Abstract
The alydid stinkbug Riptortus pedestris is specifically associated with a beneficial Burkholderia symbiont in the midgut crypts. Exceptional among insect-microbe mutualistic associations, the Burkholderia symbiont is not vertically transmitted but orally acquired by nymphal insects from the environment every generation. Here we experimentally investigated the process of symbiont acquisition during the nymphal development of R. pedestris. In a field population, many 2nd instar nymphs were Burkholderia free, while all 3rd, 4th, and 5th instar nymphs were infected. When reared on soil-grown potted soybean plants, Burkholderia acquisition occurred at a drastically higher frequency in the 2nd instar than in the other instars. Oral administration of cultured Burkholderia cells showed that 2nd and 3rd instar nymphs are significantly more susceptible to the symbiont infection than 1st, 4th, and 5th instar nymphs. Histological observations revealed rudimentary midgut crypts in the 1st instar, in contrast to well-developed midgut crypts in the 2nd and later instars. These results indicate that R. pedestris acquires the Burkholderia symbiont from the environment mainly during the 2nd instar period and strongly suggest that the competence for the symbiont infection is developmentally regulated by the host side. Potential mechanisms involved in infection competence and possible reasons why the infection preferentially occurs in the 2nd instar are discussed.
INTRODUCTION
Insects consist of over one million species and represent the most diverse animal group in the terrestrial ecosystem (18). Notably, many of them harbor microbial symbionts within their gut, body cavity, or cells. In particular, insects that feed exclusively on restricted diets, such as plant sap, vertebrate blood, or woody materials, tend to possess highly developed symbiotic systems, wherein the symbionts provide essential nutrients and/or help food digestion for their hosts. In many cases, the host insects are highly dependent on their microbial partners and vice versa: the hosts suffer retarded growth, mortality, and/or sterility when deprived of the symbionts, while the symbionts cannot survive without the hosts (5, 7, 10).
To ensure the occurrence of pivotal associations with the microbial mutualists, such insects have evolved a variety of mechanisms for symbiont transmission from mother to offspring. In aphid-Buchnera, mealybug-Tremblaya, louse-Riesia, and other intracellular symbiotic associations, the symbiont transmission to the next generation occurs transovarially within the maternal body during the process of oogenesis or embryogenesis (7, 14, 23, 38, 48). In gut extracellular symbiotic associations in many stinkbugs, anobiid beetles, and others, newborn nymphs establish the symbiont transmission via oral ingestion of symbiont cells that are either smeared on the egg surface or encased in symbiont capsules (1, 7, 22, 25, 26, 28, 31, 43). Among diverse insect-microbe mutualisms, symbionts are generally transmitted vertically at the initial stages of the host development: prenatally to oocytes in intracellular associations and postnatally to newborns in gut extracellular associations.
Apart from the insects, however, vertical transmission of beneficial symbiont is not necessarily common (6). Instead, environmental acquisition of microbial mutualists is universally found among marine invertebrates and land plants, as in the squid-Vibrio luminescent symbioses (40), the coral-Symbiodinium photosynthetic symbioses (2), the tubeworm-Endoriftia chemoautotrophic symbioses (11), the legume-Rhizobium and alder-Frankia nitrogen-fixing symbioses (4, 15, 42), and many others. In the marine and soil ecosystems, free-living symbionts are protected from environmental stresses such as UV irradiation and desiccation that must be detrimental to free-living symbionts in the terrestrial ecosystem, which may account for the paucity of environmental acquisition of mutualistic symbionts among insects.
In this context, it is notable that environmental symbiont acquisition was discovered in an insect-microbe mutualism wherein the symbiont is a group of soil bacteria. The bean bug Riptortus pedestris (or Riptortus clavatus as a synonym) (Fig. 1A and B), which belongs to the family Alydidae, is a serious pest of leguminous crops in eastern Asia (49). R. pedestris possesses numerous crypts in the posterior midgut, in which a specific betaproteobacterial symbiont of the genus Burkholderia is harbored (32). When deprived of the gut symbiont, R. pedestris suffers a marked reduction in body size and weight, indicating the beneficial nature of the symbiosis (30). However, unlike the above-mentioned stinkbugs, R. pedestris does not transmit the Burkholderia symbiont vertically, but acquires free-living bacteria present in the rhizosphere of leguminous plants every generation (30). A recent extensive survey of diverse stinkbugs suggests that environmental acquisition of the soil-derived Burkholderia symbiont is not restricted to R. pedestris but found in many allied stinkbug species of the superfamilies Lygaeoidea and Coreoidea (29).
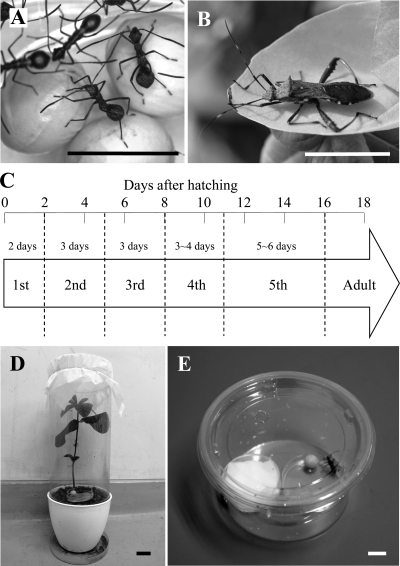
Fig. 1.
