Abstract
Adenosine is produced primarily by the metabolism of ATP and mediates its physiological actions by interacting primarily with adenosine receptors (ARs) on the plasma membranes of different cell types in the body. Activation of these G protein-coupled receptors promotes activation of diverse cellular signaling pathways that define their tissue-specific functions. One of the major actions of adenosine is cytoprotection, mediated primarily via two ARs - A1 (A1AR) and A3 (A3AR). These ARs protect cells exposed to oxidative stress and are also regulated by oxidative stress. Stress-mediated regulation of ARs involves two prominent transcription factors - activator protein-1 (AP-1) and nuclear factor (NF)-κB – that mediate the induction of genes important in cell survival. Mice that are genetically deficient in the p50 subunit of NF-κB (i.e., p50 knock-out mice) exhibit altered expression of A1AR and A2AAR and demonstrate distinct behavioral phenotypes under normal conditions or after drug challenges. These effects suggest an important role for NF-κB in dictating the level of expression of ARs in vivo, in regulating the cellular responses to stress, and in modifying behavior.
Keywords: Adenosine, adenosine receptor, p50 knockout mice, NF-κB, sleep, caffeine.
INTRODUCTION
Adenosine is an important inhibitory neuromodulator in the central nervous system (CNS). The major source of adenosine is ATP, a neurotransmitter or co-transmitter in the CNS; in the periphery, ATP is also released from mast cells, basophils and endothelial cells as a result of cellular damage. Other precursors of adenosine include ADP (released in large amounts by activated platelets) and cyclic AMP (a second messenger in most cells). Because the estimated ratio of ATP:AMP under normoxic condition is approximately 50:1, a small decrease in total ATP is expected to produce a large increase in concentrations of AMP and adenosine [1].
Adenine nucleotides are degraded by a series of ectonucleotidases. One such enzyme, 5’-nucleotidase, catalyzes the conversion of AMP to adenosine. This enzyme occurs both extracellularly (attached to the plasma membrane by glycosyl-phosphatidylinositol anchors) and in the cytosol [2]. In the CNS, it is located on glia and astrocytes near synaptic terminals. Adenosine is rapidly cleared from the extracellular space through a bi-directional facilitated transporter and/or through metabolism by adenosine deaminase to inosine. Inhibition of the activity of this transporter leads to increased extracellular adenosine and reduced excitatory synaptic transmission in rat hippocampal slices [3], affirming a CNS depressant action of the nucleoside.
AR subtypes include A1, A2a, A2b and A3 [4]. The A2AAR is localized to the striatum, where it inhibits dopaminergic neurons [5]. Expression of the A3AR is low in rat CNS [6]; however, recent findings implicate A3AR in modulating CNS injury [7]. The A1AR is the predominant AR subtype in the CNS. Autoradiographical studies indicate wide distribution of the A1AR in CNS, particularly in cortex, hippocampus and thalamus [8]. The A1AR, is the primary mediator of the neuroprotective action of adenosine.
1. ADENOSINE AND ADENOSINE RECEPTORS AND CYTOPROTECTION
Adenosine is released in response to ischemic stress and activates cells in the vicinity of its release site, thus serving a paracrine role. Extracellular concentrations of adenosine correlate with cerebral blood flow. For example, a transient increase in adenosine occurs when flow falls below 25 ml 100g–1 min–1 [9]. Increased adenosine confers protection against transient cerebral ischemia. For example, administration of 2-chloroadenosine protected rats from ischemia-induced hippocampal cell loss [10]. Another adenosine analog, cyclohexyladenosine, protected against cerebral ischemia in gerbils [11] and transient ischemia in rats [12]. Cyclohexyladenosine also provided protection to the hippocampus and striatum after 30 min of bilateral carotid occlusion [12]. Rats treated with caffeine, which increases A1AR expression in the brain, were more resistant to ischemia, underscoring the protective role of this receptor subtype [13]. In contrast, prolonged agonist treatment reduced A1AR expression and exacerbated the damage created by a subsequent ischemic episode [14]. Overall, these studies implicate the A1AR in neuroprotection under ischemic conditions.
