:12. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Parsons MP, Hirasawa M. ATP-sensitive potassium channel-mediated lactate effect on orexin neurons: implications for brain energetics during arousal. J Neurosci. 2010;30(24):8061–70. doi: 10.1523/JNEUROSCI.5741-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moriguchi T, Sakurai T, Nambu T, Yanagisawa M, Goto K. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci Lett. 1999;264(1–3):101–4. doi: 10.1016/s0304-3940(99)00177-9. [DOI] [PubMed] [Google Scholar]
- 116.Cai XJ, Evans ML, Lister CA, Leslie RA, Arch JR, Wilson S, Williams G. Hypoglycemia activates orexin neurons and selectively increases hypothalamic orexin-B levels: responses inhibited by feeding and possibly mediated by the nucleus of the solitary tract. Diabetes. 2001;50(1):105–12. doi: 10.2337/diabetes.50.1.105. [DOI] [PubMed] [Google Scholar]
- 117.Diano S, Horvath B, Urbanski HF, Sotonyi P, Horvath TL. Fasting activates the nonhuman primate hypocretin (orexin) system and its postsynaptic targets. Endocrinology. 2003;144(9):3774–8. doi: 10.1210/en.2003-0274. [DOI] [PubMed] [Google Scholar]
- 118.Kurose T, Ueta Y, Yamamoto Y, Serino R, Ozaki Y, Saito J, Nagata S, Yamashita H. Effects of restricted feeding on the activity of hypothalamic Orexin (OX)-A containing neurons and OX2 receptor mRNA level in the paraventricular nucleus of rats. Regul Pept. 2002;104(1–3):145–51. doi: 10.1016/s0167-0115(01)00340-8. [DOI] [PubMed] [Google Scholar]
- 119.Silva JP, von Meyenn F, Howell J, Thorens B, Wolfrum C, Stoffel M. Regulation of adaptive behaviour during fasting by hypothalamic Foxa2. Nature. 2009;462(7273):646–50. doi: 10.1038/nature08589. [DOI] [PubMed] [Google Scholar]
- 120.Broberger C, De Lecea L, Sutcliffe JG, Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402(4):460–74. [PubMed] [Google Scholar]
- 121.Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, Guan JL, Wang QP, Funahashi H, Sakurai T, et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology. 2003;144(4):1506–12. doi: 10.1210/en.2002-220788. [DOI] [PubMed] [Google Scholar]
- 122.Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143(1):155–62. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- 123.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38(5):701–13. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 124.Szentirmai E, Kapas L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R575–85. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- 125.Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1427–35. doi: 10.1152/ajpregu.00479.2002. [DOI] [PubMed] [Google Scholar]
- 126.Acuna-Goycolea C, van den Pol A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J Neurosci. 2004;24(37):8141–52. doi: 10.1523/JNEUROSCI.1607-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sawchenko PE. Central connections of the sensory and motor nuclei of the vagus nerve. J Auton Nerv Syst. 1983;9(1):13–26. doi: 10.1016/0165-1838(83)90129-7. [DOI] [PubMed] [Google Scholar]
- 128.Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int J Obes Relat Metab Disord. 2001;25 (Suppl 5):S42–7. doi: 10.1038/sj.ijo.0801912. [DOI] [PubMed] [Google Scholar]
- 129.Zhang YP, Zhu JN, Chen K, Li HZ, Wang JJ. Neurons in the rat lateral hypothalamic area integrate information from the gastric vagal nerves and the cerebellar interpositus nucleus. Neurosignals. 2005;14(5):234–43. doi: 10.1159/000088639. [DOI] [PubMed] [Google Scholar]
- 130.Scott JW, Pfaffmann C. Olfactory input to the hypothalamus: electrophysiological evidence. Science. 1967;158(808):1592–4. doi: 10.1126/science.158.3808.1592. [DOI] [PubMed] [Google Scholar]
- 131.Scott JW, Leonard CM. The olfactory connections of the lateral hypothalamus in the rat, mouse and hamster. J Comp Neurol. 1971;141(3):331–44. doi: 10.1002/cne.901410305. [DOI] [PubMed] [Google Scholar]
- 132.Scott JW, Pfaffmann C. Characteristics of responses of lateral hypothalamic neurons to stimulation of the olfactory system. Brain Res. 1972;48:251–64. doi: 10.1016/0006-8993(72)90182-5. [DOI] [PubMed] [Google Scholar]
- 133.Scott JW, Chafin BR. Origin of olfactory projections to lateral hypothalamus and nuclei gemini of the rat. Brain Res. 1975;88(1):64–8. doi: 10.1016/0006-8993(75)90948-8. [DOI] [PubMed] [Google Scholar]
- 134.Norgren R. Gustatory responses in the hypothalamus. Brain Res. 1970;21(1):63–77. doi: 10.1016/0006-8993(70)90021-1. [DOI] [PubMed] [Google Scholar]
