TO THE EDITOR
Calcipotriol is used as a first-line topical agent in the treatment of psoriasis. Inhibition of keratinocyte proliferation, repression of growth signals, and modulation of T-cell signaling have been proposed to contribute to the clinical effects of calcipotriol (Scott et al., 2001). However, the precise mechanism of action of calcipotriol and related vitamin D analogs remains poorly understood. Recent studies have implicated vitamin D in autophagy induction in a variety of cell types including MCF-7 breast carcinoma cells (Hoyer-Hansen et al., 2007), human myeloid leukemia cells (Wang et al., 2008), and human monocytes (Yuk et al., 2009). During autophagy, intracellular contents are enveloped in double-layered membrane vesicles that fuse with lysosomes for degradation (Mizushima et al., 2010). This “recycling” process is highly regulated and may be activated by a variety of stimuli including infections, starvation, misfolded proteins, and mitochondrial stress. In this study we find that calcipotriol, a commonly prescribed topical analog of vitamin D, also induces autophagy in both HeLa cells and keratinocytes.
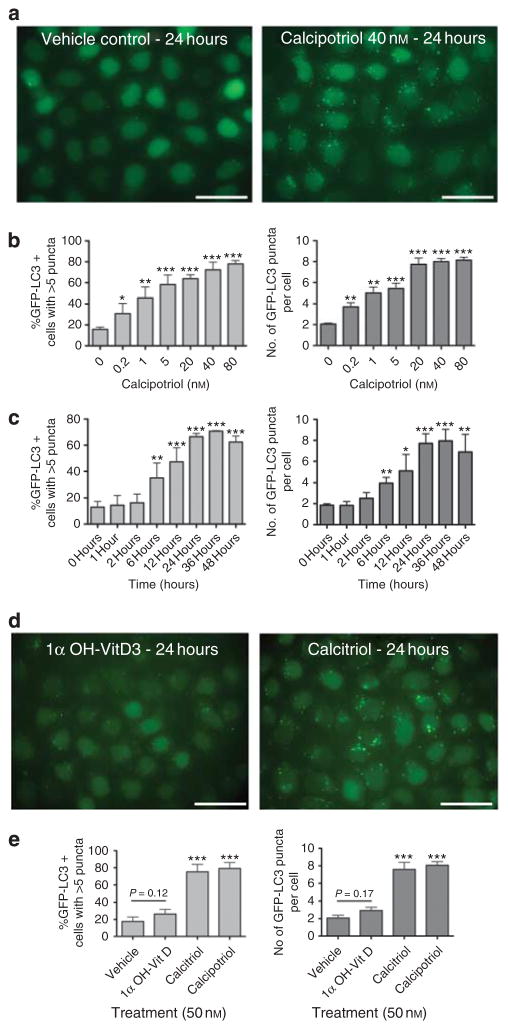
The induction of autophagy is characterized by specific histological and biochemical changes (Mizushima et al., 2010). Under basal conditions, microtubule- associated protein 1 light chain 3 beta (LC3) is a diffuse cytosolic protein. After autophagy induction, LC3 is proteolytically cleaved, lipidated, and localizes to autophagosomal membranes, forming punctate subcellular structures. Using a HeLa cell line that expresses a green fluorescent protein-LC3 (GFP-LC3) fusion protein (Orvedahl et al., 2010), we assessed whether calcipotriol alters the subcellular distribution of LC3. Indeed, calcipotriol treatment (40 nM for 24 hours) caused a striking induction of GFP-LC3 puncta when compared with the vehicle control (Figure 1a). At doses as low as 0.2 nM, calcipotriol induced a significant increase in both the percentage of cells with >5 puncta and the average number of GFP-LC3 puncta per cell (Figure 1b). Maximal effects of calcipotriol were reached at ~40 nM. Previous studies have suggested that vitamin D-induced autophagy is dependent on cathelicidin-mediated transcription (Yuk et al., 2009). Consistent with this, time course experiments demonstrated that GFP-LC3 puncta were not significantly increased until 6 hours after calcipotriol treatment (Figure 1c). The increase of GFP-LC3 puncta was greatest at ~24–36 hours after treatment with calcipotriol.