Several mechanisms have been proposed to contribute to the cytoprotective role of adenosine. The major mechanism involves activation of presynaptic A1AR to decrease release of excitatory neurotransmitters such as glutamate [15-17]. These presynaptic A1AR activate K+ conductance, leading to hyperpolarization [18, 19] and inhibition of Ca2+ influx into the nerve terminal [12]. Such actions reduce neuronal excitability and firing rate [20]. Adenosine-mediated activation of a voltage-dependent Cl- conductance that is distinct from that activated by GABA can also contribute to neuronal hyperpolarization [21]. Adenosine also acts postsynaptically to reduce NMDA receptor-induced synaptic amplification and hyperpolarizes astrocytes [22], thereby facilitating glutamate uptake by these cells. In addition, adenosine acts as a vasodilator in most vascular beds and augments cerebral blood flow [23]. A more delayed protective action of the A1AR is evident in its role in scar formation and subsequent tissue remodeling [24].
In contrast to the A1AR, activation of the A2AAR exacerbates neuronal damage, as inferred from various observations of a protective role of A2AAR blockade in different models of ischemia [25-29]. In addition, genetic deletion of the A2AAR protected against transient focal brain ischemia in mice [30]. The proposed mechanism(s) underlying the protective action of A2AAR blockade could be broadly classified as inhibition of glutamate toxicity and reduction in inflammation. The primary action of A2AAR activation is suggested to be a reduction of glutamate transport into [31] or its release from astrocytes [32]. The A2AAR has also been implicated in the recruitment and activation of microglia in the brain in response to injury or stress (for review, see 29), supporting therapeutic potential for receptor blockade by antagonists.
Studies focusing on the A3AR in the brain indicate that its activation can cause either protection from or exacerbation of neuronal injury. For example, activation of the A3AR reduces ischemic preconditioning and promotes synaptic failure produced by oxygen and glucose deprivation in the rat hippocampus [7]. This apparent toxicity of A3AR agonists was associated with rapid receptor desensitization after the ischemic episode. In contrast, A3AR agonists were protective after a shorter period of ischemia (2-5 min) in the absence of significant A3AR desensitization [33]. However, activation of the A3AR reduced ischemic brain damage after transient ligation of the middle cerebral artery [34]. The A3AR has also been implicated in protecting cultured astrocytes from hypoxic damage [35]. The basis of these differing roles of the A3AR is not clear, but could be related to the degree of receptor activation [36]. Accordingly, a lower level of A3AR activation may confer protection, whereas a higher level of activation produces cytotoxicity [36].
2. ADENOSINE RECEPTOR AND OXIDATIVE STRESS
Over the past several years, our laboratory has studied mechanism(s) underlying the cytoprotective effects of adenosine. We have shown that adenosine contributes to cytoprotection by enhancing the activities of antioxidant enzymes (e.g., superoxide dismutase, catalase and glutathione peroxidase) via activation of their protein kinase C-mediated phosphorylation [37]. This mechanism could reduce the level of reactive oxygen species (ROS), which could be harmful to the cell, appears to be relevant in vivo, and may account for adenosine-induced reduction of lipid peroxidation in the cochlea [38]. The nonselective adenosine analog (R-phenylisopropyladenosine) protects cochlear explants from damage induced by cisplatin [39] and chinchilla cochlea from noise-induced loss of hair cells, presumably via the A1AR [40]. Activation of the A1AR also protects against cisplatin-induced toxicity [41] and noise-induced hearing loss [42].
The A1AR is responsive to oxidative stress. Not only does adenosine suppress ROS generation, but ROS induce A1AR expression. This feedback induction of the A1AR boosts the A1AR response during high oxidative stress and is mediated by the stress-regulated transcription factor, nuclear factor-κB [43]. NF-κB-dependent transcription is a novel mechanism by which cellular oxidative stress modulates A1AR and G protein-coupled receptors. This mechanism could influence drug responses in cells under oxidative stress. In addition, NF-κB has similar influences on A2AAR expression [44]. Details of these findings and their behavioral manifestations in mice in response to drug challenges are discussed below.
