Abstract
Hopanoids and sterols are members of a large group of cyclic triterpenoic compounds that have important functions in many prokaryotic and eukaryotic organisms. They are biochemically synthesized from linear precursors (squalene, 2,3-oxidosqualene) in only one enzymatic step that is catalyzed by squalene-hopene cyclase (SHC) or oxidosqualene cyclase (OSC). SHCs and OSCs are related in amino acid sequences and probably are derived from a common ancestor. The SHC reaction requires the formation of five ring structures, 13 covalent bonds, and nine stereo centers and therefore is one of the most complex one-step enzymatic reactions. We summarize the knowledge of the properties of triterpene cyclases and details of the reaction mechanism of Alicyclobacillus acidocaldarius SHC. Properties of other SHCs are included.
INTRODUCTION
Hopene and related hopanoids are pentacyclic triterpenoids occurring in a wide range of Gram-positive and Gram-negative bacteria. They were discovered about 40 years ago as constituents of bacteria (8, 18, 19, 28). The name “hopene” refers to Hopea trees, which have pentacyclic triterpenes in the tree gum, and John Hope, who was the eponym for the description of the genus Hopea by Linné. Hopanoids are components of the cytoplasm membrane and are synthesized from isopentenyl units by using either the mevalonate pathway that is common in eukaryotes and in some Gram-positive prokaryotes or the mevalonate-independent 1-deoxy-d-xylulose-5-phosphate (DXP) pathway that is used in most prokaryotes (40, 42, 45, 81). Prenyltransferases catalyze stepwise head-to-tail additions of isopentenyl diphosphates to dimethylallyl diphosphate, resulting in geranyl diphosphate (C10), farnesyl diphosphate (C15), and geranylgeranyl diphosphate (C20) (for a review, see reference 104). Two farnesyl diphosphate units can be tail-to-tail condensed to squalene and then cyclized to hopene by the key enzyme of hopanoid biosynthesis, squalene-hopene cyclase (SHC; EC 5.4.99.17) (1, 37, 71, 106) (Fig. 1). Eukaryotes have sterols instead of hopanoids. Biosynthesis of sterols is also derived from squalene that is epoxidized to 2,3-oxidosqualene prior to the cyclization reaction by 2,3-oxidosqualene cyclases (OSCs) in a reaction similar to that of SHCs. SHCs and OSCs are distantly related in amino acid sequences, and both contain five to eight copies of a so-called QW motif, which seems to be unique to this enzyme class (26). Both can cyclize a variety of substrate analogs. However, the lipophilic entrance channel of OSCs rejects, for example, squalene without epoxide function before entering the active site, resulting in a substrate selection that is more specific than that of SHCs. SHCs accept both squalene as well as 2,3-oxidosqualene (57). Synthesis of hopene from squalene involves formation of five ring structures accompanied by the alteration of 13 covalent bonds and the generation of nine stereo centers. Therefore, the cyclization reaction catalyzed by SHCs (and OSCs) is one of the most complex enzymatic one-step reactions (14, 22, 105). All efforts to perform the SHC-catalyzed reaction chemically failed so far (2).
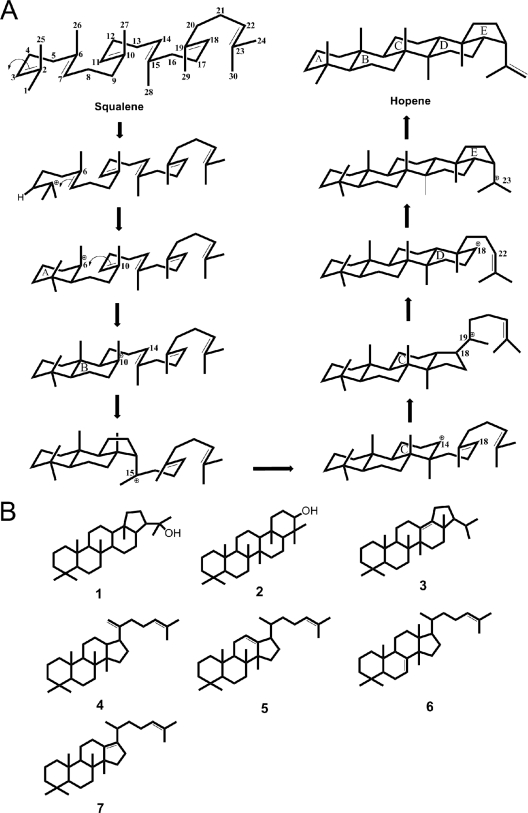
Fig. 1.
Overall mechanism of the polycyclization reaction of SHCs and structures of squalene cyclization products. (A) The polycyclization reaction of squalene to hopene by SHCs (modified from reference 89). (B) Structures of other squalene polycyclization products: hopanol (1), tetrahymanol (by squalene-tetrahymanol cyclase) (2), and minor hydrocarbons generated from squalene by A. acidocaldarius SHC (3–7).
FUNCTIONS OF HOPANOIDS
Hopanoids (and sterols) integrate in biological membranes and increase structural order at temperatures above the phase transition temperature of phospholipid membranes. Due to the rigid ring structures, hopanoids have a condensing effect on phospholipid layers and reduce permeability of membranes (42). Hopanoids therefore have stabilizing functions in bacterial membranes comparable to sterols in eukaryotes. The increase of hopanoid concentration in membranes of Alicyclobacillus acidocaldarius at high temperatures and at acidic conditions and in membranes of Zymomonas mobilis at elevated ethanol concentrations is in agreement with the stabilizing function of hopanoids at different growth conditions (61, 81). However, the relationship between ethanol concentration and hopanoid concentration in Z. mobilis membranes is controversial (30, 49). Recent experiments with shc mutants of Rhodopseudomonas palustris supported the assumption of a stabilizing effect of hopanoids on membrane integrity (97), but other functions for cyclic triterpenoids are discussed as well. For example, nitrogen-fixing actinomycetes of the genus Frankia form nitrogenase-containing vesicles which are surrounded by multilamellate lipid envelopes containing hopanoids. It is assumed that the lipid envelope serves as an oxygen barrier to protect nitrogenase from exposure to oxygen (7, 44, 53). Recently, it was found that the presence of polycyclic terpenoids in Bacillus subtilis correlated with increased resistance of spores against reactive oxygen species (9). Another example for a notable function of hopanoids was described for Streptomyces coelicolor, which synthesizes hopanoids during formation of aerial hyphae to alleviate stress in aerial mycelium by diminishing water permeability across the membrane (73). In conclusion, hopanoids certainly have a strong impact on the barrier properties of biological membranes at different culture conditions but apparently can have several other specific functions in many bacteria, most of which probably have not been identified yet. Construction of deletion mutants and phenotype analysis will be necessary to identify specific functions in selected species. Sterols also occur in a large variety of derivates with different functions in addition to membrane stabilization, e.g., such as antibiotics (squalamine in shark stomach) (48) or hormones (e.g., progesterone, pregnenolone, testosterone) in mammalians.
