Abstract
Grape seed extract (GSE) is reported to have many pharmacological benefits, including antioxidant, anti-inflammatory, anticarcinogenic, and antimicrobial properties. However, the effect of this inexpensive rich source of natural phenolic compounds on human enteric viruses has not been well documented. In the present study, the effect of commercial GSE, Gravinol-S, on the infectivity of human enteric virus surrogates (feline calicivirus, FCV-F9; murine norovirus, MNV-1; and bacteriophage MS2) and hepatitis A virus (HAV; strain HM175) was evaluated. GSE at concentrations of 0.5, 1, and 2 mg/ml was individually mixed with equal volumes of each virus at titers of ∼7 log10 PFU/ml or ∼5 log10 PFU/ml and incubated for 2 h at room temperature or 37°C. The infectivity of the recovered viruses after triplicate treatments was evaluated by standardized plaque assays. At high titers (∼7 log10 PFU/ml), FCV-F9 was significantly reduced by 3.64, 4.10, and 4.61 log10 PFU/ml; MNV-1 by 0.82, 1.35, and 1.73 log10 PFU/ml; MS2 by 1.13, 1.43, and 1.60 log10 PFU/ml; and HAV by 1.81, 2.66, and 3.20 log10 PFU/ml after treatment at 37°C with 0.25, 0.50, and 1 mg/ml GSE, respectively (P < 0.05) in a dose-dependent manner. GSE treatment of low titers (∼5 log10 PFU/ml) at 37°C also showed viral reductions. Room-temperature treatments with GSE caused significant reduction of the four viruses, with higher reduction for low-titer FCV-F9, MNV-1, and HAV compared to high titers. Our results indicate that GSE shows promise for application in the food industry as an inexpensive novel natural alternative to reduce viral contamination and enhance food safety.
INTRODUCTION
Grapes are one of the world's leading fruit crops, with production rates at more than 50 million tons a year (34). Grape seeds, which are by-products of wine and the grape juice industries, are shown to contain large quantities of phenolic compounds such as gallic acid and monomeric flavan-3-ols catechin, epicatechin, gallocatechin, epigallocatechin, and epicatechin-3-O-gallate, as well as dimeric, trimeric, and polymeric proanthocyanidins (PAC) (37). Grape seed extract (GSE) reportedly has many pharmacological and health benefits that include antioxidant, cardioprotective, hepatoprotective, neuroprotective, anti-inflammatory, antidiabetic, anticarcinogenic, and antiaging effects (28, 47, 48).
Recently, GSE has gained increasing attention in the food industry because of its associated antimicrobial properties. Rhodes et al. (31) showed that GSE at a concentration of 0.25 mg/ml decreased Listeria monocytogenes from 106 to 107 CFU/ml to undetectable levels within 10 min. Kao et al. (20) found that GSE at 1 mg/ml could cause 99% inhibition in the growth of Staphylococcus aureus. GSE has also been shown to have antibacterial activity against many other epidemiologically significant food-borne bacterial pathogens such as Escherichia coli O157:H7, Salmonella enterica serovar Enteritidis, Salmonella enterica serovar Typhimurium, etc. (2). Besides bacteria, GSE has also been shown to inhibit the replication and expression of human immunodeficiency virus type 1 (HIV-1) (25, 26).
The antiviral effect of GSE against human enteric viruses has not been explored. There are several documented human norovirus (NoV) and hepatitis A virus (HAV) outbreaks associated with the consumption of ready-to-eat foods, including salads, sandwiches, bakery products, salad dressing, raspberries, and oysters from contaminated waters (24, 33, 35, 44, 45, 51). Thus, effective mitigation and control strategies are of great importance to reduce food-borne viral illness as well as to help increase the shelf life of food products.
Like other natural antimicrobials, GSE is not reported to exhibit toxicity or adverse health effects at the dose of 50 to 100 mg per day (16, 36, 38, 42, 43, 49). GSE is known to be nonmutagenic, nonclastogenic, and nonaneugenic (49). The numerous health benefits and antimicrobial properties (28, 47, 48) of GSE along with the fact that grape seeds are inexpensive waste or by-products from the wine and juice industry make it a promising alternative control strategy for food industry applications.
In the present study, the effect of commercially available GSE, Gravinol-S, at 0.25, 0.5, and 1.0 mg/ml on the infectivity of hepatitis A virus (HAV; strain HM175) and noroviral surrogates was studied at two temperatures (room temperature and 37°C) using two viral titers (∼7 and ∼5 log10 PFU/ml). Gravinol-S was obtained from OptiPure; it was prepared from specifically selected grape seeds and processed using natural grain alcohol. It contains a minimum of 95% flavonol, of which 82% is oligomeric proanthocyanidins (OPCs), 12% being the highly active monomeric OPCs. As cell culture systems for propagation and infectivity studies of human NoV remain lacking, surrogates such as feline calicivirus (FCV-F9) (40), murine norovirus (MNV-1) (46), and MS2 bacteriophage (10), which can be assayed for infectivity, were used to determine the effects of GSE. The infectivity of the viruses after 2 h of treatment was evaluated using standardized plaque assays and compared to untreated controls. To gain further insights and understand the mechanism of action of GSE, CRFK, RAW 264.7, and FRhK4 cells were treated with GSE at various concentrations prior to or after viral infection using the respective viruses in order to determine if GSE has an effect on viral adsorption or viral replication, respectively.
MATERIALS AND METHODS
Viruses, bacterial hosts, and cell lines.
