Abstract
We report the use of antimicrobial hemolymph proteins from the model host Galleria mellonella as an inhibitor for various Listeria strains, providing a novel source for antilisterial therapeutics. We also have shown that specific virulence-associated genes known to mediate antimicrobial resistance of Listeria in mammalian models indicated a similar function in Galleria.
TEXT
The Gram-positive bacterium Listeria monocytogenes is able to cause food-borne infections in humans, such as listeriosis, which develops into fatal sepsis, meningitis, and meningoencephalitis (7, 12, 15). Listeriae are ubiquitously distributed in the environment and are resistant to extreme food-manufacturing processes. Recently, L. monocytogenes contamination in a food plant in Canada resulted in 23 casualties, along with 57 confirmed cases (Public Health Agency of Canada; www.phac-aspc.gc.ca). The pathogenic potential of Listeria is further attributed to a growing number of strains resistant to antimicrobial compounds and particularly to antibiotics (5, 19). Thus, it has become extremely important to identify novel sources for antilisterial therapeutics of both clinical and industrial interest.
The mechanisms of bacterial resistance against cationic antimicrobial peptides (CAMPs) are not clearly understood, and there are only few reports relating antimicrobial sensitivity of Listeria (5, 19). Identification of the two-component system virR/virS in Listeria revealed its dual role in virulence and resistance against CAMPs (13, 20). It is known that the transcriptional regulator VirR independently regulates expression of mprF and the genes comprising the dlt operon, which are well known for providing resistance against CAMPs of both animal and bacterial origin (2, 3, 20, 22). MprF synthesizes the lysylphosphatidyl glycerol membrane phospholipids (22), and the dlt operon (comprising of the genes dltA, dltB, dltC, and dltD)-encoded proteins are mainly responsible for adding d-alanine residues to the cell wall-associated lipotechoic acids (LTAs) (1). Both of these candidate genes maintain the positive charge balance of the bacterial cell wall, facilitating CAMP resistance.
Recently, we reported that following innate immune induction, the hemolymph of the lepidopteran greater wax moth Galleria mellonella produces antimicrobials which inhibited L. monocytogenes growth (16). Using the Galleria model, we have represented the comparative virulence attributes of different Listeria species and L. monocytogenes serotypes, similar to the findings with other mammalian models. However, Galleria can resist septic L. monocytogenes infection, and a very high 50% lethal dose (LD50) value (106 CFU/larva) is required for larval mortality. Here we report that the different Listeria species and L. monocytogenes serotypes are sensitive to the induced antimicrobial hemolymph proteins of Galleria. Accordingly, our findings demonstrate a similar role of virulence-associated genes like virR, dltB, and mprF in antimicrobial resistance against Galleria hemolymph, comparable to the earlier findings using mammalian models (1, 13, 22).
Preparation of bacterial cultures, rearing of Galleria, injection methods, RNA isolation, reverse transcriptase PCR (RT-PCR), and antimicrobial assays were performed as described by Mukherjee et al. (16). For RT-PCR analysis, we used appropriate primers to amplify the housekeeping gene 18S RNA, lysozyme, insect metalloproteinase inhibitor (IMPI), defensin 1, and defensin 2, as described previously (16). In the case of cecropin D amplification, we used the primers cecropin D-forward (5′-GCCATGTTCTTCACCACGAC-3′) and -reverse (5′-TCAGTCACCGCCTTTAATGAT-3′), respectively.
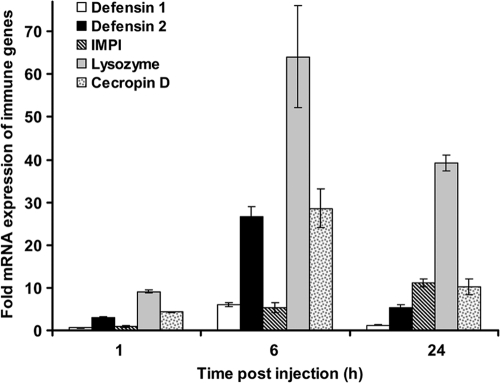
It is known that innate immune activation leads to production of antimicrobial peptides in Galleria (6, 23). Following administration of immune elicitors such as heat-killed Listeria, we found induction of antimicrobial peptide-encoding genes like galiomycin (defensin 1), gallerimycin (defensin 2), IMPI, lysozyme, and cecropin D (Fig. 1). Transcriptional activation is represented as the fold change of immune-related genes in challenged Galleria relative to those in unchallenged larvae and normalized using the housekeeping gene 18S. The amounts of immune-related mRNAs of gallerimycin and lysozyme were found to be induced about 3.0-fold and 9.1-fold at 1 h postinjection, respectively. At 6 h postinjection, we determined increased mRNA levels of IMPI (∼5.2-fold), galiomycin (∼6.0-fold), gallerimycin (∼26-fold), cecropin D (∼28-fold), and lysozyme (∼64-fold). The amounts of induced mRNA for IMPI, cecropin D, and lysozyme were found to be 11-fold, 10-fold, and 39-fold after 24 h postinjection. After this time point, the hemolymph samples were collected and tested for their antimicrobial activities against human-pathogenic L. monocytogenes.
