Abstract
Five batch cultures of Bacillus subtilis were subjected to evolution in the laboratory for 6,000 generations under conditions repressing sporulation in complex liquid medium containing glucose. Between generations 1,000 and 2,000, variants with a distinct small-colony morphology arose and swept through four of the five populations that had been previously noted for their loss of sporulation (H. Maughan et al., Genetics 177:937-948, 2007). To better understand the nature of adaptation in these variants, individual strains were isolated from one population before (WN715) and after (WN716) the sweep. In addition to colony morphology, strains WN715 and WN716 differed in their motility, aerotaxis, and cell morphology. Competition experiments showed that strain WN716 had evolved a distinct fitness advantage over the ancestral strain and strain WN715 during growth and the transition to the postexponential growth phase, which was more pronounced when WN715 was present in the coculture. Microarray analyses revealed candidate genes in which mutations may have produced some of the observed phenotypes. For example, loss of motility in WN716 was accompanied by decreased transcription of all flagellar, motility, and chemotaxis genes on the microarray. Transcription of alsS and alsD was also lower in strain WN716, and the predicted loss of acetoin production and enhanced acetate production was confirmed by high-performance liquid chromatography (HPLC) analysis. The results suggested that the derived colony morphology of strain WN716 was associated with increased fitness, the alteration of several metabolic pathways, and the loss of a typical postexponential-phase response.
Microbes have been instrumental in the efforts of biologists to understand evolutionary processes, such as genetic drift (9, 31, 32) and adaptation (6, 15), and the relative roles of recombination and mutation in generating diversity (7, 13, 19, 50). Real-time evolution experiments with microbes in the laboratory, where population size and selective regimes can be manipulated with ease, have contributed significantly to these fields of study (reviewed in reference 6). We initiated an evolution experiment in the laboratory to study how sporulation might affect adaptation in Bacillus subtilis (22–24). Sporulation, a relatively complex developmental process found in several genera of bacteria belonging to the Firmicutes phylum, is triggered by the transition from exponential growth to the postexponential growth phase upon nutrient deprivation. The complexity of sporulation is highlighted by the hundreds of genes that are expressed during sporulation, the many hours the process takes for completion (39), and the numerous other functions that are concomitantly coregulated during the vegetative-to-postexponential growth transition, including competence (4), motility (1), extracellular enzyme synthesis (43), and biofilm formation (37).
Our evolution experiment with Bacillus subtilis consisted of 10 populations that evolved for 6,000 generations. Five populations, referred to as nonsporulating populations, were cultured in a rich medium to repress the induction of sporulation; the other five populations, referred to as the sporulating populations, were cultivated in typical sporulation-inducing medium (42), and spores were selected by heat shocking the inoculum at 80°C for 10 min before daily transfer (23, 24). Several interesting observations have resulted from these experiments, all of which highlight the rapid rate of adaptation and phenotypic degradation that occurred when B. subtilis was continuously cultured in a nutrient-rich environment. First, all 10 populations exhibited an increase in fitness and mutation rate compared to their respective ancestor (23). Second, all 10 populations accumulated auxotrophs (23). Third, sporulation declined in all five populations with relaxed selection for sporulation, with two populations completely losing the ability to sporulate by the end of the 6,000-generation experiment (24). Furthermore, microarray experiments in one population at generation 6,000 showed that genes whose products are involved in sporulation initiation were not transcribed, indicating that the sporulation defect in this population was manifested at the very earliest stage of development (22). Because mutations in genes whose products act early in sporulation are often pleiotropic, we reasoned that identification of additional altered pathways would guide our investigations into the genetic and physiological basis for the loss of sporulation.
During the course of the B. subtilis evolution experiment, we observed a distinct small-colony morphology that appeared and subsequently became fixed in four of the five nonsporulating populations. Because sporulation had decreased the most in these four populations, we reasoned that investigating the evolution of the cells that had gained the small-colony morphology could illuminate our studies to determine the physiological and genetic causes of sporulation loss. This paper describes the phenotypic characterization of cells with the derived small-colony morphology from three of the nonsporulating populations and further in-depth physiological and transcriptomic characterization of a variant from one of the populations. Our experiments showed that cells with the small-colony morphology, compared to coexisting cells with ancestral colony morphology, exhibited the following: (i) higher fitness that was more pronounced in the presence of these coexisting cells; (ii) altered metabolism that resulted in acidification of the culture medium; and (iii) continued growth during postexponential phase accompanied by downregulation of motility.
MATERIALS AND METHODS
Bacterial strains and media.
B. subtilis strains used in this study are listed in Table 1. Strains WN624 (trpC2 amyE::spc) and WN628 (trpC2 amyE::cat) are congenic except for their respective antibiotic resistance markers and were the ancestral strains used in our experiments of evolution in the laboratory (23). For long-term evolution (23), strain WN624 was cultivated under conditions repressing sporulation in liquid R medium, which is a modified version of Schaeffer sporulation medium (SSM) (42) containing Difco nutrient broth (0.8%), KCl (0.1%), MgCl2 (0.025%), and glucose (1.0%) (final concentrations given in the parentheses) as described previously (23, 24). R medium was used for plating by the addition of agar to a final concentration of 1.7%. For motility screening, R medium was solidified by the addition of agar to a final concentration of 0.3%. Selection of antibiotic resistance markers was performed by the addition of either spectinomycin (Spc) (25 μg/ml final concentration) or chloramphenicol (Cm) (5 μg/ml final concentration) to the medium as appropriate. Luria-Bertani (LB) medium (25) with appropriate antibiotics added was used for routine plating. The cells were cultivated in liquid medium on a rotary shaker with vigorous aeration (300 rpm). The optical density of cells was monitored with a Klett-Summerson photometer fitted with a number 66 (red) filter or with a spectrophotometer set at 660 nm. Under these conditions, 1 optical density at 660 nm (OD660) unit equals 100 Klett units. All cultures were incubated at 37°C.
Table 1.
B. subtilis strains used in this study
| Strain | Genotype or phenotypea | Reference |
|---|---|---|
| WN624 | trpC2 amyE::spc | 22 |
| WN628 | trpC2 amyE::cat | 22 |
| WN699 | Presweep LCM strain from culture 624E; trpC2 amyE::spc | This study |
| WN700 | Postsweep SCV strain from culture 624E; trpC2amyE::spc | This study |
| WN715 | Presweep LCM strain from culture 624A; trpC2 amyE::cat | This study |
| WN716 | Postsweep SCV strain from culture 624A; trpC2 amyE::spc | This study |
| WN717 | Presweep LCM strain from culture 624B; trpC2 amyE::spc | This study |
| WN718 | Postsweep SCV strain from culture 624B; trpC2 amyE::spc | This study |
LCM, large-colony morphology; SCV, small-colony variant.
Experimental conditions for experiments studying evolution in the laboratory.
Five replicate cultures of strain WN624, designated replicates 624A through 624E, were cultivated in 10 ml of liquid R medium in 125-ml flasks. At 24-hour intervals, replicate cultures of 624A to 624E were diluted 1:100 into fresh R medium and incubation continued. At weekly (∼50-generation) intervals, an aliquot of each replicate culture was mixed with an equal volume of 50% (vol/vol) glycerol and stored at −70°C for use in further experiments and for the preservation of each population throughout evolution.
Microbiological methods.
Cell morphology was observed by either phase-contrast microscopy of living cells or bright-field microscopy of heat-fixed cells after staining with methylene blue (11). Motility in individual cells was observed by phase-contrast microscopy. Sporulation frequency was determined in heat-shocked liquid cultures (heat shocked at 80°C for 10 min) as described previously (36). Qualitative methyl red Voges-Proskauer (MR-VP) assays were performed as described previously (11). Acetoin was further quantified by a modified Voges-Proskauer assay as described previously (34). Transformation experiments were performed in two-stage competence medium as described previously (3), and the source of transforming DNA was plasmid pWN162, which carries the wild-type trpC gene (51).
Competition experiments.
In order to perform competition experiments with ancestral and evolved strains from population 624A, the resident amyE::spc cassette in the large-colony morphology (LCM) presweep strain was replaced by an amyE::cat cassette conferring resistance to Cm (46), and the resulting presweep LCM strain was designated WN715 (Table 1). Pairs of strains to be compared were inoculated simultaneously into 10 ml of liquid R medium without antibiotic. The cultures were treated as described above for evolution experiments, i.e., diluted daily 1:100 into fresh R medium. At daily intervals, duplicate aliquots were removed from the cultures, diluted serially 10-fold, and plated on LB medium containing either Spc or Cm; colonies were counted after incubation overnight at 37°C as described previously (35).
