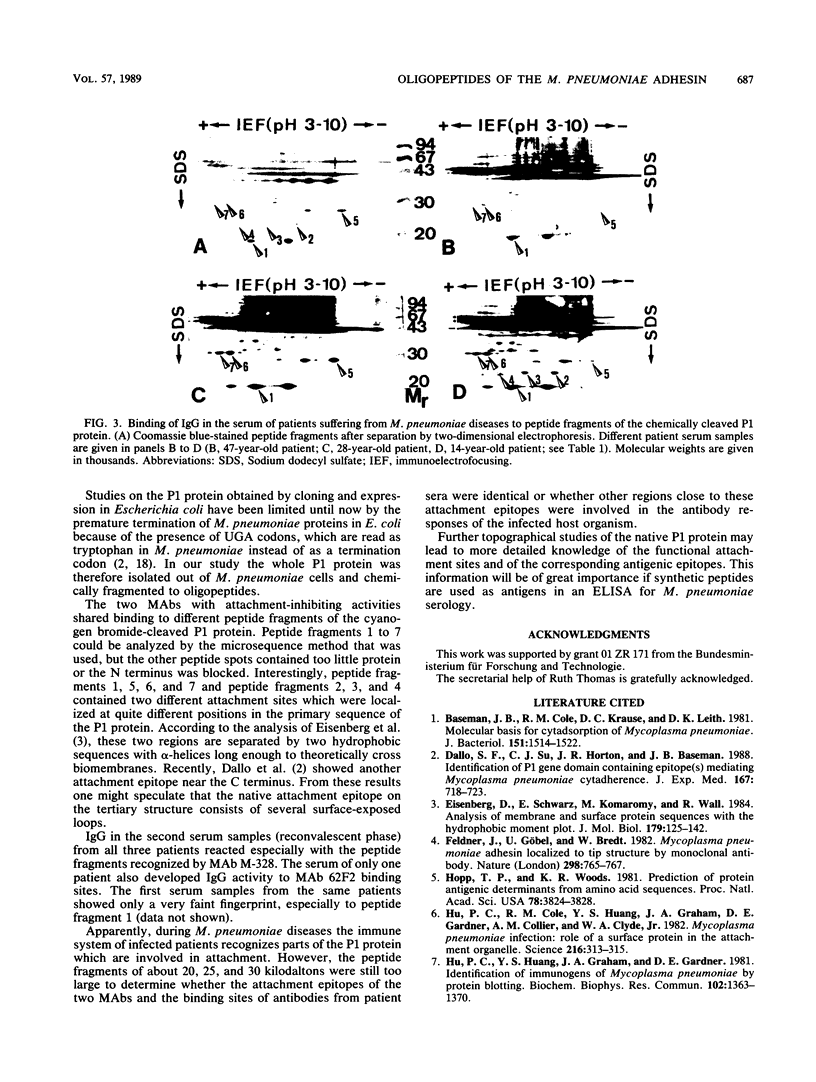
Abstract
The adherence protein (P1 protein) of Mycoplasma pneumoniae was purified by electroelution and cleaved with cyanogen bromide. The resulting peptides were separated by two-dimensional electrophoresis. Spots reacting in Western immunoblots with two attachment-inhibiting monoclonal antibodies were isolated, and the amino-terminal ends of these peptides were microsequenced. The two monoclonal antibodies had different binding sites. One was associated with the amino-terminal region of the whole P1 protein beginning at amino acid position 237, and the other was associated with amino acid position 702, which was localized approximately in the middle of the P1 amino acid sequence. Serum samples from three M. pneumoniae-infected patients were tested by Western blotting against the cyanogen bromide peptide pattern. All three serum samples reacted with peptide fragments beginning at amino acid position 702, but the serum of only one patient also had antibodies against the oligopeptides beginning at amino acid position 237. These results indicate that the corresponding epitopes of the P1 protein are also immunogenic if they are presented at the surface of the infecting organism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallo S. F., Su C. J., Horton J. R., Baseman J. B. Identification of P1 gene domain containing epitope(s) mediating Mycoplasma pneumoniae cytoadherence. J Exp Med. 1988 Feb 1;167(2):718–723. doi: 10.1084/jem.167.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Feldner J., Göbel U., Bredt W. Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody. Nature. 1982 Aug 19;298(5876):765–767. doi: 10.1038/298765a0. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Huang Y. S., Graham J. A., Gardner D. E. Identification of immunogens of Mycoplasma pneumoniae by protein blotting. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1363–1370. doi: 10.1016/0006-291x(81)90273-4. [DOI] [PubMed] [Google Scholar]
- Inamine J. M., Denny T. P., Loechel S., Schaper U., Huang C. H., Bott K. F., Hu P. C. Nucleotide sequence of the P1 attachment-protein gene of Mycoplasma pneumoniae. Gene. 1988 Apr 29;64(2):217–229. doi: 10.1016/0378-1119(88)90337-x. [DOI] [PubMed] [Google Scholar]
- Jacobs E., Bennewitz A., Bredt W. Reaction pattern of human anti-Mycoplasma pneumoniae antibodies in enzyme-linked immunosorbent assays and immunoblotting. J Clin Microbiol. 1986 Mar;23(3):517–522. doi: 10.1128/jcm.23.3.517-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E., Clad A. Electroelution of fixed and stained membrane proteins from preparative sodium dodecyl sulfate-polyacrylamide gels into a membrane trap. Anal Biochem. 1986 May 1;154(2):583–589. doi: 10.1016/0003-2697(86)90033-3. [DOI] [PubMed] [Google Scholar]
- Jacobs E., Fuchte K., Bredt W. A 168-kilodalton protein of Mycoplasma pneumoniae used as antigen in a dot enzyme-linked immunosorbent assay. Eur J Clin Microbiol. 1986 Aug;5(4):435–440. doi: 10.1007/BF02075700. [DOI] [PubMed] [Google Scholar]
- Jacobs E., Schöpperle K., Bredt W. Adherence inhibition assay: a specific serological test for detection of antibodies to Mycoplasma pneumoniae. Eur J Clin Microbiol. 1985 Apr;4(2):113–118. doi: 10.1007/BF02013574. [DOI] [PubMed] [Google Scholar]
- Leith D. K., Trevino L. B., Tully J. G., Senterfit L. B., Baseman J. B. Host discrimination of Mycoplasma pneumoniae proteinaceous immunogens. J Exp Med. 1983 Feb 1;157(2):502–514. doi: 10.1084/jem.157.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottspeich F. Identification of the phenylthiohydantoin derivatives of amino acids by high pressure liquid chromatography, using a ternary, isocratic solvent system. Hoppe Seylers Z Physiol Chem. 1980 Dec;361(12):1829–1834. doi: 10.1515/bchm2.1980.361.2.1829. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Schaper U., Chapman J. S., Hu P. C. Preliminary indication of unusual codon usage in the DNA coding sequence of the attachment protein of Mycoplasma pneumoniae. Isr J Med Sci. 1987 May;23(5):361–367. [PubMed] [Google Scholar]
- Su C. J., Tryon V. V., Baseman J. B. Cloning and sequence analysis of cytadhesin P1 gene from Mycoplasma pneumoniae. Infect Immun. 1987 Dec;55(12):3023–3029. doi: 10.1128/iai.55.12.3023-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]