(A) 2nd instar nymphs; (B) adult male of Riptortus pedestris; (C) developmental time course of Burkholderia-infected R. pedestris; (D) soybean pot for rearing of R. pedestris on a soil-grown soybean plant and a soybean seed; (E) plastic container for rearing of R. pedestris on a soybean seed and 0.05% ascorbic acid-containing water. Bars in photos represent 1 cm.
Owing to the environmental symbiont acquisition, it is expected that R. pedestris and allied stinkbugs have been freed from the developmental constraint that symbiont infection must be vertically established either during oogenesis or upon egg hatching. Potentially, the host insect can acquire the symbiont at any point during the postembryonic developmental course. Here we experimentally investigated the process of symbiont acquisition in R. pedestris that has five nymphal instars before adult molting (Fig. 1C). Interestingly, we identified a specific developmental window for symbiont acquisition at the 2nd instar stage, indicating morphogenetic and physiological mechanisms of the host side to regulate the establishment of the symbiosis.
MATERIALS AND METHODS
Insects.
Nymphs and adults of R. pedestris were collected in a field of the adzuki bean, Vigna angularis, in Tsukuba, Ibaraki, Japan, in September 2009. In the field, many 2nd, 3rd, 4th, and 5th instar nymphs and adults were obtained from adzuki bean plants, whereas no 1st instar nymphs were found. Previous studies documented that adult females of R. pedestris tend to lay eggs on gramineous plants, and nymphs migrate to leguminous host plants after hatching (35). For experimental works, we used a laboratory stock of R. pedestris that had originally been collected from a field of the soybean Glycine max in Tsukuba, Ibaraki, Japan, and had been maintained on soybean seeds and DWA (distilled water containing 0.05% ascorbic acid) at 25°C under a long-day regimen (16 h of light and 8 h of dark).
Bacterial strains.
A Burkholderia symbiont strain, RPE64, was isolated from midgut crypts of a field-collected R. pedestris (29). A rifampin-resistant spontaneous mutant strain of the Burkholderia symbiont, RPE75, was obtained from RPE64 by being cultured on a yeast extract-glucose (YG) agar plate (29) containing 100 μg/ml of rifampin at 26°C for 3 days. These symbiont strains were cultured at 26°C on YG agar plates and stored as frozen stocks at −80°C.
Dissection and DNA extraction.
Insects were surface sterilized with 70% ethanol and dissected with fine forceps and microscissors under a dissection microscope in a petri dish filled with a phosphate-buffered saline (PBS: 137 mM NaCl, 8.1 mM Na2HPO4, 2.7 mM KCl, 1.5 mM KH2PO4 [pH 7.5]). The dissected midgut crypts were either immediately subjected to DNA extraction by a standard procedure with proteinase K digestion and phenol-chloroform extraction (47) or preserved in acetone until use (13).
Diagnostic PCR.
Diagnostic PCR detections were conducted with AmpliTaq Gold DNA polymerase (Applied Biosystems) and its supplemented buffer system under a temperature profile of 95°C for 10 min followed by 30 cycles of 95°C for 30 s, 55°C (16S rRNA gene of the Burkholderia symbiont) or 48°C (COI gene) for 1 min, and 72°C for 1 min. Primers are listed in Table 1.
Table 1.
Primer sets used for diagnostic and quantitative PCR
| Target organism(s) | Target gene | Primer name | Nucleotide sequence (5′→3′) | Product size (bp) | Annealing temp (°C) | Purpose | Source or reference |
|---|---|---|---|---|---|---|---|
| Stinkbug | Mitochondrial cytochrome oxidase I gene (COI) | LCO1490 | GGTCAACAAATCATAAAGATATTGG | 650 | 48 | Diagnostic PCR | 12 |
| HCO2198 | TAAACTTCAGGGTGACCAAAAAATCA | ||||||
| Burkholderia symbiont | 16S rRNA gene | Burk16SF | TTTTGGACAATGGGGGCAAC | 750 | 55 | Diagnostic PCR | 32 |
| Burk16SR | GCTCTTGCGTAGCAACTAAG | ||||||
| Burkholderia spp. | dnaA | BurkDnaA-F | GGCCTSGGCAAGACSCAYCT | 800 | 69 | Cloning | This study |
| BurkDnaA-R | GTSGTRTGRTCGCGGCCGCC | ||||||
| Burkholderia symbiont | dnaA | BSdnaA-F | AGCGCGAGATCAGACGGTCGTCGAT | 150 | 60 | Quantitative PCR | This study |
| BSdnaA-R | TCCGGCAAGTCGCGCACGCA |
Quantitative PCR.