Microarray procedures.
Overnight cultures of strains WN715 and WN716 were inoculated into R medium from frozen stocks. The following day, a 0.1-ml aliquot of the overnight culture was inoculated into 9.9 ml of R medium containing the appropriate antibiotic. Optical density readings were taken at 15-min intervals. When exponential growth had ceased, RNA was isolated using Tri-Reagent (Sigma-Aldrich, St. Louis, MO). RNA was labeled by adding water and B. subtilis gene-specific primers (Eurogentec, Belgium) to the RNA samples and incubating the samples at 70°C for 10 min, after which the sample was snap-frozen in liquid nitrogen. To each thawed sample, 400 U Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA), 0.6 μM dithiothreitol (DTT), and the appropriate volume of 50× amino allyl-deoxynucleoside triphosphate (dNTP) mix (Sigma-Aldrich) and 5× Superscript II buffer (Invitrogen) were added. This mixture was incubated at 42°C for 3 h, and the reaction was stopped by adding 10 μM NaOH and 5 μM EDTA and incubating the mixture at 65°C for 15 min, followed by the addition of 10 μM HCl. Samples were cleaned of all excess primer, enzymes, and dNTPs using QIAquick purification columns (Qiagen, Valencia, CA) and speed vacuum dried before coupling of Cy3 or Cy5 dye. Dye coupling was done by resuspending the cDNA in 0.45 μM Na2CO3 and adding the appropriate dye, followed by incubation in the dark for 1 h at room temperature. Dye cleanup was again performed using QIAquick purification columns (Qiagen), and the samples were dried in a Speedvac.
Glass microarray slides (Eurogentec) were spotted in duplicate with 4,096 PCR products, each representing an annotated B. subtilis open reading frame (ORF) (18). For hybridization of samples to each slide, both Cy3- and Cy5-labeled samples were resuspended in 50 μM EDTA and denatured at 98°C for 10 min. One hundred forty microliters of hybridization buffer 3 (Invitrogen) was added to each sample, and the samples were loaded onto the slides. Hybridization was performed for 16 h at 42°C, followed by washes at 50°C for 2 min each in medium-stringency wash buffer, high-stringency wash buffer, and postwash buffer in a Gene TAC hybridization station using the manufacturer's reagents. Scanning of slides on an Applied Precision Array Worx scanner was performed immediately following hybridization. Raw and normalized intensities were extracted from scanned images using SoftWorx Tracker.
Analyses of fermentation products.
High-performance liquid chromatography (HPLC) analyses of fermentation products were performed essentially as described previously (34, 49). Briefly, cells were removed from culture samples by centrifugation in a microcentrifuge, culture supernatants were passed through a 0.2-μm nylon filter, and H2SO4 was added to a final concentration of 4 mM. Fermentation products were analyzed using a Hewlett-Packard 1100 HPLC fitted with an Aminex HPX-87H column (Bio-Rad Laboratories, Hercules, CA), a filter photometric detector (210 nm), and a refractive index detector connected in series. The column temperature was 45°C, and the flow rate of eluant (4 mM H2SO4) was 0.4 ml/min. The chromatograms were processed using Chemstation software, and the concentration of each analyte was determined by peak area integration after the compounds were identified on the basis of the retention times of pure standards.
Statistical analyses.
All assays were performed on either duplicate or triplicate cultures. Basic statistical parameters were determined and analysis of variance (ANOVA) was performed using commercial statistical software (Kaleidagraph). Differences with P ≤ 0.05 were considered statistically significant.
Microarray data accession number.
The complete set of microarray data has been deposited in the Gene Expression Omnibus (GEO) database at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE24018 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE24018).
RESULTS AND DISCUSSION
Appearance and fixation of variants with a distinct colony morphology.
In the course of 6,000 generations of evolution of the nonsporulating populations 624A to 624E in R medium (sporulation-repressing medium), a small-colony variant (SCV) swept to fixation in four of the five populations (624A, 624B, 624D, and 624E), replacing the typical opaque B. subtilis large-colony morphotype (LCM) (Table 2). Interestingly, the trajectory of each sweep was unique in its onset, duration, and time of final fixation of the SCV (Table 2). The SCV was first detected in populations 624A and 624E at approximately generation 1,330, and within 200 to 500 generations, became the only colony morphotype present in these two populations (Table 2). In population 624B, the SCV was first noted somewhat later, at ∼1,972 generations, and required more than 1,000 generations to become fixed (Table 2). In population 624D, the SCV appeared at generation 2,079 and dominated the population in less than 200 generations but could not be detected after a further 600 generations; however, another SCV appeared a second time at generation 4,214 and became fixed within 400 generations (Table 2). Despite the differences in trajectories, parallel evolution of SCVs occurred in 4 of the 5 populations, suggesting that something about the small-colony morphology, or a correlated phenotype, was selectively advantageous. Because the sweeps in populations 624A, 624B, and 624E exhibited characteristics of “hard” population sweeps, i.e., sudden appearance and rapid fixation of a mutant phenotype in a relatively constant environment (28), we chose to further characterize isolates preceding and following each of these 3 population sweeps.
Table 2.
Generations at which small-colony variants swept evolving populations 624A to 624E
| Culture | Generation when SCV exhibited the followinga: |
||||
|---|---|---|---|---|---|
| First appearance | First sweep | First loss | Second appearance | Final fixation | |
| 624A | 1,330 | NA | NA | NA | 1,792 |
| 624B | 1,972 | NA | NA | NA | 3,066 |
| 624C | * | * | * | * | * |
| 624D | 2,079 | 2,275 | 2,884 | 4,214 | 4,662 |
| 624E | 1,330 | NA | NA | NA | 1,547 |
NA, not applicable. Asterisks indicate that the small-colony variant (SCV) never appeared.
Prior to each population sweep, evolving cultures 624A, 624B, and 624E consisted of a homogenous population of LCMs indistinguishable from cultures of the ancestral strains. A typical SCV arising immediately after population sweeps of cultures 624A, 624B, and 624E was chosen, streak purified, and given a strain designation (Table 1). In addition, an isolate exhibiting the typical LCM immediately preceding each population sweep was also recovered from the frozen stock collection, streak purified, and given a strain designation (Table 1).
Characteristics of LCM and SCV strains.
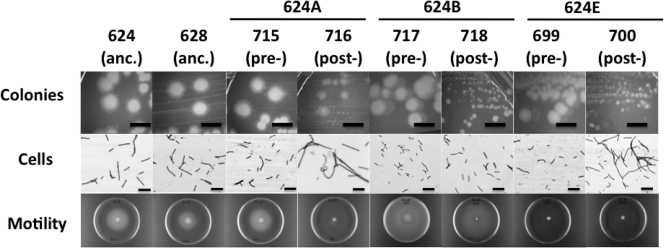
The morphologies and growth characteristics of LCM and SCV strains from populations 624A, 624B, and 624E were compared to the ancestral strains. Regarding colony morphologies, presweep strains WN699, WN715, and WN717 from cultures 624E, 624A, and 624B, respectively, exhibited a typical B. subtilis colony morphology indistinguishable from the colony morphology of ancestral strains WN624 and WN628 (Fig. 1), and the respective postsweep strains WN700, WN716, and WN718 all exhibited the SCV phenotype as expected (Fig. 1). Regarding cell morphology, microscopic examination of overnight cultures of cells grown in liquid R medium revealed that the ancestral strains WN624 and WN628, as well as presweep LCM strains WN699, WN715, and WN717, all exhibited typical B. subtilis cell morphology, i.e., mostly single rod-shaped cells, 2 to 4 μm in length (Fig. 1). In contrast, cells of postsweep SCV strains WN700 and WN716 formed long filaments (Fig. 1). However, the cell morphology of postsweep SCV strain WN718 was characteristic of ancestral and presweep strains (Fig. 1), suggesting that the SCV phenotype and the cell filamentation phenotype result from different evolutionary events. Motility of cells was analyzed by phase-contrast microscopy (data not shown) and by spotting cells on R motility agar plates. Ancestral strains WN624 and WN628 were strongly motile on R medium (Fig. 1). In the case of population 624A, presweep LCM strain WN715 was motile, but SCV strain WN716 was nonmotile on plates containing R medium (Fig. 1). (Note that motility was not completely lost from strain WN716, as cells from overnight cultures grown in liquid LB medium were observed to exhibit running and tumbling motility [data not shown].) In the case of population 624B, both pre- and postsweep LCM and SCV strains WN717 and WN718 were motile on R medium, and in the case of population 624E, motility was not observed in either presweep LCM strain WN699 or postsweep SCV strain WN700 (Fig. 1). Together, these observations indicate that populations 624A, 624B, and 624E have embarked upon similar evolutionary pathways but that the order of particular events occurring during their evolution have been different. The observations suggest that the three different phenotypes observed—colony morphology, cell filamentation, and motility—are likely the result of (at least) three different mutational events, rather than one mutation with pleiotropic effects.