Figure 1. Calcipotriol-induced accumulation of GFP-LC3 puncta (autophagosomes).
(a) HeLa-GFP-LC3 cells were treated with 40 nM calcipotriol (Sigma, St Louis, MO) or ethanol vehicle for 24 hours. Cells were fixed in 3% paraformaldehyde (PFA) and imaged. (b, c) Effects of (b) doses and (c) durations of calcipotriol treatment on GFP-LC3 puncta. For dose titration, cells were treated for 24 hours. For time course, cells were treated with 40 nM calcipotriol. Bar graphs show percentage of cells with >5 puncta (light gray) and average number of puncta per cell (dark gray). (c) Effects of calcitriol (Cayman Chemical, Ann Arbor, MI) or 1α-hydroxyvitamin D3 (Sigma) on GFP-LC3 accumulation in HeLa-GFP-LC3 cells. (d) Quantitation of effects of vitamin D analogs. In b, c, and e, >100 cells were scored for each sample. Results represent mean±SD for triplicate samples. Similar results observed in three independent experiments. *P<0.05, **P<0.01, ***P<0.001 versus control condition; Student’s t-test. Scale bar = 50μm.
Vitamin D and its analogs are thought to activate transcription through binding and activation of nuclear vitamin D receptors (VDRs) (Scott et al., 2001). The ability of 1α, 25-dihydroxyvitamin D3 (calcitriol) and calcipotriol to induce VDR-dependent transcription is similar (Masuda et al., 1994); however, 1α-hydroxyvitamin D3 (alfacalcidol), a calcitriol precursor, has a significantly decreased ability to induce transcription and fails to inhibit keratinocyte proliferation (Takahashi et al., 2003; Zhang et al., 2010). Whereas calcitriol at 50 nM significantly increased the numbers of GFP-LC3 puncta, 1α-hydroxyvitamin D3 did not (Figure 1d and e). Thus, the ability of each vitamin D analog to induce autophagy appears to correlate with its ability to induce VDR-dependent transcription.
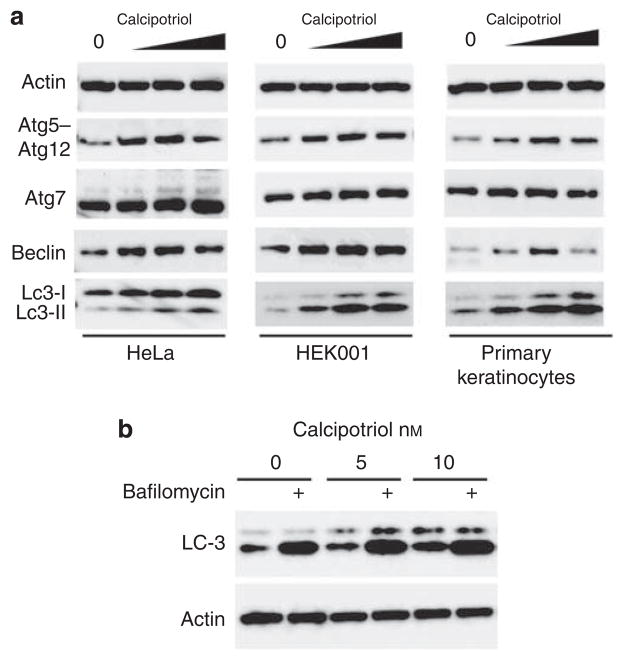
In addition to the quantitation of GFP-LC3 puncta, autophagy induction may also be monitored biochemically by the conversion of LC3 from the cytosolic (LC3-I) to the lipidated (LC3-II) form (Mizushima et al., 2010). We assessed changes in LC3-II conversion following treatment with increasing concentrations of calcipotriol (Figure 2a). In HeLa cells, increasing concentrations of calcipotriol resulted in increased levels of LC3-II. Importantly, both an E6/E7-immortalized human keratinocyte cell line (HEK001) and normal human keratinocytes (NHKs) demonstrated increased levels of LC3-II in response to calcipotriol. Thus, in both HeLa cells and keratinocytes, calcipotriol induces autophagy.