Fig. 1.
Semiquantitative induction of immune-responsive genes in Galleria after challenge with heat-killed bacteria. The transcription levels of defensin 1, defensin 2, IMPI, lysozyme, and cecropin D following injection of heat-killed L. monocytogenes were determined by quantitative real time RT-PCR analysis and are shown relative to the expression levels of mock-injected animals. Values were normalized on expression levels of the housekeeping gene 18S and represent the results of three independent determinations ± SD.
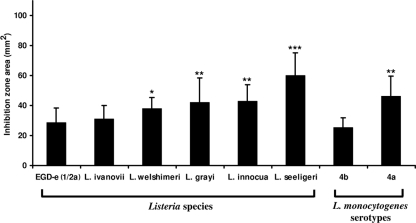
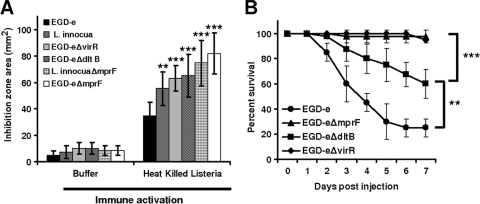
Induction of CAMPs and their secretion into the hemolymph resulted in growth inhibition of Listeria in brain heart infusion medium (BHI). All Listeria species tested in this study were sensitive to the antimicrobial hemolymph proteins of Galleria (Fig. 2). Nonpathogenic Listeria, such as Listeria seeligeri (21), showed highest sensitivity, followed by L. innocua (8), L. grayi (10), and L. welshimeri (9) with respect to pathogenic L. monocytogenes (Fig. 2). No significant difference in growth inhibition was recorded between pathogenic L. monocytogenes and Listeria ivanovii. Both were least sensitive to the induced antimicrobials of Galleria. Among the different L. monocytogenes serotypes, the 4b strain, which is mainly responsible for Listeria epidemics, causes high mortality rates in Galleria and shows only low sensitivity to the hemolymph, along with the 1/2a strain. In contrast, the 4a serotype was significantly more sensitive to hemolymph antimicrobials than the other L. monocytogenes serotypes (Fig. 2). We also investigated the role of specific virulence-associated genes in L. monocytogenes, virR, mprF, and dltB, against the antimicrobial hemolymph proteins using isogenic deletion mutants (see the supplemental material). We found that virR, mprF, and dltB in L. monocytogenes are important factors to counteract the induced antimicrobial activities of Galleria hemolymph. The ΔvirR, ΔmprF, and ΔdltB mutants showed significantly higher antimicrobial sensitivities than their isogenic wild-type strain, EGD-e (Fig. 3 A). However, deletion of mprF from nonpathogenic L. innocua (18) (see the supplemental material) also resulted in a more sensitive strain than its wild type (Fig. 3A).
Fig. 2.
The hemolymph of preimmune activated larvae produces antimicrobial effectors that inhibit the growth of various Listeria spp. Growth of the nonpathogenic Listeria species L. seeligeri, L. innocua, L. grayi, and L. welshimeri is significantly inhibited with respect to that of the pathogenic strain EGD-e. There were no significant changes in growth inhibition between L. ivanovii (P < 0.2) and the L. monocytogenes serotypes 4b (P < 0.2) and 1/2a. Only the L. monocytogenes serotype 4a shows high sensitivity toward antimicrobial effectors from Galleria hemolymph. A hemolymph sample of an individual larva was tested for all Listeria species, and each repetition contained hemolymph samples from at least 20 larvae. Results represent mean values for at least three independent experiments per strain using 20 larvae (*, P < 0.05; **, P < 0.005; ***, P < 0.0005).
Fig. 3.