PURIFICATION AND PROPERTIES OF SHCs
Only a few SHCs have been purified and biochemically studied so far (Tables 1 and 2). Pioneer work was done by Poralla and coworkers about 30 years ago (for a review, see reference 71). The SHC of Alicyclobacillus acidocaldarius (formerly Bacillus acidocaldarius [103]) is the first prokaryotic SHC that was purified (56, 87) and for which the gene was cloned and sequenced (55). It has become a model enzyme for the whole group of SHCs, and the numbering of amino acids will refer to A. acidocaldarius SHC in this review. Poralla and coworkers were the first to confirm that purified SHC of A. acidocaldarius is able to catalyze the complete cyclization reaction of squalene to hopene in vitro. They showed that SHC in vivo is a membrane-associated protein and can be solubilized from cell extracts by nonionic detergents, such as Triton X-100 or octylthioglucopyranoside (87). However, only small amounts of SHC could be purified from A. acidocaldarius. To obtain larger amounts of SHC protein, the shc gene was heterologously expressed in Escherichia coli. E. coli is an ideal host, because it does not synthesize hopanoids or sterols, indicating the lack of respective triterpene cyclases (TTCs) in this species. However, even after overexpression of SHCs, whole cells did not show cyclase activity. Apparently, the outer membrane of E. coli is impermeable for squalene. Therefore, cell extracts or purified enzyme has to be used for determination of cyclase activity (55, 56). Using highly purified SHC preparations without lipid contaminants, it was possible to observe 12 minor products in addition to hopene and hopanol, with relative amounts of about 0.5% of hopene. To date, the structure of five of these products could be identified (see structures 3 to 7 of Fig. 1B) (62). Interestingly, these side products were also detected in the hydrocarbon fraction of A. acidocaldarius, suggesting that there are no artifacts of in vitro analyses (71). In 1994, shc of R. palustris was cloned and sequenced (44), and the protein was purified and compared to the A. acidocaldarius SHC (Table 1). Interestingly, the R. palustris enzyme is less hydrophobic, and its activity is inhibited by detergents that can be used for solubilization of A. acidocaldarius SHC (44). However, the SHC of Methylococcus capsulatus can be solubilized by Triton X-100 and Tween 80 without loss of activity, but ionic detergents, such as 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), sodium-taurodeoxycolate, and different glucopyranosides, inhibit this enzyme more or less completely (95). The optimal pH for the reaction of most SHCs is around pH 6.5. The optimal temperature varies from 30°C to 60°C, depending on the species from which the SHC is purified. Interestingly, the SHC of M. capsulatus does not accept oxidosqualene as substrate. In contrast, the Bradyrhizobium japonicum SHC shows 38% to 43% similarity to eukaryotic oxidosqualene cyclases and can also convert oxidosqualene. For overview on biochemical properties of well-studied SHCs, see Table 1. The highest amino acid similarity of B. japonicum SHC was found to a Zymomonas mobilis SHC (43, 68, 69). The genetic context of these two SHCs has been analyzed in more detail. The Z. mobilis and B. japonicum shc genes (synonym, hpnF) are part of a gene cluster containing six open reading frames (ORFs) (hpnABCDEF) or four ORFs (hpnCDEF), respectively. The deduced amino acid sequences of the products of the latter showed 58% to 62% amino acid similarity to the corresponding Z. mobilis proteins. One ORF encodes for a squalene synthase (hpnC), while some of the other ORFs (hpnA, hpnB) probably are necessary for biosynthesis of the complex sugar-containing side chains of hopanoids (69, 77). Recently, hopanoid biosynthesis genes and genes responsible for hopene side chain formation in Methylobacterium extorquens AM1 were investigated (10). To date, only a small amount of information is available about the genetic context and regulation of SHC expression and cyclase activity.
Table 1.
Biochemical characteristics of purified SHCsa
| Species | Molecular mass (kDa) | pH/temperature optimum (°C) | 2,3-Oxidosqualene cyclization | Tetrahymanol formation | Remark | Reference(s) |
|---|---|---|---|---|---|---|
| Alicyclobacillus acidocaldarius | 71.6 | 6/60 | Yes | No | Km of ∼3-16 μM | 16, 38, 56, 57, 86, 87 |
| Bradyrhizobium japonicum | 76.3 | 6.5/28 | Yes | No | 43, 57, 68, 69 | |
| Methylococcus capsulatus | 74.1 | 6.8/40 | No | No | 78, 95 | |
| Rhodopseudomonas palustris | 72.3 | 6.5/30 | – | No | Active between pH 5 and pH 8 | 44 |
| Streptomyces peucetius | 74.1 | 6.8/35 | – | – | 29 | |
| Tetrahymena thermophila | 72* | 7.0/30 | – | Yes | Km of ∼18 μM | 80 |
| Zymomonas mobilis | 74.1 | 6.0/30 | – | – | 77 |
The molecular masses were calculated based on the amino acid sequence if not otherwise indicated. 2,3-Oxidosqualene and tetrahymanol were analyzed as substrate/product, respectively. Cyclization of squalene is catalyzed by all SHCs (not included in table). –, substrate/product was not investigated; *, estimated from SDS-PAGE.
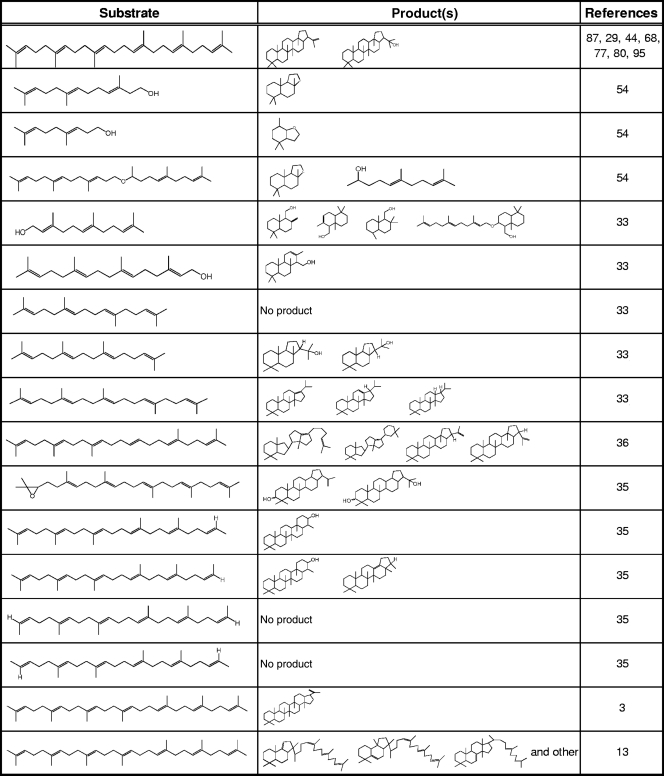
Table 2.
Product pattern of alternative substrates using A. acidocaldarius SHC
OCCURRENCE OF SHCs
The high similarity of the cyclization reaction catalyzed by SHCs and OSCs makes triterpene cyclases (TTCs) good candidates for evolutionary studies on phylogenetic relatedness of these enzymes in prokaryotic and eukaryotic organisms. Blasting the A. acidocaldarius SHC sequence resulted in several hundreds of potential SHC sequences, from which the top 100 sequences revealed amino acid identities of 39% to 64% and ≥90% coverage of the query sequence. Most shc genes have a coding potential for proteins of 70 to 75 kDa; OSCs are slightly larger than SHCs (78 to 85 kDa). Similar results were obtained with human OSC as the query sequence. The identified sequences include OSCs from organisms like fungi as well as plant species. Apparently, TTCs are widely distributed in living organisms and may be derived from a common ancestor. The conservation of TTCs is most obviously reflected in the conservation of structural elements, as depicted in the crystal structures of A. acidocaldarius SHC and of human OSC (94, 99, 101). Both structures consist of two α-barrel domains and show a large catalytic cavity with a channel entrance orientated to a membrane-inserted part of the enzyme (for details, see the next section). A DXDD motif located in the active-site cavity, which is involved in priming the polycyclization reaction by protonation (Fig. 1), is common to all SHCs. The corresponding XXDCX motif of eukaryotic OSCs is located in a position homologous to the DXDD motif in SHCs and is also involved in initiation of the polycyclization reaction (94, 98). Both enzyme types share a 16-amino-acid-long nontandem repeat known as the QW motif (see below) in addition to the presence of a large catalytic cavity in the protein center. Frickey and Kannenberg proposed that TTCs originate from a common ancestor from whom the majority of TTCs diverged in two major groups: the bacterial group, whose members use squalene as a substrate, and the eukaryotic group, cyclizing 2,3-oxidosqualene (26). For most of the proteins, vertical gene transfer from the common ancestor is assumed. However, some lateral gene transfer seems to have taken place. For example, a eukaryote-like OSC sequence is present in the translated genome of the myxobacterium Stigmatella, and a typical bacterial SHC-like sequence is present in an ancestral Pezizomycotina fungus (26). The planctomycete Gemmata obscuriglobus even produces sterols as well as hopanoids. Interestingly, the sterols were found in their eukaryotic-like nuclear membranes (64). Further investigations about the cellular functions of cyclic triterpenes are needed to understand their specific functions and the advantages and disadvantages of hopanoids or sterols for their host.
Hopanoids are excellent biomarkers for geologists and palaeobiologists, as they reflect the local microbial communities at the time of sedimentation. Hopanoids and corresponding degradation products can be found in modern sediments, oils of all geologic ages, as well as in Achaean rocks (11, 12, 23, 60). Rohmer and colleagues suggested that approximately 50% of all bacteria contain hopanoids (79). However, their data were based on studies of about 90 cultured bacterial organisms. Analysis of the genome sequences of more than 600 bacteria revealed that only 10% contained shc genes and suggested a lower abundance of hopanoid biosynthesis in bacteria (25). Studies including noncultivable organisms, using PCR and metagenomic data from different ecosystems, confirmed that in most communities not more than 10% and in aquatic systems less than 5% of the bacterial species are able to produce hopanoids (65). Recently, a new and apparently ubiquitous group of hopanoid producers whose members also possess SHCs but have no identifiable close relatives was identified by metagenomic analyses (66, 67). If we take into consideration that the majority of bacterial species remains uncultivable, this reflects how little is known about the phenotypes of hopanoid-producing bacteria (39, 75). Further work has to be performed to estimate the distribution and frequency of hopanoids produced by SHCs and the conservation of this enzyme group.
