Abstract
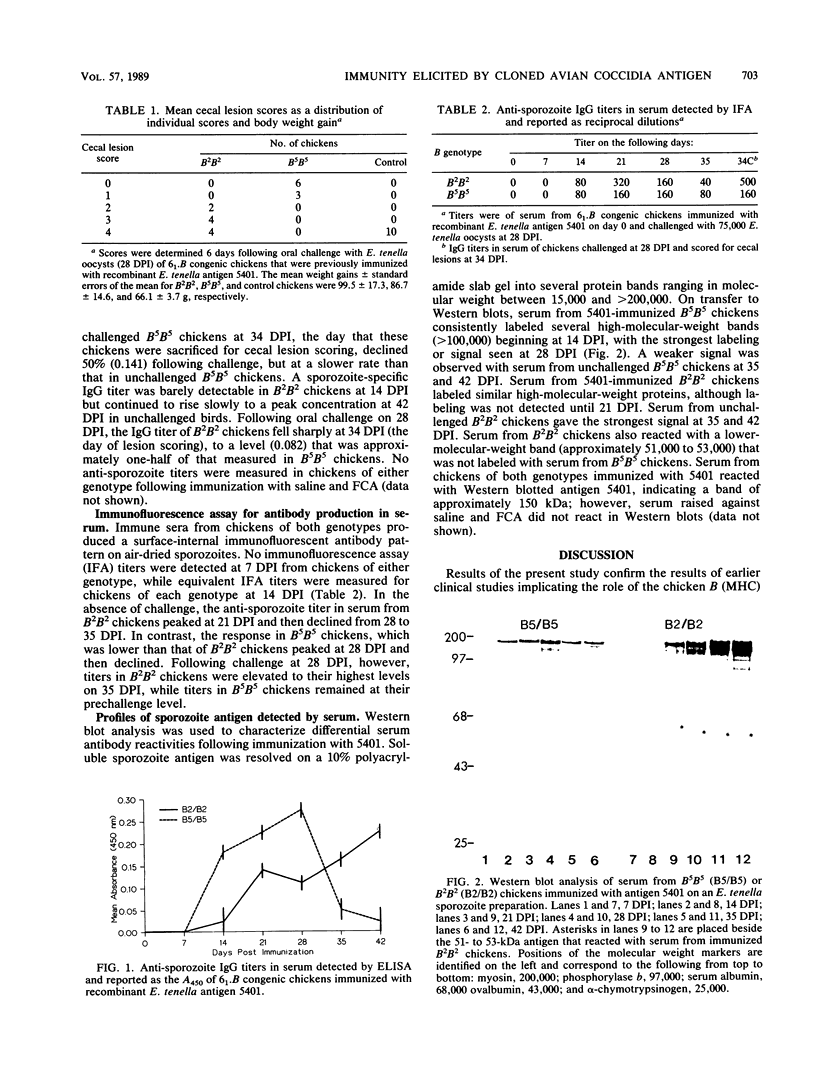
The immunogenicity of a recombinant Eimeria tenella coccidial antigen was studied in 6(1).B congenic chickens derived from B2B2 and B5B5 parents segregating for haplotypes B2 and B5. Five-week-old chickens were immunized with 2.4 micrograms of recombinant protein (designated 5401) in Freund complete adjuvant and challenged with 75,000 oocysts at 28 days postimmunization (DPI) to determine the degree of elicited protective immunity. Serum samples were collected weekly for 5 weeks postimmunization for analysis by enzyme-linked immunosorbent assay, immunofluorescence assay, and Western blotting. Lesion scores following oocyst challenge were significantly reduced in B5B5 chickens compared with those in B2B2 chickens. Immunization induced a sporozoite-specific immunoglobulin G (IgG) titer in serum detected by the enzyme-linked immunosorbent assay that peaked at 28 DPI, the day of challenge, in B5B5 chickens and at 42 DPI in B2B2 chickens. After challenge, this titer declined for each genotype. Anti-sporozoite IgG detected by the immunofluorescence assay attained a peak titer at 21 DPI in B2B2 chickens and 28 DPI in B5B5 chickens. Serum from immunized B5B5 chickens reacted strongly in Western blots with several high-molecular-weight (greater than 100,000), soluble proteins prepared from sporozoites. Serum from B2B2 chickens reacted with similar proteins as well as with a 51- to 53-kilodalton protein that was not labeled by serum from B5B5 chickens. These results demonstrate further the role of host genetics on anticoccidial immunity and suggest that a peak anti-sporozoite IgG titer in B5B5 chickens on the day of challenge may signal a state of immunocompetence to that challenge.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRILES W. E., McGIBBON W. H., IRWIN M. R. On multiple alleles effecting cellular antigens in the chicken. Genetics. 1950 Nov;35(6):633–652. doi: 10.1093/genetics/35.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles W. E., Briles R. W. Identification of haplotypes of the chicken major histocompatibility complex (B). Immunogenetics. 1982;15(5):449–459. doi: 10.1007/BF00345904. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Antigen presenting cells and mechanisms of antigen presentation. Crit Rev Immunol. 1985;5(3):263–316. [PubMed] [Google Scholar]
- Clare R. A., Strout R. G., Taylor R. L., Jr, Aeed P. A. Bile and serum immunoglobulin levels during primary and secondary infections with Eimeria tenella in chickens. Vet Parasitol. 1987 Jun;25(1):33–38. doi: 10.1016/0304-4017(87)90062-8. [DOI] [PubMed] [Google Scholar]
- Clare R. A., Strout R. G., Taylor R. L., Jr, Collins W. M., Briles W. E. Major histocompatibility (B) complex effects on acquired immunity to cecal coccidiosis. Immunogenetics. 1985;22(6):593–599. doi: 10.1007/BF00430307. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Danforth H. D., Augustine P. C. Use of hybridoma antibodies and recombinant DNA technology in protozoan vaccine development. Avian Dis. 1986 Jan-Mar;30(1):37–42. [PubMed] [Google Scholar]
- Davis P. J., Porter P. A mechanism for secretory IgA-mediated inhibition of the cell penetration and intracellular development of Eimeria tenella. Immunology. 1979 Mar;36(3):471–477. [PMC free article] [PubMed] [Google Scholar]
