Abstract
The paraventricular nucleus (PVN) of the hypothalamus has been described as the "autonomic master controller". It co-ordinates critical physiological responses through control of the hypothalamic-pituitary-adrenal (HPA)-axis, and by modulation of the sympathetic and parasympathetic branches of the central nervous system. The PVN comprises several anatomical subdivisions, including the parvocellular/ mediocellular subdivision, which contains neurones projecting to the medulla and spinal cord. Consensus indicates that output from spinally-projecting sympathetic pre-autonomic neurones (SPANs) increases blood pressure and heart rate, and dysfunction of these neurones has been directly linked to elevated sympathetic activity during heart failure. The influence of spinally-projecting SPANs on cardiovascular function high-lights their potential as targets for future therapeutic drug development. Recent studies have demonstrated pharmacological control of these spinally-projecting SPANs with glutamate, GABA, nitric oxide, neuroactive steroids and a number of neuropeptides (including angiotensin, substance P, and corticotrophin-releasing factor). The underlying mechanism of control appears to be a state of tonic inhibition by GABA, which is then strengthened or relieved by the action of other modulators. The physiological function of spinally-projecting SPANs has been subject to some debate, and they may be involved in physiological stress responses, blood volume regulation, glucose regulation, thermoregulation and/or circadian rhythms. This review describes the pharmacology of PVN spinally-projecting SPANs and discusses their likely roles in cardiovascular control.
Keywords: Blood pressure, penile erection, GABA, PVN, paraventricular nucleus, hypothalamus, cardiovascular, sympathetic, parvocellular mediocellular, neuropeptides, oxytocin, substance P, tachykinin, vasopressin, angiotensin, pharmacology.
1. INTRODUCTION
The paraventricular nucleus (PVN) of the hypothalamus is a critical regulator of numerous endocrine and autonomic functions [1-5]; Loewy [6] referred to it as being the “autonomic master controller”. Many of these autonomic functions are served by spinally-projecting neurones (Fig. 1), a number of which have been shown to be involved in cardiovascular regulation [7]. These and other spinally-projecting sympathetic pre-autonomic neurones (SPANs) in the PVN are therefore attractive targets for pharmacological manipulation of both the cardiovascular system and potentially, other autonomic systems.
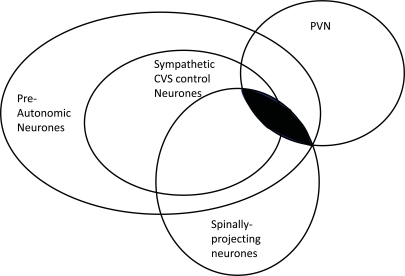
Fig. (1).
Spinally-projecting sympathetic pre-autonomic neurones of the paraventricular nucleus of the hypothalamus (PVN). The PVN consists of several sub-populations of neurones some of which project to the spinal cord. There are likely to be each combination indicated in the Venn diagram. This review focuses on the shaded area; spinally-projecting sympathetic pre-autonomic neurones (spinallyprojecting SPANs) of the PVN. Many of these target the cardiovascular system (CVS).
The cardiovascular system is subject to numerous levels of control. Although the basic functions of the heart and blood vessels are controlled locally, the whole system is centrally regulated by the brain [8, 9]. Part of the central regulation comes from the medulla, which allows rapid adaptation to changing homeostatic challenges. Anticipatory regulation, however, allows for an increase in cardiac output just prior to increased systemic oxygen demand. This was termed the “preparatory reflex” [10] and appears to be the highest level of cardiovascular control. The process is largely attributed to an increase in sympathetic activity, and was first identified by Walter Cannon [11]. This reflex is now more commonly referred to as the “fight-or-flight” response, the “stress response”, or the “defence reaction”. Inappropriate activation of this system may lead to hypertension and could account for the increased cardiac death resulting from stressors such as exercise [12], anger [13] or emotional shock [14]. Furthermore, some aspects of this pathway appear to be activated in chronic heart failure [15]. Forebrain regions involved in cardiovascular regulation were discovered early in the 20th century by stimulation or ablation of specific areas [16, 17]. Later, several areas of the hypothalamus (Fig. 2) were shown to be of particular importance [e.g., 18, 19, 20]. These include areas we now know as the PVN and the dorsomedial hypothalamus (DMH), although both were poorly defined at the time. The PVN comprises approximately 21,500 neurones, approximately three quarters of which make up the parvocellular subdivision [21], which includes both spinally-projecting SPANs and hypothalamic-pituitary-adrenal (HPA) axis control neurones [3, 6, 22].
Estimates suggest that up to 2000 neurones project directly from the PVN to the intermediolateralis (IML) of the spinal cord [23-25], and terminate close to sympathetic pre-ganglionic neurones [26, 27] and are, in this review, collectively termed spinally-projecting sympathetic pre-autonomic neurones (spinally-projecting SPANs). SPAN cell bodies are found most densely in a region of the PVN termed the parvocellular subnucleus, particularly in a subdivision referred to by some authors as the mediocellular region [21].
Spinally-projecting SPANs are strongly implicated in cardiovascular control [7, 28-30], but their primary role is unknown (Fig. 3). Several hypotheses have been proposed such as; circadian regulation of blood pressure [31], blood-volume regulation [24, 25] and the cardiovascular response to psychological stress [32]. Furthermore, elevated sympathetic activity is associated with congestive heart failure [15, 33, 34], and may also be associated with diminished GABA sensitivity of PVN neurones [15, 35, 36]. This review will discuss the neuropharmacology and function of spinally-projecting SPANs of the PVN. In the majority of cases these experiments have focussed on control of the rat cardiovascular system, although recently, study of spinally-projecting SPANs has broadened to entirely new systems such as glucose regulation, thermoregulation and penile erection.
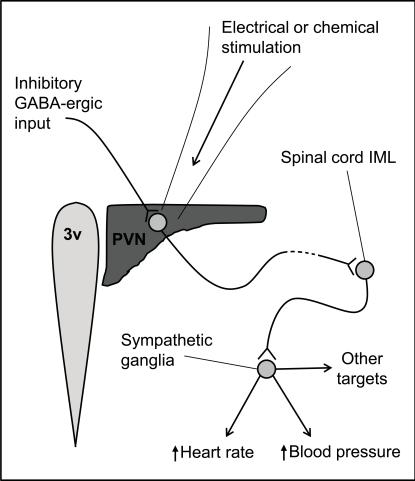
Fig. (3).
Actions of spinally-projecting SPANs originating in the paraventricular nucleus (PVN) of the hypothalamus. The PVN lies alongside the 3rd ventricle (3v), and contains a number of neurones which project to the intermediolateralis (IML) of the spinal cord. When stimulated with excitatory neurotransmitters or electrical current, they activate sympathetic pre-ganglionic neurones in the IML which, in turn, increase heart rate and blood pressure and glucose secretion. They may also increase TSH release. These PVN neurones are tonically inhibited by GABA input. Please see main text for references.
2. ELECTROPHYSIOLOGY OF SPINALLY-PROJECTING SPANS
A number of in vitro studies have investigated the electrophysiological properties of PVN neurones [37-40]. They show that parvocellular neurones (termed “PVN type II” neurones) express a slowly inactivating delayed rectifier potassium conductance. Conversely, the neurosecretory magnocellular neurones of the PVN (termed “PVN type I” neurones) appear to express a rapidly inactivating (A-type) potassium conductance. Fewer studies have been conducted on identified spinally-projecting SPANs; medulla-projecting neurones show strong inward rectification and “A-type” potassium conductance [41, 42] and spinally-projecting SPANs show a slowly inactivating potassium conductance [43]. More recent studies have also identified ATP dependent potassium channels [44, 45], which may serve to couple glucose levels to sympathetic activity. Pharmacological characterisation of the potassium channels involved is possible using potassium channels inhibitors [46-53], although confirmation requires immunohistochemical or RT-PCR approaches since most of these inhibitors lack high selectivity. When recorded from in vitro most spinally-projecting SPANs fire action potentials spontaneously [41, 54], but they are apparently quiescent in vivo [55-58]. This implies that the tonic inhibition of spinally-projecting SPANs may be, in part at least, lost in the preparation of brain-slices for in vitro recording.
3. NEUROTRANSMITTERS RELEASED BY SPANS
Discussion of the neuropharmacology of SPANs can include neurotransmitters released by the neurones and neurotransmitters acting upon them. The first of these questions has been approached by the use of retrograde/anterograde labelling, trans-synaptic tracing, immunohistochemistry and in situ hybridisation. Using these techniques it has been demonstrated that spinally-projecting SPANs express met-endorphin (20%), dynorphin (up to 40%), oxytocin (up to 40%) and vasopressin (up to 40%) [59-61]. However, a significant proportion also produce dopamine, met-enkephalin (10%), leu-enkephanlin, somatostatin, angiotensin II and atrial natriuretic peptide [62-65]. This clearly implies that some SPANs express more than one neuropeptide neurotransmitter. Interesting questions which remain unanswered include: what combinations of neurotransmitters are expressed by particular neurones, and, do different neurotransmitters serve different functions? For example, the closely related neuropeptides oxytocin and vasopressin are commonly co-expressed [see for example 66].
The anatomical evidence that vasopressin acts a SPAN neurotransmitter is supported by functional experiments in which PVN neurones were stimulated, producing an immediate increase in renal sympathetic nerve activity (rSNA) and mean arterial blood pressure [67]. However, these effects were prevented by lower thoracic intrathecal preloading with the vasopressin 1a (V1a)-receptor antagonist d(CH2)5 [Tyr(me)2,arg8vasopressin [67]. Interestingly, since oxytocin exerts effects both through the oxytocin receptor (OT-R) and through the V1a–R [68], some of the effects attributed to oxytocin may in fact be mediated by the latter. However, oxytocin is clearly important as a neurotransmitter of SPANs, since both vasopressin and oxytocin levels increase in the spinal cord when the PVN is stimulated [69]. Additionally, the tachycardia associated with PVN stimulation is reduced by the highly selective OT-R antagonists d(CH2)5[Tyr(Me)2, Orn8]-oxytocin and L-368,899 [70]. One explanation suggested for the apparent disparity of the effects of oxytocin and vasopressin antagonists is that SPAN neurotransmission in the upper thoracic spinal cord is mediated via oxytocin, but in the lower thoracic cord it is mediated by vasopressin [30].
Dopamine has also been strongly implicated as a SPAN neurotransmitter [65], but its activity is complex. Several reports demonstrate that dopamine, and its mimetic apomorphine, excite sympathetic pre-ganglionic neurones in rats (ie. the neurones that SPANs would act upon) [71-74]. However, evidence also suggests that it may serve as an inhibitor of sympathetic activity [75]. Data supporting the function of the other putative SPAN neurotransmitters described above is, so far, more disparate. For example, intrathecal glutamate antagonists reduce the increases in rSNA seen following chemical stimulation of the PVN [75] suggesting that the excitatory amino acid glutamate may act as a SPAN output neurotransmitter. However, whilst enkephalins and angiotensin II are both produced by SPANs [59, 60, 62-64] and modulate sympathetic pre-ganglionic neurones [76-78] in rats, pharmacological block of their respective receptors (at the level of the spinal cord) has not been shown directly to prevent the sympathetic effects of PVN stimulation. This may be because SPANs expressing these neurotransmitters innervate pre-ganglionic neurones targeting less well characterised sympathetic effectors in the viscera [79] or simply that appropriate experiments to detect such effects have yet to be conducted. The amino acid γ-aminobutyric acid (GABA) is the most abundant inhibitory neurotransmitter in the central nervous system [80]. Recent analysis of both retrograde and trans-neuronal labelling data shows that whilst spinally-projecting SPANs receive GABA-ergic synapses, they do not contain GABA themselves [81].
In addition to the pathways leading from the PVN to the IML thence to the heart and blood vessels there are pathways projecting from the superchiasmatic nucleus (SCN), to the thyroid and to the liver, via the PVN [82-84]. Many neurones in this pathway express thyroid stimulating hormone (TSH) [82], possibly in the PVN spinally-projecting SPANs themselves.