Feline calicivirus FCV-F9 and Crandell Reese feline kidney (CRFK) cells, as well as bacteriophage MS2 and its host E. coli B-15597, were purchased from ATCC (Manassas, VA). Murine norovirus, MNV-1, was kindly provided as a gift by Skip Virgin (Washington University, St. Louis, MO), and RAW 264.7 cells were obtained from the University of Tennessee at Knoxville. Hepatitis A virus (HAV; strain HM175) and fetal rhesus monkey kidney (FRhK4) cells were kindly provided by our collaborator, Kalmia Kniel (University of Delaware).
CRFK, RAW 264.7, and FRhK4 cells were maintained in Dulbecco's modified Eagle's medium/Ham's F-12 medium (DMEM-F12; HyClone Laboratories, Logan, UT) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone Laboratories) and 1× Anti-Anti (Antibiotic-Antimycotic; Invitrogen, Grand Island, NY) at 37°C in an atmosphere containing 5% CO2. E. coli B-15597 was grown in 3% Trypticase soy broth (TSB) at 37°C.
Propagation of viruses.
CRFK, RAW 264.7, and FRhK4 cells with ∼90% confluence in cell culture flasks were washed with phosphate-buffered saline (PBS; pH 7.4) twice before adding FCV-F9, MNV-1, and HAV stocks to their respective cell monolayers. The infected cells were then incubated until >90% cell lysis in a water-jacketed CO2 incubator at 37°C. E. coli B-15597 host in TSB containing 0.1% glucose, 2 mM CaCl2, and 10 μg/ml thiamine was used for the propagation of bacteriophage MS2 at 37°C for 18 h. All three viruses were recovered by centrifugation at 5,000 × g for 10 min, followed by filtration through 0.2-μm filters, aliquoted, and stored at −80°C until use.
Cytotoxicity determination of GSE on CRFK, RAW 264.7, and FRhK4 cell lines.
GSE at concentrations of 0.1 to 1.0 mg/ml was each added to individual wells of confluent CRFK, RAW 264.7, or FRhK4 cells in 6-well plates and incubated for 2 h under 5% CO2. The solution was then aspirated, and the cells were overlaid with complete DMEM containing 0.75% agar and incubated further for 2 to 8 days. Cytopathic effects were determined by both visual inspection under the optical microscope and neutral red staining. Each experiment was run in duplicate and replicated twice.
Antiviral effects of GSE.
GSE, Gravinol-S, was obtained as a gift from OptiPure, Chemco Industries (Los Angeles, CA). GSE solution was made by dissolving the supplied powder in water and ethanol, filtering it through a 0.2-μm filter, and further diluting it aseptically to 0.5 (pH 5.04), 1.0 (pH 5.05), and 2.0 (pH 5.40) mg/ml in sterile deionized distilled water. Each GSE solution was mixed with an equal volume of each virus to reach titers of ∼7 and ∼5 log10 PFU/ml (the resulting pH of the virus-GSE mixture was 7.32 to 7.40 after addition of GSE at the above three concentrations) and incubated at room temperature or 37°C for 2 h. Individual viruses mixed with sterile deionized distilled water were also incubated at both temperatures for 2 h and used as the untreated controls. After incubation, treated viruses and controls were neutralized in DMEM containing 10% FBS for FCV-F9, MNV-1, and HAV and in TSB containing 3% beef extract for MS2. Each treatment was replicated three times. Plaque assays for evaluating the infectivity of the viruses were carried out in duplicate as described below.
Infectious plaque assays.
Procedures for the plaque assays for FCV-F9, MNV-1, and HAV were similar. CRFK, RAW 264.7, and FRhK4 cells were added to 6-well plates at 0.5 × 106 to 1 × 106 cells/well and incubated at 37°C in an atmosphere containing 5% CO2 until becoming confluent. Serially diluted treated and untreated (control) FCV-F9, MNV-1, and HAV at 0.5 ml each were then added to their respective confluent host cells, CRFK, RAW 264.7, and FRhK4, respectively, and incubated for 2 h. The inoculum was then removed, and 2 ml complete DMEM containing 0.75% agarose was added to each well. Two (for FCV) to 8 days (for HAV) later, cell monolayers were stained with neutral red and plaques were counted (37).
MS2 plaque assays were performed using E. coli B-15597 as reported earlier (1). E. coli B-15597 was grown in TSB containing 0.1% glucose, 2 mM CaCl2, and 10 μg/ml thiamine for 6 h. MS2 treated with GSE or water after neutralization with TSB containing 3% beef extract was serially diluted in TSB, and 0.7 ml of diluted phage was mixed with 0.3 ml of 6-h E. coli host. The 1-ml host-virus combination was then added to 8 ml of 0.6% molten top agar, mixed and poured on tryptic soy agar (TSA) bottom agar plates, and incubated at 37°C overnight before counting.
Understanding the mechanism of action of GSE on FCV-F9, MNV-1, and HAV.
The concentrations of GSE that did not appear to cause any cytotoxic effects on the cell lines were used in this part of the study, namely, 0.4 mg/ml for CRFK, 0.2 mg/ml for RAW 264.7, and 0.6 mg/ml for FRhK4. To determine if GSE had an effect on viral adsorption or cell entry, the cells were pretreated with GSE for 1 h, and then GSE solution was aspirated, followed by viral infection for 2 h at 37°C. In order to determine if GSE had an effect on viral replication, CRFK, RAW 264.7, and FRhK4 cells were first infected with FCV-F9, MNV-1, and HAV for 2 h, and then the virus was aspirated followed by treatment with GSE solution for 1 h at 37°C. The cells were then overlaid with 2 ml complete DMEM containing 0.75% agarose. After incubation for 2 to 8 days at 37°C under 5% CO2, a second overlay containing neutral red was added followed by incubation to allow the visualization of plaques.