Figure 2. Calcipotriol affects levels of Atg5–Atg12 conjugate, Beclin 1, and LC3-II conversion in HeLa cells, HEK001 keratinocytes, and primary keratinocytes.
(a) HEK001 keratinocytes (ATCC, Manassas, VA) were grown in K-SFM without bovine pituitary extract. Primary human keratinocytes (Invitrogen, Carlsbad, CA) were grown on collagen IV-coated flasks (Becton Dickinson, Franklin Lakes, NJ) in K-SFM. Cells were treated with vehicle or calcipotriol (1, 10, 100 μM) for 24 hours. Protein concentrations of whole-cell lysates were normalized by Bradford assay. Western blots were probed for LC3 (Novus, 100–2220, Littleton, CO), Beclin 1 (Santa Cruz Biotechnology, 11427, Santa Cruz, CA), Atg5 (Novus, 110–53818), or actin (Santa Cruz, 47778). (b) HeLa cells were treated with calcipotriol (as indicated) and 100 nM bafilomycin A1 (Sigma) was added for the final 4 hours of the incubation, and whole-cell lysates were analyzed as described.
Previously, calcitriol-induced autophagy in monoyctes was associated with transcriptional upregulation of Beclin 1 and autophagy protein 5 (Atg5) (Yuk et al., 2009). Therefore, we assessed whether calcipotriol also increased the levels of these essential autophagy proteins (Figure 2a). In HeLa, HEK001, and NHK cells, calcipotriol treatment increased the levels of Beclin 1, a component of the Class III phosphatidylinositol 3-kinase complex that is essential for autophagy induction (Mizushima et al., 2010). Moreover, calcipotriol also increased the levels of Atg5–Atg12 conjugates, the proteins essential for elongation of the autophagosome (Mizushima et al., 2010). At higher concentrations of calcipotriol (>50 nM), Beclin 1 and Atg5–Atg12 protein levels decreased slightly; a phenomenon commonly seen with the strong induction of autophagy. These findings demonstrate that similar to the effects of calcitriol in monocytes, calcipotriol also upregulates the expression of key autophagy proteins in multiple cell types, including primary human keratinocytes.
The accumulation of GFP-LC3 puncta may occur through either increased induction or decreased turnover of autophagosomes. To distinguish between these possibilities, HeLa cells were treated with bafilomycin A1, a specific inhibitor of the vacuolar type H(+)-ATPase and inhibitor of autophagosome turnover (Mizushima et al., 2010) (Figure 2b). In the absence of bafilomycin, calcipotriol resulted in a dose-dependent increase in LC3-II levels. In the presence of bafilomycin, LC3-II levels in calcipotriol-treated cells underwent a further dramatic increase (Figure 2b). These observations demonstrate that increased autophagy induction, rather than decreased autophagosome turnover, is the cause of increased GFP-LC3 puncta accumulation (Figure 1b) and increased levels of LC3-II (Figure 2a), and provide further evidence that calcipotriol induces a complete autophagic response.
Presently, the mechanisms through which vitamin D analogs induce autophagy are unclear. In monocytes, calcitriol-induced cathelicidin transcriptionally upregulates Beclin 1 and Atg5 (Yuk et al., 2009); whether this transcriptional upregulation is sufficient for autophagy induction is unclear. Another possible mechanism is via vitamin D-mediated upregulation of FoxO signaling (An et al., 2010), which also upregulates autophagy (Zhao et al., 2008). Regardless of its precise mechanism, our finding that calcipotriol induces autophagy suggests that vitamin D analogs be tested in other skin diseases. Specifically, as autophagy protects against both viral and (myco)- bacterial intracellular infections (Deretic and Levine, 2009; Shoji-Kawata and Levine, 2009), calcipotriol may be useful in treating intracellular skin infections. Notably, there are several case reports of the successful use of vitamin D analogs in the treatment of recalcitrant warts (Egawa and Ono, 2004). Moreover, as autophagy limits cell proliferation (Wang and Levine, 2010), calcipotriol may be useful in the treatment of hyperproliferative skin diseases. Interestingly, there have been reports of the treatment of lamellar ichthyosis and epidermolytic hyperkeratosis with vitamin D analogs (Umekoji et al., 2008). Given the increasing recognition of the importance of vitamin D in skin biology and immunity, a more thorough understanding of the relationship between vitamin D and autophagy may provide a critical framework within which to understand its seemingly pleiotropic effects.