Fig. 1.
Phenotypes of ancestral (anc.) strains WN624 and WN628, presweep (pre-) large-colony morphotype (LCM) strains WN699, WN715, and WN717, and postsweep (post-) small-colony variant (SCV) strains WN700, WN716, and WN718. Bars, 10 μm (for cells) and 5 mm (for colonies).
SCV strains grow to higher density than LCMs in R medium.
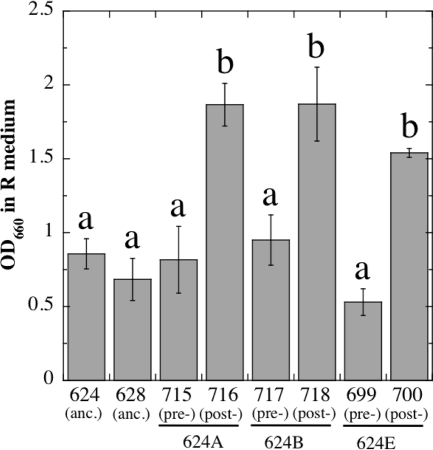
In order to sweep through and become fixed in a population, a new variant must produce more progeny, i.e., have higher fitness, than other members of the population. We first measured SCV fitness by growing triplicate cultures of all strains in liquid R medium and measuring their optical densities at 660 nm (OD660) after overnight incubation. The strains fell into 2 distinct groups by ANOVA (Fig. 2). The first group grew to OD660s of 0.5 to 1.0 and consisted of ancestral strains WN624 and WN628, as well as LCM strains WN699, WN715, and WN717 (Fig. 2). In sharp contrast, the second group all grew to significantly higher ODs (OD660s of 1.5 to 2.0) and consisted of SCV strains WN700, WN716, and WN718 (Fig. 2). Therefore, the data suggested that SCV strains evolved to more efficiently utilize R medium, perhaps through the alteration of particular metabolic pathways and/or optimizing the activity of particular enzymes.
Fig. 2.
Overnight growth in liquid R medium of ancestral (anc.) strains WN624 and WN628, presweep (pre-) LCM strains WN699, WN715, and WN717, and postsweep (post-) SCV strains WN700, WN716, and WN718. Values are averages ± standard deviations (error bars) (n = 3). Lowercase letters above the bars denote significantly different groups by ANOVA (P ≤ 0.05).
Increased competitive fitness of postsweep strain WN716.
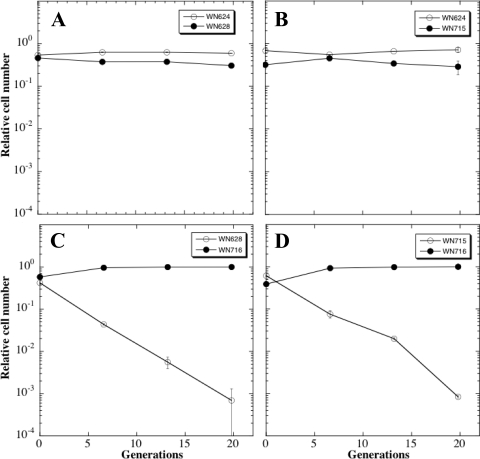
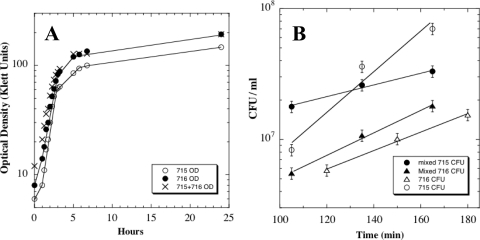
Because the SCV strain WN716 from population 624A exhibited multiple phenotypic changes (Fig. 1 and 2), we chose to study it in further detail. In order to test whether pre- and postsweep strains WN715 and WN716 from population 624A had evolved increased fitness over the ancestral strain WN624, a series of competition experiments were performed in which pairs of strains were cultivated simultaneously in R medium as described in Materials and Methods (Fig. 3). At daily intervals, the relative proportions of each strain in the populations were determined by plating on medium containing either spectinomycin (Spc) or chloramphenicol (Cm). First, we verified that the amyE::spc and amy::cat markers did not affect fitness in R medium by competing congenic ancestral strains WN624 and WN628 against each other (Fig. 3A); both antibiotic resistance markers were selectively neutral, as had been shown previously (23, 35). When ancestral strain WN624 was competed against presweep LCM strain WN715, no difference was noted in their relative proportions in the mixed population for at least 20 generations, indicating that strain WN715 had not gained a dramatic increase in fitness over the ancestor. In contrast, a marked increase in the fitness of SCV strain WN716 was observed over both ancestral strain WN628 (Fig. 3C) and LCM strain WN715 (Fig. 3D). In competition with WN716, the proportion of WN628 and WN715 cells in each mixed population declined by 1 order of magnitude each ∼7.2 and ∼8.9 generations, respectively (Fig. 3).
Fig. 3.
Competition experiments in R medium of pairs of strains. (A) Strain WN624 versus strain WN628; (B) WN624 versus WN715; (C) WN628 versus WN716; (D) WN715 versus WN716. Error bars that are not visible are smaller than the plot symbols.
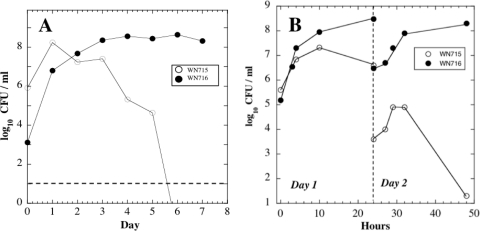
To test the notion further that SCV strain WN716 had swept the 624A population by gaining a selective advantage, a competition experiment was performed in which strains WN715 and WN716 were cultivated simultaneously in R medium without selective antibiotics, but at a WN715/WN716 input ratio of 1,000:1. Even when initially present as a minority of the mixed population, WN716 took over the culture within 6 days (∼40 generations) (Fig. 4A).
Fig. 4.
(A) Viable counts during competition of strain WN715 versus strain WN716 in liquid R medium. The two strains were inoculated at a WN715/WN716 ratio of 1,000:1. The horizontal dashed line represents 10 CFU/ml, the lower detection limit of the viability assay. (B) Viability of strains WN715 and WN716 at various times during the first 2 days of a typical competition experiment. Strains were inoculated at an initial ratio of ∼1:1. In both panels, the data are averages of duplicate experiments that differed by less than 10%.
The results from these competition experiments showed that postsweep strain WN716 had gained a clear fitness advantage in R medium, even when inoculated at a density that was orders of magnitude lower than that of presweep strain WN715. This fitness advantage extended over multiple transfers until strain WN715 was no longer detected after ∼40 generations. In the original population from which these two strains were isolated (624A), the SCV took ∼400 generations to become fixed. Why did the SCV strain WN716 take longer to fix in the 624A population, compared to our head-to-head competition experiments? It seems reasonable to speculate that the 624A population was heterogeneous, being comprised of individuals with a variety of fitnesses ranging from the fitness of LCM strain WN715 to that of SCV strain WN716. This “competition” between strains with higher fitness within an asexual population can result in a slower time to fixation for any one strain of higher fitness, a phenomenon known as clonal interference (12). Therefore, it is possible that the fixation of the SCV took longer than 40 generations because of the presence of additional variants with fitnesses higher than those of LCMs such as WN715.
What features of SCV strain WN716's growth in R medium might have led to its increased fitness? To address this question, strains WN715 and WN716 were cultivated together in R medium and cells were sampled for viable counts throughout the growth cycle for 2 days under standard evolution conditions (Fig. 4B). Two observations were made. (i) During day 1 of the experiment, it appeared that the viable counts of SCV strain WN716 increased at a faster exponential rate than LCM strain WN715. (ii) It appeared that strain WN716 continued to increase in number during the postexponential growth phase, while the number of viable cells of WN715 declined (Fig. 4B). After 1:100 dilution into fresh R medium on day 2, both strains increased in number during exponential phase, but presweep strain WN715 suffered a severe drop in viability in the postexponential growth phase; in contrast, postsweep strain WN716 again increased in viable numbers during the postexponential growth phase (Fig. 4B).
