The Diels–Alder cycloaddition represents the most powerful technology for the preparation of substituted cyclohexenes and has proven to be extremely valuable in organic synthesis.[1] However, efficient syntheses of cyclohexenes having diverse substitutions, stereochemistry, and functionalities are still challenging and continue to stimulate the development of novel cycloaddition reactions.[2] We report herein a stereoselective synthesis of highly functionalized cyclohexenones from substituted cyclopropanes through a rhodium-catalyzed 1,3-acyloxy migration and subsequent [5+1] cycloaddition. Given the well-documented strategies for the preparation of optically pure cyclopropanes,[3] this promises to be a versatile method for the synthesis of complex cyclohexenones from cyclopropanes.[4]
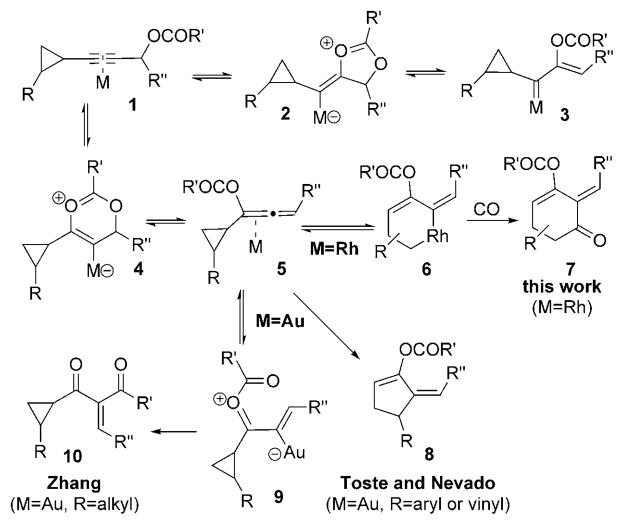
We previously reported a synthesis of highly substituted cyclobutenes from cyclopropyl metal carbenes derived from transition metal catalyzed decomposition of diazo compounds.[5,6] A more convenient and atom-economical[7] alternative for generating metal carbene intermediates would be the 1,2-acyloxy migration of propargyl esters, which has been realized using AuI,[8] PtII,[9] RuII,[10] PdII,[11] and more recently RhI,[12] the reports of which appeared while we were conducting our investigation.[13] When we searched for reaction conditions to form the cyclopropyl metal carbene 3 through 1,2-acyloxy migration, we isolated the highly functionalized cyclohexenone 7 when [{Rh(CO)2Cl}2] was employed as the catalyst (Scheme 1).
Scheme 1.
Diverse products from the metal-catalyzed acyloxy migration.
Cyclohexenone 7 was presumably generated by insertion of CO into the metallocyclohexene 6 (M = Rh),[14] which was derived from the 1,3-acyloxy migration[15] of propargyl ester 1 and subsequent ring expansion of the allenyl ester 5.[16] Gold catalysts could promote the formation of cyclopentene 8, enone 10, and some other isomerization or hydrolysis products from cyclopropane 1.[17,18] Cyclopentene 8 was derived from direct ring expansion of allene 5 when R was either an aryl or vinyl group.[17] The cyclopropane ring was not required for the transformation of allene 5 into the intermediate 9.[18] Clearly, allenyl ester 5 may undergo a variety of different reactions including isomerization (e.g., formation of 8 and 9) and hydrolysis when it was generated by π-acidic metals. We found for the first time that allenyl ester 5 could be trapped by the [{Rh(CO)2Cl}2] catalyst for a [5+1] cycloaddition. The ability of this rhodium catalyst to promote both the acyloxy migration and the subsequent cycloaddition not only increases the synthetic efficiency but also allows the development of new cycloaddition reactions.
The cyclohexenone 12a was obtained as an mixture of isomers (E/Z = ca. 1:1) in about 30% yield from 11a in the presence of 20 mol% of the [{Rh(CO)2Cl}2] catalyst in toluene at room temperature [Eq. (1); Piv = pivaloate]. The
 |
(1) |
product 12a was isolated in 93% yield by running the reaction with an attached CO balloon for 5 hours at 60°C. The temperature can be reduced to room temperature without decreasing the yield of 12a by lowering the CO pressure to between 0.1 and 0.2 atm through diluting the CO in the balloon with nitrogen.[19] Separation of the two geometric isomers of 12a and resubmitting each of them to the reaction conditions did not change the configuration, suggesting that the E/Z isomers did not equilibrate under the reaction conditions. A significant amount of the enone 13 was isolated when a cationic rhodium catalyst (e.g., [{Rh(CO)2Cl}2]/AgOTf or [Rh(cod)2]BF4) (cod = 1,5-cyclooctadiene) was employed. The addition of ligands (e.g., phosphine, phosphite, and pyridine) either decreased the conversion or completely shut down the reaction.