- 135.Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol. 1976;166(1):17–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- 136.Karadi Z, Oomura Y, Nishino H, Aou S. Olfactory coding in the monkey lateral hypothalamus: behavioral and neurochemical properties of odor-responding neurons. Physiol Behav. 1989;45(6):1249–57. doi: 10.1016/0031-9384(89)90117-0. [DOI] [PubMed] [Google Scholar]
- 137.Aou S, Takaki A, Karadi Z, Hori T, Nishino H, Oomura Y. Functional heterogeneity of the monkey lateral hypothalamus in the control of feeding. Brain Res Bull. 1991;27(3–4):451–5. doi: 10.1016/0361-9230(91)90141-6. [DOI] [PubMed] [Google Scholar]
- 138.Oomura Y, Nishino H, Karadi Z, Aou S, Scott TR. Taste and olfactory modulation of feeding related neurons in behaving monkey. Physiol Behav. 1991;49(5):943–50. doi: 10.1016/0031-9384(91)90207-5. [DOI] [PubMed] [Google Scholar]
- 139.Karadi Z, Oomura Y, Nishino H, Scott TR, Lenard L, Aou S. Responses of lateral hypothalamic glucose-sensitive and glucose-insensitive neurons to chemical stimuli in behaving rhesus monkeys. J Neurophysiol. 1992;67(2):389–400. doi: 10.1152/jn.1992.67.2.389. [DOI] [PubMed] [Google Scholar]
- 140.Burstein R, Cliffer KD, Giesler GJ., Jr Direct somatosensory projections from the spinal cord to the hypothalamus and telencephalon. J Neurosci. 1987;7(12):4159–64. doi: 10.1523/JNEUROSCI.07-12-04159.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kai Y, Oomura Y, Shimizu N. Responses of rat lateral hypothalamic neurons to periaqueductal gray stimulation and nociceptive stimuli. Brain Res. 1988;461(1):107–17. doi: 10.1016/0006-8993(88)90729-9. [DOI] [PubMed] [Google Scholar]
- 142.Zhu L, Onaka T, Sakurai T, Yada T. Activation of orexin neurones after noxious but not conditioned fear stimuli in rats. Neuroreport. 2002;13(10):1351–3. doi: 10.1097/00001756-200207190-00027. [DOI] [PubMed] [Google Scholar]
- 143.Sakamoto F, Yamada S, Ueta Y. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept. 2004;118(3):183–91. doi: 10.1016/j.regpep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 144.Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24(50):11439–48. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503(5):668–684. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- 147.Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, Sakurai T. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873(1):181–7. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- 148.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438(1–2):71–5. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 149.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 150.Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26(2):398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23(1):7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 153.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23(8):3106–11. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 155.Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285(2):908–14. [PubMed] [Google Scholar]
- 156.Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99(2):267–77. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- 157.Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 1997;132(4):350–60. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]
- 158.Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23(7):2882–8. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76(3):365–77. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 160.Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that Regulate Food Intake: Appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1436–44. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]
- 161.Stanley BG, Willett VL, 3rd, Donias HW, Dee MG, 2nd, Duva MA. Lateral hypothalamic NMDA receptors and glutamate as physiological mediators of eating and weight control. Am J Physiol. 1996;270(2 Pt 2):R443–9. doi: 10.1152/ajpregu.1996.270.2.R443. [DOI] [PubMed] [Google Scholar]
- 162.Kelly J, Alheid GF, Newberg A, Grossman SP. GABA stimulation and blockade in the hypothalamus and midbrain: effects on feeding and locomotor activity. Pharmacol Biochem Behav. 1977;7(6):537–41. doi: 10.1016/0091-3057(77)90250-7. [DOI] [PubMed] [Google Scholar]