4. NEUROTRANSMITTERS ACTING ON SPANS
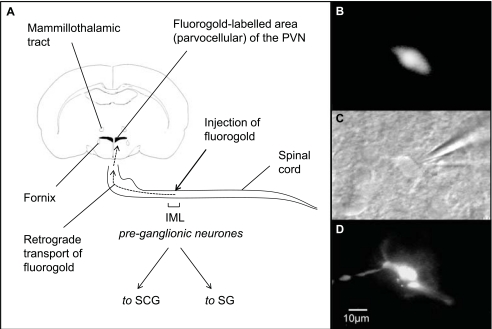
Far more studies have been published looking at the neurotransmitters expressed by SPANs than looking at neurotransmitters acting on SPANs. This is disappointing since, arguably, knowledge of the receptors expressed by a cell gives greater therapeutic potential than knowledge of the transmitters released by it. A useful approach has been the combination of retrograde labelling and patch-clamp recording. In these studies, tracer is injected into the IML of the spinal cord, and a few days later brain slices are prepared. Spinally-projecting neurones are then clearly visible and can be targeted for electrophysiological study (Fig. 4).
Fig. (4).
Methods for patch-clamping retrogradely-labelled neurones. A, the retrograde tracer fluorogold is injected into the rat intermediolateralis (IML) at level T2-T4, it is also possible to use other tracers, such as rhodamine-labelled microspheres (see Fig. 8). The IML is dense with pre-ganglionic neurones that project to the superiocervical (SCG) and stellate (SG) ganglia, and from there to the heart and blood vessels [65, 227]. The appearance of a fluorogold-labelled neurone B, prior to patch clamp recording, C, during patch-clamp, under near infrared differential interference contrast microscopy, and D, when patched with Lucifer yellow (a fluorescent dye) in the patch clamp pipette. The dye fills the neurone, and this gives re-confirmation that recording was from the appropriate cell. Reproduced from [43], with permission.
4.1. Amino Acid Neurotransmitters
A number of in vivo studies have investigated the neurotransmitters acting upon SPANs, or the receptors expressed by them, but there have been few in vitro studies on identified spinally-projecting SPANs. In vivo electrophysiological studies on cats confirmed an anticipated monosynaptic connection between the PVN and the spinal sympathetic motor area (the IML) [85]. Furthermore, electrical or chemical stimulation of the PVN was shown to generate a rapid rise of blood pressure and rSNA in conscious rats [86]. Further in vivo studies showed that PVN neurones receive tonic GABA-ergic inhibition, which maintains their spontaneous action potential discharge at relatively low rates [55, 57, 58, 87], despite additional excitatory glutaminergic input [86].
There is accumulating evidence that “tonic” inhibition is often mediated by GABA arising from extra-synaptic sources; sometimes referred to as “volume” GABA neurotransmission [88-89]. This correlates with the expression of α5-, or α6-type, and δ-type GABAA receptor subunits [90, 91]. However, this has not yet been specifically examined with spinally-projecting SPANs. It may be an important issue to explore though, since a unique pharmacological profile for extra-synaptic GABA receptors is emerging, [91-93] which may become therapeutically exploitable.
In the case of SPANs, loss of tonic inhibition would lead to an increase in sympathetic outflow. Excessive sympathetic outflow could potentially be reduced by selective activators of “volume” GABA receptors. Furthermore, the degree of tonic inhibition of sympathetic outflow is, in part at least, dependent upon the local uptake of GABA by transporter proteins, in particular GAT3 located on glial cells [94]. Therefore, there are a number of means by which parvocellular GABA inhibition could be targeted with the aim of reducing sympathetic outflow and blood pressure.
Excitation of the PVN by either glutamate or the GABAA receptor antagonist bicuculline increases blood pressure and heart rate, due to the tonic silence of SPANs [55, 56, 86, 87, 95]. This has led to many in vitro studies investigating control of SPANs to focus on the role of GABA. In fact, a number of studies have demonstrated the presence of GABAA receptor currents in the PVN [38, 87, 96-100]. The parvocellular region of the PVN, which contains the majority of SPANs, expresses a high density of GABAA α2 –subunits [101]; this was also seen in retrogradely labelled spinally-projecting SPANs [102]. Additional studies have shown spinally-projecting parvocellular neurones to be inhibited by GABA [31, 54, 103-104], as predicted by the earlier in vivo work. Interpretation of both the in vivo and in vitro work is further complicated by some frequently overlooked variables. Firstly, there is little consideration of the role of pre-synaptic GABA receptors. These are typically of the GABAB receptor subtype and have been shown to inhibit both inhibitory and excitatory input to spinally-projecting SPANs [105]. Secondly, the primary GABAA antagonist used to investigate possible GABA involvement is bicuculline or bicuculline methiodide, both of which are potassium channel blockers [106-108]. The effect of potassium channel inhibition would appear very similar to block of GABAA receptors; excitation.
Neuroactive steroids and nitric oxide (NO) are well known modulators of GABAA receptors. In the PVN, for example, tetrahydrodeoxycorticosterone (THDOC) [103] modulates spinally-projecting SPANs and NO modulates rostral ventrolateral medulla (RVLM)-projecting SPANs [109]. Both effects are mediated by modulation of GABAA receptors [110]. However, other steroid hormone receptors, such as oestrogen β-receptors [111], are expressed by SPANs, which raises the possibility that some steroids may modulate sympathetic outflow by this mechanism. Other mechanisms may also be involved, for example cortisol is known to activate SPANs at high doses via an inhibition of potassium channels [100].
SPANs are excited by the excitatory amino acid neurotransmitter glutamate [31], mediated by the activation of at least two fast ionotropic glutamate conductances. This is carried largely by α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors (i.e., sensitive to 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione, NBQX, an AMPA receptor antagonist), but with an additional component of N-methyl D-aspartate (NMDA) conductance (i.e., sensitive to the classical NMDA antagonist amino-5-phosphonovaleric acid, AP-5, [31]).
Combined electrophysiological and microdialysis studies on RVLM-projecting PVN neurones have shown that at least some of their glutaminergic input is also tonically inhibited by GABAA-receptors. Since glutaminergic neurotransmission is ubiquitous within the central nervous system, there has been particular recent focus on non-glutaminergic excitation of SPANs. This is because antagonism of the relatively sparse neuropeptide receptors could become therapeutically useful when action of amino acid receptor antagonists proves to be too widespread.
4.2. Neuropeptide Neurotransmitters
Leptin [112], angiotensin II (AGII) [113, 114], neuropeptide W [115] and each of the endogenous tachykinins [54] have all been investigated as pharmacological regulators of spinally-projecting SPANs. The strongest candidates appear to be AGII and the tachykinin substance P (SP), with both neuropeptides acting indirectly via GABA neurotransmission [54, 114].
4.2.1. Angiotensin II
The neuropeptide AGII, expressed by PVN neurones [116], acts at two principal receptors: AT1 and AT2. AT1 is the more abundant of the two [117-118] and, in rodents, is encoded by two genes: AT1A and AT1B [119]. However, it is not clear whether both of these subtypes exist in humans, nor which subtype is more important for cardiovascular control in rodents. It has been suggested that AT1B is more important for control of blood pressure in mice [120], but in situ hybridisation studies show that AT1A is expressed more richly in the parvocellular region of the rat PVN [121], the region particularly important for autonomic control. Integrative experiments showed that AGII injection into the PVN gave an AT1-dependent increase in blood pressure [122], and single-unit recording studies of antidromically-identified neurones demonstrated that this involved spinally-projecting SPANs [123]. Later, the same group demonstrated inhibition of A-type potassium currents by AGII, acting via an AT1 receptor [124]. Whilst AGII directly modulates medulla-projecting PVN neurones [125] to elevate blood pressure, spinally-projecting SPANs do not express AT1 receptors [113]. Therefore, this implies that AGII activates SPANs indirectly, and this was investigated using whole-cell recording from retrogradely labelled spinally-projecting SPANs [114]. In these experiments, AT1 receptors were found to co-localise with synaptophysin (a pre-synaptic marker protein). In addition, AGII decreased the frequency and amplitude of spontaneous inhibitory post-synaptic potentials in SPANs. This strongly suggests that AGII excites spinally-projecting SPANs by reducing the tonic GABA-ergic input.
4.2.2. Tachykinins
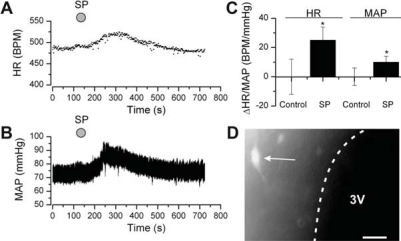
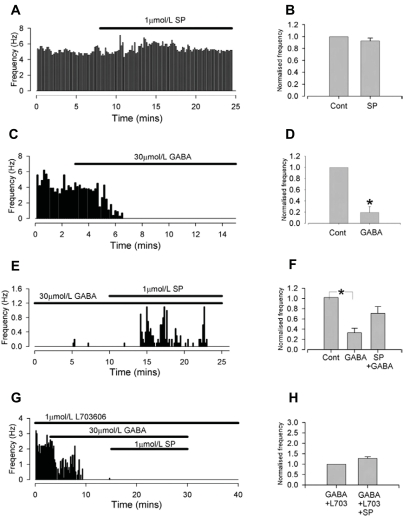
The tachykinin family of neuropeptides, which includes substance P (SP), neurokinin A (NKA) and neurokinin B (NKB), is involved in central cardiovascular control [126]. Studies have shown high levels of tachykinin receptor expression within the PVN [127-129] and in our own studies we showed that injection of SP into the PVN increases heart rate, blood pressure (Fig. 6) and rSNA [54]. These effects correlated with our observation of a strong inhibition of SPAN GABAA receptors by SP [54]. Activation of SPANs by SP in brain slice experiments was only apparent when neurones were first inhibited by GABA (Fig. 5).
Fig. (6).
Substance P (SP) injected into the PVN elicits a rise in heart rate and blood pressure. Heart rate and blood pressure were recorded in anaesthetised adult rats. Representative records of A, heart rate and B, blood pressure during intra-PVN injection of SP/FITC-SP (solution of SP and FITC-conjugated SP). C, mean data from a number of experiments. D, epifluorescent image of the medial PVN, after SP/FITC-SP intra-PVN injection. “3V” indicates the third ventricle; the white arrow indicates a parvocellular neurone labelled with FITCSP. Scale bar is 50µm. “SP” indicates SP/FITC-SP injection. Reproduced from [54], with permission.
Fig. (5).
Substance P (SP) increases the rate of action potential discharge of individual neurones in brain slices in the presence of GABA. Brain slices were taken from adult rats, and action potentials recorded from single neurones in the PVN. A, representative results showing SP applied to SPANs under control conditions; B, mean results, showing SP alone has no effect on rate of action potential discharge. C, representative results showing application of GABA; D, mean results showing that GABA inhibits spontaneous action potential frequency. E, representative results showing SP added during GABA inhibition; F, mean results showing that SP increases the rate of action potential discharge against a background of tonic GABA inhibition. G, Representative results showing SP interaction with NK1 antagonism; H, mean results showing the effects of SP are abolished by the selective NK1 antagonist L703606. Cont = control. Reproduced from [54], with permission.
All three of the principal mammalian tachykinin receptors (NK1, NK2 and NK3) are 7-transmembrane G-protein coupled receptors, although they act through a range of intracellular pathways, typically via pertussis toxin independent G-proteins [130, 131]. Of particular interest is that NK1 receptors activate protein kinase C (PKC) [131], and that PKC-dependent phosphorylation of GABAA can inhibit GABAA currents [132]. This was first shown using cultured cells expressing recombinant GABAA subunits. However, SPAN activation by SP in the PVN was also found to be both PKC and NK1 receptor dependent (Figs. 5 and 7) [54]. No effects of NKA or NKB agonists were observed (NKA4-10 and senktide, respectively). An interesting question remains; from where do the tachykinin inputs to SPANs arise? Of note, several intra-hypothalamic tachykinin pathways terminate in the PVN [133]. Furthermore, in brain slice experiments we found that SPAN activation following glutamate stimulation of the DMH was inhibited by tachykinin receptor antagonists [134]. This may prove significant since there is evidence that both the DMH and the PVN are important in the control of the cardiovascular system [135].