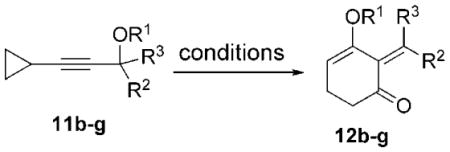
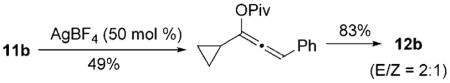
We then examined the tandem 1,3-acyloxy migration/[5+1] cycloaddition for substrates 11 b–g, which were prepared from commercially available cyclopropyl acetylene and either an aldehyde or ketone (Table 1). The reaction was still conducted under 1 atm of CO at 60°C for convenient operation and consistent results. High yields of products were isolated for substrates derived from various aldehydes and ketones. High E/Z selectivity could be obtained for propargyl esters having bulky substituents (Table 1, entries 3 and 6), and a single product was obtained for substrate 11 e (Table 1, entry 4). The cycloaddition worked well when the ester was changed from pivaloate to acetate (Table 1, entry 5). A highly functionalized product can also be prepared from 11g, which is derived from steroids (Table 1, entry 6).
Table 1.
Ring expansion of nonsubstituted cyclopropanes.[a]
 | |||
|---|---|---|---|
| Entry | Substrate | E/Z | Yield [%] |
| 1 | 11b, R1 = Piv, R2 = H, R3 =Ph | 2:1 | 95[b] |
| 2 | 11c, R1 = Piv, R2 = H, R3 =iPr | 1:1 | 87[b] |
| 3 | 11d, R1 = Piv, R2 = H, R3 =tBu | 10:1 | 92[b] |
| 4[c] | 11e R1 = Piv, R2 = R3 = Me | – | 88 |
| 5 | 11f, R1 = Ac, R2 = R3 = Me | – | 85 |
| 6 |
11g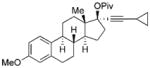
|
13:1 | 91[b] |
Reaction conditions: [{Rh(CO)2Cl}2] (5 mol%), CO (1 atm), toluene, 60°C, 5 h.
Yield of product including both isomers.
2.5 mol% catalyst, 60°C, 1 h.
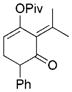
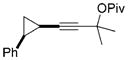
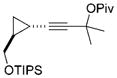
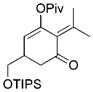
For unsymmetrically substituted cyclopropanes, the cleavage of different cyclopropane C–C σ bonds may lead to different isomers. Our research group[5] and that of others[20,21] have found that the regioselectivity for the cleavage of C–C σ bonds in cyclopropanes depends on the stereochemistry of the cyclopropane ring, the electronic properties of the substituents, and the nature of the metal catalysts. Indeed, the opposite regioselectivity was observed for trans- and cis-phenyl-substituted cyclopropanes 14 and 17, respectively (Table 2). In the case of alkyl substituents, both trans-18 and cis-21 gave product 19 as the major isomer and much higher selectivity was observed for the cis-isomer 21. The tandem reaction worked at room temperature for the cis-isomer 21 even with 1 atm of CO and a slightly higher regioselectivity was obtained at room temperature. The same trend of regioselectivity was observed for the cyclopropanes 22 and 24. Substrates having quaternary carbon atoms, such as cyclopropanes 26 and 28 yielded only one isomer. High regioselectivity was also observed for the 1,2,3-trisubstituted cyclopropane 30. The aryl and PMBOCH2 groups in cyclopropane 30 should direct the cleavage of the same C–C σ bond on the basis of regioselectivity observed for the substrates 14 and 21.
Table 2.
