Abstract
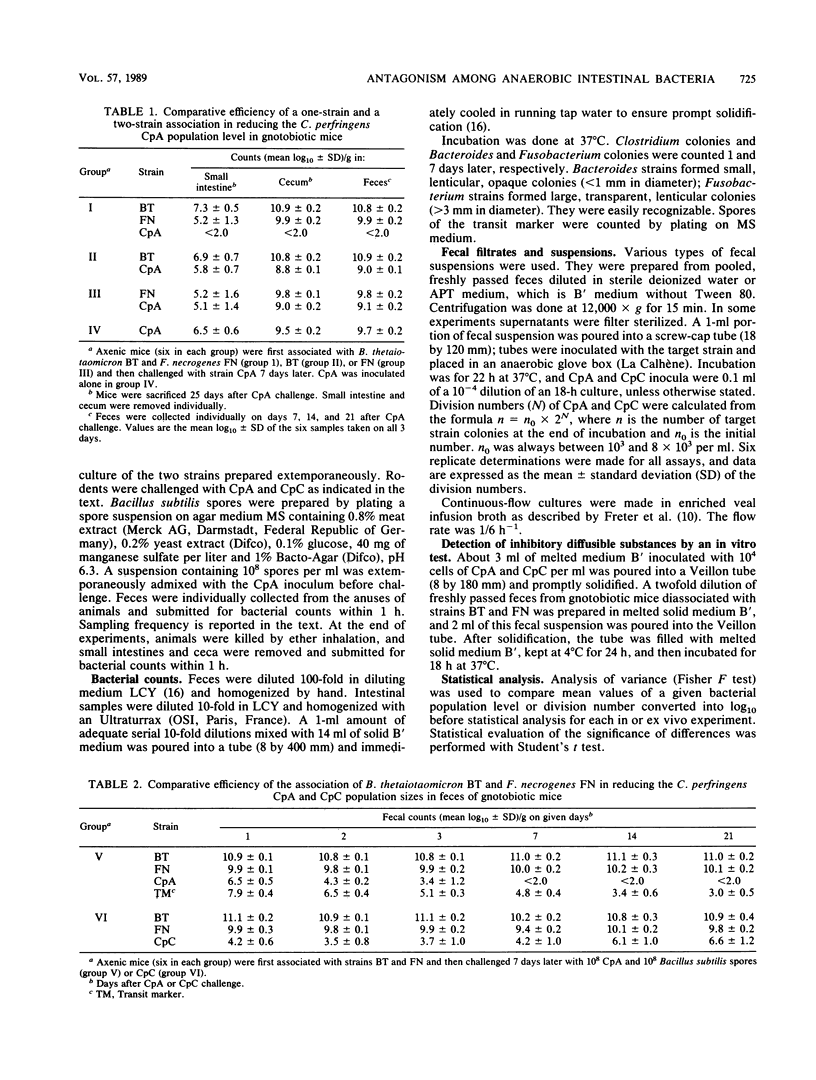
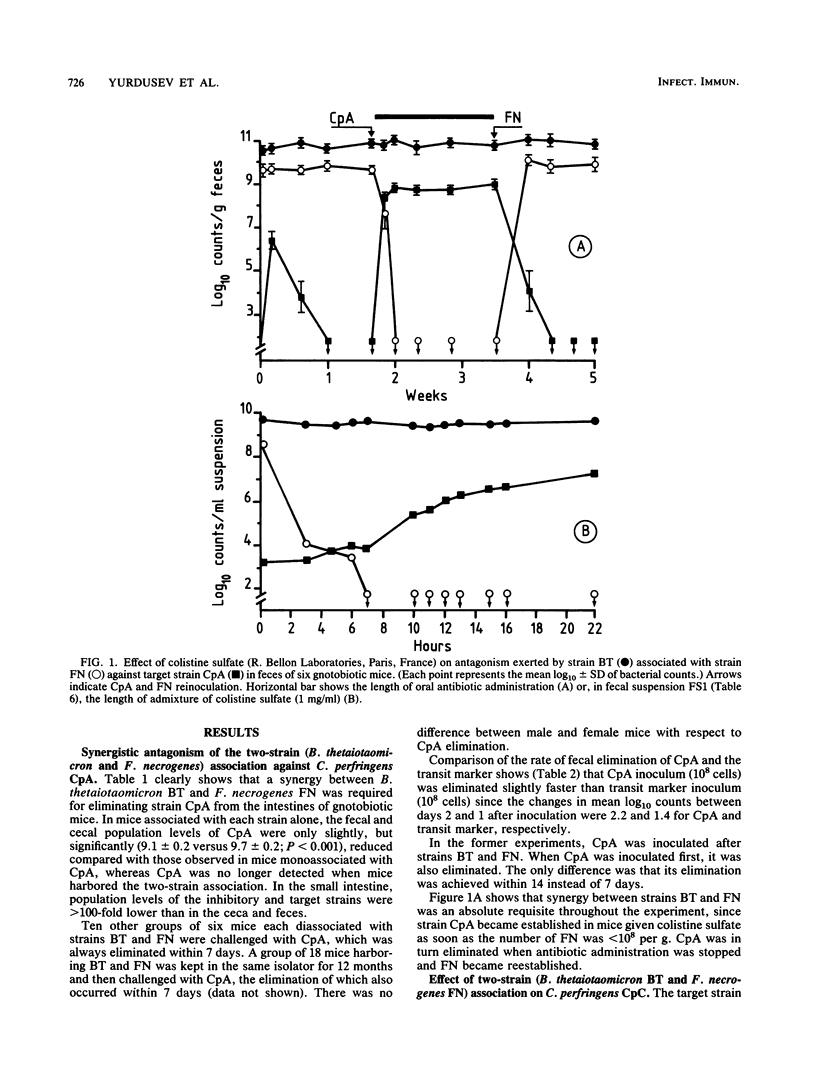
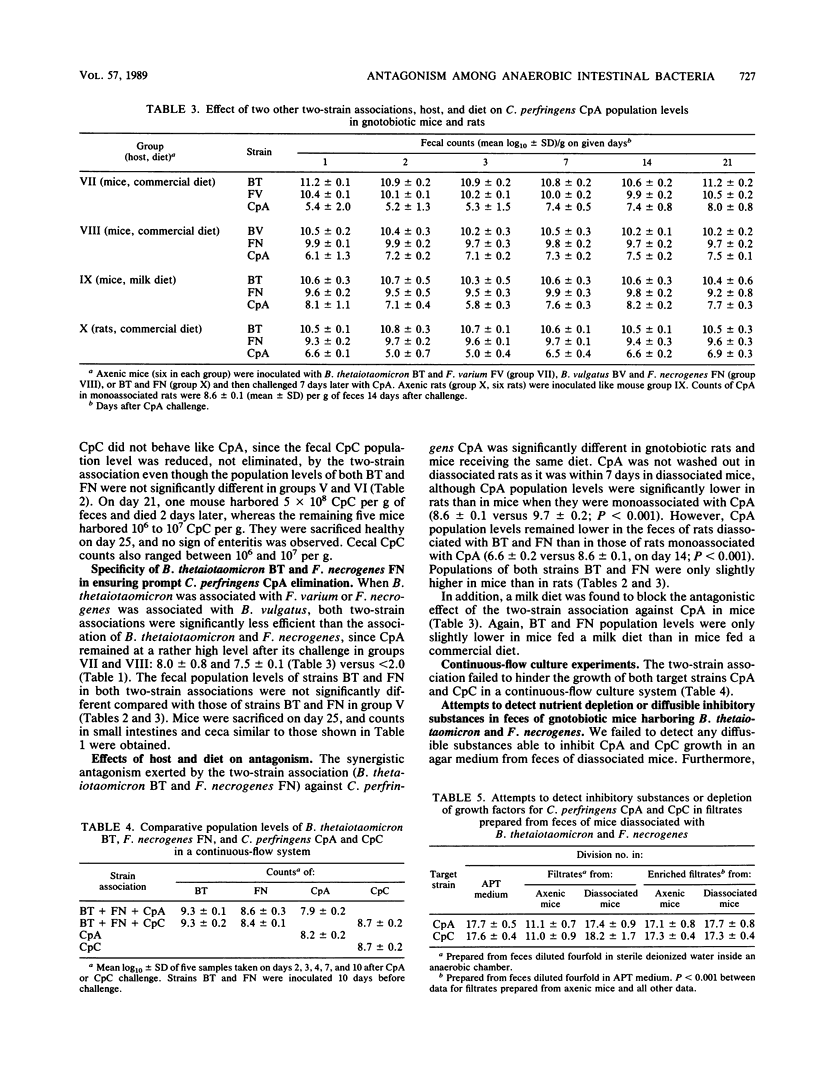
Antagonism between an association of Bacteroides thetaiotaomicron and Fusobacterium necrogenes strains and two strains of Clostridium perfringens was evidenced both in vivo in gnotobiotic mice and ex vivo in fecal suspensions incubated for 22 h at 37 degrees C. Several features of this antagonism were similar in and ex vivo. (i) An obligate and continuous synergy between B. thetaiotaomicron and F. necrogenes was required; (ii) the two C. perfringens strains did not respond to the same extent to this antagonism; and (iii) expression of the antagonism was host and diet dependent. Neither diffusible nor soluble inhibitory substances were detectable in feces of gnotobiotic mice, nor could depletion of nutrients be identified as causing antagonism in both in and ex vivo experiments. Our findings support the hypothesis that a reversible bacteriostasis induced by the inhibitory strains acting together continuously, and hindering the target strain from utilizing available nutrients, was responsible for this antagonism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ducluzeau R., Bellier M., Raibaud P. Transit Digestif de Divers Inoculums Bactériens Introduits "Per Os" Chez des Souris Axéniques et "Holoxéniques" (Conventionnelles): Effet Antagoniste de la Microflore du Tractus Gastro-Intestinal. Zentralbl Bakteriol Orig. 1970 May;213(4):533–548. [PubMed] [Google Scholar]
- Ducluzeau R., Dubos F., Raibaud P., Abrams G. D. Production of an antibiotic substance by Bacillus licheniformis within the digestive tract of gnotobiotic mice. Antimicrob Agents Chemother. 1978 Jan;13(1):97–103. doi: 10.1128/aac.13.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducluzeau R., Ladire M., Callut C., Raibaud P., Abrams G. D. Antagonistic effect of extremely oxygen-sensitive clostridia from the microflora of conventional mice and of Escherichia coli against Shigella flexneri in the digestive tract of gnotobiotic mice. Infect Immun. 