Fig. (7).
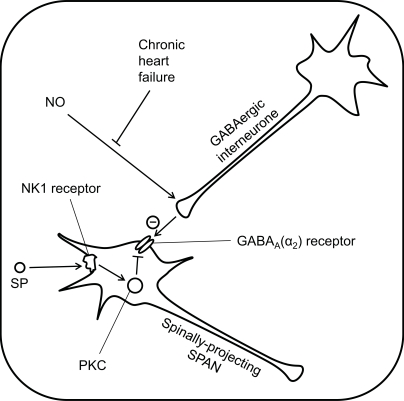
Pharmacological control of SPANs within the PVN. Spinally-projecting SPANs are tonically inhibited by GABA input, which is normally enhanced by nitric oxide (NO). However, during chronic heart failure, this potentiation of GABA is lost, leading to an overall increase in sympathetic activity. Substance P (SP) causes indirect activation of spinally-projecting SPANs by inhibition of the inhibitory GABAA receptors, via a protein kinase C (PKC)-dependent pathway.
4.2.3. Vasoctive Intestinal Peptide (VIP) and Pituitary Adenylcyclase Activating Peptide (PACAP)
Retrograde labelling evidence has demonstrated the presence of vasoctive intestinal peptide/pituitary adenylcyclase activating peptide receptors type 2 (VPAC2) on spinally-projecting SPANs [136]. PACAP(1 to 38) induced c-fos expression in PVN spinally-projecting neurones and this was abolished by a VPAC2 receptor antagonist [136]. It seems likely, from functional and viral tracing studies that these neurones are also activated by thyroid hormone (T3) [137], although receptors have not yet been identified specifically on the PVN spinally-projecting SPANs. Both the VPAC2 receptors and T3 nuclear receptors (TR) appear to be involved with control of endogenous glucose production by the liver, see below for further discussion [136, 137].
4.3. Other Receptors on Spinally-Projecting SPANs
Purinergic receptors were originally classed as either P1 or P2 subtypes [138]. The P1 receptors are now termed adenosine receptors, Ax and are seven transmembrane G-protein couple receptors [139]. The P2 receptors are the ligand-gate cation channels activated by nucleotides [139]. Examples of both families of receptors have now been identified on PVN SPANs [45, 140], but only P1 on identified spinally-projecting SPANs [45]. Adenosine is inhibitory to several types of muscle cell, by acting via A1 receptors and KATP channels [141-143]. Li et al. [45] has now established that adenosine acts on spinally-projecting SPANs in a similar manner. P2X receptors have been identified on RLVM-projecting SPANs [140], but it remains to be seen if these receptors are also expressed on spinally-projecting SPANs. There are little data supporting the presence of other receptors on spinally-projecting SPANs. Price et al. [115] used multiplex single-cell RT-PCR experiments in combination with whole-cell recording in brain slices to show inhibition of SPAN neurones by neuropeptide W. This study did not use retrograde labelling to identify SPANs, but rather electrophysiological “finger-printing” and post-hoc identification of neuronal expression of vasopressin or oxytocin. Neuropeptide W is thought of as being a regulator of feeding behaviour (see for example [144]) and in this case was found to be acting through the neuropeptide W type 1 (NPBW1) receptor.
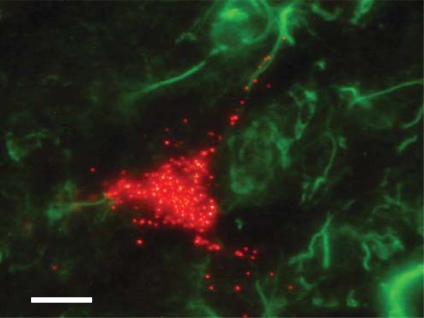
Receptors for neuropeptide FF are densely expressed in the PVN and excite both magnocellular [145] and parvocellular [146] PVN neurones. These include neurones that project to the nucleus of the solitary tract [147], but there has been no investigation of their effects specifically on spinally-projecting SPANs. Evidence also suggests that leptin [148-150] may influence SPANs of the PVN. The leptin receptor (OB-R) is highly expressed in the PVN [151], and PVN neurones are depolarised by leptin [152]. However, leptin does not increase c-fos expression in spinally-projecting SPANs [112], suggesting that the PVN neurones being activated by leptin are not SPANs. Indeed, our own immunohisto-chemical data show that OB-R is expressed by neurones immediately surrounding SPANs (unpublished data, (Fig. 8)), but not by the spinally-projecting SPANs themselves. Orexin has also been shown to depolarise neurones within the PVN, but again, this has not been shown specifically with SPANs [153]. It has been suggested that other neurotransmitters such as adrenomedullin [154] may also activate spinally-projecting SPANs, however, this has not yet been shown directly.
Fig. (8).
Spinally-projecting SPANs do not co-localise with leptin receptor (OB-R) in rat PVN. Spinally-projecting SPANs were labelled with rhodamine beads (red) using retrograde labelling (see Fig. 2) in an adult rat. A 40µm cryo-slice of the PVN was counter-stained for OB-R (green), using primary goat anti-OB-R IgG, Santa Cruz biotechnology; secondary rabbit anti-goat, Jackson. Scale bar is 10µm. Unpublished data.
5. PHYSIOLOGICAL IMPLICATIONS OF SPAN ACTIVITY
Spinally-projecting SPANs of the PVN influence the cardiovascular system [7, 155] (Fig. 3), and there is clear evidence that the functioning of these neurones is altered during heart failure [15, 35]. However, the role of these neurones in normal physiology remains controversial. There is evidence PVN SPANs are involved in physiological functions as diverse as stress, thermoregulation and penile erection.
5.1. Involvement with Stress Response
In addition to the clear increases in blood pressure observed with physical activity [156], the simple act of anticipating exercise [157] or performing mental arithmetic [158-159] is sufficient to produce a cardiovascular response. In excess, this preparatory reflex leads to increases in heart rate and blood pressure of a pathological nature [160-162]. Duan et al. showed that the stress “phenotype” could be reproduced by stimulation of the PVN [163] and non-specific chemical inhibition of the PVN prevents it [164]. Certainly, the PVN is central to the integration of the hormonal response to stress [5] and it also well known that stimulation of SPANs in the PVN exerts a powerful influence on blood pressure and heart rate and sympathetic nerve activity [55-57, 86, 95, 165-167].
In vivo experiments suggest that tachykinins play a major role in cardiovascular control and the expression of a cardiovascular stress response [126]. For example, SP is found at high levels within the PVN [168], and central tachykinins increase efferent sympathetic nerve activity [54, 169]. Furthermore, injection of tachykinin agonists into the hypothalamus [126, 170], and specifically the PVN [54, 171, 172], leads to rapid elevation of heart rate and blood pressure (Fig. 6). Crucially, central administration of tachykinin antagonists reduces the elevation of blood pressure and heart rate resulting from psychological stress of conscious rats [173]. Trans-synaptic retrograde labelling experiments show a direct coupling between the stellate ganglion (which supplies sympathetic motor neurones to the heart) and the PVN [32], implicating PVN SPANs in control of the stress response. In fact PVN SPANs were included in the set of “central command neurones” identified by Jansen et al. [32], and inferred from the work of Walter B. Cannon in the early 1900s [174].
However, some scientists now reject this idea; several lines of evidence suggest that whilst the PVN is activated by stress, and stimulation of the PVN elicits increases in blood pressure and heart rate, it is the DMH which is important for the cardiovascular response to stress [135]. This conjecture is based on the observation that injection of the GABAA antagonist bicuculline into the DMH prevents the cardiovascular response to air-jet stress, whereas injection into the PVN does not [175]. Whether this PVN SPAN-independent cardiovascular response to stress is general, or stress paradigm-specific, remains to be seen. Furthermore, since the activation of SPANs by SP involves the relief of GABAA inhibition [54], inhibition of the PVN by GABA may not be the ideal means of testing this hypothesis. Non-specific PVN lesion experiments such as Busnardo and colleagues’ recent work [164], or specific block of spinally-projecting SPAN neurotransmitter receptors may resolve the controversy.
5.2. Control of Blood Volume
It has been suggested that spinally-projecting PVN neurones may be involved in the response to changes in blood volume [24, 176, 177]. This can, to a certain extent, be differentially regulated from blood pressure, by the cardiovascular system. The atrial/venous side of the cardiovascular system is relatively compliant and “expands” to accommodate increased blood volume with relatively little change in pressure; small decreases in volume are replenished from capacitance vessels [178]. Activation of veno-atrial stretch receptors by inflation of small balloons activates afferents and results in a reflex tachycardia (equivalent to the Bainbridge reflex) [179]. Similar results can be obtained by modest changes in blood volume, which alters veno-atrial stretch receptor activity without substantially changing blood pressure. For example, removal of 2mls of blood from adult rats (~10% blood volume, by arterial cannula) has no effect on mean blood pressure, but does increase c-fos expression in PVN spinally-projecting SPANs [180]. Similar results are seen in the rat water deprivation model of Toney and colleagues [181] and cardiovascular responses to water deprivation are reduced by GABA inhibition of the PVN [182]. Combined, these data strongly support the hypothesis that spinally-projecting SPANs are involved with the control of blood volume.
5.3. Circadian Rhythm
Human blood pressure typically follows a distinct circadian rhythm, dipping overnight and rising in the morning [183]. It is notable that this rise occurs before waking up [184]. In some cases, hypertension is characterised by not just elevated blood pressure, but by a disturbed circadian pattern of blood pressure. Specifically, the usual night time blood pressure decrease fails to occur [185, 186]. This disturbance to the normal circadian cardiovascular pattern may contribute to the statistically higher number of cardiovascular deaths occurring first thing in the morning [187, 188]. Circadian rhythm is largely controlled at the level of the hypothalamus and, in particular, the suprachiasmatic nucleus (SCN) [189], which expresses a number of clock gene products [190, 191]. There are strong excitatory and inhibitory pathways from the SCN to the PVN of rats [99, 192], and, specifically, to spinally-projecting SPANs [31]. It is therefore possible that spinally-projecting SPANs play a role in circadian control of blood pressure.
5.4. Penile Erection
The PVN is involved with penile erection in rats [193]. Oxytocinergic PVN neurones, which have a positive effect on penile erection, project to the hippocampus, the medulla and, most importantly, the spinal cord (For reviews see [194, 195, 196]). A retrograde tracing study using pseudorabies virus has shown central nervous system (CNS) innervations of the penis to include sympathetic pre-ganglionic neurones and the PVN, specifically parvocellular neurones in the PVN [197]. From here, it appears likely that continuation of the sympathetic pathway involves serotonin and/or dopaminergic neurotransmission in the lower spinal cord [198, 199].
5.5. Glucose Control
Glucose-sensing by the brain requires input from a number of sensors, including the liver, carotid body and small intestine, as well as glucose-sensitive neurones in the brain [reviewed by 200]. Direct stimulation of the PVN by microinjection of glucose into the PVN causes an increase in sympathetic stimulation to brown adipose tissue (BAT) [201]. More indirect stimulation by peripheral glucose injection also activates the PVN, as shown by increased c-fos expression, particularly in identified SPANs [202, 203]. In brain slice experiments, SPANs are modulated by hypoglycaemia [44], but the mechanisms of this still need to be determined. Blood glucose levels are heavily regulated by the liver, which is under a degree of control by the autonomic nervous system. This includes sympathetic innervations projecting from the PVN [82, 84, 136, 204], which have been shown to alter blood glucose levels by influencing the liver [83, 137].