A 0.8-kb segment of dnaA was amplified from the symbiont strain RPE75 with primers BurkDnaA-F and BurkDnaA-R (Table 1) under a temperature profile of 95°C for 10 min followed by 30 cycles of 95°C for 30 s, 69°C for 1 min, and 72°C for 1 min. The PCR products were cloned and sequenced as previously described (32). Real-time quantitative PCR was performed using SYBR green and an Mx3000P quantitative PCR system (Stratagene) as described previously (34) with primers BSdnaA-F and BSdnaA-R (Table 1), which targeted a 0.15-kb region of the dnaA gene. DNA extraction was conducted with a NucleoSpin tissue kit (Macherey-Nagel), and extracted DNA was eluted in 200 μl of Tris-EDTA (TE) buffer. Each of the PCR mixtures (20 μl in total) consisted of 2 μl of 10× TaqGold buffer (Applied Biosystems), 1.2 μl of 25 mM MgCl2, 2 μl of deoxynucleoside triphosphates (dNTPs; 2 mM each dATP, dTTP, dGTP, and dCTP), 1 μl of dimethyl sulfoxide, 0.2 μl of SYBR green I (1/1,000 diluted solution) (Molecular Probes), 0.6 μl of primer mixture (5 μM each forward and reverse primers), 0.1 μl of AmpliTaq Gold DNA polymerase (Applied Biosystems), 8.9 μl of distilled water, and 4 μl of DNA sample. The PCR temperature profile was 40 cycles of 95°C for 10 s, 60°C for 15 s, and 72°C for 15 s. A standard curve for the dnaA gene was generated with standard samples that contained 10, 102, 103, 104, 105, 106, and 107 copies per reaction of the target PCR product.
Rearing experiments.
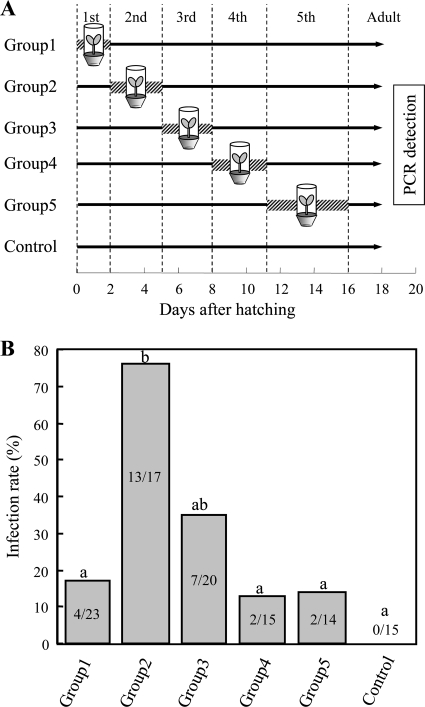
A previous study demonstrated that nymphs of R. pedestris acquire the Burkholderia symbiont when reared on soil-grown soybean plants, whereas Burkholderia infection does not occur when nymphs are reared in clean plastic containers without soil (30). Soybean pots were prepared as previously described (30), with each pot containing a soybean plant, a soybean seed, and soil from a soybean field (Fig. 1D). In each clean plastic container (8 cm in diameter, 5.5 cm in depth), a soybean seed was supplied with DWA (Fig. 1E). A total of 240 eggs of R. pedestris were randomly divided into six groups of 40 eggs, which were allocated to five experimental groups and a control group. In each of the experimental groups, each nymph was individually reared in a soybean pot during a specific instar and in a clean plastic container during the other instars. In the control group, each nymph was reared in a plastic container throughout the experimental period. Midgut crypts were dissected from the insects upon adult emergence and were subjected to DNA extraction and diagnostic PCR (see Fig. 3A).
Fig. 3.
(A) Experimental design to identify the developmental stage when the Burkholderia symbiont is acquired from the environment by nymphs of Riptortus pedestris. Shaded bars indicate the rearing periods on a soil-grown soybean plant, whereas black lines show the rearing periods in a clean plastic container without the soil. (B) Infection frequencies with the Burkholderia symbiont upon adult emergence of the treated insects. Different letters indicate statistically significant differences between the experimental groups (Fisher's exact probability tests after Bonferroni correction; P < 0.05). Each column shows the number of infected insects/the total number of insects examined.
Oral administration of cultured symbiont.