- Del Giudice G., Cooper J. A., Merino J., Verdini A. S., Pessi A., Togna A. R., Engers H. D., Corradin G., Lambert P. H. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoite repetitive epitope is I-Ab-restricted: possible implications for malaria vaccines. J Immunol. 1986 Nov 1;137(9):2952–2955. [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Maloy W. L., Hayashi Y., Fujii N., Hockmeyer W. T., Miller L. H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986 Aug 1;164(2):655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J., Reid W. M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970 Aug;28(1):30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Johnson L. W., Edgar S. A. Ea-B and Ea-C cellular antigen genes in Leghorn lines resistant and susceptible to acute cecal coccidiosis. Poult Sci. 1986 Feb;65(2):241–252. doi: 10.3382/ps.0650241. [DOI] [PubMed] [Google Scholar]
- Klesius P. H., Giambrone J. J. Adoptive transfer of delayed hypersensitivity and protective immunity to Eimeria tenella with chicken-derived transfer factor. Poult Sci. 1984 Jul;63(7):1333–1337. doi: 10.3382/ps.0631333. [DOI] [PubMed] [Google Scholar]
- Koch J., Buss E. G., Wideman R. F. Blood ionic calcium responses of hens from thick-shell and thin-shell lines to ethyleneglycol-bis-(B-aminoethylether)-N,N'-tetraacetic acid injections. Poult Sci. 1984 Jan;63(1):172–175. doi: 10.3382/ps.0630172. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Rose M. E. Mucosal transport of Eimeria tenella in the cecum of the chicken. J Parasitol. 1982 Dec;68(6):1117–1123. [PubMed] [Google Scholar]
- Mahrt J. L., Shi Y. F. Murine major histocompatibility complex and immune response to Eimeria falciformis. Infect Immun. 1988 Jan;56(1):270–271. doi: 10.1128/iai.56.1.270-271.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F. Injection versus infection: the cellular immunology of parasitism. Parasitol Today. 1987 Apr;3(4):106–111. doi: 10.1016/0169-4758(87)90047-0. [DOI] [PubMed] [Google Scholar]
- Mockett A. P., Rose M. E. Immune responses to eimeria: quantification of antibody isotypes to Eimeria tenella in chicken serum and bile by means of the ELISA. Parasite Immunol. 1986 Sep;8(5):481–489. doi: 10.1111/j.1365-3024.1986.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Nussenzweig R. S., Nussenzweig V. Development of sporozoite vaccines. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):117–128. doi: 10.1098/rstb.1984.0113. [DOI] [PubMed] [Google Scholar]
- Rose M. E. Immune responses in infections with coccidia: macrophage activity. Infect Immun. 1974 Oct;10(4):862–871. doi: 10.1128/iai.10.4.862-871.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Mockett A. P. Antibodies to coccidia: detection by the enzyme-linked immunosorbent assay (ELISA). Parasite Immunol. 1983 Sep;5(5):479–489. doi: 10.1111/j.1365-3024.1983.tb00762.x. [DOI] [PubMed] [Google Scholar]
- SCHIERMAN L. W., NORDSKOG A. W. Relationship of blood type to histocompatibility in chickens. Science. 1961 Oct 6;134(3484):1008–1009. doi: 10.1126/science.134.3484.1008. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Shen P. F., Smith E. J., Bacon L. D. The ontogeny of blood cells, complement, and immunoglobulins in 3- to 12-week-old 15I5-B congenic white Leghorn chickens. Poult Sci. 1984 Jun;63(6):1083–1093. doi: 10.3382/ps.0631083. [DOI] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Young J. F., Hockmeyer W. T., Gross M., Ballou W. R., Wirtz R. A., Trosper J. H., Beaudoin R. L., Hollingdale M. R., Miller L. H., Diggs C. L. Expression of Plasmodium falciparum circumsporozoite proteins in Escherichia coli for potential use in a human malaria vaccine. Science. 1985 May 24;228(4702):958–962. doi: 10.1126/science.2988125. [DOI] [PubMed] [Google Scholar]