5.6. Regulation of Body Fat
Two papers have shown that PVN neurones project to brown adipose tissue (BAT) via spinally-projecting sympathetic nerves [205, 206]. In both cases, pseudorabies virus was injected into interscapular brown adipose tissue and, after a number of days, immunostaining for the virus could be found in the IML and the PVN. Although this demonstrates a connection from the PVN to BAT, the mechanism remains unclear. Pharmacological stimulation of the PVN by microinjection of stimulatory amino acids has been shown to produce differing effects. Amir [207] showed an increase in sympathetic outflow to BAT, Yoshimatsu and colleagues [208] found small decreases in sympathetic activity when the anorexigenic peptide cholecystokinin (CCK) was injected into the PVN, but showed a mainly a stimulatory response to injected glutamate [209]. Conversely, Madden and Morrison [210] showed an inhibitory effect. Obviously there is a potential involvement of SPANs with regulation of body fat, but the details remain unclear.
5.7. Thermoregulation
Thermosensitivity of neurones in the PVN was first shown by Inenaga et al. [211]. Poly-synaptic viral tracing studies in rats show that PVN SPANs are directly involved in thermoregulation [212]. Since then the region has been shown to be critical in thermoregulation [213-214]. Projections from the PVN to the RVLM, which contains sympathetic pre-motor neurones, are activated following heat exposure [215]. In addition, heat exposure activates spinally-projecting PVN neurones [216]. Whether this involves a simple vasomotor responses to elevated temperature (e.g., sympathetic vasodilation of skin in humans [217] or tail in the case of rodents [212]) or some other process is unknown. The PVN also has an effect on thermogenesis, via innervations of brown adipose tissue (see above).
5.8. Involvement with Heart Failure
Whilst chronic heart failure could not be described as a function of SPANs, the relationship between NO, GABA and SPAN activity is fundamental to the development of elevated sympathetic activity seen in human patients and animal models of heart failure [15, 33-34, 218]. NO is now well established as a neuromediator in the CNS where it is synthesised by neuronal nitric oxide synthase (nNOS) [reviewed by 219, 220]. nNOS is abundantly and constitutively expressed throughout the brain, but particularly in cells of the PVN; this includes expression by pre-autonomic neurones projecting both to the IML [221] and the RVLM [222, 223]. NO appears to increase the tonic inhibition of SPANs [224] by facilitating pre-synaptic release of GABA [223]. In models where chronic heart failure has been experimentally induced in rats, there are decreased levels of nNOS expression in the PVN [225], together with evidence of reduced neuronal activity [218]. Furthermore, the degree of tonic inhibition of rSNA by GABA (albeit assessed with bicuculline) was reduced in the chronic heart failure animals [35]. When combined these data strongly suggest that the tonic inhibition of sympathetic activity is maintained by an NO-dependent local release of GABA, and that chronic heart failure leads to a loss of NO activity, and thus to a loss of inhibitory control of sympathetic neurones [36] (Fig. 7). It has recently been shown that AT1 receptor mRNA expression is increased in the PVN and, consequently, intra-PVN injection of AGII is more effective at increasing rSNA in the rat chronic heart failure model [226]. Further experiments are required to determine if the increase in angiotensin activity is causal, coincident or consequent to the decreased nNOS expression in the PVN.
6. CONCLUSIONS
Several studies have investigated the function and pharmacology of spinally-projecting SPANs in the PVN but, interestingly, we still know a great deal more about the expression of their neurotransmitters than anything else. There is relatively sparse information concerning which receptors are expressed by SPANs; GABAA, glutamate, adenosine A1 and NK1 receptors are all strong candidates, and SPANs can be indirectly modulated by NO and AGII. In terms of the modulation of SPANs, tonic inhibition is a recurrent theme and clearly central to their regulation. For example, NO, AGII and SP all appear to act by modulating this GABA tone. These neurones modulate the cardiovascular system, and it seems likely that they have other functions too. So a big question seems to be: what is the primary role of these neurones in cardiovascular function? There is evidence they become involved in the pathological increase in sympathetic activity associated with chronic heart failure, but this still leaves the function in normal, healthy animals unknown. Putative physiological functions include: volume regulation, glucose control, circadian rhythm and response to stress. Perhaps PVN SPANs play a role in all of these. The final question would then be, do the same neurones play a role in each of these functions, or does a mixed population of SPANs exist, each with a different receptor and neurotransmitter profile and each with a separate function? A great many further experiments will be required to finally answer these fascinating questions.
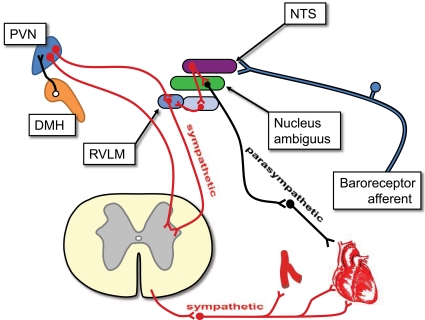
Fig. (2).
Central cardiovascular control in the rat. The cardiovascular system is largely controlled by areas of the brainstem; however, there is also strong evidence that centres in the forebrain, including the PVN, exert “top-level” control via both medulla-projecting and spinally-projecting SPANs. Afferents from carotid baroreceptors ascend via cranial nerve IX (the glossopharangeal nerve), while afferents from the aortic bodies ascend via cranial nerve X (the vagus nerve). These afferents are processed by the nucleus of the solitary tract (NTS). Parasympathetic drive emanates from the nucleus ambiguus, whilst sympathetic drive emanates from a number of locations, in particular the rostroventrolateral medulla. The role of the medulla in cardiovascular control is reviewed by [9].
ABBREVIATIONS
- 3V
= 3rd ventricle
- AGII
= Angiotensin II
- AMPA
= α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP-5
= Amino-5-phosphonovaleric acid
- AT1
= Angiotensin II type 1 receptor
- AT2
= Angiotensin II type 2 receptor
- BAT
= Brown adipose tissue
- CCK
= Cholecystokinin
- CNS
= Central nervous system
- CVS
= Cardiovascular system
- DMH
= Dorsomedial hypothalamus
- FITC
= Fluorescein isothiocyanate
- GABA
= γ-aminobutyric acid
- HPA
= Hypothalamic-pituitary-adrenal
- IML
= Intermediolateralis
- NBQX
= 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione
- NKA
= Neurokinin A
- NKB
= Neurokinin B
- NMDA
= N-methyl D-aspartate
- nNOS
= Neuronal nitric oxide synthase
- NO
= Nitric oxide
- NTS
= Nucleus of the solitary tract
- OB-R
= Leptin receptor
- OT-R
= Oxytocin receptor
- PACAP
= Pituitary adenylcyclase activating peptide
- PKC
= Protein kinase C
- rSNA
= Renal sympathetic nerve activity
- RT-PCR
= Reverse transcriptase PCR
- RVLM
= Rostral ventrolateral medulla
- SCG
= Superiocervical ganglion
- SCN
= Suprachiasmatic nucleus
- SG
= Stellate ganglion
- SP
= substance P
- SPAN
= Sympathetic pre-autonomic neurone
- T3
= Thyroid hormone
- THDOC
= Tetrahydrodeoxycorticosterone
- TSH
= Thyroid stimulating hormone
- TR
= Thyroid hormone receptor
- V1a-receptor
= Vasopressin 1a receptor
- VIP
= Vasoctive intestinal peptide
- VPAC2
= Vasoctive intestinal peptide/pituitary adenylcyclase activating peptide receptors type 2
REFERENCES
- 1.Swanson LW, Sawchenko PE, Wiegand SJ, Price JL. Separate neurons in the paraventricular nucleus project to the median-eminence and to the medulla or spinal-cord. Brain Res. 1980;198:190–195. doi: 10.1016/0006-8993(80)90354-6. [DOI] [PubMed] [Google Scholar]
- 2.Palkovits M. Interconnections between the neuroendocrine hypothalamus and the central autonomic system. Front. Neuroendocrinol. 1999;20:270–295. doi: 10.1006/frne.1999.0186. [DOI] [PubMed] [Google Scholar]
- 3.Swanson L, Kuypers H. The paraventricular nucleus of the hypothalamus: Cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J. Comp. Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 4.Swanson LW, Sawchenko PE. Hypothalamic integration - organization of the paraventricular and supraoptic nuclei. Ann. Rev. Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 5.Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 6.Loewy A. Forebrain nuclei involved in autonomic control. Prog. Brain Res. 1991;87:253–0. doi: 10.1016/s0079-6123(08)63055-1. [DOI] [PubMed] [Google Scholar]
- 7.Coote JH. Landmarks in understanding the central nervous control of the cardiovascular system. Exp. Physiol. 2007;92:3–18. doi: 10.1113/expphysiol.2006.035378. [DOI] [PubMed] [Google Scholar]
- 8.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 9.Spyer KM. Annual-review prize lecture - central nervous mechanisms contributing to cardiovascular control. J. Physiol-(London) 1994;474:1–19. doi: 10.1113/jphysiol.1994.sp019997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilton SM. The defense-arousal system and its relevance for circulatory and respiratory control. J. Exp. Biol. 1982;100:159–174. doi: 10.1242/jeb.100.1.159. [DOI] [PubMed] [Google Scholar]
- 11.Cannon WB. Bodily changes in pain, hunger, fear and rage. 2nd. New York: Appleton; 1929. [Google Scholar]
- 12.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial-infarction by heavy physical exertion - protection against triggering by regular exertion. N. Engl. J. Med. 1993;329:1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 13.Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H, Muller JE. Triggering of acute myocardial-infarction onset by episodes of anger. Circulation. 1995;92:1720–1725. doi: 10.1161/01.cir.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 14.Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. New Engl. J.Med. 1996;334:413–419. doi: 10.1056/NEJM199602153340701. [DOI] [PubMed] [Google Scholar]
- 15.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail.Rev. 2000;V5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- 16.Woodworth R, Sherrington C. A pseudaffective reflex and its spinal path. J. Physiol. 1904;31:234. doi: 10.1113/jphysiol.1904.sp001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bard P. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. Am. J. Physiol. 1928;84:490. [Google Scholar]
- 18.Bard P. Anatomical organization of central nervous system in relation to control of heart and blood vessels. Physiol. Rev. 1961;40:3–26. [PubMed] [Google Scholar]
- 19.Keller AD. Ablation and stimulation of hypothalamus - circulatory effects. Physiol. Rev. 1961;40:116–120. [PubMed] [Google Scholar]
- 20.Delgado J. Circulatory effects of cortical stimulation. Physiol. Rev. Suppl. 1960;4:146. [PubMed] [Google Scholar]
- 21.Kiss J, Martos J, Palkovits M. Hypothalamic paraventricular nucleus: A quantitative analysis of cytoarchitectonic subdivisions in the rat. J. Comp. Neurol. 1991;313:563–573. doi: 10.1002/cne.903130403. [DOI] [PubMed] [Google Scholar]
- 22.Koutcherov Y, Mai J, Ashwell K, Paxinos G. Organization of the human paraventricular hypothalamic nucleus. J. Comp. Neurol. 2000;423:299–318. [PubMed] [Google Scholar]