Were the behaviors seen in the above mixed-culture experiment due to competition between strains WN715 and WN716, or were they intrinsic properties of the two strains? To test this notion, strains WN715 and WN716 were cultivated in R medium separately and in mixed culture, and their growth parameters were determined (Fig. 5). When exponential growth rates were compared by optical density, a measurement of the increase in total cell mass of the cultures, exponential growth rates of the three cultures were essentially identical with doubling times of ∼50 min (Fig. 5A). However, at 24 h, postsweep strain WN716 had grown to a higher optical density (193 Klett units) than LCM strain WN715 (147 Klett units); furthermore, the mixed culture also had grown to 193 Klett units, characteristic of strain WN716 (Fig. 5A).
Fig. 5.
(A) Optical densities of strains WN715 (open circles) and WN716 (filled circles) grown separately in R medium and mixed cultures of strains WN715 and WN716 inoculated at an approximate ratio of 1:1 (crosses). (B) Viable counts during exponential growth of strain WN715 (circles) and WN716 (triangles) grown either separately (open symbols) or in mixed culture (filled symbols). Values are averages ± standard deviations (error bars) of duplicate experiments. Best-fit exponential lines through the data points are indicated, from which doubling times were derived. The correlation coefficient (r2) values of best-fit exponential lines were 0.954 (WN715 grown alone), 0.994 (WN716 grown alone), 0.983 (WN715 grown in mixed culture), and 0.995 (WN716 grown in mixed culture).
However, when exponential growth rates were determined by viability counts, a different picture emerged (Fig. 5B). When grown separately, the doubling times of strains WN715 and WN716 were 20 min and 45 min, respectively. The apparent lower rate of increase in the viable counts of strain WN716 was likely due to its septation defect during growth in R medium (Fig. 1). In contrast, when strains WN715 and WN716 were cocultivated in R medium, the doubling times of strains WN715 and WN716 were 68 and 36 min, respectively (Fig. 5B). In other words, strain WN716 grew slightly faster in coculture than it did separately, and strain WN715 grew dramatically more slowly in coculture than it did separately. Thus, it appeared that strain WN716 exerted a negative effect on both the exponential growth rate and on the postexponential-phase survival of strain WN715 during mixed cultivation (Fig. 4 and 5). These observations suggest that postsweep strain WN716 may be producing some product that is toxic to presweep strain WN715 or may be utilizing a nutrient required for growth more rapidly or both.
Transcription microarray profiling of strain WN715 versus WN716.
It appeared that increased competitive fitness of strain WN716 in mixed culture could be explained at least in part by (i) its faster exponential growth rate than WN715, (ii) its continued growth in postexponential phase, and (iii) the loss of viability of strain WN715 during postexponential growth phase when cocultivated with strain WN716. What might be the underlying molecular causes of these fitness advantages? We reasoned that differences in the global transcription patterns of strains WN715 and WN716 would help identify candidate genetic changes underlying the increased fitness of strain WN716 in R medium. Strains WN715 and WN716 were grown separately in R medium, and RNA was harvested from early postexponential-phase cells. This time was chosen because many of the phenotypes distinguishing LCM from SCV (e.g., filamentation, motility, continued growth) manifested themselves during early postexponential growth phase.
Because we were searching for dramatic expression differences in the transcription patterns of strains WN715 and WN716, multiple microarrays were not performed, and therefore, subtle differences in expression were not considered; we arbitrarily chose to concentrate on analyzing changes greater than 8-fold (i.e., differences of ≥3 log2 units). We separately analyzed genes and operons exhibiting increased (Table 3) or decreased (Table 4) transcription in strain WN716 and concentrated on annotated genes of known function. The entire set of microarray data can be viewed at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE24018.
Table 3.
Genes and operons of known function exhibiting increased transcription in early postexponential-phase cells of postsweep strain WN716
| Category, subcategory, and gene or operon | Function | Fold expression increase |
|---|---|---|
| Category 1. Cell envelope and cellular processes | ||
| 1.1 Cell wall | ||
| lytB | Amidase enhancer precursor (modifier protein of major autolysin) | 10 |
| 1.2 Transport/binding proteins and lipoproteins | ||
| amyC | Multiple sugar transport system permease | 13 |
| 1.8 Sporulation | ||
| cotS | Spore coat protein | 9 |
| cotV | Spore coat protein (insoluble fraction) | 12 |
| Category 2. Intermediary metabolism | ||
| 2.1 Metabolism of carbohydrates and related molecules | ||
| lacA | β-Galactosidase | 17 |
| 2.1.1 Main glycolytic pathways | ||
| pdhA | Pyruvate dehydrogenase E1 (alpha subunit) | 9 |
| pdhB | Pyruvate dehydrogenase E1 (beta subunit) | 10 |
| pdhC | Pyruvate dehydrogenase E2 subunit | 5 |
| pdhD | Pyruvate dehydrogenase E3 subunit | 10 |
| 2.2 Metabolism of amino acids and related molecules | ||
| asnH | Asparagine synthetase (glutamine hydrolyzing) | 8 |
| nasB | Assimilatory nitrate reductase, electron transfer subunit | 15 |
| 2.3 Metabolism of nucleotides and nucleic acids | ||
| purE | Phosphoribosylaminoimidazole carboxylase I | 92 |
| purK | Phosphoribosylaminoimidazole carboxylase II | 5 |
| purB | Adenylosuccinate lyase | 9 |
| purC | Phosphoribosylaminoimidazole succinocarboxamide synthetase | 6 |
| purS | Required for PurQL activity | 10 |
| purQ | Phosphoribosylformylglycinamidine synthase I | –a |
| purL | Phosphoribosylformylglycinamidine synthase II | 7 |
| purF | Phosphoribosylpyrophosphate amidotransferase | 3 |
| purM | Phosphoribosylaminoimidazole synthetase | 2 |
| purN | Phosphoribosylglycinamide formyltransferase | 1 |
| purH | Phosphoribosylaminoimidazole carboxy formyl formyltransferase/inosine-monophosphate cyclohydrolase | 1 |
| purD | Phosphoribosylglycinamide synthetase | 1 |
| pyrR | Transcriptional attenuator of the pyrimidine operon/uracil phosphoribosyltransferase | 91 |
| pyrP | Uracil permease | 17 |
| pyrB | Aspartate carbamoyltransferase catalytic chain | 52 |
| pyrC | Dihydroorotase | 30 |
| pyrAA | Carbamoyl-phosphate synthase small chain | 70 |
| pyrAB | Carbamoyl-phosphate synthase large chain | 39 |
| pyrK | Dihydroorotate dehydrogenase (electron transfer subunit) | 24 |
| pyrD | Dihydroorotate dehydrogenase (catalytic subunit) | 24 |
| pyrF | Orotidine 5′-phosphate decarboxylase | 19 |
| pyrE | Orotate phosphoribosyltransferase | 19 |
| 2.4 Metabolism of lipids | ||
| nap | Carboxylesterase NA | 8 |
| 2.6 Metabolism of phosphate | ||
| phoB | Alkaline phosphatase III | 8 |
| Category 3. Information pathways | ||
| 3.2 DNA restriction/modification and repair | ||
| dinG | DNA damage-inducible ATP-dependent DNA helicase | 126 |
| mtbP | Modification methylase Bsu | 12 |
| 3.5 RNA synthesis | ||
| 3.5.2 Transcription regulation | ||
| ctsR | Transcriptional repressor of class III stress genes | 12 |
| gerE | Transcriptional regulator required for expression of late spore coat genes | 8 |
| hrcA | Transcriptional repressor of class I heat shock genes | 20 |
| tnrA | Transcriptional pleiotropic regulator involved in global nitrogen regulation | 9 |
| 3.7 Protein synthesis | ||
| 3.7.1 Ribosomal proteins | ||
| rplL | Ribosomal protein L12 (BL9) | 9 |
| 3.8 Protein folding | ||
| groES | Class I heat shock protein (chaperonin) | 12 |
| groEL | Class I heat shock protein (chaperonin) | 12 |
| Category 4. Other functions | ||
| 4.1 Adaptation to atypical conditions | ||
| clpC | Class III stress response ATPase | 5 |
| clpE | ATP-dependent Clp protease (class III stress gene) | 44 |
| grpE | Heat shock protein (activation of DnaK) | 5 |
| dnaK | Class I heat shock protein, chaperone | 7 |
| gspA | General stress protein A | 8 |
| mcsA | Modulator of CtsR repression | 13 |
| mcsB | Modulator of CtsR repression | 8 |
| mrgA | Metalloregulation DNA-binding stress protein | 16 |
| 4.2 Detoxification | ||
| katB | Catalase 2 | 8 |
–, signal not detected.