Ring expansion of substituted cyclopropanes.[a]
| Cyclopropane | Cyclohexenones[b] | Yield [%][c] | |
|---|---|---|---|
 14 |
 15 |
 16 |
|
| 15/16 = 1:2.5[b] | 90[d] | ||
 17 |
15/16 = 3.5:1[b] | 95[d] | |
 18 |
 19 |
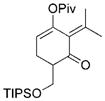 20 |
|
| 19/20 = 10:1[b] | 91 | ||
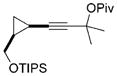 21 |
19/20 > 20:1[b,e] | 93 | |
 22 |
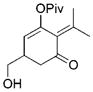 23 (12:1)[b] |
79 | |
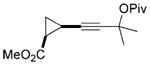 24 |
 25 |
76[f] | |
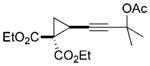 26 |
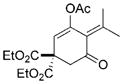 27 |
74 | |
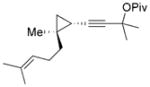 28 (87% ee) |
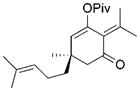 29 (87% ee) |
86 | |
 30 |
 31 (16:1)[b] |
80 | |
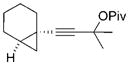 32 |
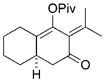 33 |
80[g] | |
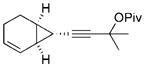 34 |
 35 |
82[g] | |
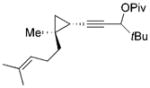 36 (d.r. = 1:1) |
 37 (E/Z = 10:1) |
74[h] |
Reaction conditions: see footnote [a] of Table 1, unless noted otherwise.
Regioisomeric ratios were determined by 1H NMR analysis of the crude reaction mixture.
Yields are of the major isomer (isolated) unless noted otherwise.
Yield is for 15 and 16 combined.
The reaction was run at RT. A 16:1 ratio was observed at 60°C.
Other unidentified by-products were observed.
Products without CO insertion were also isolated. See text for details.
80°C, 24 h. TIPS = triisopropylsilyl.
The chirality of cyclopropane 28 was completely transferred to cyclohexenone 29,[22] thus confirming that cyclohexenones can be enantioselectively synthesized from optically pure cyclopropanes. The highly functionalized decalin rings 33 and 35 were also prepared in high yields from bicyclic substrates with high regioselectivity. Five-membered ring isomerization products were isolated in 19% and 11% yields from substrates 32 and 34, respectively under the standard reaction conditions.[20] Interestingly, no five-membered ring isomerization product was observed in the monocyclic system even in the absence of a CO atmosphere.
Cleavage of the less hindered C–C σ bonds occurred selectively for most cyclopropanes in Table 2. The substituent that is cis to the propargyl ester generally has a more significant steric effect than the corresponding trans substituent. For cyclopropanes 14 and 34, the C–C σ bonds that are adjacent to aryl or vinyl groups were selectively cleaved presumably because of the electronic effect of the adjacent π system.[5,20,21] Relatively low regioselectivities observed for the phenyl-substituted cyclopropanes 14 and 17 may be the result of competing steric and electronic effects.
The E/Z ratio is 10:1 for the product 37 when a mixture of cyclopropane 36 (d.r. = 1:1) is employed as the starting material. We demonstrated that the two Z/E isomers of cyclohexenone 12a did not equilibrate under the reaction conditions. If the RhI-catalyzed 1,3-acyloxy migration of the propargyl ester is stereospecific and the configuration of the resulting allene is stable under the reaction conditions, one would expect a 1:1 E/Z ratio for 37. Our result suggests that either the 1,3-acyloxy migration is not stereospecific or the two diastereomeric allenes can interconvert, which is similar to what was observed for gold catalysts.[17]
In summary, we have developed an efficient and highly selective method for the synthesis of polysubstituted cyclohexenones. Regioselective cleavage of C–C σ bonds can be achieved for various substituted cyclopropanes. The π-acid property of the [{Rh(CO)2Cl}2] catalyst and its ability to readily undergo oxidative addition/reductive elimination will lead to a number of opportunities for mechanistic investigations, the design of new reactions, and target-oriented synthesis of natural products and pharmaceutical agents, such as cyclohexenone-containing compounds.[23]
Supplementary Material
Footnotes
We thank the NIH (R01GM088285) and the University of Wisconsin for funding.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201006881.
Contributor Information
Dongxu Shu, Department of Chemistry, University of Wisconsin Madison, WI 53706-1322 (USA).
Xiaoxun Li, The School of Pharmacy, University of Wisconsin Madison, WI 53705-2222 (USA).
Dr. Min Zhang, The School of Pharmacy, University of Wisconsin Madison, WI 53705-2222 (USA)
Patrick J. Robichaux, Department of Chemistry, University of Wisconsin Madison, WI 53706-1322 (USA)
Prof. Dr. Weiping Tang, The School of Pharmacy, University of Wisconsin Madison, WI 53705-2222 (USA).