STRUCTURE-FUNCTION RELATIONSHIPS OF SHCs
An important milestone for elucidation of the biochemical mechanism of the SHC reaction was the solving of the A. acidocaldarius SHC structures with and without bound substrate analogue 2-azasqualene by Wendt and coworkers (100, 101). Crystallized A. acidocaldarius SHC is a homodimer with a dimension of 50 by 70 Å (70 kDa per monomer). Each subunit consists of α-helical domains that build up a dumbbell-shaped structure (Fig. 2). The first domain consists of a regular (α/α)6 barrel structure, whereas the second domain shows an α-barrel structure in a less periodic manner. Comparisons of the two domains suggest that the second domain may have been developed after gene duplication of the first domain.
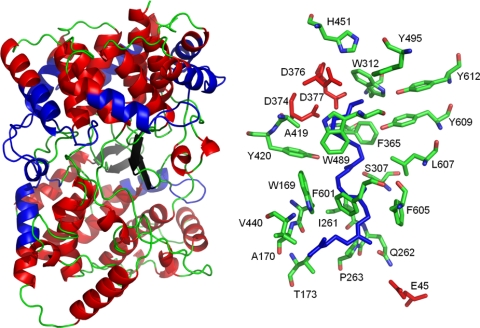
Fig. 2.
Structure and active site of the A. acidocaldarius SHC. Dumbbell-shaped structure of chain A (left) with a more structured α-barrel structure in domain 1 (upper part). The α-helices are shown in red, β-sheets in black, and loops in green. The QW motifs in the junction of helices and loops are in blue. Amino acids (sticks) of the active site together with substrate analogue 2-azasqualene (blue) are shown on the right. The aspartate residues of the DXDD motif important for the protonation initiated reaction are shown in red, as well as the glutamate residue 45, responsible for the final deprotonation. Images were generated on the basis of pDB entry 1ump by Pymol program, version 0.99rc6.
When the SHC from A. acidocaldarius was purified for the first time, it was assumed that the enzyme is attached to the inner side of the cytoplasm membrane by interactions of hydrophobic residues with the phospholipids (87). Analysis of the crystal structure confirmed that SHCs are only partially integrated in the membrane and obviously do not span the whole lipid bilayer (monotopic membrane protein). The membrane-binding part of the enzyme is a nonpolar region that is encircled by positive-charged amino acids enforcing the anchoring of the enzyme to the negatively charged surface of the phospholipid membrane. A protruding part in the center of this region contains a lipophilic channel and directs the substrate to the active-site cavity inside the protein. The channel and cavity are separated by a narrow constriction buildup of four amino acids (in the case of A. acidocaldarius SHC, D376, F166, C435, and F434) that appear to block access to the active site (Fig. 2). Nevertheless, the residues C435 and F434 are part of a loop that seems to be flexible enough to permit passage of the substrate and the product (57–59, 100). The presence of a channel directing toward the membrane is an important advantage, because squalene is hydrophobic and probably enters the enzyme after solubilization in the membrane (102). In OSCs, the channel is in addition at least partly responsible for substrate specificity (94). Mutagenesis and inhibition studies suggested that the channel constriction plays a critical role in substrate recognition (58, 59). This is rather plausible, because the epoxide edge of the substrate has to be orientated toward the protonating apparatus, but the active-site cavity of OSCs is more spatially restricted than that of SHCs, making it unlikely to turn the substrate inside the active-site cavity (17). The orientation of squalene in the active site of SHCs is less crucial, because the substrate is symmetric.
A large central cavity represents the catalytic site in A. acidocaldarius SHC that takes up and orientates the squalene molecule (Fig. 2). Analysis of the catalytic properties of SHC muteins obtained by site-directed mutagenesis led to a variety of alternative products caused by early truncations of the polycyclization cascade and/or aberrant cyclization products (Table 3). Such products can have a stereo configuration opposite to that of the normal hopene product. This emphasizes the importance of the correct folding of the substrate prior to the cyclization reaction that is ensured by the spatial form of the active-site cavity (31, 32). The active-site cavity is made up of a bulky hydrophobic central core and polar areas at the top (around D376) and bottom (around E45) parts (101). D376 is part of the DXDD motif, which is typical for all SHCs known to date. D374, D376, and D377 represent this motif in A. acidocaldarius. D376 and D377 are essential for SHC activity (24), whereas mutation of Asp374 only lowers polycyclization activity (83). The polar hydrogen bonding network around E45, consisting of Q262, E45, E93, and R127, and a water molecule at the bottom of the active-site cavity are involved in the final deprotonation step (101). The nonpolar central core is surrounded by aromatic residues facing the cavity to stabilize the cationic intermediates during the polycyclization reaction. They are important for proper folding of the substrate to ensure the formation of the correct product isomer during the whole cyclization cascade (94, 101).
Table 3.
Overview of cyclization products of squalene with A. acidocaldarius SHC muteins
| Mutation(s) | Product pattern | Reference(s) |
|---|---|---|
| Y606A/W23V/W495V/W522V/W533A/W591L/W78S/E35Q/E197Q/D530N/T378A | Same product pattern and activity as the wild type | 50, 84 |
| Y612F/D376E/D376G/D377E/D377G/D377Q/E45A/E45D/F365W/T41A/E93A/R127Q/W133A/Y267A/F434A/F437A/W258L/D350N/D421N/D442N/H451R/D447N/D377N/D313N/E535Q/D374E | Same product pattern as the wild type with less enzyme activity | 16, 24, 27, 83, 86 |
| D376E + D377E/D376Q/D376R/D377R/E45K/W406V/W417A/D377C + V380E + V381A/D376C + C435S/D374I/D374V/H451F | No enzyme activity | 16, 24, 47, 83 |
| F605A | hop-22(29)-ene; hopanol; podioda-8,17,21-triene; podioda-7,17,21-triene; 17-epi-dammara-20(21),24-diene; dammara-13(17),24-diene; eupha-7,24-diene; 20-hydroxy-17-epi-dammarene; hop-17(21)-ene; hop-(16)17-ene; neohop-13(18)-ene; neohop-12(13)-ene | 31 |
| Q262G/Q262A/P263G/P263A | hop-22(29)-ene; hopanol; hop-21(22)-ene | 86 |
| Y609Fa | α-polypodatetraene; dammara-13(17),24-diene; 17-isodammara-12,24-diene; eupha-7,24-diene; dammara-20(21),24-diene; 17-isodammara-20(21),24-diene; hopene; diaplopterol | 27 |
| D377C/D377N/Y612A | hop-(22)29-ene; hopanol; 3-deoxyhomologe of achilleol A | 37, 83 |
| F601A | hop-(22)29-ene; hopanol; 6-6-5-fused tricycles; 6-6-6-5-fused tetracycle | 37 |
| F605A | 6-6-5-fused tricyclic; 6-6-6-5-fused tetracyclic; 6-6-6-6-5-fused pentacyclic skeletons | 37 |
| F365A | 6-6-fused bicyclic skeleton | 37 |
| Y420A | 6-6-fused bicyclic; 6-6-5-fused tricyclic skeletons | 37 |
| Y609A/Y612A/L607K | 6-6-5-fused tricyclic skeleton | 37 |
| Y609Fa | 6-6-fused bicycle; 6-6-6-5-fused tetracyclic skeletons | 37 |
| I261A | 6-6-5-fused tricycle; 6-6-6-5-fused tetracycle; 6-6-6-6-5-fused pentacyclic skeletons | 37 |
| W169F/W169H/W489A/F605K | 6-6-6-5-fused tetracyclic skeleton | 37 |
Phenotype of Y609F mutein is contrarily described in two publications.