Statistical analysis.
Results from the treatments and controls were statistically analyzed using analysis of variance (ANOVA) with SAS software (version 9.2; SAS Institute, Cary, NC) and Tukey's test on a completely randomized design with three replications.
RESULTS
Determination of cytotoxicity of GSE on CRFK, RAW 264.7, and FRhK4 cell lines.
GSE was found to be cytotoxic at concentrations that exceeded 0.6 mg/ml for CRFK, 0.4 mg/ml for RAW 264.7, and 0.8 mg/ml for FRhK4. As the highest concentration of GSE for the direct viral contact treatment was 1 mg/ml, the maximum concentration of GSE added to the cell lines during plaque assays was no more than 0.1 mg/ml after neutralizing the treatment by 10-fold dilution in DMEM containing 10% FBS, which is less than the GSE threshold concentration for causing cytotoxic effect on all three cell lines. Thus, the plaque assays using these cell lines at the tested GSE concentrations were valid to determine the antiviral effects of GSE against FCV-F9, MNV-1, and HAV.
Reduction in titers of FCV-F9, MNV-1, bacteriophage MS2, and HAV by GSE.
Incubation of FCV-F9, MNV-1, MS2, and HAV with GSE at concentrations of 0.25, 0.5, and 1.0 mg/ml for 2 h at either 37°C or room temperature decreased the titer of all the four tested viruses (Table 1). The titer reduction was found to be dependent on the virus type and the concentration of GSE.
Table 1.
Effect of grape seed extract (GSE) against feline calicivirus (FCV-F9), murine norovirus (MNV-1), MS2 bacteriophage, and hepatitis A virus (HAV) at both high (∼7-log10-PFU) and low (∼5-log10-PFU) titers after 2 h of incubation at 37°C or room temperaturea
| Virus | Treatment | 37°C |
Room temp |
||||||
|---|---|---|---|---|---|---|---|---|---|
| High titer (log10 PFU/ml) |
Low titer (log10 PFU/ml) |
High titer (log10 PFU/ml) |
Low titer (log10 PFU/ml) |
||||||
| Recovered titer | Reduction | Recovered titer | Reduction | Recovered titer | Reduction | Recovered titer | Reduction | ||
| FCV-F9 | Water | 6.93 ± 0.17 A | 0 | 4.98 ± 0.08 A | 0 | 6.99 ± 0.16 A | 0 | 5.01 ± 0.06 A | 0 |
| 0.25 mg/ml GSE | 3.29 ± 0.35 B | 3.64 | 0.00 ± 0.00 B | 4.98 | 4.21 ± 0.21 B | 2.78 | 0.00 ± 0.00 B | 5.01 | |
| 0.5 mg/ml GSE | 2.83 ± 0.73 B | 4.10 | 0.00 ± 0.00 B | 4.98 | 3.69 ± 0.06 BC | 3.30 | 0.00 ± 0.00 B | 5.01 | |
| 1 mg/ml GSE | 2.32 ± 0.63 B | 4.61 | 0.00 ± 0.00 B | 4.98 | 3.19 ± 0.30 C | 3.80 | 0.00 ± 0.00 B | 5.01 | |
| MNV-1 | Water | 6.93 ± 0.12 A | 0 | 4.82 ± 0.09 A | 0 | 6.86 ± 0.07 A | 0 | 4.86 ± 0.06 A | 0 |
| 0.25 mg/ml GSE | 6.11 ± 0.18 B | 0.82 | 3.33 ± 0.42 AB | 1.49 | 6.42 ± 0.12 B | 0.44 | 3.49 ± 0.22 B | 1.37 | |
| 0.5 mg/ml GSE | 5.58 ± 0.21 BC | 1.35 | 3.10 ± 0.64 B | 1.72 | 6.14 ± 0.08 B | 0.72 | 3.38 ± 0.37 B | 1.48 | |
| 1 mg/ml GSE | 5.20 ± 0.32 C | 1.73 | 2.85 ± 0.67 B | 1.97 | 5.80 ± 0.21 C | 1.06 | 3.19 ± 0.38 B | 1.67 | |
| MS2 | Water | 7.04 ± 0.08 A | 0 | 5.17 ± 0.02 A | 0 | 7.14 ± 0.05 A | 0 | 5.15 ± 0.06 A | 0 |
| 0.25 mg/ml GSE | 5.91 ± 0.18 B | 1.13 | 3.82 ± 0.08 B | 1.35 | 6.11 ± 0.13 B | 1.03 | 4.12 ± 0.08 B | 1.03 | |
| 0.5 mg/ml GSE | 5.61 ± 0.24 B | 1.43 | 3.65 ± 0.12 B | 1.52 | 6.05 ± 0.12 B | 1.09 | 3.99 ± 0.13 B | 1.16 | |
| 1 mg/ml GSE | 5.44 ± 0.33 B | 1.60 | 3.32 ± 0.13 C | 1.85 | 5.48 ± 0.13 C | 1.66 | 3.99 ± 0.18 B | 1.16 | |
| HAV | Water | 6.67 ± 0.04 A | 0 | 5.26 ± 0.12 A | 0 | 6.59 ± 0.04 A | 0 | 5.25 ± 0.07 A | 0 |
| 0.25 mg/ml GSE | 4.86 ± 0.16 B | 1.81 | 3.40 ± 0.13 B | 1.86 | 5.73 ± 0.10 B | 0.86 | 2.85 ± 0.09 B | 2.40 | |
| 0.5 mg/ml GSE | 4.01 ± 0.09 C | 2.66 | 3.00 ± 0.09 C | 2.26 | 5.37 ± 0.05 C | 1.22 | 2.63 ± 0.09 C | 2.62 | |
| 1 mg/ml GSE | 3.47 ± 0.07 D | 3.20 | 2.37 ± 0.17 D | 2.89 | 4.69 ± 0.13 D | 1.90 | 2.24 ± 0.11 D | 3.01 | |
Each treatment was replicated three times, and plaque assays for evaluating the infectivity of the viruses were carried out in duplicate. Within each column for each virus, different letters denote significant differences between treatments (P < 0.05). At P < 0.01, only low-titer MNV-1 reduction after GSE treatment at 37°C and only high-titer MNV-1 reduction by 0.25-mg/ml GSE treatment at room temperature were not significantly different.