Table 4.
Genes and operons of known function exhibiting decreased transcription in early postexponential-phase cells of SCV strain WN716
| Category, subcategory, and gene or operon | Function(s)a | Fold expression decreaseb |
|---|---|---|
| Category 1. Cell envelope and cellular processes | ||
| 1.1 Cell wall | ||
| lytA | Involved in secretion of major autolysin LytC | 7 |
| lytC | N-Acetylmuramoyl-l-alanine amidase; major autolysin; involved in motility and cell separation | 9 |
| lytD | N-Acetylglucosaminidase, major autolysin | 7 |
| 1.2 Transport/binding proteins and lipoproteins | ||
| Oligopeptide ABC transport system | ||
| appD | ATP-binding protein | 4 |
| appF | ATP-binding protein | 6 |
| appA | Substrate-binding protein | 10 |
| appB | Permease | 5 |
| appC | Permease | 6 |
| feuA | Iron transport substrate-binding protein | 11 |
| feuB | Iron transport permease (integral membrane protein) | 1 |
| feuC | Iron transport permease (integral membrane protein) | 20 |
| Glycine betaine/proline/choline transport | ||
| opuBA | ATP-binding protein | – |
| opuBB | Permease | 4 |
| opuBC | Substrate-binding protein | 13 |
| opuBD | Permease | – |
| Glycine betaine/carnitine/choline transport | ||
| opuCA | ATP-binding protein | 7 |
| opuCB | Permease | – |
| opuCC | Substrate-binding protein | 19 |
| opuCD | Permease | 3 |
| Oligopeptide transport system (competence, sporulation initiation) | ||
| oppA | Substrate-binding protein | 6 |
| oppB | Permease | 8 |
| oppC | Permease | 8 |
| oppD | ATP-binding protein | 2 |
| oppF | ATP-binding protein | 10 |
| 1.3 Sensors (signal transduction) | ||
| cheB | Chemotaxis response regulator | 17 |
| cheA | Chemotaxis sensor kinase | 8 |
| 1.4 Membrane bioenergetics (electron transport chain, ATP synthase | ||
| Cytochrome caa3 oxidase | ||
| ctaA | Required for biosynthesis | 2 |
| ctaB | Assembly factor | 3 |
| ctaC | Subunit II | 6 |
| ctaD | Subunit I | 1 |
| ctaE | Subunit III | 13 |
| ctaF | Subunit IV | 2 |
| ctaG | Required for biosynthesis | 2 |
| resA | Cytochrome c biogenesis protein, similar to thioredoxin | 12 |
| resB | Cytochrome c biogenesis protein | 4 |
| resC | Cytochrome c biogenesis protein | 4 |
| 1.5 Motility and chemotaxis | ||
| mcpB | Methyl-accepting chemotaxis protein | 11 |
| tlpA | Methyl-accepting chemotaxis protein | 432 |
| mcpA | Methyl-accepting chemotaxis protein | 20 |
| flgB | Flagellar basal body rod protein | 11 |
| flgC | Flagellar basal body rod protein | 2 |
| fliE | Flagellar hook-basal body complex protein | 19 |
| fliF | Flagellar basal body M-ring protein | 12 |
| fliG | Flagellar motor switch protein | 40 |
| fliH | Flagellar assembly protein | 16 |
| fliI | Flagellum-specific ATP synthase | 12 |
| fliJ | Flagellar protein required for basal body formation | 27 |
| fliK | Flagellar hook-length control protein | 17 |
| flgE | Flagellar hook protein | 15 |
| fliM | Flagellar motor switch protein | 13 |
| fliY | Flagellar motor switch protein | 28 |
| cheY | Chemotaxis response regulator | 15 |
| fliZ | Protein required for flagellum formation | 14 |
| fliP | Protein required for flagellum formation | 25 |
| fliQ | Protein required for flagellum formation | – |
| fliR | Protein required for flagellum formation | 24 |
| flhB | Flagellum-associated protein | 2 |
| flhA | Flagellum-associated protein | 18 |
| flhF | Flagellum-associated protein | 13 |
| ylxH | Similar to flagellar switch protein | 20 |
| cheW | Purine-binding chemotaxis protein | 10 |
| cheC | CheY-P phosphatase | 5 |
| cheD | Flagellar motor switch protein | 8 |
| yvyF | Similar to flagellar protein | 6 |
| flgM | Anti-sigma-D factor | 10 |
| yvyG | Similar to flagellar protein | 16 |
| flgK | Flagellar hook-associated protein 1 | 10 |
| flgL | Flagellar hook-associated protein 3 | 22 |
| hag | Flagellin | 13 |
| yvyC | Similar to flagellar protein | – |
| fliD | Flagellar hook-associated protein 2 | 19 |
| fliS | Flagellar protein | 12 |
| fliT | Flagellar protein | 24 |
| flhO | Flagellar basal body rod protein | 8 |
| flhP | Flagellar hook-basal body protein | 9 |
| 1.6 Protein secretion | ||
| lytA | Membrane-bound protein, secretion of LytC | 7 |
| 1.8 Sporulation | ||
| rapA | Response regulator aspartate phosphatase (Spo0F∼P) | 21 |
| phrA | RapA inhibitor | 22 |
| rapC | Response regulator aspartate phosphatase | 7 |
| phrC | RapC inhibitor; competence and sporulation stimulating factor (CSF) | 17 |
| rapF | Response regulator aspartate phosphatase | 10 |
| phrF | RapF inhibitor | 12 |
| cotW | Spore coat protein (insoluble fraction) | 12 |
| spoIID | Required for dissolution of asymmetric septum | 11 |
| spoIIAB | Anti-sigma factor serine kinase | 11 |
| spoVID | Required for assembly of spore coat | 9.5 |
| sspF | Minor α/β-type small acid-soluble spore protein | 44 |
| 1.10 Transformation/competence | ||
| comC | Late competence protein | 15 |
| comS | Competence protein (embedded in srfAB) | 39 |
| comX | Competence pheromone precursor | 8 |
| Category 2. Intermediary metabolism | ||
| 2.1 Metabolism of carbohydrates and related molecules | ||
| 2.1.1 Specific pathways | ||
| alsS | α-Acetolactate synthase, acetoin production | 122 |
| alsD | α-Acetolactate decarboxylase, acetoin production | 87 |
| kdgR | Repressor of kdg operon, LacI family | 1 |
| kdgK | 2-Keto-3-deoxygluconate kinase (pectin utilization) | 2 |
| kdgA | 2-Keto-3-deoxyphosphogluconate aldolase (pectin utilization) | 13 |
| kdgT | 2-Keto-3-deoxygluconate permease (pectin utilization) | 2 |
| 2.2 Metabolism of amino acids and related molecules | ||
| mpr | Extracellular metalloprotease | 8 |
| 2.5 Metabolism of coenzymes and prosthetic groups | ||
| bioW | 6-Carboxylhexanoate-CoA ligase | 5 |
| bioA | AdoMet-8-amino-7-oxononanoate aminotransferase | 6 |
| bioF | 8-Amino-7-oxononanoate synthase | 4 |
| bioD | Dethiobiotin synthetase | 16 |
| bioB | Biotin synthetase | 14 |
| bioI | Biotin synthesis cytochrome P450-like enzyme | 19 |
| Category 3. Information pathways | ||
| 3.1 DNA replication | ||
| dnaA | Initiation of chromosome replication | 13 |
| dnaN | DNA polymerase III beta subunit | 7 |
| 3.2 DNA restriction/modification and repair | ||
| alkA | DNA-3-methyladenine glycosidase | 10 |
| adaA | Methylphosphotriester-DNA alkyltransferase/transcriptional activator of adaAB operon | 2 |
| adaB | O6-methylguanine-DNA methyltransferase | 8 |
| Category 4. Other functions | ||
| 4.2 Detoxification | ||
| mmr | Methylenomycin A resistance protein | 17 |
| 4.3 Antibiotic production | ||
| pksD | Polyketide synthesis | 148 |
| pksS | Polyketide synthesis | 8.2 |
| srfAA | Surfactin synthetase subunit 1 | 17 |
| srfAB | Surfactin synthetase subunit 2 | 24 |
| srfAC | Surfactin synthetase subunit 3 | 24 |
| srfAD | Surfactin synthetase subunit 4 | 25 |
AdoMet, S-adenosylmethionine.