A 16-amino-acid-long nontandem repeat with a consensus motif called the QW motif is another typical structural element present in all TTCs. Its characteristic is the regular occurrence of Q and W in the (R/K)-(A/G)-X3-(F/W/Y)-L-X3-Q-X3-G-X-W sequence (X3 indicates XXX). Up to eight repeats of the motif can be found in all SHCs known to date. It was assumed that the aromatic rings of the conserved tryptophan residues of this motif could stabilize the intermediary carbocations (72, 102). This would be in agreement with other models of active centers with carbocation intermediates (21, 41, 88). However, the SHC structure revealed that all QW motifs are located at the C terminus of an exposed α-helix, with the eponymous part of the motif (Q-X3-G-X-W) generating the loop structure (see Fig. 2). Interactions between the QW motifs stabilize the protein and help the enzyme to maintain its integrity (101). Mutagenesis experiments revealed the importance of QW motifs for thermostability of SHC (82–84). This is necessary because the cyclization reaction is highly exergonic, with 40 to 50 kcal/mol (72). This amount is significantly above that of the average protein stabilization energy (70, 72). The crystal structure of SHC with cocrystallized 2-azasqualene showed that the wall between the channel and dimer surface is rather flexible and might be appreciably enlarged by melting and displacing the mobile peptide. The produced energy may be channeled and used to release the bulky hopene into the membrane without changing the overall structure of the protein (76). Truncated analogs of the QW motif and a similar active-site motif (DDTA) occur in an ent-copalyl synthase, a kaurene synthase A, and an abietadiene synthase (51, 90, 96). These diterpene cyclases have geranylgeranyl pyrophosphate as a substrate but use a cyclization mechanism similar to that of TTCs. Normally, mono- and sesquiterpene cyclases initiate the polycyclization reaction by dephosphorylation of the activated substrate, leading to the first carbocation. However, these diterpene cyclases generate the first carbocation by protonation- like SHCs and OSCs (see the next section). The exact reaction mechanism of these diterpene cyclases without any more similarities to the complex structure of TTCs still remains unclear. The intricate structure of SHC correlates with its very low turnover rate (1.98 turnovers/s) (50). Taking into account that SHCs first channel the substrate into the catalytic cave, fold the compound correctly, promote five cyclization reactions, and export the bulky product, this is conceivable.
REACTION MECHANISM
The formation of the pentacyclic hopene molecule from linear squalene in a one-step reaction is one of the most complex biochemical reactions in nature. The respective enzyme has (i) to take care of provision of a Brønsted acid strong enough to initiate the polycyclization, (ii) to enforce the correct conformation of the intermediates in case of nonchair conformations, (iii) to protect the cationic intermediates against damage by nucleophilic attacks through functional groups of the enzyme or water, and (iv) to accelerate the reaction by stabilizing carbocations in an electron-rich environment.
Formation of squalene carbocation.
The initial reaction catalyzed by SHCs is the protonation of the terminal double bond of squalene. The conserved DXDD motif of SHCs is essential for this protonation reaction (94, 98). In A. acidocaldarius SHC, Asp376 of this motif is hydrogen bonded to His451, and an additional hydrogen bond exists to an ordered water molecule, which connects D376 to the hydroxyl group of the Y495 side chain and thus further enhances its acidity. The carboxyl groups of Asp374 and Asp377 accommodate the positive charge of the D376-H451 pair prior to proton transfer. After proton transfer to the 2,3-double bond of squalene, the D376-H451 pair loses its charge, leaving the remaining negative charge on the D374-D377 pair for stabilization of the initial cationic intermediates (24, 101). Reprotonation of D376 occurs through a water molecule bound to Y495-OH, which can transfer protons from disordered water in the solvent-accessible upper cavity of SHC (100). The presence of two terminal methyl groups at C-2 (C-1 and C-25) or C-23 (C-24 and C-30) of squalene is essential for the reaction. Experiments with C-1;C-25- or C-24;C-30-bisnorsqualene (squalene lacking both terminal methyl groups at one side) showed that the reaction was always starting from the other terminal end, and substrates lacking all methyl groups at both terminal sides were never cyclized by A. acidocaldarius SHC (35). The high electrophilic character of the catalytic domain enables nearly all SHCs to cyclize both enantiomers of 2,3-oxidosqualene (5). This property has been shown only for the OSC of M. capsulatus, which harbors an SHC as well as an OSC that is able to accept both enantiomers (78). Other OSCs from animals, yeast, and plants accept only the (S)-enantiomer of 2,3-oxidosqualene as a substrate (6). The less hydrophobic character of their catalytic domains probably is responsible for their inability to initiate the polycyclization of squalene (25).
Polycyclization.
Once the carbocation is formed, the generation of the polycyclus is pushed on. Hoshino postulated a polycyclization reaction in the following 8 steps (Fig. 1) (35). First, the A ring is formed by proton attack of the terminal double bond donated by the DXDD motif. A second ring closure gives the B ring, followed by a third cyclization round yielding the 5-carbon-atom C ring by Markovnikov closure. After ring expansion to a 6-carbon-atom C ring, a thermodynamically favored 5-carbon-atom D ring is formed and transformed in a 6-carbon-atom ring, too. The last ring closure gives the final 6-6-6-6-5 ring system, and in the end the double bond of hopene is introduced during the final deprotonation reaction (see Fig. 1) (16, 31, 32, 46, 62, 63, 85).
It is generally accepted that the cyclization reaction is initiated by protonation. However, it is still an open question whether the overall process is formed via discrete carbocation intermediates as described above or by a concerted, highly asynchronous ring-forming reaction (20, 89, 93). Reinert and coworkers published the three-dimensional structure of the A. acidocaldarius SHC with the cocrystallized substrate analogue 2-azasqualene (76). The authors suggested a barrier-free ring closure of the A to D rings followed by a pause at the 6-6-6-5 tetracyclic tertiary carbocation before concerted ring expansion of ring D and formation of the E ring. In contrast to this, Hoshino and Sato analyzed SHC muteins and isolated not only tricyclic but also bicyclic and monocyclic products, which are postulated to represent intermediates occurring during the formation of the A to C rings (Table 3) (37, 50). However, there is some agreement that the 6-6-5 carbocation is transiently formed in the overall squalene cyclization process. This was shown by using mutant SHCs or diols of squalene as the substrate (4, 32). A comparable intermediate also occurred during the conversion of squalene oxide to lanosterol by OSCs (15) and was further approved through results of computational modeling (89).
Independently from the discussion about the exact reaction cascade catalyzed by SHCs and OSCs, the importance of the structure of the active site for conformational and stereochemical properties of the product is widely accepted. It is assumed that the intermediately formed carbocations are stabilized by interactions of the transient local positive charge of the substrate with π-electrons provided by aromatic residues of the enzyme (21, 41). The tryptophan residues conserved among prokaryotic SHCs greatly contribute to the acceleration of the polycyclization reaction, at least at low temperatures (83). The steric bulk of the active-site residues direct the folding conformation, ensuring the correct stereochemistry of the hopene product during the reaction cascade, as it makes the catalytic cave exceptionally compact (37). This is a problem when using site-directed mutagenesis to explore the importance of cation-π interactions that might play a role for the termination reaction and the ring enlargement. Even slightly different van der Waals radii of active-site-lining amino acids lead to a modified product pattern. The use of unnatural amino acids avoids this problem: Morikubo and coworkers showed that cation-π interactions occupy a key position in the catalytic mechanism by the use of mono-, di-, and trifluorophenylalanines (50). These unnatural amino acids have van der Waals radii similar to those of phenylalanine but have an extremely higher electronegativity, anticipating a slower reaction rate with a normal bell-shaped profile. Measurements of the polycyclization activity of muteins harboring corresponding unnatural amino acids corroborated this assumption. The cation-π interactions in conjunction with the meandering conformation of the substrate effectively foster the propagation of carbocyclic rings and are crucial for an efficient termination reaction and ring enlargement process in cyclic triterpene biosynthesis (50).
Final deprotonation.
At the end of the A. acidocaldarius SHC reaction, the positive charge is neutralized by direct deprotonation of the polycyclic intermediate leading to hopene (diploptene [main product]) or by the addition of water to diplopterol (hopanol [minor by-product]) (70, 74, 76, 98). Hoshino and coworkers estimated that the final deprotonation reaction occurs exclusively from the Z-methyl group C-24 of squalene (35, 37). If the substrate lacks the terminal methyl groups at one end, large amounts of 6-6-6-6-6-fused pentacyclic rings are formed, but no hopane is produced (38). This demonstrates that the isopropylidene moiety is required for initializing the polycyclization as well as for finalizing the reaction.
ALTERNATIVE SUBSTRATES
Hoshino and coworkers invested much effort on the enzymatic conversion of squalene analogues by SHCs (Table 2). Using norsqualene lacking a methyl group of the squalene backbone, they got important information regarding the polycyclization pathway (see above). The central methyl group (C-27 bound to C-10) was found to be crucial for the normal polycyclization pathway, as it ensures proper folding in the all chair conformation during the cyclization reaction (52). The loss of a methyl group at the alternative terminal ends created an optional pentacyclic ring system with 6-6-6-6-6-fused rings in contrast to the normal 6-6-6-6-5 ring systems of hopene (32, 38). Thus, the methyl groups at the terminal and at the central positions play crucial roles for the proper folding of the substrate during formation of hopene.