References
- 1.For selected reviews, see: Takao K, Munakata R, Tadano K. Chem Rev. 2005;105:4779. doi: 10.1021/cr040632u.Winkler JD. Chem Rev. 1996;96:167. doi: 10.1021/cr950029z.Danishefsky SJ. Acc Chem Res. 1981;14:400.
- 2.For a review on transition metal mediated cycloadditions, see: Lautens M, Klute W, Tam W. Chem Rev. 1996;96:49. doi: 10.1021/cr950016l.
- 3.Lebel H, Marcoux JF, Molinaro C, Charette AB. Chem Rev. 2003;103:977. doi: 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]
- 4.For a recent review on transition metal mediated reactions of cyclopropanes, see: Rubin M, Rubina M, Gevorgyan V. Chem Rev. 2007;107:3117. doi: 10.1021/cr050988l.
- 5.Xu H, Zhang W, Shu D, Werness JB, Tang W. Angew Chem. 2008;120:9065. doi: 10.1002/anie.200803910. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:8933. [Google Scholar]
- 6.For related work, see: Barluenga J, Riesgo L, Lopez LA, Rubio E, Tomas M. Angew Chem. 2009;121:7705. doi: 10.1002/anie.200903902.Angew Chem Int Ed. 2009;48:7569.
- 7.Trost BM. Science. 1991;254:1471. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- 8.a) Johansson MJ, Gorin DJ, Staben ST, Toste FD. J Am Chem Soc. 2005;127:18002. doi: 10.1021/ja0552500. [DOI] [PubMed] [Google Scholar]; b) Gorin DJ, Dube P, Toste FD. J Am Chem Soc. 2006;128:14480. doi: 10.1021/ja066694e. [DOI] [PubMed] [Google Scholar]; c) Gorin DJ, Watson IDG, Toste FD. J Am Chem Soc. 2008;130:3736. doi: 10.1021/ja710990d. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Li G, Zhang G, Zhang L. J Am Chem Soc. 2008;130:3740. doi: 10.1021/ja800001h. [DOI] [PubMed] [Google Scholar]
- 9.a) Mainetti E, Mouries V, Fensterbank L, Malacria M, Marco-Contelles J. Angew Chem. 2002;114:2236. [PubMed] [Google Scholar]; Angew Chem Int Ed. 2002;41:2132. [Google Scholar]; b) Harrak Y, Blaszykowski C, Bernard M, Cariou K, Mainetti E, Mouries V, Dhimane AL, Fensterbank L, Malacria M. J Am Chem Soc. 2004;126:8656. doi: 10.1021/ja0474695. [DOI] [PubMed] [Google Scholar]; c) Prasad BAB, Yoshimoto FK, Sarpong R. J Am Chem Soc. 2005;127:12468. doi: 10.1021/ja053192c. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Pujanauski BG, Prasad BAB, Sarpong R. J Am Chem Soc. 2006;128:6786. doi: 10.1021/ja061549m. [DOI] [PubMed] [Google Scholar]; e) Ji K, Shu X, Chen J, Zhao S, Zheng Z, Lu L, Liu X, Liang Y. Org Lett. 2008;10:3919. doi: 10.1021/ol8015463. [DOI] [PubMed] [Google Scholar]
- 10.a) Miki K, Ohe K, Uemura S. J Org Chem. 2003;68:8505. doi: 10.1021/jo034841a. [DOI] [PubMed] [Google Scholar]; b) Miki K, Ohe K, Uemura S. Tetrahedron Lett. 2003;44:2019. [Google Scholar]; c) Tenaglia A, Marc S. J Org Chem. 2006;71:3569. doi: 10.1021/jo060276a. [DOI] [PubMed] [Google Scholar]
- 11.a) Rautenstrauch V. Tetrahedron Lett. 1984;25:3845. [Google Scholar]; b) Rautenstrauch V. J Org Chem. 1984;49:950. [Google Scholar]
- 12.a) Shibata Y, Noguchi K, Tanaka K. J Am Chem Soc. 2010;132:7896. doi: 10.1021/ja102418h. [DOI] [PubMed] [Google Scholar]; b) Brancour C, Fukuyama T, Ohta Y, Ryu I, Dhimane AL, Fensterbank L, Malacria M. Chem Commun. 2010;46:5470. doi: 10.1039/c0cc00747a. [DOI] [PubMed] [Google Scholar]
- 13.For recent reviews, see: Miki K, Uemura S, Ohe K. Chem Lett. 2005;34:1068.Marion N, Nolan SP. Angew Chem. 2007;119:2806–2809.Angew Chem Int Ed. 2007;46:2750–2752. doi: 10.1002/anie.200604773.F4rstner A, Davies PW. Angew Chem. 2007;119:3478–3519.Angew Chem Int Ed. 2007;46:3410–3449. doi: 10.1002/anie.200604335.Hashmi ASK. Chem Rev. 2007;107:3180. doi: 10.1021/cr000436x.Hashmi ASK. Angew Chem. 2008;120:6856–6858.Angew Chem Int Ed. 2008;47:6754–6756. doi: 10.1002/anie.200802517.Jimenez-Nunez E, Echavarren AM. Chem Rev. 2008;108:3326. doi: 10.1021/cr0684319.Gorin DJ, Sherry BD, Toste FD. Chem Rev. 2008;108:3351. doi: 10.1021/cr068430g.