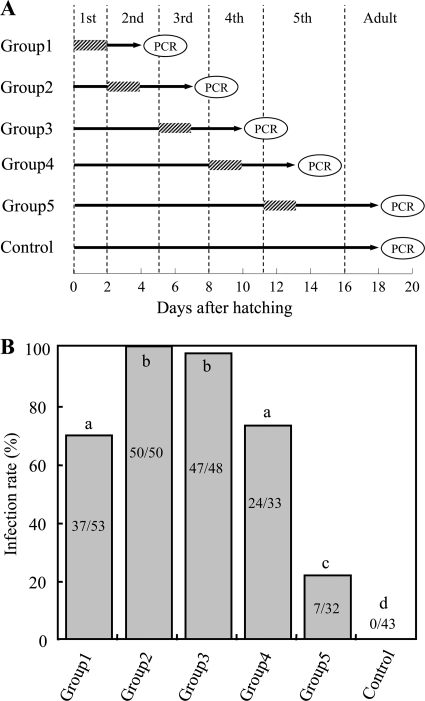
The symbiont strain RPE75 was grown to an early log phase in YG-RIF (YG medium containing 10 μg/ml rifampin) on a gyratory shaker (150 rpm) at 26°C. Colony formation units (CFU) were estimated by plating the cultured media on YG-RIF agar plates. The symbiont cells were harvested by centrifuging the cultured media, suspended in DWA, and adjusted to 107 CFU/ml in DWA. Each nymph of R. pedestris was individually reared in the clean plastic container as described above. Each nymph was fed with the symbiont-containing DWA during the first 2 days of a specific instar, while symbiont-free DWA was provided throughout the experiment, except for this 2-day period. Two days after the nymphs molted to the next instar, midgut crypts were dissected from them and were subjected to DNA extraction and diagnostic PCR for the symbiont infection. In the control treatment, nymphs were provided with symbiont-free DWA throughout the experimental period until adulthood (see Fig. 4A).
Fig. 4.
(A) Experimental design to identify the developmental stage when nymphs of Riptortus pedestris are susceptible to infection with the Burkholderia symbiont. Shaded bars indicate the periods when the insects were supplied with water containing Burkholderia, whereas black lines show the periods when the insects were supplied with sterilized water. (B) Infection frequencies with the Burkholderia symbiont 2 days after molting to the next instar. Different letters indicate statistically significant differences between the experimental groups (Fisher's exact probability tests after Bonferroni correction; P < 0.05). Each column shows the number of infected insects/the total number of insects examined.
Histological observations.
The whole midgut was dissected from each insect in PBS as described above and photographed by a digital camera (EC3; Leica) connected to a dissection microscope (S8APO; Leica). The dissected tissues were fixed with 4% paraformaldehyde for 10 min at room temperature, washed in PBS twice, incubated in PBS containing 0.1% Triton X-100 for 5 min, stained with 0.5 μM SYTOX Green and 5 U/ml of Alexa Fluor 568 phalloidin (Molecular Probes) in PBS for 20 min, washed in PBS twice, and mounted on silane-coated glass slides. Phase-contrast and confocal images were taken by a light microscope (AX70; Olympus) and a laser-scanning microscope (TCS SP2 ABOS; Leica), respectively.
Statistical analysis.
Fisher's exact probability test, chi-square test, and Kendall's rank correlation test were performed using the program R v2.11.1 (45).
Nucleotide sequence accession number.
The nucleotide sequence of the dnaA gene has been deposited in the DDBJ/EMBL/GenBank databases under accession no. AB605612.
RESULTS
Burkholderia infection in field-collected nymphs.
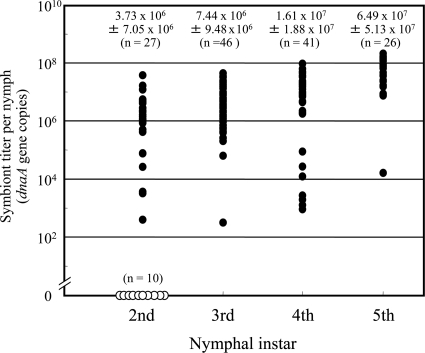
Figure 2 shows infection titers of the Burkholderia symbiont in the dissected midgut of field-collected nymphs of R. pedestris. Quantitative PCR targeting symbiont dnaA gene revealed that 27% (10/37) of 2nd instar nymphs were Burkholderia free, while all 3rd, 4th, and 5th instar nymphs were Burkholderia positive. The symbiont titers in the infected insects increased as the host developmental stages proceeded (Kendall's rank correlation test; τ = 0.443, P < 0.0001).
Fig. 2.
Infection titers of the Burkholderia symbiont in field-collected nymphs of Riptortus pedestris. Infection titers in numbers of symbiont dnaA gene copies per insect are shown. Filled dots and open dots indicate infected and uninfected insects, respectively. Means ± standard deviations (sample sizes) are shown on the plots. In the 2nd instar, uninfected insects were excluded from the calculation.
Preferential Burkholderia acquisition by 2nd instar nymphs.
The acquisition process of the Burkholderia symbiont was investigated during the nymphal development of R. pedestris. Figure 3A shows the experimental scheme of rearing on soil-grown soybean plants and rearing in clean plastic containers at different developmental stages. When 1st instar nymphs were exposed to soil-grown soybean plants, the Burkholderia infection was observed in 17.4% of the insects. In contrast, when 2nd instar nymphs were similarly treated, the Burkholderia infection was observed at a significantly higher frequency of 76.5%. In the subsequent instars, the Burkholderia acquisition declined to significantly lower frequencies: 35.0%, 13.3% and 14.3% in the 3rd, 4th and 5th instar treatments, respectively. No Burkholderia acquisition occurred in the control treatment without exposure to the soil-grown soybean plants (Fig. 3B). Mortality rates of the insects were not significantly different between the experimental groups (see Table S1 in the supplemental material).