A. acidocaldarius SHC accepts a variety of alternative substrates, such as truncated analogs of squalene with lengths of 11 to 29 carbon atoms (Table 2). Diverse analogue substrates were transformed to products with di-, sesqui-, and sesterterpene skeletons. For example, the C31 compound homofarnesol-1,5,9-trimethyl-4,8-decadienyl-ether is converted into two products, ambroxan (Ambroxane) and 1,5,9-trimethyl-4,8-decadienol, whereas polycyclization of the C16 substance E,E-homofarnesol results exclusively in ambroxan (54). Even a C20 isoprenoid harboring an indole or a pyrrole ring was cyclized by A. acidocaldarius SHC (91, 92). The smallest described substrate of SHC so far is homogeraniol (C11), which can be converted into octahydro-4,4,7-trimethylbenzofurane (54). A smaller C10 analogue, like geraniol, was not accepted as a substrate by SHC (33). It is speculated that this is caused by the absence of a methyl group at C-10 (36). However, unpublished data from our lab suggest that at least Z. mobilis SHC is able to use monoterpenes as substrates for cyclization. We already mentioned the importance of the active-site cavity for correct folding and cyclization of the substrate. Interestingly, this cavity can accept alternative substrates that are even bigger than squalene. For example, 26- and 27-methylidensqualene are converted into novel unnatural pentacyclic C31 polyisoprenoids, a dammarene derivative with a 6-6-6-5+6 ring system and a 26-methylidene-hop-22(29)-ene, respectively (91). Even C35 squalene analogues do not function as inhibitors for SHC activity, although there is some controversy about the product of the conversion of C35 heptapolyprene. Abe and coworkers reported that this substrate was converted into a novel 6-6-6-6-6-5-fused hexacyclic ring system with 10% yield as a single product (3). However, Cheng and Hoshino stated that formation of a hexacyclic scaffold from the C35 heptaprolyprene is impossible (13). Instead, a large amount of tri- and tetracyclic compounds was produced, suggesting that not the entire carbon skeleton can be accommodated by the active-site cavity. This is in agreement with the fact that a multiring system larger than a pentacycle has not yet been found as a product of an enzymatic reaction (34). A C33-bisnorheptaprenoid was also tested as a substrate for conversion into a hexacyclic product. However, again no hexacyclic product was observed. A variety of mono- (∼13%), bi- (∼7%), tri- (∼35%), tetra- (∼43%), and pentacyclic (∼2%) skeletons were identified. The absence of hexacyclic products suggests that the reaction cavity is limited and not large enough to shelter more than five carbon cycles (13). However, SHCs tolerate a variety of truncated and elongated substrate analogues, reflecting broad substrate range of the enzyme, at least in vitro. This is contrary to the OSCs, in which the substrate specificity is given at least partially by the nature of the entrance to the catalytic cave, resulting in a selection before cyclization.
OUTLOOK
Bacterial SHCs came into focus of scientific interest more than 40 years ago, and up to now an impressive mass of data has been collected. Nevertheless, there are still many questions that need to be answered. In particular, the cellular functions of the produced hopanoids need further investigation. The specific appearance of hopanoids at some phases of organism life cycles or during adaptations to stress suggests a tight control of expression and/or activity of the corresponding enzymes. However, studies on the regulation of SHC expression are rare. In the case of Z. mobilis SHC, it was found that the addition of ethanol to the growth medium leads to enhanced production of the enzyme (49). How this increase is regulated is still unknown. Much work was spent on deciphering the complex polycyclization mechanism. Despite much progress, the reaction mechanism is still under discussion, as the exact chronology of the polycyclization is not completely solved. The problem is the tight folding control by the shape and spatial dimension of the catalytic site cavity, which complicates mutagenesis studies. Alternative products after mutagenesis can be a result of changes in the reaction mechanism as well as of different folding of the substrate. The use of unnatural amino acids with comparable structures but different substitutions of ligands may help to overcome this problem in the future. Even some fundamental questions remain unexplored. It is still unclear how the bulky product is set free from the active-site cavity. It is speculated that the energy released during the polycyclization reaction could be important for rearrangements of the enzyme. However, experimental data are still missing.
Further investigations should also focus on the broad substrate range of the enzyme, which makes SHCs an interesting tool for producing novel, unnatural cyclic products. SHCs can polycyclize substances in a one-step reaction and therefore may become an interesting biocatalyst for biotechnological processes. The tight stereo control during the reaction is a great advantage of this enzyme in comparison to chemically produced compounds.
ACKNOWLEDGMENTS
This work was supported by a grant of the Bundesministerium für Bildung und Forschung (BMBF) and by BASF-SE.
We thank M. Breuer (BASF) and B. Hauer (University of Stuttgart) for fruitful discussions.
Biographies

Gabriele Siedenburg is a postdoctoral researcher in the group of Dieter Jendrossek at the University of Stuttgart, Germany. She studied biology at the University of Osnabrück in Germany and obtained her diploma in 2004. During her Ph.D. in the department of applied genetics of microorganisms managed by Professor Hildgund Schrempf at the University of Osnabrück, she got insights into the fascinating world of streptomycetes. Under the direction of Dr. Darío Ortiz de Orúe Lucana, her Ph.D. thesis focused on a new kind of a two-component regulatory system in Streptomyces reticuli, using a third protein component for recognition of redox stress signals. After finishing her Ph.D. thesis in 2008, she started working on an industry-university joint project at the University of Stuttgart, analyzing the potential of squalene-hopene cyclases as industrial biocatalysts.

Dieter Jendrossek is a professor of microbiology and biochemistry at the University of Stuttgart. He studied biology in Göttingen, Germany, and obtained his Ph.D. in microbiology (1988) in Hans-Günther Schlegel's department (Alexander Steinbüchel's lab) on the function of fermentative enzymes in the strict aerobe Ralstonia eutropha H16. He became a group leader in 1989 and started his own research on biodegradation of biopolymers, such as polyhydroxybutyrate (PHB), other polyhydroxyalkanoates (PHAs), and polyisoprene (rubber). In 1995, he finished his habilitation and performed a short-term stay in Hildgund Schrempf's lab in Osnabrück, Germany, where he learned basic methods of how to handle and work with streptomycetes. He moved to Stuttgart University in 1999, where he continued his research on biopolymers and added PHB granule formation in PHA-accumulating bacteria and the biochemistry of acyclic monoterpenes in pseudomonads to his research interests. Recently, he started a project on microbial squalene-hopene cyclases as promising biocatalysts for novel cyclization reactions.
Footnotes
Published ahead of print on 29 April 2011.