- 23.Shafton A, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998;801:239–243. doi: 10.1016/s0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- 24.Lovick T, Malpas S, Mahony M. Renal vasodilatation in response to acute volume load is attenuated following lesions of parvocellular neurones in the paraventricular nucleus in rats. J. Auton. Nerv. Syst. 1993;43:247–255. doi: 10.1016/0165-1838(93)90331-n. [DOI] [PubMed] [Google Scholar]
- 25.Pyner S, Coote J. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- 26.Motawei K, Pyner S, Ranson RN, Kamel M, Coote JH. Terminals of paraventricular spinal neurones are closely associated with adrenal medullary sympathetic preganglionic neurones: Immunocytochemical evidence for vasopressin as a possible neurotransmitter in this pathway. Exp. Brain Res. 1999;126:68–76. doi: 10.1007/s002210050717. [DOI] [PubMed] [Google Scholar]
- 27.Ranson RN, Motawei K, Pyner S, Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp. Brain Res. 1998;120:164–172. doi: 10.1007/s002210050390. [DOI] [PubMed] [Google Scholar]
- 28.Coote JH. Cardiovascular function of the paraventricular nucleus of the hypothalamus. Biol. Signals. 1995;4:142–149. doi: 10.1159/000109434. [DOI] [PubMed] [Google Scholar]
- 29.Badoer E. Cardiovascular role of parvocellular neurons in the paraventricular nucleus of the hypothalamus. News Physiol. Sci. 1996;11:43–47. [Google Scholar]
- 30.Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: Implications for cardiovascular regulation. J. Chem. Neuroanat. 2009;38:197–208. doi: 10.1016/j.jchemneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Cui LN, Coderre E, Renaud LP. Glutamate and gaba mediate suprachiasmatic nucleus inputs to spinal-projecting paraventricular neurons. Am. J. Physiol-Regul. Integr. Comp. Physiol. 2001;281:R1283–R1289. doi: 10.1152/ajpregu.2001.281.4.R1283. [DOI] [PubMed] [Google Scholar]
- 32.Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: Basis of the fight-or-flight response. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson DW, Berg WJ, Sanders JS. Clinical and hemodynamic correlates of sympathetic-nerve activity in normal humans and patients with heart-failure - evidence from direct microneurographic recordings. J. Am. Coll. Cardiol. 1990;16:1125–1134. doi: 10.1016/0735-1097(90)90544-y. [DOI] [PubMed] [Google Scholar]
- 34.Vahid-Ansari F, Leenen FHH. Pattern of neuronal activation in rats with chf after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 1998;44:H2140–H2146. doi: 10.1152/ajpheart.1998.275.6.H2140. [DOI] [PubMed] [Google Scholar]
- 35.Zhang K, Li YF, Patel KP. Reduced endogenous gaba-mediated inhibition in the pvn on renal nerve discharge in rats with heart failure. Am. J. Physiol. Regul. Integ. Comp. Physiol. 2002;282:R1006–R1015. doi: 10.1152/ajpregu.00241.2001. [DOI] [PubMed] [Google Scholar]
- 36.Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: The altered inhibitory mechanisms. Acta Physiol. Scand. 2003;177:17–26. doi: 10.1046/j.1365-201X.2003.01043.x. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman NW, Tasker JG, Dudek FE. Immunohistochemical differentiation of electrophysiologically defined neuronal populations in the region of the rat hypothalamic paraventricular nucleus. J. Comp. Neurol. 1991;307:405–416. doi: 10.1002/cne.903070306. [DOI] [PubMed] [Google Scholar]
- 38.Tasker JG, Dudek FE. Local inhibitory synaptic inputs to neurones of the paraventricular nucleus in slices of rat hypothalamus. J. Physiol. (Lond) 1993;469:179–192. doi: 10.1113/jphysiol.1993.sp019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luther JA, Halmos KC, Tasker JG. A slow transient potassium current expressed in a subset of neurosecretory neurons of the hypothalamic paraventricular nucleus. J. Neurophysiol. 2000;84:1814–1825. doi: 10.1152/jn.2000.84.4.1814. [DOI] [PubMed] [Google Scholar]
- 40.Luther JA, Tasker JG. Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J. Physiol-London. 2000;523:193–209. doi: 10.1111/j.1469-7793.2000.t01-1-00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J. Physiol-London. 2001;537:161–177. doi: 10.1111/j.1469-7793.2001.0161k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sonner PM, Stern JE. Role of a-type potassium currents on the excitability and firing activity of rvlm-projecting pvn neurons. FASEB J. 2005;19:A599–A599. [Google Scholar]
- 43.Barrett-Jolley R, Pyner S, Coote JH. Measurement of voltage-gated potassium currents in identified spinally- projecting sympathetic neurones of the paraventricular nucleus. J. Neurosci. Methods. 2000;102:25–33. doi: 10.1016/s0165-0270(00)00271-5. [DOI] [PubMed] [Google Scholar]
- 44.Lewis R, Mills AF, Barrett-Jolley R. Models of paraventricular nucleus (pvn) sympathetic neurone modulation by glucose and hypoglycaemia. Biophys. J. 2010;98:140a–140a. [Google Scholar]
- 45.Li DP, Chen SR, Pan HL. Adenosine inhibits paraventricular pre-sympathetic neurons through atp-dependent potassium channels. J. Neurochem. 2010;113:530–542. doi: 10.1111/j.1471-4159.2010.06618.x. [DOI] [PubMed] [Google Scholar]
- 46.Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003;44:1–7. doi: 10.1016/s0028-3908(02)00339-8. [DOI] [PubMed] [Google Scholar]
- 47.Kirsch GE, Narahashi T. 3, 4-diaminopyridine - potent new potassium channel blocker. Biophys. J. 1978;22:507–512. doi: 10.1016/S0006-3495(78)85503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jan LY, Jan YN. Annual review prize lecture - voltage-gated and inwardly rectifying potassium channels. J. Physiol-London. 1997;505:267–282. doi: 10.1111/j.1469-7793.1997.267bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia ML, Galvez A, Garciacalvo M, King VF, Vazquez J, Kaczorowski GJ. Use of toxins to study potassium channels. J. Bioenerg. Biomembr. 1991;23:615–646. doi: 10.1007/BF00785814. [DOI] [PubMed] [Google Scholar]
- 50.Edwards G, Weston AH. The pharmacology of atp-sensitive potassium channels. Ann. Rev. Pharmacol. Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- 51.Choi KL, Aldrich RW, Yellen G. Tetraethylammonium blockade distinguishes 2 inactivation mechanisms in voltage-activated k+ channels. Proc. Natl. Acad. Sci. USA. 1991;88:5092–5095. doi: 10.1073/pnas.88.12.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Candia S, Garcia ML, Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance ca-2+-activated k+ channel. Biophys. J. 1992;63:583–590. doi: 10.1016/S0006-3495(92)81630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrett-Jolley R, Dart C, Standen NB. Direct block of native and cloned (kir2.1) inward rectifier k+ channels by chloroethylclonidine. Br. J. Pharmacol. 1999;128:760–766. doi: 10.1038/sj.bjp.0702819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Womack MD, Morris R, Gent TC, Barrett-Jolley R. substance p targets sympathetic control neurons in the paraventricular nucleus. Circ. Res. 2007;100:1650–1658. doi: 10.1161/CIRCRESAHA.107.153494. [DOI] [PubMed] [Google Scholar]
- 55.Badoer E, Ng CW, De Matteo R. Tonic sympathoinhibition arising from the hypothalamic pvn in the conscious rabbit. Brain Res. 2002;947:17–24. doi: 10.1016/s0006-8993(02)02901-3. [DOI] [PubMed] [Google Scholar]
- 56.Martin D, Haywood J. Hemodynamic responses to paraventricular nucleus disinhibition with bicuculline in conscious rats. Am. J. Physiol. Heart Circ. Physiol. 1993;265:H1727. doi: 10.1152/ajpheart.1993.265.5.H1727. [DOI] [PubMed] [Google Scholar]
- 57.Martin DS, Segura T, Haywood JR. Cardiovascular responses to bicuculline in the paraventricular nucleus of the rat. Hypertension. 1991;18:48–55. doi: 10.1161/01.hyp.18.1.48. [DOI] [PubMed] [Google Scholar]
- 58.Park JB, Stern JE. A tonic, gabaa receptor-mediated inhibitory postsynaptic current restrains firing activity in preautonomic and magnocellular neuroendocrine neurons of the paraventricular nucleus of the hypothalamus (pvn) FASEB J. 2005;19:A599–A599. [Google Scholar]
- 59.Hallbeck M, Blomqvist A. Spinal cord-projecting vasopressinergic neurons in the rat paraventricular hypothalamus. J. Comp. Neurol. 1999;411:201–211. [PubMed] [Google Scholar]
- 60.Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J. Comp. Neurol. 2001;433:222–238. doi: 10.1002/cne.1137. [DOI] [PubMed] [Google Scholar]
- 61.Hallbeck M. Dynorphin mrna-expressing neurons in the rat paraventricular hypothalamic nucleus project to the spinal cord. Neurosci. Lett. 2000;285:161–164. doi: 10.1016/s0304-3940(00)01093-4. [DOI] [PubMed] [Google Scholar]
- 62.Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to the spinal-cord in the rat. J. Comp. Neurol. 1988;272:579–604. doi: 10.1002/cne.902720410. [DOI] [PubMed] [Google Scholar]
- 63.Jansen ASP, Wessendorf MW, Loewy AD. Transneuronal labeling of cns neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion. Brain Res. 1995;683:1–24. doi: 10.1016/0006-8993(95)00276-v. [DOI] [PubMed] [Google Scholar]
- 64.Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal-cord in the rat. J. Comp. Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- 65.Strack AM, Sawyer WB, Platt KB, Loewy AD. Cns cell groups regulating the sympathetic outflow to adrenal-gland as revealed by trans-neuronal cell body labeling with pseudorabies virus. Brain Res. 1989;491:274–296. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]
- 66.Xi D, Kusano K, Gainer H. Quantitative analysis of oxytocin and vasopressin messenger ribonucleic acids in single magnocellular neurons isolated from supraoptic nucleus of rat hypothalamus. Endocrinology. 1999;140:4677–4682. doi: 10.1210/endo.140.10.7054. [DOI] [PubMed] [Google Scholar]
- 67.Malpas SC, Coote JH. Role of vasopressin in sympathetic response to paraventricular nucleus stimulation in anesthetized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994;266:R228–R236. doi: 10.1152/ajpregu.1994.266.1.R228. [DOI] [PubMed] [Google Scholar]
- 68.Sermasi E, Coote JH. Oxytocin acts at v-1 receptors to excite sympathetic preganglionic neurons in neonate rat spinal-cord in-vitro. Brain Res. 1994;647:323–332. doi: 10.1016/0006-8993(94)91331-5. [DOI] [PubMed] [Google Scholar]
- 69.Pittman Q, Riphagen C, Lederis K. Release of immunoassayable neurohypophyseal peptides from rat spinal cord, in vivo. Brain Res. 1984;300:321–326. doi: 10.1016/0006-8993(84)90842-4. [DOI] [PubMed] [Google Scholar]
- 70.Yang Z, Han DD, Coote JH. Cardiac sympatho-excitatory action of pvn-spinal oxytocin neurones. Auton. Neurosci-Basic Clin. 2009;147:80–85. doi: 10.1016/j.autneu.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 71.Gladwell SJ, Coote JH. Inhibitory and indirect excitatory effects of dopamine on sympathetic preganglionic neurones in the neonatal rat spinal cord in vitro. Brain Res. 1999;818:397–407. doi: 10.1016/s0006-8993(98)01330-4. [DOI] [PubMed] [Google Scholar]
- 72.Gladwell SJ, Coote JH. Fast excitatory post synaptic potentials and their response to catecholaminergic antagonists in rat sympathetic preganglionic neurones in vitro. Neurosci. Lett. 1999;268:89–92. doi: 10.1016/s0304-3940(99)00395-x. [DOI] [PubMed] [Google Scholar]
- 73.Gladwell SJ, Pyner S, Barnes NM, Coote JH. D-1-like dopamine receptors on retrogradely labelled sympathoadrenal neurones in the thoracic spinal cord of the rat. Exp. Brain Res. 1999;128:377–382. doi: 10.1007/s002210050857. [DOI] [PubMed] [Google Scholar]
- 74.Lahlou S. Mechanisms underlying the cardiovascular responses to spinal dopamine receptor stimulation by apomorphine in anesthetized rats. Neurosci. Lett. 2003;335:187–191. doi: 10.1016/s0304-3940(02)01190-4. [DOI] [PubMed] [Google Scholar]