1977 Aug;17(2):415–424. doi: 10.1128/iai.17.2.415-424.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducluzeau R., Raibaud P. Interaction between Escherichia coli and Shigella flexneri in the digestive tract of "gnotobiotic" mice. Infect Immun. 1974 Apr;9(4):730–733. doi: 10.1128/iai.9.4.730-733.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Iflah Y., Raibaud P., Rousseau M. Antagonisms among isogenic strains of Escherichia coli in the digestive tracts of gnotobiotic mice. Infect Immun. 1981 Dec;34(3):957–969. doi: 10.1128/iai.34.3.957-969.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Brickner H., Botney M., Cleven D., Aranki A. Mechanisms that control bacterial populations in continuous-flow culture models of mouse large intestinal flora. Infect Immun. 1983 Feb;39(2):676–685. doi: 10.1128/iai.39.2.676-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Brickner H., Fekete J., Vickerman M. M., Carey K. E. Survival and implantation of Escherichia coli in the intestinal tract. Infect Immun. 1983 Feb;39(2):686–703. doi: 10.1128/iai.39.2.686-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Stauffer E., Cleven D., Holdeman L. V., Moore W. E. Continuous-flow cultures as in vitro models of the ecology of large intestinal flora. Infect Immun. 1983 Feb;39(2):666–675. doi: 10.1128/iai.39.2.666-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENTGES D. J., FRETER R. In vivo and in vitro antagonism of intestinal bacteria against Shigella flexneri. I. Correlation between various tests. J Infect Dis. 1962 Jan-Feb;110:30–37. doi: 10.1093/infdis/110.1.30. [DOI] [PubMed] [Google Scholar]