REFERENCES
- 1. Abe I. 2007. Enzymatic synthesis of cyclic triterpenes. Nat. Prod. Rep. 24: 1311–1331 [DOI] [PubMed] [Google Scholar]
- 2. Abe I., Rohmer M., Prestwich G. D. 1993. Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem. Rev. 93: 2189–2206 [Google Scholar]
- 3. Abe I., Tanaka H., Noguchi H. 2002. Enzymatic formation of an unnatural hexacyclic C35 polyprenoid by bacterial squalene cyclase. J. Am. Chem. Soc. 124: 14514–14515 [DOI] [PubMed] [Google Scholar]
- 4. Abe T., Hoshino T. 2005. Enzymatic cyclizations of squalene analogs with threo- and erythro-diols at the 6,7- or 10,11-positions by recombinant squalene cyclase. Trapping of carbocation intermediates and mechanistic insights into the product and substrate specificities. Org. Biomol. Chem. 3: 3127–3139 [DOI] [PubMed] [Google Scholar]
- 5. Anding C., Rohmer M., Ourisson G. 1976. Letter: nonspecific biosynthesis of hopane triterpenes in a cell-free system from Acetobacter rancens. J. Am. Chem. Soc. 98: 1274–1275 [DOI] [PubMed] [Google Scholar]
- 6. Barton D. H. R., Jarman T., Watson K. C., Widdowson D. A. 1975. Investigations on the biosynthesis of steroids and terpenoids. Part XII. Biosynthesis of 3-beta-hydroxy-triterpenoids and -steroids from (3S)-2,3-epoxy-2,3-dihydrosqualene. J. Chem. Soc. Perkin 1 1975: 1134–1138 [DOI] [PubMed] [Google Scholar]
- 7. Berry A. M., et al. 1993. Hopanoid lipids compose the Frankia vesicle envelope, presumptive barrier of oxygen diffusion to nitrogenase. Proc. Natl. Acad. Sci. U. S. A. 90: 6091–6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bird C. W., et al. 1971. Steroids and squalene in Methylococcus capsulatus grown on methane. Nature 230: 473–474 [DOI] [PubMed] [Google Scholar]
- 9. Bosak T., Losick R. M., Pearson A. 2008. A polycyclic terpenoid that alleviates oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 105: 6725–6729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradley A. S., Pearson A., Saenz J. P., Marx C. J. 2010. Adenosylhopane: the first intermediate in hopanoid side chain biosynthesis. Org. Geochem. 41: 1075–1081 [Google Scholar]
- 11. Brocks J. J., Logan G. A., Buick R., Summons R. E. 1999. Archean molecular fossils and the early rise of eukaryotes. Science 285: 1033–1036 [DOI] [PubMed] [Google Scholar]
- 12. Brocks J. J., et al. 2005. Biomarker evidence for green and purple sulphur bacteria in a stratified Palaeoproterozoic sea. Nature 437: 866–870 [DOI] [PubMed] [Google Scholar]
- 13. Cheng J., Hoshino T. 2009. Cyclization cascade of the C33-bisnorheptaprenoid catalyzed by recombinant squalene cyclase. Org. Biomol. Chem. 7: 1689–1699 [DOI] [PubMed] [Google Scholar]
- 14. Corey E. J., Matsuda S. P., Bartel B. 1993. Isolation of an Arabidopsis thaliana gene encoding cycloartenol synthase by functional expression in a yeast mutant lacking lanosterol synthase by the use of a chromatographic screen. Proc. Natl. Acad. Sci. U. S. A. 90: 11628–11632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corey E. J., et al. 1995. New insights regarding the cyclization pathway for sterol biosynthesis from (S)-2,3-oxidosqualene. J. Am. Chem. Soc. 117: 11819–11820 [Google Scholar]
- 16. Dang T., Prestwich G. D. 2000. Site-directed mutagenesis of squalene-hopene cyclase: altered substrate specificity and product distribution. Chem. Biol. 7: 643–649 [DOI] [PubMed] [Google Scholar]
- 17. Dehmlow H., et al. 2003. Synthesis and structure-activity studies of novel orally active non-terpenoic 2,3-oxidosqualene cyclase inhibitors. J. Med. Chem. 46: 3354–3370 [DOI] [PubMed] [Google Scholar]
- 18. De Rosa M., Gambacorta A., Minale L. 1971. Bacterial triterpenes. Chem. Comm. 1971: 619–620 [Google Scholar]
- 19. De Rosa M., Gambacorta A., Minale L., Bu'Lock J. D. 1973. Isoprenoids of Bacillus acidocaldarius. Phytochemistry 12: 1117–1123 [Google Scholar]
- 20. Dewar M. J. S., Jie C. 1992. Mechanisms of pericyclic reactions: the role of quantitative theory in the study of reaction mechanisms Acc. Chem. Res. 25: 537–543 [Google Scholar]
- 21. Dougherty D. A. 1996. Cation-pi interactions in chemistry and biology: a new view of benzene, Phe, Tyr, and Trp. Science 271: 163–168 [DOI] [PubMed] [Google Scholar]
- 22. Eschenmoser A., Ruzicka L., Jeger O., Arigoni D. 1955. Zur kenntnis der triterpene. 190. Mitteilung. Eine stereochemische interpretation der biogenetischen isoprenregel bei den triterpenen. Helv. Chim. Acta 38: 1890–1904 [Google Scholar]
- 23. Farrimond P., Head I. M., Innes H. E. 2000. Environmental influence on the biohopanoid composition of recent sediments. Geochim. Cosmochim. Acta 64: 2985–2992 [Google Scholar]
- 24. Feil C., Süssmuth R., Jung G., Poralla K. 1996. Site-directed mutagenesis of putative active-site residues in squalene-hopene cyclase. Eur. J. Biochem. 242: 51–55 [DOI] [PubMed] [Google Scholar]
- 25. Fischer W. W., Pearson A. 2007. Hypothesis for the origin and early evolution of triterpenoid cyclases. Geobiology 5: 19–34 [DOI] [PubMed] [Google Scholar]
- 26. Frickey T., Kannenberg E. 2009. Phylogenetic analysis of the triterpene cyclase protein family in prokaryotes and eukaryotes suggests bidirectional lateral gene transfer. Environ. Microbiol. 11: 1224–1241 [DOI] [PubMed] [Google Scholar]
- 27. Füll C., Poralla K. 2000. Conserved Tyr residues determine functions of Alicyclobacillus acidocaldarius squalene-hopene cyclase. FEMS Microbiol. Lett. 183: 221–224 [DOI] [PubMed] [Google Scholar]
- 28. Gelpi E., Schneider H., Mann J., Oró J. 1979. Hydrocarbons of geochemical significance in microscopical algae. Phytochemistry 9: 603–612 [Google Scholar]
- 29. Ghimire G. P., Oh T. J., Lee H. C., Sohng J. K. 2009. Squalene-hopene cyclase (Spterp25) from Streptomyces peucetius: sequence analysis, expression and functional characterization. Biotechnol. Lett. 31: 565–569 [DOI] [PubMed] [Google Scholar]
- 30. Hermans M. A., Neuss B., Sahm H. 1991. Content and composition of hopanoids in Zymomonas mobilis under various growth conditions. J. Bacteriol. 173: 5592–5595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoshino T., Abe T., Kouda M. 2000. Unnatural natural triterpenes produced by altering isoleucine into alanine at position 261 in hopene synthase and the importance of having the appropriate bulk size at this position for directing the stereochemical destiny during the polycyclization cascade. Chem. Commun. (Camb.) 2000: 441–442 [Google Scholar]
- 32. Hoshino T., Kouda M., Abe T., Ohashi S. 1999. New cyclization mechanism for squalene: a ring-expansion step for the five-membered C-ring intermediate in hopene biosynthesis. Biosci. Biotechnol. Biochem. 63: 2038–2041 [DOI] [PubMed] [Google Scholar]
- 33. Hoshino T., Kumai Y., Kudo I., Nakano S., Ohashi S. 2004. Enzymatic cyclization reactions of geraniol, farnesol and geranylgeraniol, and those of truncated squalene analogs having C20 and C25 by recombinant squalene cyclase. Org. Biomol. Chem. 2: 2650–2657 [DOI] [PubMed] [Google Scholar]
- 34. Hoshino T., Kumai Y., Sato T. 2009. Reviewing the polyolefin cyclization reaction of the C(35) polyprene catalyzed by squalene-hopene cyclase. Chemistry 15: 2091–2100 [DOI] [PubMed] [Google Scholar]
- 35. Hoshino T., Nakano S., Kondo T., Sato T., Miyoshi A. 2004. Squalene-hopene cyclase: final deprotonation reaction, conformational analysis for the cyclization of (3R,S)-2,3-oxidosqualene and further evidence for the requirement of an isopropylidene moiety both for initiation of the polycyclization cascade and for the formation of the 5-membered E-ring. Org. Biomol. Chem. 2: 1456–1470 [DOI] [PubMed] [Google Scholar]
