Abstract
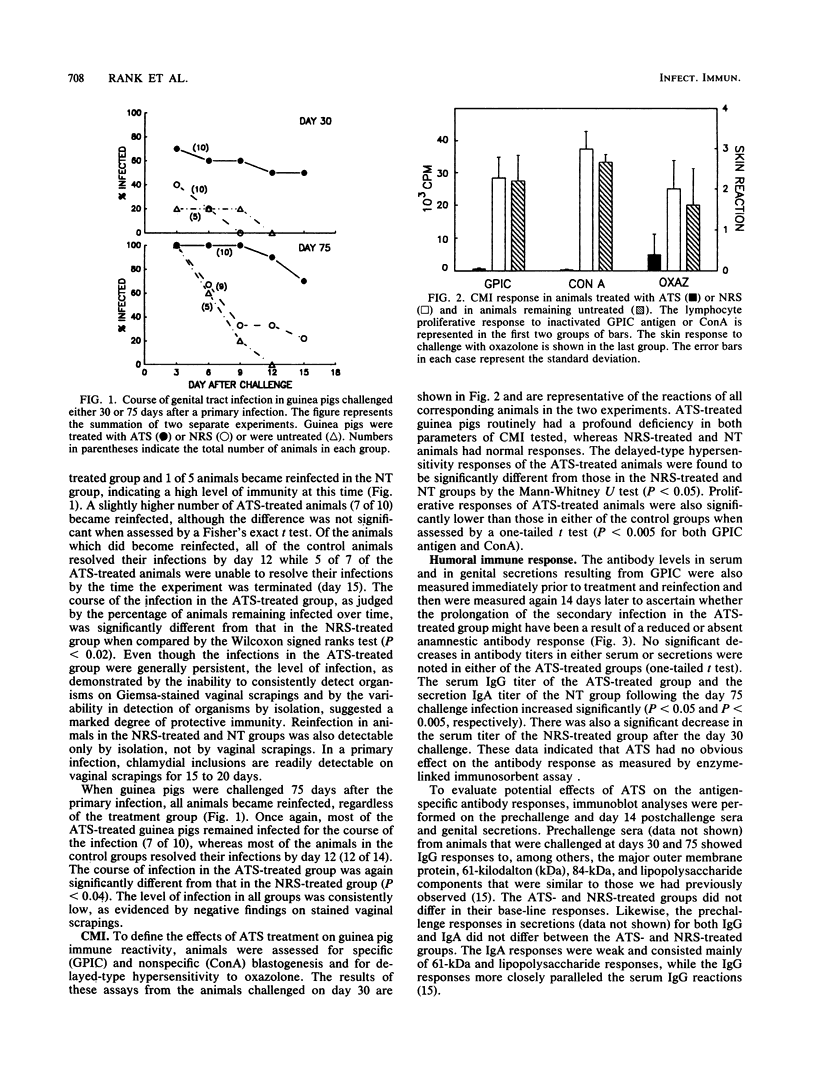
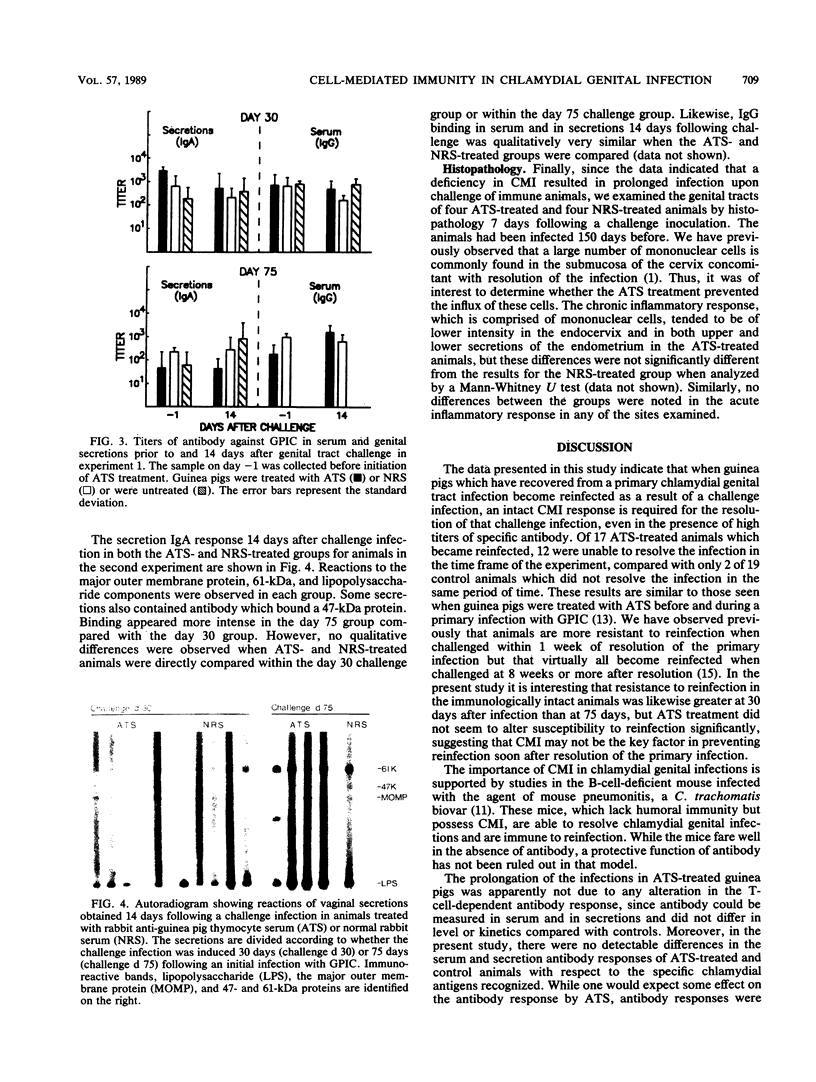
Guinea pigs which have recovered from a genital infection with the agent of guinea pig inclusion conjunctivitis demonstrate strong immunity to reinfection for a short period of time but then become susceptible to reinfection. The secondary infection is markedly shortened in duration and decreased in intensity. Previous studies have indicated an important role for humoral immunity in resistance to and in recovery from reinfection. However, the contribution of cell-mediated immunity to immunity toward or recovery from a secondary infection is not clear. Guinea pigs were infected in the genital tract with guinea pig inclusion conjunctivitis and were challenged at either 30 or 75 days after the primary infection. Prior to challenge, one group of animals were injected with rabbit anti-guinea pig thymocyte serum (ATS) while control groups received either normal rabbit serum or no treatment. Treatment was continued daily for the course of the experiment. On day 30, ATS-treated guinea pigs had a slightly higher rate of reinfection, and generally the infection persisted longer than in controls. On day 75, all animals became reinfected upon challenge, but control animals resolved their infections in 3 to 9 days. In contrast, most ATS-treated animals remained infected throughout the course of the experiment. Although the animals became reinfected, the levels of chlamydiae were much lower than those observed during the primary infection. ATS treatment abrogated T-cell responses, but serum and secretory antibody responses remained normal. Histopathological examination revealed some decrease in mononuclear infiltration of endocervical and uterine tissues in ATS-treated animals. These data indicate that previously infected guinea pigs require both cell-mediated immunity and humoral immunity for resolution of a challenge infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barron A. L., White H. J., Rank R. G., Soloff B. L. Target tissues associated with genital infection of female guinea pigs by the chlamydial agent of guinea pig inclusion conjunctivitis. J Infect Dis. 1979 Jan;139(1):60–68. doi: 10.1093/infdis/139.1.60. [DOI] [PubMed] [Google Scholar]
- Batteiger B. E., Rank R. G. Analysis of the humoral immune response to chlamydial genital infection in guinea pigs. Infect Immun. 1987 Aug;55(8):1767–1773. doi: 10.1128/iai.55.8.1767-1773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Martin D. H., Kuo C. C., Wang S. P., Stevens C. E., Hubbard T., Holmes K. K. Cellular immune response during uncomplicated genital infection with Chlamydia trachomatis in humans. Infect Immun. 1981 Oct;34(1):98–104. doi: 10.1128/iai.34.1.98-104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Grubbs B., Marshall T. J., Schachter J., Williams D. M. Gamma interferon-mediated cytotoxicity related to murine Chlamydia trachomatis infection. Infect Immun. 1988 Aug;56(8):2023–2027. doi: 10.1128/iai.56.8.2023-2027.