3. NUCLEAR FACTOR κB FUNCTION AND REGULATION
The transcription factor NF-κB, first described for its role in the transcription of the immunoglobulin κ light chain in B lymphocytes [45], mediates gene expression in response to a variety of stimuli. The mammalian NF-κB family consists of five members, p65 (RelA), RelB, c-Rel, p50/p105 (NF-κB1) and p52/p100 (NF-κB2), all of which possess a common Rel homology domain [46]. The Rel homology domain is a conserved region of 300 amino acids present at the N-terminal of these proteins and serves multiple functions, including DNA binding, dimerization, and interaction with the inhibitory subunit, IκB. The Rel domain also contains the nuclear localization sequence that allows nuclear translocation of the subunits after NF-κB activation [47]. In the inactive state, NF-κB exists in the cytoplasm as a homo- or heterodimer bound to IκB. The associated IκB can become phosphorylated secondary to stimuli that activate IκB kinase (IKK). Phosphorylation of IκB results in its ubiquitination and degradation, which releases the dimer for nuclear translocation and stimulation of the transcription of genes that contain the decameric κB consensus sequence.
The p65/p50 combination is the most abundant NF-κB dimer in most cells. Other transcriptionally active dimers are p65/p65, p50/c-Rel and p65/c-Rel. Some dimer combinations, such as p50/p50 and p52/p52 are believed to be inactive or repressive [48]. However, these dimers can stimulate transcription by binding to an IκB-like nuclear protein, BCL-3 [49]. The p50 subunit lacks the transcriptional activation domain that is present in p65 and c-Rel subunits, which likely accounts for property of transcriptional repression by the p50 homodimer [50].
In addition to IκB phosphorylation, dissociation, and degradation, other mechanisms can activate NF-κB. The p-100 mediated pathway involves an NF-κB inducing kinase (NIK) and IKK1. IKK1 phosphorylates p100, which leads to its ubiquitination and degradation to form the p52 subunit. The p52 subunit can then dimerize with RelB, and the dimer can enter the nucleus. In a similar fashion, constitutive processing of p105 to produce p50 occurs in the cytoplasm, allowing formation of p50/p50 dimers, which can then enter the nucleus [51].
4. NUCLEAR FACTOR κB REGULATION OF A1AR AND A2AAR EXPRESSION
4.1. Adenosine A1 Receptor
Early studies in our laboratories indicated that the A1AR is dynamically regulated by oxidative stress. In effect, the A1AR serves as a sensor of oxidative stress. These observations were initially derived from studying the effect of the chemotherapeutic agent cisplatin on the expression of A1AR in the chinchilla cochlea [38]. Cisplatin damages outer hair cells of the cochlea and produces significant hearing loss. We observed significant induction of A1AR in the cochlea prior to the induction of outer hair cell death. This induction likely represents a mechanism for combating, albeit ineffectively, the toxic effect of cisplatin in the cochlea.
To delineate the mechanism responsible for A1AR induction, we examined the role of NF-κB, which is known to be induced by oxidative stress. Using the inhibitor pyrrolidine dithiocarbamate (PDTC), we showed that cisplatin-induced induction of A1AR was directly related to activation of NF-κB. Induction of the A1AR also occurred in response to other chemotherapeutic agents (e.g., doxorubicin, mitroxantrone) that increase the production of ROS. Cisplatin-induced A1AR induction in cells was blunted by incubation with the ROS scavenger catalase or the inhibition of NF-κB with dexamethasone. Examination of the promoter of the human A1AR gene suggested the presence of an NF-κB consensus sequence located 623 base pairs upstream of the start site of promoter A construct (pBLPnif/PmtA) [52]. Transfection of a plasmid construct containing this promoter and firefly luciferase reporter gene in ductus deferens tumor (DDT) cells allowed us to demonstrate NF-κB dependent regulation of promoter activity [43]. These data suggest that oxidative stress, through activation of NF-κB, could induce the expression of the A1AR. Such a process would likely aid in cytoprotection under conditions of increased oxidative stress.