As shown in Table 1, incubation of high viral titers (∼7 log10 PFU/ml) at 37°C with 0.25, 0.5, and 1.0 mg/ml GSE for 2 h showed FCV-F9 titer reductions of 3.64, 4.10, and 4.61 log10 PFU/ml; MNV-1 titer reductions of 0.82, 1.35, and 1.73 log10 PFU/ml; MS2 titer reductions of 1.13, 1.43, and 1.60 log10 PFU/ml; and HAV titer reductions of 1.81, 2.66, and 3.20 log10 PFU/ml, respectively. When low-titer viruses (∼5 log10 PFU/ml) were treated with GSE at 0.25, 0.5, and 1.0 mg/ml at 37°C for 2 h, FCV-F9 titers were reduced to undetectable levels; MNV-1 titers were reduced by 1.49, 1.72, and 1.97 log10 PFU/ml; MS2 titers were reduced by 1.35, 1.52, and 1.85 log10 PFU/ml; and HAV titers were reduced by 1.86, 2.26, and 2.89 log10 PFU/ml, respectively.
At room temperature, high-titer FCV-F9 was reduced by 2.78, 3.30, and 3.80 log10 PFU/ml and low-titer FCV-F9 was reduced to undetectable levels by 0.25, 0.5, and 1.0 mg/ml GSE, respectively. High-titer MNV-1 was reduced by 0.44, 0.72, and 1.06 log10 PFU/ml, and low-titer MNV-1 was reduced by 1.37, 1.48, and 1.67 log10 PFU/ml using 0.25, 0.5, and 1.0 mg/ml GSE at room temperature, respectively. High-titer MS2 was reduced by 1.03, 1.09, and 1.66 log10 PFU/ml, and low-titer MS2 was reduced by 1.03, 1.16, and 1.16 log10 PFU/ml with 0.25, 0.5, and 1.0 mg/ml GSE at room temperature, respectively. High-titer HAV was reduced by 0.86, 1.22, and 1.90 log10 PFU/ml, and low-titer HAV was reduced by 2.40, 2.62, and 3.01 log10 PFU/ml with 0.25, 0.5, and 1.0 mg/ml GSE at room temperature, respectively (Table 1).
Statistical analysis showed that, overall, GSE treatment at room temperature for 2 h caused greater reduction on low titers than high titers for only FCV-F9, MNV-1, and HAV (P < 0.05), with no difference in reduction between high- and low-titer MS2. However, when 37°C was used, overall there did not appear to be significant differences in reduction between low- and high-titer GSE-treated viruses. Thus, titers played a role for reduction by GSE treatment only at room temperature. Also, statistical analysis revealed that when comparing GSE treatment at 37°C to room temperature, significantly higher reduction of only high-titer FCV-F9, MNV-1, and HAV was obtained (P < 0.05), except for FCV-F9 treated with 0.5 mg/ml or 1 mg/ml GSE. For low-titer FCV-F9, MNV-1, and MS2, the reductions were almost the same at both temperatures when treated with GSE. Thus, temperature played a role mainly for high-titer viruses where GSE treatments at 37°C caused greater titer reduction than at room temperature.
Among the four tested viruses, FCV-F9 showed the highest reduction in viral titers, followed by HAV. The titer reductions between MNV-1 and MS2 by GSE treatment were similar. The antiviral effect of GSE was found to be dose dependent with increasing concentrations of GSE resulting in increased antiviral effects.
Understanding the mechanisms of action of GSE on FCV-F9, MNV-1, and HAV.
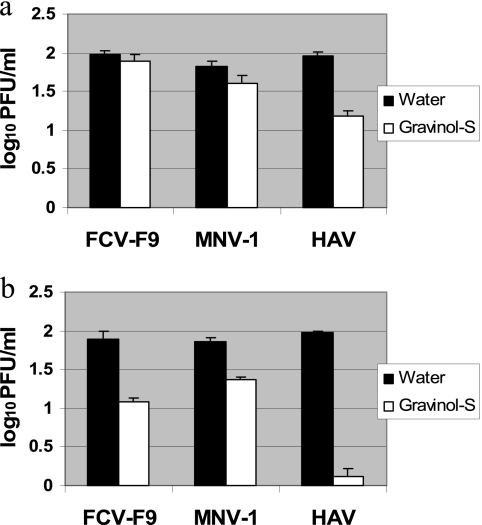
To determine if GSE had an effect on viral replication, confluent CRFK, RAW 264.7, and FRhK4 cells were infected with FCV-F9, MNV-1, and HAV for 2 h, followed by treating the infected cells with various concentrations of GSE solution for 1 h. As described above, GSE was found to be cytotoxic when the concentration exceeded 0.6 mg/ml for CRFK, 0.4 mg/ml for RAW 264.7, and 0.8 mg/ml for FRhK4. Therefore, this experiment was conducted by adding 0.4 mg/ml GSE for CRFK, 0.2 mg/ml GSE for RAW 264.7, and 0.6 mg/ml GSE for FRhK4 after viral infection of the cell lines. The titers of GSE-treated and untreated FCV-F9, MNV-1, and HAV are shown in Fig. 1a. FCV-F9, MNV-1, and HAV titers were reduced by 0.08, 0.23, and 0.78 log10 PFU/ml, respectively, after postinfection treatment with GSE. This indicated that GSE had minor effects on the replication of FCV-F9, MNV-1, and HAV.