Growth inhibition and survival of listerial isogenic mutants compared to their wild types in the Galleria mellonella infection model. (A) In response to the hemolymph samples obtained from larvae injected with heat-killed Listeria, pathogenic EGD-e and its isogenic mutants EGD-eΔmprF, EGD-eΔvirR, and EGD-eΔdltB, along with L. innocua and L. innocuaΔmprF, showed significant high growth inhibitions in comparison to the hemolymph samples obtained from larvae injected with buffer (P < 0.0005). Significantly high growth inhibition was observed for EGD-eΔmprF, followed by EGD-eΔvirR and EGD-eΔdltB, with respect to the pathogenic EGD-e strain. Also, the mprF deletion strain of L. innocua showed high sensitivity to the activated hemolymph proteins of Galleria in comparison to that of its wild-type strain (P < 0.0005). A hemolymph sample of an individual larva was tested for all Listeria mutants, and each repetition contained hemolymph samples from at least 20 larvae. Results represent mean values of at least three independent determinations ± SD (*, P < 0.05; **, P < 0.005; ***, P < 0.0005). (B) Inoculation with 106 CFU/larvae EGD-eΔvirR (⧫) and EGD-eΔmprF (▴) resulted in significantly lower mortality rates of Galleria larvae than infection with the wild type EGD-e strain (•). The EGD-eΔdltB (▪) strain caused only a partial attenuation in Galleria, as shown by intermediate mortality rates in respect to infection with EGD-e. Results represent mean values of at least three independent determinations ± SD from each of 20 animals per treatment (*, P < 0.05; **, P < 0.005; ***, P < 0.0005).
Our infection studies with Galleria gave similar results, as was previously demonstrated in the mouse model. The strains EGD-eΔvirR and EGD-eΔmprF (at a 106 CFU/larva concentration) were strongly attenuated, whereas EGD-eΔdltB showed only a partial reduction in pathogenicity compared to the wild type (Fig. 3B). It has been shown that that deletion of the dlt operon from Bacillus cereus resulted in reduced survival of Galleria larvae upon oral infection (2). The varied in vitro and in vivo survivability of EGD-eΔdltB in Galleria hemolymph and a whole-animal model possibly indicates the specialized role of other virulence-associated proteins, like MprF, in providing resistance against antimicrobial host defense, such as phagocytosis and cationic antimicrobial peptides.
Confirming the antimicrobial effects of Galleria hemolymph, we have tested the effect of cecropin D, a CAMP of the greater wax moth (4, 11), against pathogenic EGD-e and its isogenic deletion mutants, EGD-eΔmprF, EGD-eΔdltB, and EGD-eΔvirR. Cecropin is known for exhibiting strong antimicrobial activities, especially against Gram-positive bacteria (4, 11). Cecropin D was chemically synthesized by standard Merrifield solid-phase peptide synthesis (14, 17), purified by reverse-phase high-performance liquid chromatography (RP-HPLC), and controlled by electrospray ionization mass spectrometry (ESI-MS) (see Fig. S1A and B in the supplemental material). Purity was >98%, and the molecular weight (MW) was found to be 4,256.2 (theoretical MW, 4,255.9). Antimicrobial assays were performed as described by Cytryńska et al. and Kim et al. (4, 11). Growth inhibition in the presence of cecropin D was observed for all tested strains in BHI (Fig. 4 A, B, C and D). However, the direct lethality associated with the growth inhibitions of Listeria following cecropin D treatment is yet to be reported. Similarly, the human cationic peptide LL37 and bacterial polymyxin B showed strong growth arrest of pathogenic EGD-e and its isogenic mutants, EGD-eΔmprF, EGD-eΔdltB, and EGD-eΔvirR, in BHI (see Fig. S2A and B in the supplemental material). The antilisterial effects of cationic peptides such as cecropin D indicate their great potential as new therapeutics and justify more efforts for the identification and production of other novel antimicrobial candidates for treating bacterial infections.
Fig. 4.
Growth of the L. monocytogenes EGD-e wild type (A) or of the deletion mutant EGD-eΔdltB (B), EGD-eΔmprF (C), or EGD-eΔvirR (D) in the presence of the Galleria cationic peptide cecropin D. Bacteria were cultured in BHI broth supplemented with 160 μM cecropin D, and the optical density at 600 nm (OD600) was measured hourly. Results represent mean values of at least three independent determinations ± SD (*, P < 0.05; **, P < 0.005; ***, P < 0.0005).
Conclusion.
We report the contributions of the mprF, virR, and dltB genes to CAMP resistance in Galleria mellonella, indicating the involvement of the members comprising the VirR regulon for antimicrobial resistance of Listeria. The varied sensitivities of different Listeria species and serotypes to the hemolymph proteins advocate for the development of Galleria-derived antimicrobial peptides for therapeutic use to counteract the development of multidrug-resistant pathogens.
Supplementary Material
Acknowledgments
We thank Martina Hudel and Meike Fischer for excellent technical assistance.
The project was funded by the German Ministry of Education and Research through the ERANET program grants SPATELIS and sncRNAomics to T.H. and T.C. and through the LOEWE program of the state Hessia by the collaborative research project Insect Biotechnology to A.V., T.H., and T.C. We acknowledge financial support of K.M. by Rainer Fischer from RWTH Aachen, Germany.
We have no financial conflict of interest.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 29 April 2011.