High susceptibility of 2nd and 3rd instar nymphs to Burkholderia infection.
The infection competence for the Burkholderia symbiont was investigated during the nymphal development of R. pedestris. Figure 4A shows the experimental scheme of oral administration of the cultured Burkholderia cells to nymphs at different developmental stages. In all the experimental groups, it was confirmed that all nymphs ingested the symbiont-containing water (see Table S2 in the supplemental material). The highest infection frequencies, 100% and 97.9%, were observed in the 2nd and 3rd instar treatments, respectively. These infection frequencies were significantly higher than the infection frequencies in the 1st, 4th, and 5th instar treatments: 70.0%, 72.7%, and 21.9%, respectively. No Burkholderia infection occurred in the control treatment (Fig. 4B). In all experimental groups, no mortality was observed during the period from symbiont administration to DNA extraction (Table S2).
Histological configuration of nymphal midgut crypts.
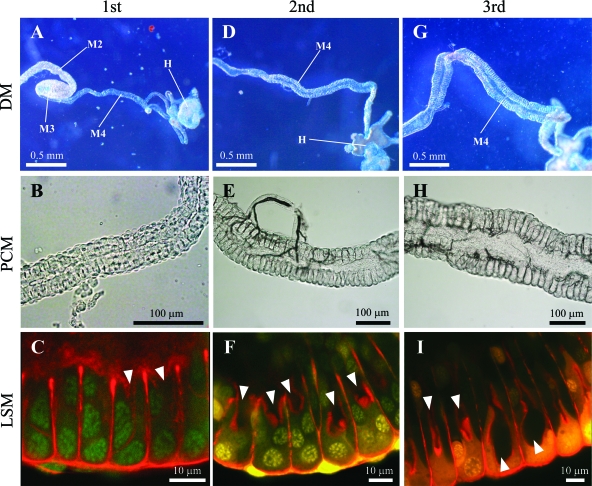
Development of the midgut crypts was observed in uninfected insects. Dissection of the nymphal alimentary tract revealed an undeveloped symbiotic organ in the 1st instar: the midgut 4th section was very thin and thread-like (Fig. 5A), wherein the crypts were tiny and rudimentary (Fig. 5B and C). In the 2nd instar, in contrast, the midgut 4th section was thicker, and two rows of the crypts with lumen were evidently seen (Fig. 5D, E, and F). In the 3rd instar and on, the midgut crypts were further developed (Fig. 5G, H, and I).
Fig. 5.
Development of midgut symbiotic organs in uninfected nymphs of Riptortus pedestris. (A to C) 1st instar nymph; (D to F) 2nd instar nymph; (G to I) 3rd instar nymph. Dissection microscopic (DM), phase-contrast microscopic (PCM), and laser-scanning microscopic (LSM) images are shown. In the LSM images, nuclei and cytoskeleton underlining the cell membrane were visualized in green/yellow and red, respectively. Arrowheads indicate the lumen of the midgut crypts. Abbreviations: M2, midgut 2nd section; M3, midgut 3rd section; M4, midgut 4th section with crypts (symbiotic organ); H, hindgut.
DISCUSSION
From these results, we conclude that a specific developmental window exists in the 2nd instar for successful establishment of the R. pedestris-Burkholderia symbiosis. Among diverse insect-microbe mutualisms, symbiont infections generally occur at initial stages of the host development. To our knowledge, the symbiont acquisition at a later developmental stage in R. pedestris is exceptional among insect-microbe mutualistic symbioses. Another exception is known from termites, wherein gut symbiotic bacteria and protists, which are responsible for cellulose digestion, are acquired after every molt of each insect via feeding of anal excrement from the colony mates (21, 24).
Possible involvement of midgut morphogenesis and physiology in the nymphal development.
Histological observations revealed that the midgut crypts, the location of the Burkholderia infection, are rudimentary in the 1st instar but well-developed in the 2nd and later instars (Fig. 5). The morphogenetic aspect is probably involved in the low susceptibility to the Burkholderia infection in the 1st instar. There are two alternative possibilities as to why the midgut crypts are atrophied and unsusceptible to the Burkholderia infection in the 1st instar. One possibility is a developmental constraint: the symbiotic organ is immature in the 1st instar and not competent for the symbiont infection. The other possibility is a regulation mechanism over the symbiont infection: the 1st instar nymphs may suppress the development of the midgut crypts to limit the symbiont infection. Note that, in many other groups of stinkbugs, 1st instar nymphs ingest symbiotic bacteria upon hatching and promptly establish the infection (1, 7, 22, 25, 26, 28, 31, 43). In the 3rd instar and on, by contrast, the morphogenetic aspect cannot account for the low susceptibility to the Burkholderia infection: despite the highly developed midgut crypts (Fig. 5G, H, and I), the infection competence declined in the 3rd, 4th, and 5th instars (Fig. 3B and 4B). There is no doubt that some physiological or functional changes occur in the midgut crypts between the 2nd and 3rd instars that attenuate the susceptibility to the symbiont infection. Taken together, we suggest that R. pedestris regulates Burkholderia infection by means of morphogenetic and physiological mechanisms, thereby restricting the symbiont acquisition to the 2nd instar period.