- 75.Yang Z, Wheatley M, Coote JH. Neuropeptides, amines and amino acids as mediators of the sympathetic effects of paraventricular nucleus activation in the rat. Exp. Physiol. 2002;87:663–674. doi: 10.1113/eph8702439. [DOI] [PubMed] [Google Scholar]
- 76.Gwyn P, Logan SD. Direct actions of l-enkephalin on neonatal rat sympathetic preganglionic neurones via delta opioid receptors in vitro. Br. J. Pharmacol. 1997;122:350P. doi: 10.1038/sj.bjp.0701127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lewis DI, Coote JH. Angiotensin-ii in the spinal-cord of the rat and its sympathoexcitatory effects. Brain Res. 1993;614:1–9. doi: 10.1016/0006-8993(93)91010-p. [DOI] [PubMed] [Google Scholar]
- 78.Suter C, Coote JH. Intrathecally administered angiotensin-ii increases sympathetic activity in the rat. J. Auton. Nervous Syst. 1987;19:31–37. doi: 10.1016/0165-1838(87)90142-1. [DOI] [PubMed] [Google Scholar]
- 79.Llewellyn-Smith IJ, DiCarlo SE, Collins HL, Keast JR. Enkephalin-immunoreactive interneurons extensively innervate sympathetic preganglionic neurons regulating the pelvic viscera. J. Comp. Neurol. 2005;488:278–289. doi: 10.1002/cne.20552. [DOI] [PubMed] [Google Scholar]
- 80.Macdonald RL, Olsen RW. Gaba(a) receptor channels. Ann. Rev. Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 81.Watkins ND, Cork SC, Pyner S. An immunohistochemical investigation of the relationship between neuronal nitric oxide synthase, gaba and presympathetic paraventricular neurons in the hypothalamus. Neuroscience. 2009;159:1079–1088. doi: 10.1016/j.neuroscience.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 82.Kalsbeek A, Fliers E, Franke AN, Wortel J, Buijs RM. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology. 2000;141:3832–3841. doi: 10.1210/endo.141.10.7709. [DOI] [PubMed] [Google Scholar]
- 83.Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic gabaergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J. Neurosci. 2004;24:7604–7613. doi: 10.1523/JNEUROSCI.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.la Fleur SE, Kalsbeek A, Wortel J, Buijs RM. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 2000;871:50–56. doi: 10.1016/s0006-8993(00)02423-9. [DOI] [PubMed] [Google Scholar]
- 85.Yamashita H, Inenaga K, Koizumi K. Possible projections from regions of paraventricular and supraoptic nuclei to the spinal-cord - electrophysiological studies. Brain Res. 1984;296:373–378. doi: 10.1016/0006-8993(84)90077-5. [DOI] [PubMed] [Google Scholar]
- 86.Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am. J. Physiol. 1989;256:R1325–R1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- 87.Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and gaba systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2847–H2856. doi: 10.1152/ajpheart.00625.2005. [DOI] [PubMed] [Google Scholar]
- 88.Brickley SG, CullCandy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of gaba(a) receptors. J. PhysiolLond. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of gaba(a) receptors. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 90.Glykys J, Mann EO, Mody I. Which gaba(a) receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active gaba(a) receptors: Modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 92.Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL. Taurine is a potent activator of extrasynaptic gaba(a) receptors in the thalamus. J. Neurosci. 2008;28:106–115. doi: 10.1523/JNEUROSCI.3996-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mody I, Glykys J, Wei WZ. A new meaning for "Gin & tonic": Tonic inhibition as the target for ethanol action in the brain. Alcohol. 2007;41:145–153. doi: 10.1016/j.alcohol.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park JB, Jo JY, Zheng H, Patel KP, Stern JE. Regulation of tonic gaba inhibitory function, presympathetic neuronal activity and sympathetic outflow from the paraventricular nucleus by astroglial gaba transporters. J. Physiol. London. 2009;587:4645–4660. doi: 10.1113/jphysiol.2009.173435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schlenker E, Barnes L, Hansen S, Martin D. Cardiorespiratory and metabolic responses to injection of bicuculline into the hypothalamic paraventricular nucleus (pvn) of conscious rats. Brain Res. 2001;895:33–40. doi: 10.1016/s0006-8993(01)02011-x. [DOI] [PubMed] [Google Scholar]
- 96.Barrett-Jolley R. Nipecotic acid directly activates gaba(a)-like ion channels. Br. J. Pharmacol. 2001;133:673–678. doi: 10.1038/sj.bjp.0704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boudaba C, Szabo K, Tasker JG. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J. Neurosci. 1996;16:7151–7160. doi: 10.1523/JNEUROSCI.16-22-07151.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fenelon VS, Sieghart W, Herbison AE. Cellular localization and differential distribution of gabaa receptor subunit proteins and messenger rnas within hypothalamic magnocellular neurons. Neurosci. 1995;64:1129–1143. doi: 10.1016/0306-4522(94)00402-q. [DOI] [PubMed] [Google Scholar]
- 99.Hermes ML, Coderre EM, Buijs RM, Renaud LP. (Gaba and glutamate mediate rapid neurotransmission from suprachiasmatic nucleus to hypothalamic paraventricular nucleus in rat. J. Physiol.(Lond) 1996;496(Pt 3):749–757. doi: 10.1113/jphysiol.1996.sp021724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zaki A, Barrett-Jolley R. Rapid neuromodulation by cortisol in the rat paraventricular nucleus: An in vitro study. Br. J. Pharmacol. 2002;137:87–97. doi: 10.1038/sj.bjp.0704832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fritschy JM, Mohler H. Gaba(a)-receptor heterogeneity in the adult-rat brain - differential regional and cellular-distribution of 7 major subunits. J. Comp. Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 102.Barrett-Jolley R, Pyner S. Inhibition of rat spinally projecting pvn neurones by the neurosteroid tetrahydroxycorticosterone. J. Physiol. London. 2003:548P. [Google Scholar]
- 103.Womack MD, Pyner S, Barrett-Jolley R. Inhibition by [alpha]-tetrahydrodeoxycorticosterone (thdoc) of pre-sympathetic parvocellular neurones in the paraventricular nucleus of rat hypothalamus. Br. J. Pharmacol. 2006;149:600–607. doi: 10.1038/sj.bjp.0706911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li DP, Chen SR, Pan HL. Nitric oxide inhibits spinally projecting paraventricular neurons through potentiation of presynaptic gaba release. J. Neurophysiol. 2002;88:2664–2674. doi: 10.1152/jn.00540.2002. [DOI] [PubMed] [Google Scholar]
- 105.Chen Q, Pan HL. Regulation of synaptic input to hypothalamic presympathetic neurons by gaba(b) receptors. Neuroscience. 2006;142:595–606. doi: 10.1016/j.neuroscience.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 106.Khawaled R, Bruening-Wright A, Adelman J, Maylie J. Bicuculline block of small-conductance calcium-activated potassium channels. Pflügers Archiv. Eur. J. Physiol. 1999;438:314–321. doi: 10.1007/s004240050915. [DOI] [PubMed] [Google Scholar]
- 107.Strobaek D, Jorgensen TD, Christophersen P, Ahring PK, Olesen SP. Pharmacological characterization of small-conductance ca2+-activated k+ channels stably expressed in hek 293 cells. Br. J. Pharmacol. 2000;129:991–999. doi: 10.1038/sj.bjp.0703120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Druzin M, Haage D, Johansson S. Bicuculline free base blocks voltage-activated k+ currents in rat medial preoptic neurons. Neuropharmacology. 2004;46:285–295. doi: 10.1016/j.neuropharm.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 109.Stern JE, Li YJ, Sonner P. Nitric oxide modulates firing activity of preautonomic neurons in the paraventricular nucleus (pvn) of the hypothalamus. FASEB J. 2002;16:A501–A501. [Google Scholar]
- 110.Stern JE, Zhang W. Preautonomic neurons in the paraventricular nucleus of the hypothalamus contain estrogen receptor beta. Brain Res. 2003;975:99–109. doi: 10.1016/s0006-8993(03)02594-0. [DOI] [PubMed] [Google Scholar]
- 111.Zhang WF, Stern JE. Preautonomic neurons in the paraventricular nucleus (pvn) of the hypothalamus express estrogen receptor-b immunoreactivity. FASEB J. 2002;16:A501–A501. [Google Scholar]
- 112.Badoer E, Ryan A. Effects of leptin on spinally projecting neurons in the pvn of the hypothalamus. Brain Res. 1999;844:210–215. doi: 10.1016/s0006-8993(99)01902-2. [DOI] [PubMed] [Google Scholar]
- 113.Oldfield BJ, Davern PJ, Giles ME, Allen AM, Badoer E, McKinley MJ. Efferent neural projections of angiotensin receptor (at1) expressing neurones in the hypothalamic paraventricular nucleus of the rat. J. Neuroendocrinol. 2001;13:139–146. doi: 10.1046/j.1365-2826.2001.00597.x. [DOI] [PubMed] [Google Scholar]
- 114.Li DP, Chen SR, Pan HL. Angiotensin ii stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J. Neurosci. 2003;23:5041–5049. doi: 10.1523/JNEUROSCI.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Price CJ, Samson WK, Ferguson AV. Neuropeptide w has cell phenotype-specific effects on the excitability of different subpopulations of paraventricular nucleus neurones. J. Neuroendocrinol. 2009;21:850–857. doi: 10.1111/j.1365-2826.2009.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lind RW, Swanson LW, Bruhn TO, Ganten D. The distribution of angiotensin-ii-immunoreactive cells and fibers in the paraventriculo-hypophysial system of the rat. Brain Res. 1985;338:81–89. doi: 10.1016/0006-8993(85)90250-1. [DOI] [PubMed] [Google Scholar]
- 117.Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (grac), 3rd edition. Br. J. Pharmacol. 2008;153:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Allen AM, MacGregor DP, McKinley MJ, Mendelsohn FAO. Angiotensin ii receptors in the human brain. Regul. Peptides. 1999;79:1–7. doi: 10.1016/s0167-0115(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 119.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. Xxiii. The angiotensin II receptors. Pharmacol. Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 120.Oliverio MI, Best CF, Kim HS, Arendshorst WJ, Smithies O, Coffman TM. Angiotensin II responses in at(1a) receptor-deficient mice: A role for at(1b) receptors in blood pressure regulation. Am. J. Physiol. Renal. Physiol. 1997;41:F515–F520. doi: 10.1152/ajprenal.1997.272.4.F515. [DOI] [PubMed] [Google Scholar]
- 121.Lenkei Z, Palkovits M, Corvol P, LlorensCortes C. Expression of angiotensin type-1 (at1) and type-2 (at2) receptor mrnas in the adult rat brain: A functional neuroanatomical review. Front. Neuroendocrinol. 1997;18:383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- 122.Bains JS, Potyok A, Ferguson AV. Angiotensin-ii actions in paraventricular nucleus - functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res. 1992;599:223–229. doi: 10.1016/0006-8993(92)90395-p. [DOI] [PubMed] [Google Scholar]
- 123.Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal-cord receive excitatory input from the subfornical organ. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1995;268:R625–R633. doi: 10.1152/ajpregu.1995.268.3.R625. [DOI] [PubMed] [Google Scholar]
- 124.Li Z, Ferguson AV. Electrophysiological properties of paraventricular magnocellular neurons in rat brain slices: Modulation of I-A by angiotensin II. Neuroscience. 1996;71:133–145. doi: 10.1016/0306-4522(95)00434-3. [DOI] [PubMed] [Google Scholar]