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Krueger D. A. Lymphokine-mediated inhibition of Chlamydia replication in mouse fibroblasts is neutralized by anti-gamma interferon immunoglobulin. Infect Immun. 1983 Dec;42(3):1152–1158. doi: 10.1128/iai.42.3.1152-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna L., Kerlan R., Senyk G., Stites D. P., Juster R. P., Jawetz E. Immune responses to chlamydial antigens in humans. Med Microbiol Immunol. 1982;171(1):1–10. doi: 10.1007/BF02122702. [DOI] [PubMed] [Google Scholar]
- Hough A. J., Jr, Rank R. G. Induction of arthritis in C57B1/6 mice by chlamydial antigen. Effect of prior immunization or infection. Am J Pathol. 1988 Jan;130(1):163–172. [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Schachter J. Failure to detect cell-mediated cytotoxicity against Chlamydia trachomatis-infected cells. Infect Immun. 1983 Mar;39(3):1271–1274. doi: 10.1128/iai.39.3.1271-1274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvigstad E., Hirschberg H. Lack of cell-mediated cytotoxicity towards Chlamydia trachomatis infected target cells in humans. Acta Pathol Microbiol Immunol Scand C. 1984 Jun;92(3):153–159. doi: 10.1111/j.1699-0463.1984.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Ramsey K. H., Soderberg L. S., Rank R. G. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988 May;56(5):1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Barron A. L. Effect of antithymocyte serum on the course of chlamydial genital infection in female guinea pigs. Infect Immun. 1983 Aug;41(2):876–879. doi: 10.1128/iai.41.2.876-879.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Barron A. L. Humoral immune response in acquired immunity to chlamydial genital infection of female guinea pigs. Infect Immun. 1983 Jan;39(1):463–465. doi: 10.1128/iai.39.1.463-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Barron A. L. Specific effect of estradiol on the genital mucosal antibody response in chlamydial ocular and genital infections. Infect Immun. 1987 Sep;55(9):2317–2319. doi: 10.1128/iai.55.9.2317-2319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Batteiger B. E., Soderberg L. S. Susceptibility to reinfection after a primary chlamydial genital infection. Infect Immun. 1988 Sep;56(9):2243–2249. doi: 10.1128/iai.56.9.2243-2249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., White H. J., Barron A. L. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1979 Nov;26(2):573–579. doi: 10.1128/iai.26.2.573-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell K., Daniel K., Blazkovec A. A. Effects of anti-lymphocyte, anti-macrophage and anti-thymocyte serum IgG on the immune response. Int Arch Allergy Appl Immunol. 1971;41(2):286–301. doi: 10.1159/000230527. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Byrne G. I., Grubbs B., Marshal T. J., Schachter J. Role in vivo for gamma interferon in control of pneumonia caused by Chlamydia trachomatis in mice. Infect Immun. 1988 Nov;56(11):3004–3006. doi: 10.1128/iai.56.11.3004-3006.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]