–, signal not detected.
Genes transcribed at higher levels in postsweep strain WN716.
A number of genes involved in central metabolism and biosynthesis were more highly transcribed in postsweep strain WN716 (Table 3). Enhanced expression was noted of the pdhABCD operon encoding pyruvate dehydrogenase (PDH) (Table 3), which converts pyruvate into acetyl coenzyme A (acetyl-CoA) fueling diverse pathways such as the Krebs cycle, fatty acid biosynthesis, and fermentation pathways (45). Because R medium contains a high concentration of glucose, pyruvate accumulation might be expected, thus leading to upregulation of pdhABCD expression. Also, operons encoding purine (purEKBCSQL) and pyrimidine (pyrR pyrPBC-pyrAA-pyrAB-pyrKDFE) biosynthetic pathways were more highly transcribed in strain WN716 (Table 3). Increased purine and pyrimidine biosynthesis appears consistent with continued growth of strain WN716 in the postexponential phase, which would be expected to require continued synthesis of nucleic acids. Collectively, the data suggest that WN716 cells continue to perform central metabolism and nucleotide biosynthesis into the postexponential phase, consistent with their continued growth in stationary phase (Fig. 4 and 5). Continued stationary-phase growth has also been shown to be selectively advantageous in evolving batch culture populations of Escherichia coli (5, 20), and the growth of strain WN716 may be somewhat analogous to so-called GASP (growth advantage in stationary phase) mutants observed in evolving E. coli cultures (8). Interestingly, a number of genes and operons whose products are involved in cellular stress responses (ctsR-mcsA-mcsB-clpC, clpE, gspA, hrcA-grpE-dnaK, katE, mrgA, groES-groEL, dinG) were also more highly transcribed in early postexponential-phase cells of strain WN716 (Table 3), suggesting that its growth in flask culture produces some alteration to its environment that is stressful to cells.
Genes transcribed at lower levels in postsweep strain WN716.
It was observed that the lytA and lytC genes of the lytABC operon, and the monocistronic lytD gene were all downregulated in strain WN716 (Table 4). (Note, however, that for unknown reasons, the lytB gene within the lytABC operon was observed to be upregulated 10-fold [Table 3]). LytC and LytD are major autolysins that are secreted by LytA involved in cell separation and motility (2); thus, lowered transcription of these genes could potentially underlie the filamentous and nonmotile phenotype of strain WN716 (Fig. 1). In addition, examination of the microarray data revealed that essentially all of the operons encoding proteins involved in flagellar structure, assembly, motility, and chemotaxis were also transcribed at much lower levels in strain WN716 (Table 4). The fact that the lyt and flagellar operons all belong to the sigD regulon (14) led us to speculate that a mutation lowering sigma-D activity or sigD expression may be responsible for the nonmotile, filamentous phenotype. In support of the latter notion, examination of the microarray data revealed that sigD transcription was ∼3-fold lower in postsweep strain WN716 than in presweep strain WN715 (see complete microarray data). However, sequencing of the sigD promoter and coding region showed that strains WN715 and WN716 and the ancestor strain WN624 were all identical at this locus (data not shown), indicating that structural changes in SigD itself are likely not involved in decreased transcription of flagellar/chemotaxis/motility operons in strain WN716.
Although at present the exact reason for downregulation of flagellar, motility, and chemotaxis genes is unknown, the results suggest a possible reason why strain WN716 might grow faster and to a higher density in early postexponential phase. Production and operation of the flagella, rotary motor, and chemotaxis proteins exert a significant drain on a cell in both biosynthetic capacity and energetics (26). Loss of the motility and chemotactic apparatus by WN716 cells in a shaking flask culture, where the establishment of chemical gradients is prevented, may therefore result in increased allocation of energy and resources for growth, i.e., increased fitness (27).
In addition to autolysin and motility genes, the expression of a number of other genes encoding cell surface-associated functions were transcribed at lower levels in strain WN716, such as the feuABC iron transport operon and the opuBA-opuBB-opuBC-opuBD and opuCA-opuCB-opuCC-opuCD operons involved in transport of osmoprotectants, such as glycine betaine, proline, choline, or carnitine (16). It is conceivable that in the environment of R medium, these functions may be of decreased importance. Genes encoding functions for the cell surface-associated synthesis and assembly of cytochromes (ctaA, ctaBCDEFG, resABC) were also transcribed at lower levels (Table 4). Lowered transcription of these surface-associated functions may reflect general alterations of the cell surface associated with the filamentation phenotype of strain WN716 or to a switch in metabolism away from oxidative phosphorylation toward fermentation in response to the presence of glucose and a decrease in oxygen concentration in early postexponential phase. Transcription of ctaA, ctaBCDEFG, resABC, and lyt genes is known to be under the control of the oxygen-responsive two-component environmental sensor-response regulator pair ResD-ResE (10, 33, 47). Transcript levels of resD and resE were not observed to be different for strain WN715 versus strain WN716 (see complete microarray data), but microarrays give no information on whether these genes contain mutations altering structure or activity; hence, further examination of the resDE region by sequencing is warranted.
Competence for genetic transformation is a process under a complex set of controls that overlap with sporulation initiation and other transition state functions (4). Examination of the microarray data revealed that the competence genes comC, comX, and comS (embedded in the srf operon) were transcribed at lower levels in postsweep strain WN716, suggesting a competence defect. Therefore, we tested the abilities of strain WN715 and WN716 cells to be transformed from tryptophan auxotrophy to prototrophy (Trp− to Trp+). Strains WN715 and WN716 exhibited 3.36 × 105 and <20 Trp+ transformants per microgram of DNA, respectively, confirming the competence defect in postsweep strain WN716 that was predicted by the microarray data.
Transcript levels in strain WN716 were much lower for the oligopeptide permease operons appDFABC and oppABCDF, which are involved both in competence development and sporulation initiation (4, 30, 38). In addition, three rap-phr operons (rapA-phrA, rapC-phrC, and rapF-phrF) were observed to be downregulated in strain WN716 (Table 4). The rap-phr operons encode Rap phosphatases of phosphorylated Spo0F (Spo0F∼P) and Rap-inhibitory Phr peptides involved in regulation of the sporulation initiation phosphorelay and competence (4, 38, 40, 44), suggesting that in addition to genetic competence, sporulation may also be impaired in strain WN716. This prediction was tested by culturing strains WN715 and WN716 in sporulation medium and assaying sporulation efficiency at 24 h (36). The sporulation efficiencies of strains WN715 and WN716 were 0.41 and <1.42 × 10−6, respectively, confirming that WN716 was indeed sporulation deficient as predicted by the microarray data.
The synthesis of extracellular antibiotics is a hallmark of B. subtilis entrance into the postexponential growth phase. Genes encoding extracellular surfactin (srfAA, srfAB, srfAC, and srfAD) and polyketide antibiotic (pksD and pksS) and were observed to be transcribed at lower levels in strain WN716 (Table 4). Furthermore, in an earlier communication, we documented the physical loss of the lipopeptide antibiotic plipistatin biosynthetic genes ppsC and ppsD by their deletion during long-term evolution under relaxed selection for sporulation (22), indicating that under batch culture cultivation in R medium, synthesis of extracellular antibiotics is likely not selectively advantageous.
Taken together, the microarray data indicated that SCV strain WN716 failed to induce expression of suites of genes normally activated during the transition state from exponential- to postexponential-phase development (e.g., environmental sensing, competence, motility, sporulation initiation, and extracellular antibiotic production). These results are consistent with our previous observations that transition state functions were lost after 6,000 generations of evolution in the absence of selective pressure for sporulation (22).
Alteration of fermentative pathways in strain WN716.