- 125.Cato MJ, Toney GM. Angiotensin ii excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: An in vitro patch-clamp study in brain slices. J. Neurophysiol. 2005;93:403–413. doi: 10.1152/jn.01055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Culman J, Itoi K, Unger T. Hypothalamic tachykinins. Mediators of stress responses? Ann. N.Y. Acad. Sci. 1995;771:204–218. doi: 10.1111/j.1749-6632.1995.tb44682.x. [DOI] [PubMed] [Google Scholar]
- 127.Ding YQ, Shigemoto R, Takada M, Ohishi H, Nakanishi S, Mizuno N. Localization of the neuromedin k receptor (nk3) in the central nervous system of the rat. J. Comp. Neurol. 1996;364:290–310. doi: 10.1002/(SICI)1096-9861(19960108)364:2<290::AID-CNE8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 128.Koutcherov Y, Ashwell KWS, Paxinos G. The distribution of the neurokinin b receptor in the human and rat hypothalamus. Neuroreport. 2000;11:3127–3131. doi: 10.1097/00001756-200009280-00018. [DOI] [PubMed] [Google Scholar]
- 129.Shults CW, Buck SH, Burcher E, Chase TN, O'Donohue TL. Distinct binding sites for substance p and neurokinin a (substance k): Co-transmitters in rat brain. Peptides. 1985;6:343–345. doi: 10.1016/0196-9781(85)90060-9. [DOI] [PubMed] [Google Scholar]
- 130.Nakajima Y, Tsuchida K, Negishi M, Ito S, Nakanishi S. Direct linkage of three tachykinin receptors to stimulation of both phosphatidylinositol hydrolysis and cyclic amp cascades in transfected chinese hamster ovary cells. J. Biol. Chem. 1992;267:2437–2442. [PubMed] [Google Scholar]
- 131.Quartara L, Maggi CA. The tachykinin nk1 receptor. Part i: Ligands and mechanisms of cellular activation. Neuropeptides. 1997;31:537–563. doi: 10.1016/s0143-4179(97)90001-9. [DOI] [PubMed] [Google Scholar]
- 132.Connolly CN, Kittler JT, Thomas P, Uren JM, Brandon NJ, Smart TG, Moss SJ. Cell surface stability of gamma-aminobutyric acid type a receptors - dependence on protein kinase c activity and subunit composition. J. Biol. Chem. 1999;274:36565–36572. doi: 10.1074/jbc.274.51.36565. [DOI] [PubMed] [Google Scholar]
- 133.Bittencourt JC, Benoit R, Sawchenko PE. Distribution and origins of substance-p-immunoreactive projections to the paraventricular and supraoptic nuclei - partial overlap with ascending catecholaminergic projections. J. Chem. Neuroanat. 1991;4:63–78. doi: 10.1016/0891-0618(91)90032-8. [DOI] [PubMed] [Google Scholar]
- 134.Womack MD, Barrett-Jolley R. Activation of paraventricular nucleus neurones by the dorsomedial hypothalamus via a tachykinin pathway in rats. Exp. Physiol. 2007;92:671–676. doi: 10.1113/expphysiol.2007.037457. [DOI] [PubMed] [Google Scholar]
- 135.DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress part renaissance, part revolution. Pharmacol. Biochem. Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 136.Yi C-X, la Fleur SE, Fliers E, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim. Biophys. Acta - Mol. Basis Dis. 2010;1802:416–431. doi: 10.1016/j.bbadis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 137.Klieverik LP, Janssen SF, van Riel A, Foppen E, Bisschop PH, Serlie MJ, Boelen A, Ackermans MT, Sauerwein HP, Fliers E, Kalsbeek A. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5966–5971. doi: 10.1073/pnas.0805355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- 139.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 140.Cham JL, Owens NC, Barden JA, Lawrence AJ, Badoer E. P2x purinoceptor subtypes on paraventricular nucleus neurones projecting to the rostral ventrolateral medulla in the rat. Exp. Physiol. 2006;91:403–411. doi: 10.1113/expphysiol.2005.032409. [DOI] [PubMed] [Google Scholar]
- 141.Kirsch GE, Codina J, Birnbaumer L, Brown AM. Coupling of atp-sensitive k+ channels to a1-receptors by g-proteins in rat ventricular myocytes. Am. J. Physiol. 1990;259:H820–H826. doi: 10.1152/ajpheart.1990.259.3.H820. [DOI] [PubMed] [Google Scholar]
- 142.Dart C, Standen NB. Adenosine-activated potassium current in smooth-muscle cells isolated from the pig coronary-artery. J. Physiol-London. 1993;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barrett-Jolley R, Comtois A, Davies NW, Stanfield PR, Standen NB. Effect of adenosine and intracellular gtp on k-atp channels of mammalian skeletal muscle. J. Membr. Biol. 1996;152:111–116. doi: 10.1007/s002329900090. [DOI] [PubMed] [Google Scholar]
- 144.Mondal MS, Yamaguchi H, Date Y, Shimbara T, Toshinai K, Shimomura Y, Mori M, Nakazato M. A role for neuropeptide w in the regulation of feeding behavior. Endocrinology. 2003;144:4729–4733. doi: 10.1210/en.2003-0536. [DOI] [PubMed] [Google Scholar]
- 145.Jhamandas JH, MacTavish D, Harris KH. Neuropeptide ff (npff) control of magnocellular neurosecretory cells of the rat hypothalamic paraventricular nucleus (pvn) Peptides. 2006;27:973–979. doi: 10.1016/j.peptides.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 146.Jhamandas JH, Simonin F, Bourguignon JJ, Harris KH. Neuropeptide ff and neuropeptide vf inhibit gabaergic neurotransmission in parvocellular neurons of the rat hypothalamic paraventricular nucleus. Am. J. Physiol-Regul. Integr. Compar. Physiol. 2007;292:R1872–R1880. doi: 10.1152/ajpregu.00407.2006. [DOI] [PubMed] [Google Scholar]
- 147.Jhamandas JH, MacTavish D. Central administration of neuropeptide ff causes activation of oxytocin paraventricular hypothalamic neurones that project to the brainstem. J. Neuroendocrinol. 2003;15:24–32. doi: 10.1046/j.1365-2826.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 148.Maratos-Flier E. The long reach of leptin. Nat. Med. 2008;14:604–606. doi: 10.1038/nm0608-604. [DOI] [PubMed] [Google Scholar]
- 149.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 150.Cone RD. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 151.Meister B, Hakansson ML. Leptin receptors in hypothalamus and circumventricular organs. Clin. Exp. Pharmacol. Physiol. 2001;28:610–617. doi: 10.1046/j.1440-1681.2001.03493.x. [DOI] [PubMed] [Google Scholar]
- 152.Powis JE, Bains JS, Ferguson AV. Leptin depolarizes rat hypothalamic paraventricular nucleus neurons. Am. J. Physiol-Regul. Integrat. Comp. Physiol. 1998;274:R1468–R1472. doi: 10.1152/ajpregu.1998.274.5.R1468. [DOI] [PubMed] [Google Scholar]
- 153.Shirasaka T, Miyahara S, Kunitake T, Jin QH, Kato K, Takasaki M, Kannan H. Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. Am. J. Physiol-Regul. Integr. Comp. Physiol. 2001;281:R1114–R1118. doi: 10.1152/ajpregu.2001.281.4.R1114. [DOI] [PubMed] [Google Scholar]
- 154.Shan J, Krukoff T. Intracerebroventricular adrenomedullin stimulates the hypothalamic-pituitary-adrenal axis, the sympathetic nervous system and production of hypothalamic nitric oxide. J. Neuroendocrinol. 2001;13:975–984. doi: 10.1046/j.1365-2826.2001.00721.x. [DOI] [PubMed] [Google Scholar]
- 155.Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin. Exp. Pharmacol. Physiol. 2001;28:95–99. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- 156.Herd JA. Cardiovascular response to stress in man. Annu. Rev. Physiol. 1984;46:177–185. doi: 10.1146/annurev.ph.46.030184.001141. [DOI] [PubMed] [Google Scholar]
- 157.Everson SA, Kaplan GA, Goldberg DE, Salonen JT. Anticipatory blood pressure response to exercise predicts future high blood pressure in middle-aged men. Hypertension. 1996;27:1059–1064. doi: 10.1161/01.hyp.27.5.1059. [DOI] [PubMed] [Google Scholar]
- 158.Berdina NA, Kolenko OL, Kotz IM, Kuznetzov AP, Rodionov IM, Savtchenko AP, Thorevsky VI. Increase in skeletal muscle performance during emotional stress in man. Circ. Res. 1972;30:642–650. doi: 10.1161/01.res.30.6.642. [DOI] [PubMed] [Google Scholar]
- 159.Gillespie RD. The relative influence of mental and muscular work on the pulse-rate and blood-pressure. J. Physiol-London. 1924;58:425–432. doi: 10.1113/jphysiol.1924.sp002144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Herd JA. Cardiovascular response to stress. Physiol. Rev. 1991;71:305–330. doi: 10.1152/physrev.1991.71.1.305. [DOI] [PubMed] [Google Scholar]
- 161.Folkow B. Mental stress and its importance for cardiovascular disorders; physiological aspects, "From-mice-to-man". Scand. Cardiovasc. J. 2001;35:163–172. [PubMed] [Google Scholar]
- 162.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 163.Duan YF, Winters R, McCabe PM, Green EJ, Huang Y, Schneiderman N. Cardiorespiratory components of defense reaction elicited from paraventricular nucleus. Physiol. Behav. 1997;61:325–330. doi: 10.1016/s0031-9384(96)00410-6. [DOI] [PubMed] [Google Scholar]
- 164.Busnardo C, Tavares RF, Resstel LBM, Elias LLK, Correa FMA. Paraventricular nucleus modulates autonomic and neuroendocrine responses to acute restraint stress in rats. Autonomic Neurosci. doi: 10.1016/j.autneu.2010.06.003. In Press. [DOI] [PubMed] [Google Scholar]
- 165.Li YF, Wang W, Mayhan WG, Patel KP. Angiotensin-mediated increase in renal sympathetic nerve discharge within the pvn: Role of nitric oxide. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006;290:R1035–R1043. doi: 10.1152/ajpregu.00338.2004. [DOI] [PubMed] [Google Scholar]
- 166.Martin DS, Haywood JR. Sympathetic nervous-system activation by glutamate injections into the paraventricular nucleus. Brain Res. 1992;577:261–267. doi: 10.1016/0006-8993(92)90282-e. [DOI] [PubMed] [Google Scholar]
- 167.Martin DS, Haywood JR, Thornhill JA. Stimulation of the hypothalamic paraventricular nucleus causes systemic venoconstriction. Brain Res. 1993;604:318–324. doi: 10.1016/0006-8993(93)90383-x. [DOI] [PubMed] [Google Scholar]
- 168.Jessop DS, Chowdrey HS, Biswas S, Lightman SL. substance-p and substance-k in the rat hypothalamus following monosodium glutamate lesions of the arcuate nucleus. Neuropeptides. 1991;18:165–170. doi: 10.1016/0143-4179(91)90109-v. [DOI] [PubMed] [Google Scholar]
- 169.Unger T, Becker H, Petty M, Demmert G, Schneider B, Ganten D, Lang RE. Differential-effects of central angiotensin-ii and substance-p on sympathetic-nerve activity in conscious rats - implications for cardiovascular adaptation to behavioral-responses. Circ. Res. 1985;56:563–575. doi: 10.1161/01.res.56.4.563. [DOI] [PubMed] [Google Scholar]
- 170.Itoi K, Jost N, Badoer E, Tschope C, Culman J, Unger T. Localization of the substance p-induced cardiovascular responses in the rat hypothalamus. Brain Res. 1991;558:123–126. doi: 10.1016/0006-8993(91)90727-d. [DOI] [PubMed] [Google Scholar]
- 171.Nakayama Y, Takano Y, Saito R, Kamiya H. Central pressor actions of tachykinin nk-3 receptor in the paraventricular nucleus of the rat hypothalamus. Brain Res. 1992;595:339–342. doi: 10.1016/0006-8993(92)91069-q. [DOI] [PubMed] [Google Scholar]
- 172.Takano Y, Nakayama Y, Matsumoto T, Saito R, Kamiya H. The mechanism of central pressor actions of tachykinin nk-3 receptor in the paraventricular nucleus of the hypothalamus in rats. Regul. Peptides. 1993;46:360–363. doi: 10.1016/0167-0115(93)90086-n. [DOI] [PubMed] [Google Scholar]
- 173.Culman J, Klee S, Ohlendorf C, Unger T. Effect of tachykinin receptor inhibition in the brain on cardiovascular and behavioral responses to stress. J. Pharmacol. Exp. Ther. 1997;280:238–246. [PubMed] [Google Scholar]
- 174.Cannon WB. New York & London: D. Appleton & Company; 1927. Bodily changes in pain hunger, fear and rage - an account of recent researches into the function of emotional excitement. [Google Scholar]
- 175.Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Res. 1996;742:219–224. doi: 10.1016/s0006-8993(96)01011-6. [DOI] [PubMed] [Google Scholar]
- 176.Pyner S, Deering J, Coote JH. Right atrial stretch induces renal nerve inhibition and c-fos expression in parvocellular neurones of the paraventricular nucleus in rats. Exp. Physiol. 2002;87:25–32. doi: 10.1113/eph8702279. [DOI] [PubMed] [Google Scholar]