- 36. Hoshino T., Ohashi S. 2002. Importance of the methyl group at C(10) of squalene for hopene biosynthesis and novel carbocyclic skeletons with 6/5+5/5+(6) ring system(s). Org. Lett. 4: 2553–2556 [DOI] [PubMed] [Google Scholar]
- 37. Hoshino T., Sato T. 2002. Squalene-hopene cyclase: catalytic mechanism and substrate recognition. Chem. Commun. (Camb.) 2002: 291–301 [DOI] [PubMed] [Google Scholar]
- 38. Hoshino T., Shimizu K., Sato T. 2004. Deletion of the Gly600 residue of Alicyclobacillus acidocaldarius squalene cyclase alters the substrate specificity into that of the eukaryotic-type cyclase specific to (3S)-2,3-oxidosqualene. Angew. Chem. Int. Ed. Engl. 43: 6700–6703 [DOI] [PubMed] [Google Scholar]
- 39. Hugenholtz P., Pace N. R. 1996. Identifying microbial diversity in the natural environment: a molecular phylogenetic approach. Trends Biotechnol. 14: 190–197 [DOI] [PubMed] [Google Scholar]
- 40. Hunter W. N. 2007. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J. Biol. Chem. 282: 21573–21577 [DOI] [PubMed] [Google Scholar]
- 41. Johnson W. S., Lindell S. D., Steele J. 1987. Rate enhancement of biomimetic polyene cyclizations by a cation-stabilizing auxiliary. J. Am. Chem. Soc. 109: 5852–5853 [Google Scholar]
- 42. Kannenberg E., Poralla K. 1999. Hopanoid biosynthesis and function in bacteria. Naturwissenschaften 86: 168–176 [Google Scholar]
- 43. Kannenberg E. L., Perzl M., Müller P., Härtner T., Poralla K. 1996. Hopanoid lipids in Bradyrhizobium and other plant-associated bacteria and cloning of the Bradyrhizobium japonicum squalene-hopene cyclase gene. Plant Soil 186: 107–112 [Google Scholar]
- 44. Kleemann G., Kellner R., Poralla K. 1994. Purification and properties of the squalene-hopene cyclase from Rhodopseudomonas palustris, a purple non-sulfur bacterium producing hopanoids and tetrahymanol. Biochim. Biophys. Acta 1210: 317–320 [DOI] [PubMed] [Google Scholar]
- 45. Lichtenthaler H. K. 2000. Non-mevalonate isoprenoid biosynthesis: enzymes, genes and inhibitors. Biochem. Soc. Trans. 28: 785–789 [PubMed] [Google Scholar]
- 46. Merkofer T., Pale-Grosdemange C., Wendt K. U., Rohmer M., Poralla K. 1999. Altered product pattern of a squalene-hopene cyclase by mutagenesis of active site residues. Tetrahedron Lett. 40: 2121–2124 [Google Scholar]
- 47. Milla P., et al. 2002. Thiol-modifying inhibitors for understanding squalene cyclase function. Eur. J. Biochem. 269: 2108–2116 [DOI] [PubMed] [Google Scholar]
- 48. Moore K. S., et al. 1993. Squalamine: an aminosterol antibiotic from the shark. Proc. Natl. Acad. Sci. U. S. A. 90: 1354–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moreau R. A., Powell M. J., Fett W. F., Whitaker B. D. 1997. The effect of ethanol and oxygen on the growth of Zymomonas mobilis and the levels of hopanoids and other membrane lipids. Curr. Microbiol. 35: 124–128 [DOI] [PubMed] [Google Scholar]
- 50. Morikubo N., et al. 2006. Cation-pi interaction in the polyolefin cyclization cascade uncovered by incorporating unnatural amino acids into the catalytic sites of squalene cyclase. J. Am. Chem. Soc. 128: 13184–13194 [DOI] [PubMed] [Google Scholar]
- 51. Morrone D., et al. 2009. Gibberellin biosynthesis in bacteria: separate ent-copalyl diphosphate and ent-kaurene synthases in Bradyrhizobium japonicum. FEBS Lett. 583: 475–480 [DOI] [PubMed] [Google Scholar]
- 52. Nakano S., Ohashi S., Hoshino T. 2004. Squalene-hopene cyclase: insight into the role of the methyl group on the squalene backbone upon the polycyclization cascade. Enzymatic cyclization products of squalene analogs lacking a 26-methyl group and possessing a methyl group at C7 or C11. Org. Biomol. Chem. 2: 2012–2022 [DOI] [PubMed] [Google Scholar]
- 53. Nalin R., et al. 2000. High hopanoid/total lipids ratio in Frankia mycelia is not related to the nitrogen status. Microbiology 146 (Pt. 11): 3013–3019 [DOI] [PubMed] [Google Scholar]
- 54. Neumann S., Simon H. 1986. Purification, partial characterization and substrate specificity of a squalene cyclase from Bacillus acidocaldarius. Biol. Chem. Hoppe-Seyler 367: 723–729 [DOI] [PubMed] [Google Scholar]
- 55. Ochs D., Kaletta C., Entian K. D., Beck-Sickinger A., Poralla K. 1992. Cloning, expression, and sequencing of squalene-hopene cyclase, a key enzyme in triterpenoid metabolism. J. Bacteriol. 174: 298–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ochs D., Tappe C. H., Gartner P., Kellner R., Poralla K. 1990. Properties of purified squalene-hopene cyclase from Bacillus acidocaldarius. Eur. J. Biochem. 194: 75–80 [DOI] [PubMed] [Google Scholar]
- 57. Oliaro-Bosso S., et al. 2005. Analogs of squalene and oxidosqualene inhibit oxidosqualene cyclase of Trypanosoma cruzi expressed in Saccharomyces cerevisiae. Lipids 40: 1257–1262 [DOI] [PubMed] [Google Scholar]
- 58. Oliaro-Bosso S., Schulz-Gasch T., Balliano G., Viola F. 2005. Access of the substrate to the active site of yeast oxidosqualene cyclase: an inhibition and site-directed mutagenesis approach. Chembiochem 6: 2221–2228 [DOI] [PubMed] [Google Scholar]
- 59. Oliaro-Bosso S., et al. 2005. Access of the substrate to the active site of squalene and oxidosqualene cyclases: comparative inhibition, site-directed mutagenesis and homology-modelling studies. Biochem. Soc. Trans. 33: 1202–1205 [DOI] [PubMed] [Google Scholar]
- 60. Ourisson G., Allbrecht P. 1992. Hopanoids. 1. Geohopanoids: the most abundant natural products on earth. Acc. Chem. Res. 25: 398–402 [Google Scholar]
- 61. Ourisson G., Rohmer M., Poralla K. 1987. Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu. Rev. Microbiol. 41: 301–333 [DOI] [PubMed] [Google Scholar]
- 62. Pale-Grosdemange C., Feil C., Rohmer M., Poralla K. 1998. Kationische zwischenstufen und fehlerhafte kontrolle während der enzymatischen cyclisierung von squalen zu hopanoiden. Angew. Chem. 110: 2355–2358 [Google Scholar]
- 63. Pale-Grosdemange C., Merkofer T., Rohmer M., Poralla K. 1999. Production of bicyclic and tricyclic triterpenes by mutated squalene-hopene cyclase. Tetrahedron Lett. 40: 6009–6012 [Google Scholar]
- 64. Pearson A., Budin M., Brocks J. J. 2003. Phylogenetic and biochemical evidence for sterol synthesis in the bacterium Gemmata obscuriglobus. Proc. Natl. Acad. Sci. U. S. A. 100: 15352–15357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pearson A., Flood Page S. R., Jorgenson T. L., Fischer W. W., Higgins M. B. 2007. Novel hopanoid cyclases from the environment. Environ. Microbiol. 9: 2175–2188 [DOI] [PubMed] [Google Scholar]
- 66. Pearson A., et al. 2009. Diversity of hopanoids and squalene-hopene cyclases across a tropical land-sea gradient. Environ. Microbiol. 11: 1208–1223 [DOI] [PubMed] [Google Scholar]
- 67. Pearson A., Rusch D. B. 2009. Distribution of microbial terpenoid lipid cyclases in the global ocean metagenome. ISME J. 3: 352–363 [DOI] [PubMed] [Google Scholar]
- 68. Perzl M., Müller P., Poralla K., Kannenberg E. L. 1997. Squalene-hopene cyclase from Bradyrhizobium japonicum: cloning, expression, sequence analysis and comparison to other triterpenoid cyclases. Microbiology 143 (Pt. 4): 1235–1242 [DOI] [PubMed] [Google Scholar]
- 69. Perzl M., et al. 1998. Cloning of conserved genes from Zymomonas mobilis and Bradyrhizobium japonicum that function in the biosynthesis of hopanoid lipids. Biochim. Biophys. Acta 1393: 108–118 [DOI] [PubMed] [Google Scholar]
- 70. Poralla K. 2004. Profound insights into squalene cyclization. Chem. Biol. 11: 12–14 [DOI] [PubMed] [Google Scholar]
- 71. Poralla K. 2001. The changing path of hopanoid research: from condensing lipids to new membrane enzymes, p. 263–284 In Braun V., Götz F. (ed.), The microbial fundamentals of biotechnology. Wiley-VCH, Weilheim, Germany [Google Scholar]