On glucose-containing medium, B. subtilis is a mixed-acid fermenter, converting pyruvate into lactate, acetate, ethanol, acetoin, and 2,3-butanediol as fermentative end products (reviewed in reference 33). The pathway leading from pyruvate to acetoin in B. subtilis is well established and results from conversion of pyruvate to α-acetolactate by the enzyme α-acetolactate synthase (ALS) encoded by the alsS gene, followed by conversion of α-acetolactate to acetoin by alsD-encoded α-acetolactate decarboxylase (ALDC) (41). We observed that the genes that belonged to the alsSD operon had the lowest transcript levels in strain WN716 compared to strain WN715 (Table 4). Upregulation of the pdhABCD operon (Table 3) would be predicted to result in increased accumulation of acetyl-CoA, while simultaneous downregulation of alsSD would block the pyruvate-to-acetoin pathway. This combination of events would be predicted to result in significantly enhanced production of acids, particularly acetate, over acetoin, and concomitant lowering of the pH in R medium.
This prediction was first tested simply by performing the classic methyl red Voges-Proskauer (MR-VP) test (11) on overnight cultures of ancestral strains WN624 and WN628, as well as presweep LCM and postsweep SCV isolates from cultures 624A, 624B, and 624E. Both ancestral strains and the presweep strains WN699, WN715, and WN717 all produced acetoin by the VP test and did not produce significant acid by the MR test, and the supernatants from their overnight cultures were pH 7 (Table 5). In contrast, postsweep strains WN700, WN716, and WN718 did not produce acetoin by the VP test and did acidify the culture medium as judged by the MR test; furthermore, the pH of the R medium in which the postsweep strains had grown overnight had dropped to pH 4.5 to 6.0 (Table 5).
Table 5.
Results of the MR-VP assay and pH values for supernatants of cultures grown overnight in R mediuma
| Strain | Population | Sweep status of strain | MR result | VP result | pH |
|---|---|---|---|---|---|
| WN624 | Ancestor | − | + | 7.0 | |
| WN628 | Ancestor | − | + | 7.0 | |
| WN715 (LCM) | 624A | Presweep | − | + | 7.0 |
| WN716 (SCV) | 624A | Postsweep | + | − | 4.5 |
| WN717 (LCM) | 624B | Presweep | − | + | 7.0 |
| WN718 (SCV) | 624B | Postsweep | + | − | 6.0 |
| WN699 (LCM) | 624E | Presweep | − | + | 7.0 |
| WN700 (SCV) | 624E | Postsweep | + | − | 4.5 |
MR, methyl red assay for acid; V-P, Voges-Proskauer assay for acetoin.
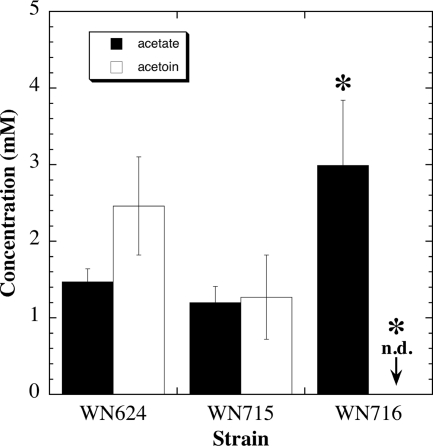
In order to quantify and compare acetoin and acid production between strains, the culture supernatants of ancestral strain WN624, presweep strain WN715, and postsweep strain WN716 were subjected to analysis by HPLC (34). Strains WN624 and WN715 produced both acetate and acetoin in similar quantities; in contrast, strain WN716 produced about twice as much acetate, and acetoin was not detected (Fig. 6). (Note that after 48 h in R medium, none of the three strains produced detectable levels of lactate [data not shown].) Thus, the fermentation patterns of WN624, WN715, and WN716 were consistent with the observations of the microarray data (Table 4) and the MR-VP results (Table 5).
Fig. 6.
Concentrations of acetate and acetoin in culture supernatants of strains WN624, WN715, and WN716. The cells were grown for 48 h in R medium plus the appropriate antibiotic, and samples were quantified by HPLC as described in Materials and Methods. Data are averages ± standard deviations (n = 3). Asterisks denote significant difference from strain WN624 by ANOVA (P ≤ 0.05). Acetoin was not detected (n.d.) for strain WN716 by HPLC.
Decreased transcription of the alsSD operon suggests that a mutation may have occurred in the alsSD promoter or cis-regulatory region, or in the adjacent gene alsR, which encodes a positive regulator of alsSD transcription (41). Sequence analysis of this region is under way.
It has been proposed that acetoin production in cells acts to maintain intracellular pH homeostasis (17, 41, 48). Thus, it might be predicted that loss of acetoin production and increase in acetate production would lead to acid stress. It is possible that postsweep strain WN716 is better able to cope with pH stress than presweep strain WN715, as evidenced by its increased transcription of various stress response genes such as groES, groEL, clpC, clpE, grpE, dnaK, gspA, mcsA, mcsB, mrgA, and katB, as well as transcriptional regulators of stress responses such as hrcA and ctsR (Table 3). Interestingly, these genes were recently shown to be part of the transcriptional response to acetic acid stress in B. cereus (29). It is also noteworthy that acid production and acid tolerance are common strategies used by the lactobacilli and other lactic acid bacteria that result in promotion of their own growth and simultaneous inhibition of less-acid-tolerant competitors (21).
Altered metabolism and increased fitness in postsweep strain WN716.
To understand how the observed gene expression changes led to the evolution of increased fitness in SCV strain WN716, it is important to interpret these results in the context of the nutrient-rich, constantly mixed selective environment of shaking flask cultivation in R medium, where an excess of glucose inhibits sporulation initiation and promotes fermentation. Therefore, cells evolving under these conditions may have increased their fitness during long-term propagation by the following: (i) more-rapid exponential growth, (ii) continued growth in postexponential phase, (iii) loss of energetically costly motility and chemotactic functions, and (iv) modification of their environment to favor their own survival and growth at the expense of potential competitors. These are indeed the properties observed in the evolution of strain WN716 during optimization of its fitness in R medium. It should be stressed here that increased fitness of strain WN716 in R medium would necessarily result in this strain becoming less of a “generalist” and more of a “niche specialist” at the cost of lowering its adaptability to environmental change (i.e., plasticity). Indeed, loss of transcriptome plasticity was previously documented in a generation 6,000 population that also evolved in R medium (22). The loss of motility, competence, sporulation, extracellular antibiotic production, etc., would place strain WN716 at a severe selective disadvantage in the natural soil habitat of B. subtilis.
The multilocus nature of the transcriptional changes observed in strain WN716, affecting entire regulons, strongly indicates that mutations affecting higher-order levels in the regulatory hierarchy may have occurred during the evolution of WN716. Experimental effort is currently directed toward whole-genome sequencing of strains WN624, WN715, and WN716, in order to pinpoint which regulator(s) is/are involved. These sequence data will enable an integrated view of the mutations affecting the evolution of the phenotypes presented here, as well as auxotrophy and sporulation, as previously described (23, 24).
ACKNOWLEDGEMENTS
We thank K.T. Shanmugam for assistance with early aspects of the HPLC analyses, the Genomic Analysis and Technology Core (GATC) at the University of Arizona for microarray hybridization, and the 3 anonymous reviewers for insightful comments.
This work was supported by an NSF-IGERT fellowship to H.M. and by a grant from the NASA Astrobiology: Exobiology and Evolutionary Biology program (NNX08AO15G) to W.L.N.
Footnotes
Published ahead of print on 29 April 2011.