Fig. 1.
Recovered titers of feline calicivirus (FCV-F9), murine norovirus (MNV-1), and hepatitis A virus (HAV) after treating confluent Crandell Reese feline kidney (CRFK) cells, RAW 264.7 cells, and fetal rhesus monkey kidney (FRhK4) cells with grape seed extract (GSE) and water (control). (a) Confluent CRFK, RAW 264.7, and FRhK4 cell layers were treated with water or GSE after viral infection with FCV-F9, MNV-1, or HAV, respectively. (b) Confluent CRFK, RAW 264.7, and FRhK4 cell layers were treated with water or GSE prior to viral infection with FCV-F9, MNV-1, or HAV, respectively. The concentrations of GSE used for CRFK, RAW 267.4, and HAV cells were 0.4 mg/ml, 0.2 mg/ml, and 0.6 mg/ml, respectively. The initial viral titer for all three viruses was ∼2 log10 PFU/ml.
To understand if GSE had an effect on viral adsorption, CRFK, RAW 264.7, or FRhK4 cells were pretreated with GSE at concentrations of 0.4 mg/ml, 0.2 mg/ml, and 0.6 mg/ml, respectively, for 1 h followed by infection by FCV-F9, MNV-1, and HAV for 2 h. As shown in Fig. 1b, FCV-F9 titers were reduced by 0.81 log10 PFU/ml with 0.4 mg/ml GSE, MNV-1 titers were reduced by 0.50 log10 PFU/ml with 0.2 mg/ml GSE, and HAV titers were reduced by 1.85 log10 PFU/ml with 0.6 mg/ml GSE. These data show that GSE had a greater effect on viral adsorption (or viral binding) than on viral replication. However, overall these pre- and postinfection treatments were still relatively less effective compared to direct contact of the virus with GSE for 2 h.
DISCUSSION
The present study clearly showed that GSE was effective in reducing the titers of FCV-F9, MNV-1, MS2 bacteriophage, and HAV in a dose-dependent manner, where increasing concentrations of GSE showed increased reduction in viral titers. GSE at 1 mg/ml after 2 h of incubation at 37°C decreased high-titer viruses (∼7 log10 PFU/ml) by 4.61 log10 PFU/ml for FCV-F9, 1.73 log10 PFU/ml for MNV-1, 1.60 log10 PFU/ml for MS2, and 3.20 log10 PFU/ml for HAV. Thus, FCV-F9 seems to be the most sensitive among the four tested viruses to GSE treatment, followed by HAV.
Though the antibacterial effects of GSE have been well studied (2, 4, 19, 29, 31), there is very little documentation on its antiviral effects. Nair et al. (25, 26) studied the antiviral effect of GSE on HIV-1 and showed that GSE significantly downregulated the expression of the HIV-1 coreceptors. Matias et al. (23) evaluated the effect of extract obtained from winemaking by-products (composed of both grape skin and seeds) on adenovirus type 5 infection and found that the extract at a concentration of 0.8 mg/ml caused a 5-log10-50% tissue culture infective dose (TCID50)/ml reduction in total infectious adenovirus type 5. Resveratrol (RV) is a nonflavonoid polyphenol that is present in both seed and skin of grapes, being produced in response to physiological stimuli and environmental stress (22). It has been shown that RV is effective against a wide variety of viruses (6). RV is reported to strongly inhibit the in vitro and in vivo replication of influenza virus (30) and also has strong antiviral activity against herpes simplex virus type 1 (11–14), polyomavirus (3), and varicella-zoster virus (15).
The commercial GSE, Gravinol-S, contains 95% flavonol, of which 82% is oligomeric proanthocyanidins, with 12% being the highly active monomeric proanthocyanidins (37). Therefore, we could compare our results with the antiviral effects of proanthocyanidins (PAC) obtained from other sources. Iwasawa et al. (18) studied the effect of proanthocyanidin purified from the fruit of Zanthoxylum piperitum (Japan pepper) against FCV-F9 and coxsackievirus and showed that after 10 s of contact, FCV titer was reduced by 1 to 2 log10 PFU/ml, and coxsackievirus titer was reduced by 0.35 to 0.43 log10 PFU/ml with 0.5 and 1 mg/ml proanthocyanidin, respectively. Cheng et al. (8) studied the effect of proanthocyanidin A-1 from Vaccinium vitis-idaea (lingonberry) against herpes simplex virus type 2 (HSV-2) and showed that 63 μM PAC-A1 decreased HSV-2 titers by 1 log10 PFU/ml after 1 h of incubation at 37°C. Previous studies in our laboratory have shown that PAC from cranberry extract at 0.6 mg/ml after 1 h of incubation at room temperature decreased titers of human norovirus surrogates, FCV-F9 and MNV-1, as well as MS2 and φX174 bacteriophages by ∼7, 1.22, 1.00, and 2.63 log10 PFU/ml, respectively, when initial viral titers were ∼7 log10 PFU/ml and by 5.02, 2.95, 0.96, and 4.98 log10 PFU/ml, respectively, when initial viral titers were ∼5 log10 PFU/ml (41). In comparison, the titer reduction of FCV-F9 and MNV-1 by 1 mg/ml GSE at room temperature seems to be less than that obtained by cranberry PAC at 0.6 mg/ml. However, based on commercial retail pricing, GSE is currently 3 times less expensive than cranberry PAC.