REFERENCES
- 1. Abachin E., et al. 2002. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43:1–14 [DOI] [PubMed] [Google Scholar]
- 2. Abi Khattar Z., et al. 2009. The dlt operon of Bacillus cereus is required for resistance to cationic antimicrobial peptides and for virulence in insects. J. Bacteriol. 191:7063–7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chatterjee S. S., et al. 2006. Invasiveness is a variable and heterogeneous phenotype in Listeria monocytogenes serotype strains. Int. J. Med. Microbiol. 296:277–286 [DOI] [PubMed] [Google Scholar]
- 4. Cytryńska M., Mak P., Zdybicka-Barabas A., Suder P., Jakubowicz T. 2007. Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides 28:533–546 [DOI] [PubMed] [Google Scholar]
- 5. Davis J. A., Jackson C. R. 2009. Comparative antimicrobial susceptibility of Listeria monocytogenes, L. innocua, and L. welshimeri. Microb. Drug Resist. 15:27–32 [DOI] [PubMed] [Google Scholar]
- 6. De Verno P. J., Chadwick J. S., Aston W. P., Dunphy G. B. 1984. The in vitro generation of an antibacterial activity from the fat body and hemolymph of non-immunized larvae of Galleria mellonella. Dev. Comp. Immunol. 8:537–546 [DOI] [PubMed] [Google Scholar]
- 7. Fleming D. W., et al. 1985. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N. Engl. J. Med. 312:404–407 [DOI] [PubMed] [Google Scholar]
- 8. Glaser P., et al. 2001. Comparative genomics of Listeria species. Science 294:849–852 [DOI] [PubMed] [Google Scholar]
- 9. Hain T., et al. 2006. Whole-genome sequence of Listeria welshimeri reveals common steps in genome reduction with Listeria innocua as compared to Listeria monocytogenes. J. Bacteriol. 188:7405–7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hain T., et al. 2007. Pathogenomics of Listeria spp. Int. J. Med. Microbiol. 297(7–8):541–557 [DOI] [PubMed] [Google Scholar]
- 11. Kim C., et al. 2004. Purification and cDNA cloning of a cecropin-like peptide from the great wax moth, Galleria mellonella. Mol. Cells 17:262–266 [PubMed] [Google Scholar]
- 12. Linnan M. J., et al. 1988. Epidemic listeriosis associated with Mexican-style cheese. N. Engl. J. Med. 319:823–828 [DOI] [PubMed] [Google Scholar]
- 13. Mandin P., et al. 2005. VirR, a response regulator critical for Listeria monocytogenes virulence. Mol. Microbiol. 57:1367–1380 [DOI] [PubMed] [Google Scholar]
- 14. Merrifield B. 1997. Concept and early development of solid-phase peptide synthesis. Methods Enzymol. 289:3–13 [DOI] [PubMed] [Google Scholar]
- 15. Midelet-Bourdin G., et al. 2006. Modification of a virulence-associated phenotype after growth of Listeria monocytogenes on food. J. Appl. Microbiol. 101:300–308 [DOI] [PubMed] [Google Scholar]
- 16. Mukherjee K., et al. 2010. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl. Environ. Microbiol. 76:310–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sabatino G., Papini A. M. 2008. Advances in automatic, manual and microwave-assisted solid-phase peptide synthesis. Curr. Opin. Drug Discov. Dev. 11:762–770 [PubMed] [Google Scholar]
- 18. Schaferkordt S., Chakraborty T. 1995. Vector plasmid for insertional mutagenesis and directional cloning in Listeria spp. Biotechniques 19:720–725 [PubMed] [Google Scholar]
- 19. Schwaiger K., Schmied E. M., Bauer J. 2009. Comparative analysis on antibiotic resistance characteristics of Listeria spp. and Enterococcus spp. isolated from laying hens and eggs in conventional and organic keeping systems in Bavaria, Germany. Zoonoses Public Health 57:171–180 [DOI] [PubMed] [Google Scholar]
- 20. Staubitz P., Neumann H., Schneider T., Wiedemann I., Peschel A. 2004. MprF-mediated biosynthesis of lysylphosphatidylglycerol, an important determinant in staphylococcal defensin resistance. FEMS Microbiol. Lett. 231:67–71 [DOI] [PubMed] [Google Scholar]
- 21. Steinweg C., et al. 2010. Complete genome sequence of Listeria seeligeri, a nonpathogenic member of the genus Listeria. J. Bacteriol. 192:1473–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thedieck K., et al. 2006. The MprF protein is required for lysinylation of phospholipids in listerial membranes and confers resistance to cationic antimicrobial peptides (CAMPs) on Listeria monocytogenes. Mol. Microbiol. 62:1325–1339 [DOI] [PubMed] [Google Scholar]
- 23. Vilcinskas A., Jegorov A., Landa Z., Matha V. 1999. Effects of beauverolide L and cyclosporin A on humoral and cellular immune response of the greater wax moth, Galleria mellonella. Comp. Biochem. Physiol. 122C:83–92 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.