- 72. Poralla K., et al. 1994. A specific amino acid repeat in squalene and oxidosqualene cyclases. Trends Biochem. Sci. 19: 157–158 [DOI] [PubMed] [Google Scholar]
- 73. Poralla K., Muth G., Hartner T. 2000. Hopanoids are formed during transition from substrate to aerial hyphae in Streptomyces coelicolor A3(2). FEMS Microbiol. Lett. 189: 93–95 [DOI] [PubMed] [Google Scholar]
- 74. Rajamani R., Gao J. 2003. Balancing kinetic and thermodynamic control: the mechanism of carbocation cyclization by squalene cyclase. J. Am. Chem. Soc. 125: 12768–12781 [DOI] [PubMed] [Google Scholar]
- 75. Rappe M. S., Giovannoni S. J. 2003. The uncultured microbial majority. Annu. Rev. Microbiol. 57: 369–394 [DOI] [PubMed] [Google Scholar]
- 76. Reinert D. J., Balliano G., Schulz G. E. 2004. Conversion of squalene to the pentacarbocyclic hopene. Chem. Biol. 11: 121–126 [DOI] [PubMed] [Google Scholar]
- 77. Reipen I. G., Poralla K., Sahm H., Sprenger G. A. 1995. Zymomonas mobilis squalene-hopene cyclase gene (shc): cloning, DNA sequence analysis, and expression in Escherichia coli. Microbiology 141 (Pt. 1): 155–161 [DOI] [PubMed] [Google Scholar]
- 78. Rohmer M., Bouvier P., Ourisson G. 1980. Non-specific lanosterol and hopanoid biosynthesis be a cell-free system from the bacterium Methylococcus capsulatus. Eur. J. Biochem. 112: 557–560 [DOI] [PubMed] [Google Scholar]
- 79. Rohmer M., Bouvier-Nave P., Ourisson G. 1984. Distribution of hopanoid triterpenes in prokaryotes. J. Gen. Microbiol. 130: 1137–1150 [Google Scholar]
- 80. Saar J., Kader J. C., Poralla K., Ourisson G. 1991. Purification and some properties of the squalene-tetrahymanol cyclase from Tetrahymena thermophila. Biochim. Biophys. Acta 1075: 93–101 [DOI] [PubMed] [Google Scholar]
- 81. Sahm H., Rohmer M., Bringer-Meyer S., Sprenger G. A., Welle R. 1993. Biochemistry and physiology of hopanoids in bacteria. Adv. Microb. Physiol. 35: 247–273 [DOI] [PubMed] [Google Scholar]
- 82. Sato T., Hoshino T. 2001. Catalytic function of the residues of phenylalanine and tyrosine conserved in squalene-hopene cyclases. Biosci. Biotechnol. Biochem. 65: 2233–2242 [DOI] [PubMed] [Google Scholar]
- 83. Sato T., Hoshino T. 1999. Functional analysis of the DXDDTA motif in squalene-hopene cyclase by site-directed mutagenesis experiments: initiation site of the polycyclization reaction and stabilization site of the carbocation intermediate of the initially cyclized A-ring. Biosci. Biotechnol. Biochem. 63: 2189–2198 [DOI] [PubMed] [Google Scholar]
- 84. Sato T., Hoshino T. 1999. Kinetic studies on the function of all the conserved tryptophans involved inside and outside the QW motifs of squalene-hopene cyclase: stabilizing effect of the protein structure against thermal denaturation. Biosci. Biotechnol. Biochem. 63: 1171–1180 [DOI] [PubMed] [Google Scholar]
- 85. Sato T., Kanai Y., Hoshino T. 1998. Overexpression of squalene-hopene cyclase by the pET vector in Escherichia coli and first identification of tryptophan and aspartic acid residues inside the QW motif as active sites. Biosci. Biotechnol. Biochem. 62: 407–411 [DOI] [PubMed] [Google Scholar]
- 86. Sato T., Kouda M., Hoshino T. 2004. Site-directed mutagenesis experiments on the putative deprotonation site of squalene-hopene cyclase from Alicyclobacillus acidocaldarius. Biosci. Biotechnol. Biochem. 68: 728–738 [DOI] [PubMed] [Google Scholar]
- 87. Seckler B., Poralla K. 1986. Characterization and partial purification of squalene-hopene cyclase from Bacillus acidocaldarius. Biochim. Biophys. Acta 881: 356–363 [Google Scholar]
- 88. Shi Z., Buntel C. J., Griffin J. H. 1994. Isolation and characterization of the gene encoding 2,3-oxidosqualene-lanosterol cyclase from Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 91: 7370–7374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Smentek L., Hess B. A. 2010. Compelling computational evidence for the concerted cyclization of the ABC rings of hopene from protonated squalene. J. Am. Chem. Soc. 132: 17111–17117 [DOI] [PubMed] [Google Scholar]
- 90. Sun T. P., Kamiya Y. 1994. The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis. Plant Cell 6: 1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tanaka H., Noguchi H., Abe I. 2004. 1-Methylidenesqualene and 25-methylidenesqualene as active-site probes for bacterial squalene:hopene cyclase. Org. Lett. 6: 803–806 [DOI] [PubMed] [Google Scholar]
- 92. Tanaka H., Noguchi H., Abe I. 2005. Enzymatic formation of indole-containing unnatural cyclic polyprenoids by bacterial squalene:hopene cyclase. Org. Lett. 7: 5873–5876 [DOI] [PubMed] [Google Scholar]
- 93. Tantillo D. J. 2008. Recent excursions to the borderlands between the realms of concerted and stepwise: carbocation cascades in natural products biosynthesis. J. Phys. Org. Chem. 21: 561–570 [Google Scholar]
- 94. Thoma R., et al. 2004. Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase. Nature 432: 118–122 [DOI] [PubMed] [Google Scholar]
- 95. Tippelt A., Jahnke L., Poralla K. 1998. Squalene-hopene cyclase from Methylococcus capsulatus (Bath): a bacterium producing hopanoids and steroids. Biochim. Biophys. Acta 1391: 223–232 [DOI] [PubMed] [Google Scholar]
- 96. Vogel B. S., Wildung M. R., Vogel G., Croteau R. 1996. Abietadiene synthase from grand fir (Abies grandis). cDNA isolation, characterization, and bacterial expression of a bifunctional diterpene cyclase involved in resin acid biosynthesis. J. Biol. Chem. 271: 23262–23268 [DOI] [PubMed] [Google Scholar]
- 97. Welander P. V., et al. 2009. Hopanoids play a role in membrane integrity and pH homeostasis in Rhodopseudomonas palustris TIE-1. J. Bacteriol. 191: 6145–6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wendt K. U. 2006. Enzyme mechanisms for triterpene cyclization: new pieces of the puzzle. Angew. Chem. Int. Ed. Engl. 44: 3966–3971 [DOI] [PubMed] [Google Scholar]
- 99. Wendt K. U., Feil C., Lenhart A., Poralla K., Schulz G. E. 1997. Crystallization and preliminary X-ray crystallographic analysis of squalene-hopene cyclase from Alicyclobacillus acidocaldarius. Protein Sci. 6: 722–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wendt K. U., Lenhart A., Schulz G. E. 1999. The structure of the membrane protein squalene-hopene cyclase at 2.0 A resolution. J. Mol. Biol. 286: 175–187 [DOI] [PubMed] [Google Scholar]
- 101. Wendt K. U., Poralla K., Schulz G. E. 1997. Structure and function of a squalene cyclase. Science 277: 1811–1815 [DOI] [PubMed] [Google Scholar]
- 102. Wendt K. U., Schulz G. E., Corey E. J., Liu D. R. 2000. Enzyme mechanisms for polycyclic triterpene formation. Angew. Chem. Int. Ed. Engl. 39: 2812–2833 [PubMed] [Google Scholar]
- 103. Wisotzkey J. D., Jurtshuk P., Jr., Fox G. E., Deinhard G., Poralla K. 1992. Comparative sequence analyses on the 16S rRNA (rDNA) of Bacillus acidocaldarius, Bacillus acidoterrestris, and Bacillus cycloheptanicus and proposal for creation of a new genus, Alicyclobacillus gen. nov. Int. J. Syst. Bacteriol. 42: 263–269 [DOI] [PubMed] [Google Scholar]
- 104. Withers S. T., Keasling J. D. 2007. Biosynthesis and engineering of isoprenoid small molecules. Appl. Microbiol. Biotechnol. 73: 980–990 [DOI] [PubMed] [Google Scholar]
- 105. Xu R., Fazio G. C., Matsuda S. P. 2004. On the origins of triterpenoid skeletal diversity. Phytochemistry 65: 261–291 [DOI] [PubMed] [Google Scholar]
- 106. Yoder R. A., Johnston J. N. 2005. A case study in biomimetic total synthesis: polyolefin carbocyclizations to terpenes and steroids. Chem. Rev. 105: 4730–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]