To the best of our knowledge and to date, the antiviral mechanism of GSE against food-borne viruses has not been established. Nair et al. (25) studied the antiviral mechanisms of GSE against HIV and showed that GSE significantly downregulated the expression of HIV entry coreceptors and thus that GSE can interfere with the binding of the virus to the cell receptor and prevent HIV entry into the normal lymphocyte. The antiviral mechanism of tea polyphenols against influenza virus was studied, and it was found that tea polyphenols reduced viral infectivity by inhibition of influenza virus adsorption to Madin-Darby canine kidney (MDCK) cells (27), interference in viral membrane fusion (17), and suppression of viral RNA synthesis (39). Our study showed that GSE has some effect on FCV-F9, MNV-1, and HAV adsorption (we speculate due to blocking of either host cell receptors or viral binding sites) but only minor effects on FCV-F9, MNV-1, and HAV replication. Again, one must be aware that these are only speculations, and therefore, further studies on the mechanism of action of GSE are needed.
Since grape seeds are waste or by-products from the wine and grape juice industry, they are readily available and inexpensive to acquire. In addition, grape seed extract has been proven to have considerable health benefits and antimicrobial activity. These combined associated health benefits and chemopreventive properties provide great advantages for the use of GSE in the food industry. Recently, applications of GSE in the food industry have been explored. Bisha et al. (4) evaluated the potential application of GSE as a produce wash and found that at 0.125% GSE reduced L. monocytogenes by ∼2 log10 CFU from an initial titer of ∼5 log10 CFU within 2 min on tomato surfaces. They concluded that GSE-based antimicrobial wash solutions might offer an inexpensive way to inactivate L. monocytogenes on fresh produce. Also, recently Yerlikaya et al. (50) investigated the use of GSE in batter coating of shrimp and concluded that the incorporation of GSE in batter materials could improve the chemical, microbiological, and overall quality of shrimp during storage. In 2009, GSE was shown to be effectively incorporated into edible pea starch film to reduce the growth of undesirable pathogens in meat, improving meat quality and extending its shelf life (9).
GSE has been shown to have great antioxidant activities in meat products (5, 21, 32, 50). Kulkarni et al. (21) evaluated the effect of GSE at concentrations of 0.1 to 0.5 mg/ml on oxidation and color stability of precooked, frozen, reheated beef and found that GSE-containing samples retained their fresh cooked beef odor and flavor longer than controls during storage. GSE-containing samples had lower rancid odor and flavor scores than controls, as well as lower thiobarbituric acid-reactive substance values than controls. Carpenter et al. (7) also found that the addition of GSE (1,000 μg/g muscle) to cooked pork patties stored for up to 4 days at 4°C significantly increased (P < 0.05) the “a” redness values (color stability) relative to controls during storage. This increase in color was not negatively perceived by the sensory panel as determined by the sensory scores of cooked pork patties that were not significantly different from the controls.
Based on these applications of natural plant extracts such as readily available GSE, there is great potential to use this available natural ingredient as a wash/rinse for the reduction of food-borne viral titers in produce. The findings of this study demonstrating that GSE has activity at room temperature against food-borne viruses and also previous reports on the use of GSE as an antibacterial produce wash further emphasize the need to explore these natural alternatives to prevent food-borne outbreaks. However, if the wash solution temperature is increased, the GSE solution can possibly have improved antiviral effects when viral contamination/load is high. Additionally, since it is a natural bioactive compound and shown to be used in edible food items such as shrimp and edible starch films, other applications include use of GSE as or in film coatings or wraps to reduce viral contamination of produce. GSE can also be used for the protection from further viral contamination, or for coating of ready-to-eat deli items, since GSE can have potential activity after ingestion at 37°C. Thus, based on literature and this study, GSE appears to have a broad spectrum of activity, being effective against food-borne bacterial pathogens as well as food-borne viruses.
In conclusion, it has been shown that commercial GSE can reduce the titers of the tested food-borne viral surrogates. Thus, GSE shows potential to be used as a promising natural broad-spectrum alternative to increase food safety, extend shelf life, and improve food quality. GSE is easily and inexpensively available worldwide wherever grapes are grown, and its application for enhancing food safety would not require much labor, cost, or technology and should have a global market for use. Future work will focus on the application of GSE in the food industry and food processing environments to decrease viral contamination. The individual chemical components of GSE associated with antiviral activity need to be also explored, as well as further detailed studies on the mechanism of action involving transmission electron microscopy.
ACKNOWLEDGMENT
Funding for this research was provided by the TN Agricultural Experiment Station (UT-TEN-HATCH no. 00391) and is gratefully acknowledged.
Footnotes
Published ahead of print on 15 April 2011.