Environmental symbiont acquisition, relaxed developmental constraints, and the evolution of 2nd instar infection.
As for the transovarially transmitted symbionts, their infection must occur before birth. As for the egg-contaminated or capsule-transmitted symbionts, their infection should establish just after birth. In the case of termites, their social living enables a constant supply of symbiont inoculum in the colony, which must have relaxed such developmental constraints on the timing of symbiont transmission and led to the evolution of life-long symbiont acquisition (21, 24). Similarly, the evolution of 2nd instar infection in R. pedestris may be relevant to its unique mode of symbiont acquisition from the environment.
Why does the symbiont acquisition occur in the 2nd instar?
Theoretical studies have suggested that evolution of mutualism without vertical transmission is favored when the symbiont negatively affects the host at early developmental stages (17, 53). It is of interest whether experimental Burkholderia infection in the 1st instar is detrimental or, at best not beneficial, to the fitness components of R. pedestris. In this context, it should be noted that newborn nymphs of R. pedestris possess a large amount of egg yolk inside their midgut (see Fig. S1 in the supplemental material), and are able to normally molt to the 2nd instar without feeding (27, 35). Immature immunity in young insects may potentially result in early vulnerability to microbial infection and pathology (37, 46). It is notable that female adults of R. pedestris tend to lay eggs on gramineous plants, and newborn nymphs migrate to leguminous host plants after hatching (35), implying that the first instar nymphs may have a greater chance of encountering bacteria other than the Burkholderia symbiont. Such biological aspects might also have evolutionarily affected the timing of symbiont acquisition. Meanwhile, on the ground that delayed acquisition of beneficial symbiont will result in reduced fitness advantages to the host (3, 50), it is expected that acquisition of the Burkholderia symbiont in a later instar may lead to reduced performance of R. pedestris. Most of these predictions are experimentally testable and should be verified in future studies.
Competence for the symbiont infection during the host development.
The rearing experiments clearly showed that the Burkholderia acquisition preferentially occurs in the 2nd instar (Fig. 3B), whereas the patterns observed in the administration experiments were certainly similar but less evident (Fig. 4B). This apparent discrepancy is quite likely due to an experimental artifact caused by overdosing in the symbiont inoculation. In the soil environment, an enormous bacterial diversity is present, usually consisting of over 106 species of bacteria per g of soil (16). Previous studies estimated Burkholderia densities in the soil of around 105 CFU per g (33, 44, 51), which must contain both potentially symbiotic and nonsymbiotic Burkholderia strains. Hence, the Burkholderia inoculum dose in this study, 107 CFU per ml of drinking water, is over 100 times higher than the real inoculum dose under natural conditions. It should be noted that, despite such an extreme overdosing, many 1st, 4th, and 5th instar nymphs and some 3rd instar nymphs failed to establish the Burkholderia infection (Fig. 4B), which is suggestive of substantially lower infection competence of the midgut crypts in these non-2nd-instar insects. Of course, the infection doses of the Burkholderia symbiont in these nymphal stages should be rigorously investigated and established in future studies. Even the lower competence for the symbiont infection in the 3rd, 4th, and 5th instars would ensure against the risk of accidental infection failure in the 2nd instar. In a bobtail squid and a lucinid clam, the time frame for symbiont infection can be significantly extended by experimental elimination of the symbiont inoculum in the ambient seawater (19, 36), which might represent a fail-safe mechanism to ensure the symbiotic associations. In squid-Vibrio, legume-Rhizobium, and coral-Symbiodinium associations, sugar-sugar and/or sugar-lectin interactions have been identified to mediate the initial contact between the host and the symbiont cell surfaces (9, 20, 41, 52). Such mechanisms involved in the infection competence and localization during the development of the midgut crypts in early instars of R. pedestris (Fig. 5) are totally unknown and need to be investigated in future studies.
Implications for the evolution of symbiont transmission modes.
Apart from the insects, environmental acquisition of beneficial symbionts is universally found among marine invertebrates and land plants (2, 4, 11, 15, 40, 42). In the marine invertebrates, like in 2nd instar nymphs of R. pedestris through the alimentary tract to the midgut crypts, the symbiont infection is established at a particular postembryonic stage through a specific passage: in postsettlement larvae of tubeworms through penetration of the epidermis (39), in juveniles of squids through pores on the nascent symbiotic light organ (40), in sedentary larvae of the beard worm through the anus (8), etc. These observations suggest that, irrespective of the animal phyla, environmental acquisition of beneficial symbiont entails a specific developmental stage with infection competence. On the other hand, no such specific infection stages are known for legume-Rhizobium and alder-Frankia symbioses (4, 6, 15, 42), which may be relevant to the continuous root formation and the simple root morphogenesis in the plant life cycle. Ecologically, the R. pedestris-Burkholderia symbiosis can be regarded as an insect analogue of the well-known symbioses between the plants and the soil-associated microbes (30), but needless to say, there are a number of differences, including the developmental window for symbiont infection, between the insect system and the plant systems. The environmental symbiont acquisition in R. pedestris is certainly exceptional among insects, but in a broader perspective, not exceptional and will contribute to our understanding of the diversity and evolution of symbiont transmission modes.