Acknowledgments
Rhea Sumpter, Zhongju Zou, and other members of the Levine laboratory are thanked for helpful discussions.
Abbreviations
- NHK
normal human keratinocyte
- VDR
vitamin D receptor
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Contributor Information
Richard C. Wang, Email: Richard.Wang@utsouthwestern.edu.
Beth Levine, Email: Beth.Levine@utsouthwestern.edu.
References
- An BS, Tavera-Mendoza LE, Dimitrov V, et al. Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor. Mol Cell Biol. 2010;30:4890–900. doi: 10.1128/MCB.00180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–49. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa K, Ono T. Topical vitamin D3 derivatives for recalcitrant warts in three immunocompromised patients. Br J Dermatol. 2004;150:374–6. doi: 10.1111/j.1365-2133.2003.05766.x. [DOI] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Bastholm L, Szyniarowski P, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Masuda S, Strugnell S, Calverley MJ, et al. In vitro metabolism of the anti-psoriatic vitamin D analog, calcipotriol, in two cultured human keratinocyte models. J Biol Chem. 1994;269:4794–803. [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A, MacPherson S, Sumpter R, Jr, et al. Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host Microbe. 2010;7:115–27. doi: 10.1016/j.chom.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, Dunn CJ, Goa KL. Calcipotriol ointment. A review of its use in the management of psoriasis. Am J Clin Dermatol. 2001;2:95–120. doi: 10.2165/00128071-200102020-00008. [DOI] [PubMed] [Google Scholar]
- Shoji-Kawata S, Levine B. Autophagy, antiviral immunity, and viral countermeasures. Biochim Biophys Acta. 2009;1793:1478–84. doi: 10.1016/j.bbamcr.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Ibe M, Kinouchi M, et al. Similarly potent action of 1,25-dihydroxyvitamin D3 and its analogues, tacalcitol, calcipotriol, and maxacalcitol on normal human keratinocyte proliferation and differentiation. J Dermatol Sci. 2003;31:21–8. doi: 10.1016/s0923-1811(02)00136-6. [DOI] [PubMed] [Google Scholar]
- Umekoji A, Fukai K, Ishii M. A case of mosaic-type bullous congenital ichthyosiform erythroderma successfully treated with topical maxacalcitol, a vitamin D3 analogue. Clin Exp Dermatol. 2008;33:501–2. doi: 10.1111/j.1365-2230.2008.02761.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Lian H, Zhao Y, et al. Vitamin D3 induces autophagy of human myeloid leukemia cells. J Biol Chem. 2008;283:25596–605. doi: 10.1074/jbc.M801716200. [DOI] [PubMed] [Google Scholar]
- Wang RC, Levine B. Autophagy in cellular growth control. FEBS Lett. 2010;584:1417–26. doi: 10.1016/j.febslet.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–43. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chalmers MJ, Stayrook KR, et al. Hydrogen/deuterium exchange reveals distinct agonist/partial agonist receptor dynamics within vitamin D receptor/retinoid X receptor heterodimer. Structure. 2010;18:1332–41. doi: 10.1016/j.str.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Brault JJ, Schild A, et al. Coordinate activation of autophagy and the proteasome pathway by FoxO transcription factor. Autophagy. 2008;4:378–80. doi: 10.4161/auto.5633. [DOI] [PubMed] [Google Scholar]