REFERENCES
- 1. Bae J., Schwab K. J. 2008. Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Appl. Environ. Microbiol. 74:477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baydar N. G., Sagdic O., Ozkan G., Cetin S. 2006. Determination of antibacterial effects and total phenolic contents of grape (Vitis vinifera L.) seed extracts. Int. J. Food Sci. Technol. 41:799–804 [Google Scholar]
- 3. Berardi V., Ricci F., Castelli M., Galati G., Risuleo G. 2009. Resveratrol exhibits a strong cytotoxic activity in cultured cells and has an antiviral action against polyomavirus: potential clinical use. J. Exp. Clin. Cancer Res. 28:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bisha B., Weinsetel N., Brehm-Stecher B. F., Mendonca A. 2010. Antilisterial effects of gravinol-s grape seed extract at low levels in aqueous media and its potential application as a produce wash. J. Food Prot. 73:266–273 [DOI] [PubMed] [Google Scholar]
- 5. Brannan R. G. 2009. Effect of grape seed extract on descriptive sensory analysis of ground chicken during refrigerated storage. Meat Sci. 81:589–595 [DOI] [PubMed] [Google Scholar]
- 6. Campagna M., Rivas C. 2010. Antiviral activity of resveratrol. Biochem. Soc. Trans. 38:50–53 [DOI] [PubMed] [Google Scholar]
- 7. Carpenter R., O'Grady M. N., O'Callaghan Y. C., O'Brien N. M., Kerry J. P. 2007. Evaluation of the antioxidant potential of grape seed and bearberry extracts in raw and cooked pork. Meat Sci. 76:604–610 [DOI] [PubMed] [Google Scholar]
- 8. Cheng H. Y., Lin T. C., Yang C. M., Shieh D. E., Lin C. C. 2005. In vitro anti-HSV-2 activity and mechanism of action of proanthocyanidin A-1 from Vaccinium vitis-idaea. J. Sci. Food Agric. 85:10–15 [Google Scholar]
- 9. Corrales M., Han J. H., Tauscher B. 2009. Antimicrobial properties of grape seed extracts and their effectiveness after incorporation into pea starch films. Int. J. Food Sci. Technol. 44:425–433 [Google Scholar]
- 10. Dawson D. J., Paish A., Staffell L. M., Seymour I. J., Appleton H. 2005. Survival of viruses on fresh produce, using MS2 as a surrogate for norovirus. J. Appl. Microbiol. 98:203–209 [DOI] [PubMed] [Google Scholar]
- 11. Docherty J., et al. 2003. In vivo anti-herpes simplex virus activity of resveratrol, a cyclin dependent kinase gene inhibitor. Antiviral Res. 57:86 [Google Scholar]
- 12. Docherty J. J., et al. 2005. Effect of resveratrol on herpes simplex virus vaginal infection in the mouse. Antiviral Res. 67:155–162 [DOI] [PubMed] [Google Scholar]
- 13. Docherty J. J., et al. 1999. Resveratrol inhibition of herpes simplex virus replication. Antiviral Res. 43:145–155 [DOI] [PubMed] [Google Scholar]
- 14. Docherty J. J., Smith J. S., Fu M. M., Stoner T., Booth T. 2004. Effect of topically applied resveratrol on cutaneous herpes simplex virus infections in hairless mice. Antiviral Res. 61:19–26 [DOI] [PubMed] [Google Scholar]
- 15. Docherty J. J., Sweet T. J., Bailey E., Faith S. A., Booth T. 2006. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antiviral Res. 72:171–177 [DOI] [PubMed] [Google Scholar]
- 16. Gadang V. P., Hettiarachchy N. S., Johnson M. G., Owens C. 2008. Evaluation of antibacterial activity of whey protein isolate coating incorporated with nisin, grape seed extract, malic acid, and EDTA on a turkey frankfurter system. J. Food Sci. 73:M389–M394 [DOI] [PubMed] [Google Scholar]
- 17. Imanishi N., et al. 2002. Additional inhibitory effect of tea extract on the growth of influenza A and B viruses in MDCK cells. Microbiol. Immunol. 46:491–494 [DOI] [PubMed] [Google Scholar]
- 18. Iwasawa A., Niwano Y., Mokudai T., Kohno M. 2009. Antiviral activity of proanthocyanidin against feline calicivirus used as a surrogate for noroviruses, and coxsackievirus used as a representative enteric virus. Biocontrol Sci. 14:107–111 [DOI] [PubMed] [Google Scholar]
- 19. Jayaprakasha G. K., Selvi T., Sakariah K. K. 2003. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 36:117–122 [Google Scholar]
- 20. Kao T. T., et al. 2010. Grape seed extract inhibits the growth and pathogenicity of Staphylococcus aureus by interfering with dihydrofolate reductase activity and folate-mediated one-carbon metabolism. Int. J. Food Microbiol. 141:17–27 [DOI] [PubMed] [Google Scholar]
- 21. Kulkarni S., DeSantos F. A., Kattamuri S., Rossi S. J., Brewer M. S. 2011. Effect of grape seed extract on oxidative, color and sensory stability of a pre-cooked, frozen, re-heated beef sausage model system. Meat Sci. 88:139–144 [DOI] [PubMed] [Google Scholar]
- 22. Li X. D., Wu B. H., Wang L. J., Li S. H. 2006. Extractable amounts of trans-resveratrol in seed and berry skin in Vitis evaluated at the germplasm level. J. Agric. Food Chem. 54:8804–8811 [DOI] [PubMed] [Google Scholar]
- 23. Matias A. A., et al. 2010. Portuguese winemaking residues as a potential source of natural anti-adenoviral agents. Int. J. Food Sci. Nutr. 61:357–368 [DOI] [PubMed] [Google Scholar]
- 24. Mesquita J. R., Nascimento M. S. J. 2009. A foodborne outbreak of norovirus gastroenteritis associated with a Christmas dinner in Porto Portugal, December 2008. Euro Surveill. 14:19–21 [PubMed] [Google Scholar]