Supplementary Material
ACKNOWLEDGEMENTS
This study was funded by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-Oriented Industry (BRAIN). Y.K. was supported by the Research Fellowship of the Japan Society for the Promotion of Science (JSPS).
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 29 April 2011.
REFERENCES
- 1. Abe Y., Mishiro K., Takanashi M. 1995. Symbiont of brown-winged green bug, Plautia stali Scott. Jpn. J. Appl. Entomol. Zool. 39:109–115 [Google Scholar]
- 2. Baker A. C. 2003. Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu. Rev. Ecol. Evol. Syst. 34:661–689 [Google Scholar]
- 3. Bashan Y. 1986. Significance of timing and level of inoculation with rhizosphere bacteria on wheat plants. Soil Biol. Biochem. 18:297–301 [Google Scholar]
- 4. Benson D. R., Silvester W. B. 1993. Biology of Frankia strains, actinomycete symbionts of actinorhizal plants. Microbiol. Rev. 57:293–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bourtzis K., Miller T. A. 2003. Insect symbiosis. CRC Press, Boca Raton, FL [Google Scholar]
- 6. Bright M., Bulgheresi S. 2010. A complex journey: transmission of microbial symbionts. Nat. Rev. Microbiol. 8:218–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchner P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, NY [Google Scholar]
- 8. Callsen-Cencic P., Flügel H. J. 1995. Larval development and the formation of the gut of Siboglinum poseidoni Flügel & Langhof (Pogonophora, Perviata). Evidence of protostomian affinity. Sarsia 80:73–89 [Google Scholar]
- 9. De Hoff P. L., Brill L. M., Hirsch A. M. 2009. Plant lectins: the ties that bind in root symbiosis and plant defense. Mol. Genet. Genomics 282:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Douglas A. E. 1989. Mycetocyte symbiosis in insects. Biol. Rev. 64:409–434 [DOI] [PubMed] [Google Scholar]
- 11. Dubilier N., Bergin C., Lott C. 2008. Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nat. Rev. Microbiol. 6:725–740 [DOI] [PubMed] [Google Scholar]
- 12. Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3:294–299 [PubMed] [Google Scholar]
- 13. Fukatsu T. 1999. Acetone preservation: a practical technique for molecular analysis. Mol. Ecol. 8:1935–1945 [DOI] [PubMed] [Google Scholar]
- 14. Fukatsu T., Nikoh N. 2000. Endosymbiotic microbiota of the bamboo pseudococcid Antonina crawii (Insecta, Homoptera). Appl. Environ. Microbiol. 66:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gage D. J. 2004. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev. 68:280–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gans J., Wolinsky M., Dunbar J. 2005. Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science 309:1387–1390 [DOI] [PubMed] [Google Scholar]
- 17. Genkai-Kato M., Yamamura N. 1999. Evolution of mutualistic symbiosis without vertical transmission. Theor. Popul. Biol. 55:309–323 [DOI] [PubMed] [Google Scholar]
- 18. Grimaldi D., Engel M. S. 2005. Evolution of the insects. Cambridge University Press, New York, NY [Google Scholar]
- 19. Gros O., Frankiel L., Moueza M. 1998. Gill filament differentiation and experimental colonization by symbiotic bacteria in aposymbiotic juveniles of Codakia orbicularis (Bivalvia: Lucinidae). Invert. Reprod. Dev. 34:219–231 [Google Scholar]
- 20. Hirsch A. M. 1999. Role of lectins (and rhizobial exopolysaccharides) in legume nodulation. Curr. Opin. Plant Biol. 2:320–326 [DOI] [PubMed] [Google Scholar]
- 21. Honigberg B. M. 1970. Protozoa associated with termites and their role in digestion, p. 1–36 In Krishna K., Weesner F. M.(ed.), Biology of termites, vol. 2. Academic Press, New York, NY [Google Scholar]
- 22. Hosokawa T., Kikuchi Y., Meng X. Y., Fukatsu T. 2005. The making of symbiont capsule in the plataspid stinkbug Megacopta punctatissima. FEMS Microbiol. Ecol. 54:471–477 [DOI] [PubMed] [Google Scholar]
- 23. Hosokawa T., Koga R., Kikuchi Y., Meng X. Y., Fukatsu T. 2010. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. U. S. A. 107:769–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inoue T., Kitade O., Yoshimura T., Yamaoka I. 2000. Symbiotic association with protists, p. 275–288 In Abe T., Bignell D. E., Higashi M. (ed.), Termites: evolution, sociality, symbioses, ecology . Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 25. Kaiwa N., et al. 2010. Primary gut symbiont and secondary Sodalis-allied symbiont in the scutellerid stinkbug Cantao ocellatus. Appl. Environ. Microbiol. 76:3486–3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaltenpoth M., Winter S. A., Kleinhammer A. 2009. Localization and transmission route of Coriobacterium glomerans, the endosymbiont of pyrrhocorid bugs. FEMS Microbiol. Ecol. 69:373–383 [DOI] [PubMed] [Google Scholar]