- 163.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27(41):11075–82. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29(10):571–7. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 165.Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183(1):43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Carter ME, Borg JS, de Lecea L. The brain hypocretins and their receptors: mediators of allostatic arousal. Curr Opin Pharmacol. 2009;9(1):39–45. doi: 10.1016/j.coph.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493(1):72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- 168.Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res. 2000;863(1–2):71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- 169.Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(5930):1080–4. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Baird JP, Choe A, Loveland JL, Beck J, Mahoney CE, Lord JS, Grigg LA. Orexin-A hyperphagia: hindbrain participation in consummatory feeding responses. Endocrinology. 2009;150(3):1202–16. doi: 10.1210/en.2008-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Horvath TL, Diano S, van den Pol AN. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci. 1999;19(3):1072–87. doi: 10.1523/JNEUROSCI.19-03-01072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ma X, Zubcevic L, Bruning JC, Ashcroft FM, Burdakov D. Electrical inhibition of identified anorexigenic POMC neurons by orexin/hypocretin. J Neurosci. 2007;27(7):1529–33. doi: 10.1523/JNEUROSCI.3583-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shiraishi T. Hypothalamic control of gastric acid secretion. Brain Res Bull. 1988;20(6):791–7. doi: 10.1016/0361-9230(88)90093-7. [DOI] [PubMed] [Google Scholar]
- 175.Berthoud HR, Bereiter DA, Jeanrenaud B. Role of the autonomic nervous system in the mediation of LHA electrical stimulation-induced effects on insulinemia and glycemia. J Auton Nerv Syst. 1980;2(2):183–98. doi: 10.1016/0165-1838(80)90044-2. [DOI] [PubMed] [Google Scholar]
- 176.Shimazu T, Fukuda A, Ban T. Reciprocal influences of the ventromedial and lateral hypothalamic nuclei on blood glucose level and liver glycogen content. Nature. 1966;210(5041):1178–9. doi: 10.1038/2101178a0. [DOI] [PubMed] [Google Scholar]
- 177.Shimazu T, Ogasawara S. Effects of hypothalamic stimulation on gluconeogenesis and glycolysis in rat liver. Am J Physiol. 1975;228(6):1787–93. doi: 10.1152/ajplegacy.1975.228.6.1787. [DOI] [PubMed] [Google Scholar]
- 178.Shimazu T, Takahashi A. Stimulation of hypothalamic nuclei has differential effects on lipid synthesis in brown and white adipose tissue. Nature. 1980;284(5751):62–3. doi: 10.1038/284062a0. [DOI] [PubMed] [Google Scholar]
- 179.Krowicki ZK, Burmeister MA, Berthoud HR, Scullion RT, Fuchs K, Hornby PJ. Orexins in rat dorsal motor nucleus of the vagus potently stimulate gastric motor function. Am J Physiol Gastrointest Liver Physiol. 2002;283(2):G465–72. doi: 10.1152/ajpgi.00264.2001. [DOI] [PubMed] [Google Scholar]
- 180.Grabauskas G, Moises HC. Gastrointestinal-projecting neurones in the dorsal motor nucleus of the vagus exhibit direct and viscerotopically organized sensitivity to orexin. J Physiol. 2003;549(Pt 1):37–56. doi: 10.1113/jphysiol.2002.029546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Miyasaka K, Masuda M, Kanai S, Sato N, Kurosawa M, Funakoshi A. Central Orexin-A stimulates pancreatic exocrine secretion via the vagus. Pancreas. 2002;25(4):400–4. doi: 10.1097/00006676-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 182.Wu X, Gao J, Yan J, Owyang C, Li Y. Hypothalamus-brain stem circuitry responsible for vagal efferent signaling to the pancreas evoked by hypoglycemia in rat. J Neurophysiol. 2004;91(4):1734–47. doi: 10.1152/jn.00791.2003. [DOI] [PubMed] [Google Scholar]
- 183.van den Top M, Nolan MF, Lee K, Richardson PJ, Buijs RM, Davies CH, Spanswick D. Orexins induce increased excitability and synchronisation of rat sympathetic preganglionic neurones. J Physiol. 2003;549(Pt 3):809–21. doi: 10.1113/jphysiol.2002.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem Cell Biol. 2005;123(2):147–56. doi: 10.1007/s00418-005-0761-x. [DOI] [PubMed] [Google Scholar]
- 185.Kokkotou E, Jeon JY, Wang X, Marino FE, Carlson M, Trombly DJ, Maratos-Flier E. Mice with MCH ablation resist diet induced obesity through strain specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005 doi: 10.1152/ajpregu.00861.2004. [DOI] [PubMed] [Google Scholar]