- 14.For recent examples of [5+1] cycloadditions of vinyl and allenyl cyclopropanes, see: Taber DF, Kanai K, Jiang Q, Bui G. J Am Chem Soc. 2000;122:6807.Kurahashi T, de Meijere A. Synlett. 2005:2619.Iwasawa N, Owada Y, Matsuo T. Chem Lett. 1995:115.Owada Y, Matsuo T, Iwasawa N. Tetrahedron. 1997;53:11069.Murakami M, Itami K, Ubukata M, Tsuji I, Ito Y. J Org Chem. 1998;63:4. doi: 10.1021/jo9718859.For [6+1] cycloaddition of allenylcyclobutanes, see: Wender PA, Deschamps NM, Sun R. Angew Chem. 2006;118:4061 –4064. doi: 10.1002/anie.200600806.Angew Chem Int Ed. 2006;45:3957 –3960. doi: 10.1002/anie.200600806.
- 15.For selected examples of tandem reactions involving gold-, copper-, silver-, or platinum-catalyzed 1,3-acyloxy migration of propargyl esters, see: Sromek AW, Kel’in AV, Gevorgyan V. Angew Chem. 2004;116:2330. doi: 10.1002/anie.200353535.Angew Chem Int Ed. 2004;43:2280.Zhang L. J Am Chem Soc. 2005;127:16804. doi: 10.1021/ja056419c.Zhang L, Wang S. J Am Chem Soc. 2006;128:1442. doi: 10.1021/ja057327q.Zhao J, Hughes CO, Toste FD. J Am Chem Soc. 2006;128:7436. doi: 10.1021/ja061942s.Buzas A, Gagosz F. J Am Chem Soc. 2006;128:12614. doi: 10.1021/ja064223m.Marion N, Diez-Gonzalez S, de Fremont P, Noble AR, Nolan SP. Angew Chem. 2006;118:3729. doi: 10.1002/anie.200600571.Angew Chem Int Ed. 2006;45:3647.Buzas A, Istrate F, Gagosz F. Org Lett. 2006;8:1957. doi: 10.1021/ol0606839.Wang S, Zhang L. Org Lett. 2006;8:4585. doi: 10.1021/ol0618151.Barluenga J, Riesgo L, Vicente R, Lopez LA, Tomas M. J Am Chem Soc. 2007;129:7772. doi: 10.1021/ja072864r.Schwier T, Sromek AW, Yap DML, Chernyak D, Gevorgyan V. J Am Chem Soc. 2007;129:9868. doi: 10.1021/ja072446m.Zhang G, Catalano VJ, Zhang L. J Am Chem Soc. 2007;129:11358. doi: 10.1021/ja074536x.Lemiere G, Gandon V, Cariou K, Fukuyama T, Dhimane AL, Fensterbank L, Malacria M. Org Lett. 2007;9:2207. doi: 10.1021/ol070788r.Amijs CHM, Opez-Carrillo V, Echavarren AM. Org Lett. 2007;9:4021. doi: 10.1021/ol701706d.Luo TP, Schreiber SL. Angew Chem. 2007;119:8398–8401.Angew Chem Int Ed. 2007;46:8250–8253. doi: 10.1002/anie.200703276.Dudnik AS, Schwier T, Gevorgyan V. Org Lett. 2008;10:1465. doi: 10.1021/ol800229h.Shu X, Ji K, Zhao S, Zheng Z, Chen J, Lu L, Liu X, Liang Y. Chem Eur J. 2008;14:10556. doi: 10.1002/chem.200801591.Peng Y, Cui L, Zhang G, Zhang L. J Am Chem Soc. 2009;131:5062. doi: 10.1021/ja901048w.Yu M, Zhang G, Zhang L. Tetrahedron. 2009;65:1846.Lu L, Liu XY, Shu XZ, Yang K, Ji KG, Liang YM. J Org Chem. 2009;74:474. doi: 10.1021/jo802043z.Dudnik AS, Schwier T, Gevorgyan V. Tetrahedron. 2009;65:1859.Dudnik AS, Schwier T, Gevorgyan V. J Organomet Chem. 2009;694:482. doi: 10.1016/j.jorganchem.2008.08.010.Teng TM, Liu RS. J Am Chem Soc. 2010;132:9298. doi: 10.1021/ja1043837.