- 27. Kamano S. 1980. Artificial diet for rearing bean bug, Riptortus clavatus Thunberg. Jpn. J. Appl. Entomol. Zool. 24:184–188 [Google Scholar]
- 28. Kikuchi Y. 2009. Endosymbiotic bacteria in insects: their diversity and culturability. Microbes Environ. 24:195–204 [DOI] [PubMed] [Google Scholar]
- 29. Kikuchi Y., Hosokawa T., Fukatsu T. 2011. An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J. 5:446–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kikuchi Y., Hosokawa T., Fukatsu T. 2007. Insect-microbe mutualism without vertical transmission: a stinkbug acquires beneficial gut symbiont from environment every generation. Appl. Environ. Microbiol. 73:4308–4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kikuchi Y., et al. 2009. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kikuchi Y., Meng X. Y., Fukatsu T. 2005. Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae). Appl. Environ. Microbiol. 71:4035–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. King E. B., Parke J. L. 1996. Population density of the biocontrol agent Burkholderia cepacia AMMDR1 on four pea cultivars. Soil Biol. Biochem. 28:307–312 [Google Scholar]
- 34. Kono M., Koga R., Shimada M., Fukatsu T. 2008. Infection dynamics of coexisting beta- and gammaproteobacteria in the nested endosymbiotic system of mealybugs. Appl. Environ. Microbiol. 74:4175–4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leal W. S., et al. 1995. Multifunctional communication in Riptortus clavatus (Heteroptera: Alydidae): conspecific nymphs and egg parasitoid Ooencyrtus nezarae use the same adult attractant pheromone as chemical cue. J. Chem. Ecol. 21:973–985 [DOI] [PubMed] [Google Scholar]
- 36. McCann J., Stabb E. V., Millikan D. S., Ruby E. G. 2003. Population dynamics of Vibrio fischeri during infection of Euprymna scolopes. Appl. Environ. Microbiol. 69:5928–5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meister M., Richards G. 1996. Ecdysone and insect immunity: the maturation of the inducibility of the diptericin gene in Drosophila larvae. Insect Biochem. Mol. Biol. 26:155–160 [DOI] [PubMed] [Google Scholar]
- 38. Miura T., et al. 2003. A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea). J. Exp. Zool. B 295:59–81 [DOI] [PubMed] [Google Scholar]
- 39. Nussbaumer A. D., Fisher C. R., Bright M. 2006. Horizontal endosymbiont transmission in hydrothermal vent tubeworms. Nature 441:345–348 [DOI] [PubMed] [Google Scholar]
- 40. Nyholm S. V., McFall-Ngai M. J. 2004. The winnowing: establishing the squid-Vibrio symbiosis. Nat. Rev. Microbiol. 2:632–642 [DOI] [PubMed] [Google Scholar]
- 41. Nyholm S. V., Stabb E. V., Ruby E. G., McFall-Ngai M. J. 2000. Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment. Proc. Natl. Acad. Sci. U. S. A. 97:10231–10235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perret X., Staehelin C., Broughton W. J. 2000. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 64:180–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prado S. S., Rubinoff D., Almeida R. P. P. 2006. Vertical transmission of a pentatomid caeca-associated symbiont. Ann. Entomol. Soc. Am. 99:577–585 [Google Scholar]
- 44. Ramette A., LiPuma J. J., Tiedje J. M. 2005. Species abundance and diversity of Burkholderia cepacia complex in the environment. Appl. Environ. Microbiol. 71:1193–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. R Developmental Core Team 2010. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org [Google Scholar]
- 46. Reichhart J. M., et al. 1992. Insect immunity: developmental and inducible activity of the Drosophila diptericin promoter. EMBO J. 11:1469–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 48. Sasaki-Fukatsu K., et al. 2006. Symbiotic bacteria associated with stomach discs of human lice. Appl. Environ. Microbiol. 72:7349–7352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schaefer C. W., Panizzi A. R. 2000. Heteroptera of economic importance. CRC Press, Boca Raton, FL [Google Scholar]
- 50. Sohn B. K., et al. 2003. Effect of the different timing of AMF inoculation on plant growth and flower quality of chrysanthemum. Sci. Hort. 98:173–183 [Google Scholar]
- 51. Tabacchioni S., Bevivino A., Dalmastri C., Chiarini L. 2002. Burkholderia cepacia complex in the rhizosphere: a minireview. Ann. Microbiol. 52:103–117 [Google Scholar]
- 52. Wood-Charlson E. M., Hollingsworth L. L., Krupp D. A., Weis V. M. 2006. Lectin/glycan interactions play a role in recognition in a coral/dinoflagellate symbiosis. Cell. Microbiol. 8:1985–1993 [DOI] [PubMed] [Google Scholar]
- 53. Yamamura N. 1993. Vertical transmission and evolution of mutualism from parasitism. Theor. Popul. Biol. 44:95–109 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.