- 177.Deering J, Coote JH. Paraventricular neurones elicit a volume expansion-like change of activity in sympathetic nerves to the heart and kidney in the rabbit. Exp. Physiol. 2000;85:177–186. [PubMed] [Google Scholar]
- 178.Greenway CV, Lister GE. Capacitance effects and blood reservoir function in splanchnic vascular bed during non-hypotensive hemorrhage and blood-volume expansion in anesthetized cats. J. Physiol-London. 1974;237:279–294. doi: 10.1113/jphysiol.1974.sp010482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fater DC, Schultz HD, Sundet WD, Mapes JS, Goetz KL. Effects of left atrial stretch in cardiac-denervated and intact conscious dogs. Am. J. Physiol-Heart Cir. Physiol. 1982;242:H1056–1064. doi: 10.1152/ajpheart.1982.242.6.H1056. [DOI] [PubMed] [Google Scholar]
- 180.Badoer E, McKinley MJ, Oldfield BJ, McAllen RM. A comparison of hypotensive and nonhypotensive hemorrhage on fos expression in spinally projecting neurons of the paraventricular nucleus and rostral ventrolateral medulla. Brain Res. 1993;610:216–223. doi: 10.1016/0006-8993(93)91403-f. [DOI] [PubMed] [Google Scholar]
- 181.Stocker SD, Cunningham JT, Toney GM. Water deprivation increases fos immunoreactivity in pvn autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am. J. Physiol-Regul. Integrat. Comp. Physiol. 2004;287:R1172–R1183. doi: 10.1152/ajpregu.00394.2004. [DOI] [PubMed] [Google Scholar]
- 182.Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am. J. Physiol-Regul. Integr. Compar. Physiol. 2004;286:R719–R725. doi: 10.1152/ajpregu.00494.2003. [DOI] [PubMed] [Google Scholar]
- 183.Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet. 1978;1:795–797. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- 184.Degaute JP, Vandeborne P, Linkowski P, Vancauter E. Quantitative-analysis of the 24-hour blood-pressure and heart-rate patterns in young men. Hypertension. 1991;18:199–210. doi: 10.1161/01.hyp.18.2.199. [DOI] [PubMed] [Google Scholar]
- 185.Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, Porcellati C. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528–536. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 186.Pickering TG. The clinical significance of diurnal blood pressure variations. Dippers and nondippers. Circulation. 1990;81:700–702. doi: 10.1161/01.cir.81.2.700. [DOI] [PubMed] [Google Scholar]
- 187.Boari B, Salmi R, Gallerani M, Malagoni AM, Manfredini F, Manfredini R. Acute myocardial infarction: Circadian, weekly, and seasonal patterns of occurrence. Biol. Rhythm. Res. 2007;38:155–167. [Google Scholar]
- 188.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular-disease. Circulation. 1989;79:733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 189.Brown TM, Piggins HD. Electrophysiology of the suprachiasmatic circadian clock. Prog. Neurobiol. 2007;82:229–255. doi: 10.1016/j.pneurobio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 190.Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y. Circadian oscillation of a mammalian homologue of the drosophila period gene. Nature. 1997;389:512–516. doi: 10.1038/39086. [DOI] [PubMed] [Google Scholar]
- 191.Reppert SM, Weaver D. . Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 192.Hermes ML, Buijs RM, Renaud LP. Electrophysiology of suprachiasmatic nucleus projections to hypothalamic paraventricular nucleus neurons. Prog. Brain Res. 1996;111:241–252. doi: 10.1016/s0079-6123(08)60412-4. [DOI] [PubMed] [Google Scholar]
- 193.Chen KK, Chan SHH, Chang LS, Chan JYH. Participation of paraventricular nucleus of hypothalamus in central regulation of penile erection in the rat. J. Urol. 1997;158:238–244. doi: 10.1097/00005392-199707000-00078. [DOI] [PubMed] [Google Scholar]
- 194.Argiolas A, Melis MR. Neuromodulation of penile erection: An overview of the role of neurotransmitters and neuropeptides. Prog. Neurobiol. 1995;47:235–255. [PubMed] [Google Scholar]
- 195.Andersson KE, Wagner G. Physiology of penile erection. Physiol. Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- 196.Argiolas A, Melis MR. Central control of penile erection: Role of the paraventricular nucleus of the hypothalamus. Prog. Neurobiol. 2005;76:1–21. doi: 10.1016/j.pneurobio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 197.Marson L, Platt KB, Mckenna KE. Central-nervous-system innervation of the penis as revealed by the transneuronal transport of pseudorabies virus. Neuroscience. 1993;55:263–280. doi: 10.1016/0306-4522(93)90471-q. [DOI] [PubMed] [Google Scholar]
- 198.Stafford SA, Tang K, Coote JH. Activation of lumbosacral 5-ht2c receptors induces bursts of rhythmic activity in sympathetic nerves to the vas deferens in male rats. Br. J. Pharmacol. 2006;148:1083–1090. doi: 10.1038/sj.bjp.0706814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stafford SA, Bowery NG, Tang K, Coote JH. Activation by p-chloroamphetamine of the spinal ejaculatory pattern generator in anaesthetized male rats. Neuroscience. 2006;140:1031–1040. doi: 10.1016/j.neuroscience.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 200.Levin BE. Metabolic sensing neurons and the control of energy homeostasis. Physiol. Behav. 2006;89:486–489. doi: 10.1016/j.physbeh.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 201.Sakaguchi T, Bray GA. Sympathetic activity following paraventricular injections of glucose and insulin. Brain Res Bull. 1988;21:25–29. doi: 10.1016/0361-9230(88)90115-3. [DOI] [PubMed] [Google Scholar]
- 202.Carrasco M, Portillo F, Larsen PJ, Vallo JJ. Insulin and glucose administration stimulates fos expression in neurones of the paraventricular nucleus that project to autonomic preganglionic structures. J. Neuroendocrinol. 2001;13:339–346. doi: 10.1046/j.1365-2826.2001.00631.x. [DOI] [PubMed] [Google Scholar]
- 203.Dunn-Meynell AA, Govek E, Levin BE. Intracarotid glucose selectively increases fos-like immunoreactivity in paraventricular, ventromedial and dorsomedial nuclei neurons. Brain Res. 1997;748:100–106. doi: 10.1016/s0006-8993(96)01280-2. [DOI] [PubMed] [Google Scholar]
- 204.Stanley S, Pinto S, Segal J, Perez CA, Viale A, DeFalco J, Cai XL, Heisler LK, Friedman JM. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc. Natl. Acad. Sci USA. 2010;107:7024–7029. doi: 10.1073/pnas.1002790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–526. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- 206.Bamshad M, Song CK, Bartness TJ. Cns origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol-Regul Integr. Compar. Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 207.Amir S. Stimulation of the paraventricular nucleus with glutamate activates interscapular brown adipose-tissue thermogenesis in rats. Brain Res. 1990;508:152–155. doi: 10.1016/0006-8993(90)91129-5. [DOI] [PubMed] [Google Scholar]
- 208.Yoshimatsu H, Egawa M, Bray GA. Effects of cholecystokinin on sympathetic activity to interscapular brown adipose-tissue. Brain Res. 1992;597:298–303. doi: 10.1016/0006-8993(92)91486-x. [DOI] [PubMed] [Google Scholar]
- 209.Yoshimatsu H, Egawa M, Bray GA. Sympathetic-nerve activity after discrete hypothalamic injections of l-glutamate. Brain Res. 1993;601:121–128. doi: 10.1016/0006-8993(93)91702-t. [DOI] [PubMed] [Google Scholar]
- 210.Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am. J. Physiol-Regul. Integr. Comp. Physiol. . 0092;96:R831–R843. doi: 10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Inenaga K, Osaka T, Yamashita H. Thermosensitivity of neurons in the paraventricular nucleus of the rat slice preparation. Brain Res. 1987;424:126–132. doi: 10.1016/0006-8993(87)91201-7. [DOI] [PubMed] [Google Scholar]
- 212.Smith JE, Jansen ASP, Gilbey MP, Loewy AD. Cns cell groups projecting to sympathetic outflow of tail artery: Neural circuits involved in heat loss in the rat. Brain Res. 1998;786:153–164. doi: 10.1016/s0006-8993(97)01437-6. [DOI] [PubMed] [Google Scholar]
- 213.Cham JL, Badoer E. Hypothalamic paraventricular nucleus is critical for renal vasoconstriction elicited by elevations in body temperature. Am. J. Physiol-Renal. Physiol. 2008;294:F309–F315. doi: 10.1152/ajprenal.00488.2007. [DOI] [PubMed] [Google Scholar]
- 214.Chen F, Dworak M, Wang Y, Cham JL, Badoer E. Role of the hypothalamic pvn in the reflex reduction in mesenteric blood flow elicited by hyperthermia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1874–1881. doi: 10.1152/ajpregu.90384.2008. [DOI] [PubMed] [Google Scholar]
- 215.Cham JL, Badoer E. Exposure to a hot environment can activate rostral ventrolateral medulla-projecting neurones in the hypothalamic paraventricular nucleus in conscious rats. Exp. Physiol. 2008;93:64–74. doi: 10.1113/expphysiol.2007.039560. [DOI] [PubMed] [Google Scholar]
- 216.Cham JL, Klein R, Owens NC, Mathai M, McKinley M, Badoer E. Activation of spinally projecting and nitrergic neurons in the pvn following heat exposure. Am. J. Physiol-Regul. Integr. Comp. Physiol. 2006;291:R91–R101. doi: 10.1152/ajpregu.00675.2005. [DOI] [PubMed] [Google Scholar]
- 217.Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilation during body heating. J. Physiol-London. 1957;136:489–497. doi: 10.1113/jphysiol.1957.sp005775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Patel KP, Zhang K, Kenney MJ, Weiss M, Mayhan WG. Neuronal expression of fos protein in the hypothalamus of rats with heart failure. Brain Res. 2000;865:27–34. doi: 10.1016/s0006-8993(00)02186-7. [DOI] [PubMed] [Google Scholar]
- 219.Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AMG. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 220.Garthwaite J. Neural nitric-oxide signaling. Trends Neurosci. 1995;18:51–52. [PubMed] [Google Scholar]
- 221.Yamaguchi N, Okada S, Usui D, Yokotani K. Nitric oxide synthase isozymes in spinally projecting pvn neurons are involved in crf-induced sympathetic activation. Auton. Neurosci-Basic Clin. 2009;148:83–89. doi: 10.1016/j.autneu.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 222.Kantzides A, Badoer E. Nnos-containing neurons in the hypothalamus and medulla project to the rvlm. Brain Res. 2005;1037:25–34. doi: 10.1016/j.brainres.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 223.Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: Role of gaba. Neuroscience. 2003;118:585–601. doi: 10.1016/s0306-4522(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 224.Bains JS, Ferguson AV. Nitric oxide regulates nmda-driven gabaergic inputs to type i neurones of the rat paraventricular nucleus. J. Physiol-London. 1997;499:733–746. doi: 10.1113/jphysiol.1997.sp021965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang K, Zucker IH, Patel KP. Altered number of diaphorase (nos) positive neurons in the hypothalamus of rats with heart failure. Brain Res. 1998;786:219–225. doi: 10.1016/s0006-8993(97)01449-2. [DOI] [PubMed] [Google Scholar]
- 226.Zheng H, Li YF, Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the paraventricular nucleus of anesthetized rats with heart failure. Am. J. Physiol-Regul. Integr. Comp. Physiol. 2009;297:R1364–R1374. doi: 10.1152/ajpregu.00149.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of cns innervation of the sympathetic outflow demonstrated by trans-neuronal pseudorabies viral-infections. Brain Res. 1989;491:156–162. doi: 10.1016/0006-8993(89)90098-x. [DOI] [PubMed] [Google Scholar]