REFERENCES
- 1. Aizawa S.-I., Zhulin I. B., Márquez-Magaña L., Ordal G. W. 2002. Chemotaxis and motility , p. 437–452 In Sonenshein A. L., Hoch J. A., Losick R. (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 2. Blackman S. A., Smith T. J., Foster S. J. 1998. The role of autolysins during vegetative growth of Bacillus subtilis 168. Microbiology 144: 73–82 [DOI] [PubMed] [Google Scholar]
- 3. Boylan R. J., Mendelson N. H., Brooks D., Young F. E. 1972. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J. Bacteriol. 110: 281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dubnau D., Lovett C. M. 2002. Transformation and recombination, p. 453–471 In Sonenshein A. L., Hoch J. A., Losick R. (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 5. Elena S. F., Lenski R. E. 1997. Long-term experimental evolution in Escherichia coli. VII. Mechanisms maintaining genetic variability within populations. Evolution 51: 1058–1067 [DOI] [PubMed] [Google Scholar]
- 6. Elena S. F., Lenski R. E. 2003. Evolution experiments with microorganisms: the dynamics and genetic basis of adaptation. Nat. Rev. Genet. 4: 457–469 [DOI] [PubMed] [Google Scholar]
- 7. Feil E. J. 2004. Small change: keeping pace with microevolution. Nat. Rev. Microbiol. 2: 483–495 [DOI] [PubMed] [Google Scholar]
- 8. Finkel S. E., Kolter R. 1999. Evolution of microbial diversity during prolonged starvation. Proc. Natl. Acad. Sci. U. S. A. 96: 4023–4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Funchain P., et al. 2000. The consequences of growth of a mutator strain of Escherichia coli as measured by loss of function among multiple gene targets and loss of fitness. Genetics 154: 959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geng H., Zuber P., Nakano M. M. 2007. Regulation of respiratory genes by ResD-ResE signal transduction system in Bacillus subtilis. Methods Enzymol. 442: 448–464 [DOI] [PubMed] [Google Scholar]
- 11. Gerhardt P., et al. (ed.). 1981. Manual of methods for general bacteriology. ASM Press, Washington, DC [Google Scholar]
- 12. Gerrish P. J., Lenski R. E. 1998. The fate of competing beneficial mutations in an asexual population. Genetica 102/103: 127–144 [PubMed] [Google Scholar]
- 13. Guttman D. S., Dykhuizen D. E. 1994. Clonal divergence in Escherichia coli as a result of recombination, not mutation. Science 266: 1380–1383 [DOI] [PubMed] [Google Scholar]
- 14. Helmann J. D., Moran C. P., Jr 2002. RNA polymerase and sigma factors, p. 289–312 In Sonenshein A. L., Hoch J. A., Losick R. (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 15. Kassen R. 2002. The experimental evolution of specialists, generalists, and the maintenance of diversity. J. Evol. Biol. 15: 173–190 [Google Scholar]
- 16. Kempf B., Bremer E. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 170: 319–330 [DOI] [PubMed] [Google Scholar]
- 17. Kovacikova G., Wei L., Skorupski J. 2005. Dual regulation of genes involved in acetoin biosynthesis and motility/biofilm formation by the virulence activator AphA and the acetate-responsive LysR-type regulator AlsR in Vibrio cholerae. Mol. Microbiol. 57: 420–433 [DOI] [PubMed] [Google Scholar]
- 18. Kunst F., et al. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390: 249–256 [DOI] [PubMed] [Google Scholar]
- 19. Lenski R., Rose M. R., Simpson S. C., Tadler S. C. 1991. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am. Nat. 138: 1315–1341 [Google Scholar]
- 20. Lenski R. E., Travisano M. 1994. Dynamics of adaptation and diversification: a 10,000-generation experiment with bacterial populations. Proc. Natl. Acad. Sci. U. S. A. 91: 6808–6814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madigan M. T., Martinko J. M., Parker J. (ed.). 1997. Brock biology of microorganisms. Prentice Hall, Upper Saddle River, NJ [Google Scholar]
- 22. Maughan H., Birky C. W., Jr., Nicholson W. L. 2009. Transcriptome divergence and the loss of plasticity in Bacillus subtilis after 6,000 generations of evolution under relaxed selection for sporulation. J. Bacteriol. 191: 428–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maughan H., et al. 2006. The population genetics of phenotypic deterioration in experimental populations of Bacillus subtilis. Evolution 60: 686–695 [PubMed] [Google Scholar]
- 24. Maughan H., Masel J., Birky C. W., Jr., Nicholson W. L. 2007. The roles of mutation accumulation and selection in loss of sporulation in experimental populations of Bacillus subtilis. Genetics 177: 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 26. Mitchell J. G. 1991. The influence of cell size on marine bacterial motility and energetics. Microb. Ecol. 22: 227–238 [DOI] [PubMed] [Google Scholar]
- 27. Mitchell J. G. 2002. The energetics and scaling of search strategies in bacteria. Am. Nat. 160: 727–740 [DOI] [PubMed] [Google Scholar]
- 28. Mitchell-Olds T., Willis J. H., Goldstein D. B. 2007. Which evolutionary processes influence natural genetic variation for phenotypic traits? Nat. Rev. Genet. 8: 745–856 [DOI] [PubMed] [Google Scholar]
- 29. Mols M., et al. 2010. Comparative analysis of transcriptional and physiological responses of Bacillus cereus to organic and inorganic acid shocks. Int. J. Food Microbiol. 137: 13–21 [DOI] [PubMed] [Google Scholar]
- 30. Monnet V. 2003. Bacterial oligopeptide-binding proteins. Cell. Mol. Life Sci. 60: 2100–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moran N. A. 1996. Accelerated evolution and Muller's rachet in endosymbiotic bacteria. Proc. Natl. Acad. Sci. U. S. A. 93: 2873–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moran N. A., McLaughlin H. J., Sorek R. 2009. The dynamics and time scale of ongoing genomic erosion in symbiotic bacteria. Science 323: 379–382 [DOI] [PubMed] [Google Scholar]
- 33. Nakano M. M., Zuber P. 2002. Anaerobiosis, p. 393–404 In Sonenshein A. L, Hoch J. A., Losick R. (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 34. Nicholson W. L. 2008. The Bacillus subtilis ydjL (bdhA) gene encodes acetoin reductase/2,3-butanediol dehydrogenase. Appl. Environ. Microbiol. 74: 6832–6838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nicholson W. L., et al. 2010. Exploring the low-pressure limit: evolution of Bacillus subtilis in the laboratory to enhanced growth at 5 kilopascals. Appl. Environ. Microbiol. 76: 7559–7565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nicholson W. L., Setlow P. 1990. Sporulation, germination, and outgrowth, p. 391–450 In Harwood C. R., Cutting S. M. (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Sussex, England [Google Scholar]
- 37. O'Toole G., Kaplan H. B., Kolter R. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54: 49–79 [DOI] [PubMed] [Google Scholar]
- 38. Perego M., Hoch J. A. 2002. Two-component systems, phosphorelays, and regulation of their activities by phosphatases, p. 473–481 In Sonenshein A. L., Hoch J. A., Losick R. (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 39. Piggot P. J., Losick R. 2002. Sporulation genes and intercompartmental regulation, p. 483–517 In Sonenshein A. L., Hoch J. A., Losick R. (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 40. Pottathil M., Lazazzera B. A. 2003. The extracellular Phr peptide-Rap phosphatase signaling circuit of Bacillus subtilis. Frontiers Biosci. 8: D32–D45 [DOI] [PubMed] [Google Scholar]
- 41. Renna M. C., Najimudin N., Winik L. R., Zahler S. A. 1993. Regulation of the alsS, alsD, and alsR genes involved in post-exponential-phase production of acetoin. J. Bacteriol. 175: 3863–3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schaeffer P., Millet J., Aubert J.-P. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. U. S. A. 54: 704–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schallmey M., Singh A., Ward O. P. 2004. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 50: 1–17 [DOI] [PubMed] [Google Scholar]
- 44. Smits W. K., et al. 2007. Temporal separation of distinct differentiation pathways by a dual specificity Rap-Phr system in Bacillus subtilis. Mol. Microbiol. 65: 103–120 [DOI] [PubMed] [Google Scholar]
- 45. Sonenshein A. L. 2002. Krebs citric acid cycle, p. 151–162 In Sonenshein A. L., Hoch J. A., Losick R. (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 46. Steinmetz M., Richter R. 1994. Plasmids designed to alter the antibiotic resistance by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene 142: 79–83 [DOI] [PubMed] [Google Scholar]
- 47. Sun G., et al. 1996. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J. Bacteriol. 178: 1374–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsau J.-L., Guffanti A. A., Montville T. J. 1992. Conversion of pyruvate to acetoin helps to maintain pH homeostasis in Lactobacillus plantarum. Appl. Environ. Microbiol. 58: 891–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Underwood S. A., et al. 2002. Genetic changes to optimize carbon partitioning between ethanol and biosynthesis in ethanologenic Escherichia coli. Appl. Environ. Microbiol. 68: 6263–6272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vos M., Didelot X. 2009. A comparison of homologous recombination rates in bacteria and archaea. ISME J. 3: 199–208 [DOI] [PubMed] [Google Scholar]
- 51. Xue Y., Nicholson W. L. 1996. The two major DNA repair pathways, nucleotide excision repair and spore photoproduct lyase, are sufficient for the resistance of Bacillus subtilis spores to artificial UV-C and UV-B but not to solar radiation. Appl. Environ. Microbiol. 62: 2221–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]