- Hudault S., Bewa H., Bridonneau C., Raibaud P. Efficiency of various bacterial suspensions derived from cecal floras of conventional chickens in reducing the population level of Salmonella typhimurium in gnotobiotic mice and chicken intestines. Can J Microbiol. 1985 Sep;31(9):832–838. doi: 10.1139/m85-155. [DOI] [PubMed] [Google Scholar]
- Hudault S., Bridonneau C., Raibaud P. Pouvoir pathogène de différents types toxinogènes de Clostridium perfringens inoculés per os à des souris axéniques et holoxéniques. Ann Microbiol (Paris) 1983 Sep-Oct;134B(2):277–283. [PubMed] [Google Scholar]
- Hudault S., Raibaud P., Ducluzeau R., Bridonneau C. Effet antagoniste à l'égard de Clostridium perfringens exercé par des souches de Clostridium isolées de la microflore de souris holoxéniques dans le tube digestif de souris gonotoxéniques. Ann Microbiol (Paris) 1982 May-Jun;133(3):443–459. [PubMed] [Google Scholar]
- Raibaud P., Dickinson A. B., Sacquet E., Charlier H., Mocquot G. La microflore du tube digestif du rat. I. Techniques d'étude et milieux de culture proposés. Ann Inst Pasteur (Paris) 1966 Apr;110(4):568–590. [PubMed] [Google Scholar]
- Sacquet E., Raibaud P., Garnier J. Etude comparée de la microflore de l'estomac, de l'intestin grêle et du caecum du rat "holoxénique" (conventionnel), et de ses modifications à la suite de diverses interventions chirurgicales: anse aveugle jéjunale, déviations biliaires. Ann Inst Pasteur (Paris) 1971 Apr;120(4):501–524. [PubMed] [Google Scholar]
- Wilson K. H., Freter R. Interaction of Clostridium difficile and Escherichia coli with microfloras in continuous-flow cultures and gnotobiotic mice. Infect Immun. 1986 Nov;54(2):354–358. doi: 10.1128/iai.54.2.354-358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurdusev N., Nicolas J. L., Ladire M., Ducluzeau R., Raibaud P. Antagonistic effect exerted by three strictly anaerobic strains against various strains of Clostridium perfringens in gnotobiotic rodent intestines. Can J Microbiol. 1987 Mar;33(3):226–231. doi: 10.1139/m87-039. [DOI] [PubMed] [Google Scholar]