- 25. Nair M. P., et al. 2002. Grape seed extract proanthocyanidins downregulate HIV-1 entry coreceptors, CCR2b, CCR3 and CCR5 gene expression by normal peripheral blood mononuclear cells. Biol. Res. 35:421–431 [DOI] [PubMed] [Google Scholar]
- 26. Nair M. P. N., Mahajan S., Kandaswami C., Schwartz S. A. 1999. Suppression of HIV-1 entry coreceptor gene expression in peripheral blood mononuclear cells (PBMC) by defined grape seed extract proanthocyanidins. FASEB J. 13:A1023–A1023 [DOI] [PubMed] [Google Scholar]
- 27. Nakayama M., et al. 1993. Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral Res. 21:289–299 [DOI] [PubMed] [Google Scholar]
- 28. Nassiri-Asl M., Hosseinzadeh H. 2009. Review of the pharmacological effects of Vitis vinifera (grape) and its bioactive compounds. Phytother. Res. 23:1197–1204 [DOI] [PubMed] [Google Scholar]
- 29. Ozkan G., Sagdic O., Baydar N. G., Kurumahmutoglu Z. 2004. Antibacterial activities and total phenolic contents of grape pomace extracts. J. Sci. Food Agric. 84:1807–1811 [Google Scholar]
- 30. Palamara A. T., et al. 2005. Inhibition of influenza A virus replication by resveratrol. J. Infect. Dis. 191:1719–1729 [DOI] [PubMed] [Google Scholar]
- 31. Rhodes P. L., Mitchell J. W., Wilson M. W., Melton L. D. 2006. Antilisterial activity of grape juice and grape extracts derived from Vitis vinifera variety Ribier. Int. J. Food Microbiol. 107:281–286 [DOI] [PubMed] [Google Scholar]
- 32. Rojas M. C., Brewer M. S. 2007. Effect of natural antioxidants on oxidative stability of cooked, refrigerated beef and pork. J. Food Sci. 72:S282–S288 [DOI] [PubMed] [Google Scholar]
- 33. Rosenblum L. S., Mirkin I. R., Allen D. T., Safford S., Hadler S. C. 1990. A multifocal outbreak of hepatitis A traced to commercially distributed lettuce. Am. J. Public Health 80:1075–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schieber A., Stintzing F. C., Carle R. 2001. By-products of plant food processing as a source of functional compounds—recent developments. Trends Food Sci. Technol. 12:401–413 [Google Scholar]
- 35. Schmid D., et al. 2007. A foodborne norovirus outbreak due to manually prepared salad, Austria 2006. Infection 35:232–239 [DOI] [PubMed] [Google Scholar]
- 36. Shan B., Cai Y. Z., Brooks J. D., Corke H. 2009. Antibacterial and antioxidant effects of five spice and herb extracts as natural preservatives of raw pork. J. Sci. Food Agric. 89:1879–1885 [Google Scholar]
- 37. Shi J., Pohorly J., Kakuda Y. 2003. Polyphenolics in grape seeds—biochemistry and functionality. J. Med. Food 6:291–299 [DOI] [PubMed] [Google Scholar]
- 38. Sivarooban T., Hettiarachchy N. S., Johnson M. G. 2008. Physical and antimicrobial properties of grape seed extract, nisin, and EDTA incorporated soy protein edible films. Food Res. Int. 41:781–785 [Google Scholar]
- 39. Song J. M., Lee K. H., Seong B. L. 2005. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 68:66–74 [DOI] [PubMed] [Google Scholar]
- 40. Steinmann J. 2004. Surrogate viruses for testing virucidal efficacy of chemical disinfectants. J. Hosp. Infect. 56:S49–S54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Su X., Howell A. B., D'Souza D. H. 2010. The effect of cranberry juice and cranberry proanthocyanidins on the infectivity of human enteric viral surrogates. Food Microbiol. 27:535–540 [DOI] [PubMed] [Google Scholar]
- 42. Taguri T., Tanaka T., Kouno I. 2004. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull. 27:1965–1969 [DOI] [PubMed] [Google Scholar]
- 43. Theivendran S., Hettiarachchy N. S., Johnson M. G. 2006. Inhibition of Listeria monocytogenes by nisin combined with grape seed extract or green tea extract in soy protein film coated on turkey frankfurters. J. Food Sci. 71:M39–M44 [Google Scholar]
- 44. Vivancos R., et al. 2009. Food-related norovirus outbreak among people attending two barbeques: epidemiological, virological, and environmental investigation. Int. J. Infect. Dis. 13:629–635 [DOI] [PubMed] [Google Scholar]
- 45. Wadl M., et al. 2010. Food-borne norovirus-outbreak at a military base, Germany, 2009. BMC Infect. Dis. 10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wobus C. E., et al. 2004. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2:2076–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xia E. Q., Deng G. F., Guo Y. J., Li H. B. 2010. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 11:622–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yadav M., et al. 2009. Biological and medicinal properties of grapes and their bioactive constituents: an update. J. Med. Food 12:473–484 [DOI] [PubMed] [Google Scholar]
- 49. Yamakoshi J., Saito M., Kataoka S., Kikuchi M. 2002. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 40:599–607 [DOI] [PubMed] [Google Scholar]
- 50. Yerlikaya P., Gokoglu N., Topuz O. K. 2010. Use of natural plant extracts in batter coating of shrimp and their effects on the quality of shrimp during frozen storage. J. Food Processing Preservation 34:127–138 [Google Scholar]
- 51. Zomer T. P., et al. 2010. A foodborne norovirus outbreak at a manufacturing company. Epidemiol. Infect. 138:501–506 [DOI] [PubMed] [Google Scholar]