-
16.The independently prepared allene 5 yielded the same product under the standard reaction conditions in Table 1.
- 17.a) Mauleon P, Krinsky JL, Toste FD. J Am Chem Soc. 2009;131:4513. doi: 10.1021/ja900456m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Garayalde D, Gomez-Bengoa E, Huang XG, Goeke A, Nevado C. J Am Chem Soc. 2010;132:4720. doi: 10.1021/ja909013j. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Zhang L. J Am Chem Soc. 2006;128:8414. doi: 10.1021/ja062777j. [DOI] [PubMed] [Google Scholar]
- 19.Similar effects were also observed in other cycloaddition reactions: Kobayashi T, Koga Y, Narasaka K. J Organomet Chem. 2001;624:73.Wang Y, Wang J, Su J, Huang F, Jiao L, Liang Y, Yang D, Zhang S, Wender PA, Yu Z. J Am Chem Soc. 2007;129:10060. doi: 10.1021/ja072505w.
- 20.For a report on regioselective isomerization of allenylcyclopropane to cyclopentene, see: Hayashi M, Ohmatsu T, Meng YP, Saigo K. Angew Chem. 1998;110:877–879. doi: 10.1002/(SICI)1521-3773(19980403)37:6<837::AID-ANIE837>3.0.CO;2-R.Angew Chem Int Ed. 1998;37:837–839. doi: 10.1002/(SICI)1521-3773(19980403)37:6<837::AID-ANIE837>3.0.CO;2-R.
- 21.For discussions on regioselectivity of vinyl cyclopropane in metal-catalyzed cycloadditions, see: Wender PA, Dyckman AJ, Husfeld CO, Kadereit D, Love JA, Rieck H. J Am Chem Soc. 1999;121:10442.Wender PA, Dyckman AJ. Org Lett. 1999;1:2089.Liu P, Sirois LE, Cheong PHY, Yu ZX, Hartung IV, Rieck H, Wender PA, Houk KN. J Am Chem Soc. 2010;132:10127. doi: 10.1021/ja103253d.Wender PA, Nuss J, Smith DB, Suarez-Sobrino A, Vgberg J, Decosta D, Bordner J. J Org Chem. 1997;62:4908.Trost BM, Shen HC. Org Lett. 2000;2:2523. doi: 10.1021/ol0061945.Trost BM, Shen HC. Angew Chem. 2001;113:2375–2378.Angew Chem Int Ed. 2001;40:2313–2316.Trost BM, Shen HC, Horne DB, Toste ED, Steinmetz BG, Koradin C. Chem Eur J. 2005;11:2577. doi: 10.1002/chem.200401065.
- 22.The stereochemistry of the cyclopropane ring was not completely retained in the gold-catalyzed reaction. See Ref. [17] and the following reference for details: Zou Y, Garayalde D, Wang QR, Nevado C, Goeke A. Angew Chem. 2008;120:10264–10267. doi: 10.1002/anie.200804202.Angew Chem Int Ed. 2008;47:10110.
- 23.a) Stratmann K, Moore RE, Bonjouklian R, Deeter JB, Patterson GML, Shaffer S, Smith CD, Smitka TA. J Am Chem Soc. 1994;116:9935. [Google Scholar]; b) Jimenez JI, Huber U, Moore RE, Patterson GML. J Nat Prod. 1999;62:569. doi: 10.1021/np980485t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.