A1AR are also induced in response to other conditions associated with oxidative stress and inflammation. For example, A1AR mRNA and protein increased in brain after the induction of cerebral ischemia in rats [53]. In addition, nitric oxide acts through NF-κB to increase A1AR expression in a pheochromocytoma cell line (PC12 cells) and in isolated cortical neurons [54]. Noise exposure, which is associated with increased NADPH oxidase activity in the chinchilla cochlea, is also linked to the induction of the A1AR in the inner ear [55]. A1AR and A3AR were also induced in the gut in a rabbit model of ileitis [56], and A1AR was induced in kidney in association with oxidative stress produced by osmotic diuresis [57].
4.2. Adenosine A2A Receptor
Gene expression profiling in PC12 cells demonstrated that exposure to nerve growth factor (NGF) significantly reduced the expression of the A2AAR gene. The initial study reported a 3-fold decrease in A2AAR within 3 days of NGF treatment [58]. Further studies demonstrated that NGF caused rapid activation of NF-κB via the low affinity p75 NGF receptor, with a resultant reduction in A2AAR [44]. This response was mimicked by agents that activate NF-κB (e.g., ceramide, H2O2) [44]. These results support a functional role for the NF-κB consensus sites present in the A2AAR gene promoter [59]. Other mechanisms for NGF regulation of A2AAR depend on TrkA-, Src-, and Ras and are linked to the activation of extracellular regulated kinase and stress-activated protein kinase/c-Jun NH(2)-terminal kinase [60]. The involvement of multiple pathways in the regulation of the A2AAR by NGF may indicate different temporal profiles of activation of these pathways during development. In addition, the p75/NF-κB pathway could provide a general mechanism for control of A2AAR expression by neurotrophins that do not use TrkA for signaling (e.g., brain-derived neurotrophic factor).
5. A1AR EXPRESSION AND FUNCTION IN P50 KNOCKOUT MICE
To evaluate the significance of NF-κB in the regulation of the A1AR expression, we used mice with deletion of the gene for the p50 subunit of NF-κB. Although this gene knock-out (KO) renders these mice immune deficient, they are viable and able to reproduce [61]. However, deletion of the p65 subunit of NF-κB is embryonically lethal. Deletions of p65 can therefore generally be assessed only in vitro [62].
5.1. Biochemical Characterization
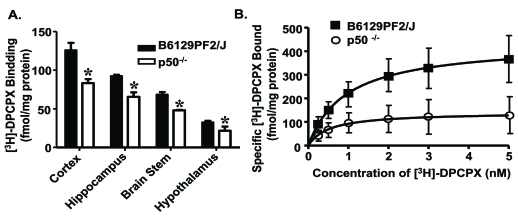
Brain cortical plasma membranes prepared from p50 KO mice demonstrated significant reductions in the levels of the A1AR, as determined by radioligand binding assays, Western blotting, immunohistochemistry, and real time polymerase chain reactions (PCR) [63] (Fig. 1). Similar reductions in A1AR were measured in the hippocampus, brain stem, hypothalamus and peripheral tissues (adrenal gland, kidney and spleen) Fig. (1). Levels of the guanine nucleotide coupling proteins (G proteins) alpha subunits (Gαi3) were also significantly reduced in the p50 KO mice, although the levels of Gαi1 were not changed, suggesting deficits in multiple components of the A1AR signaling cascade. Functionally, the deficit in A1AR/Gi protein expression was associated with reduced protection of neurons from apoptosis [63]. Overall, these findings suggest that activation of NF-κB is essential for maintaining normal A1AR expression and function and for survival of neurons in culture. Thus, A1AR promotes neuronal survival.
Fig. (1).
A1AR in brain of p50 KO mice. (A) A1AR binding in F2 and p50 KO mice (n = 4 per strain) was quantified in membrane fractions of cortex, hippocampus, brain stem, and hypothalamus using the specific A1AR antagonist [3H]-DPCPX (1 nM). Values are expressed as fmol/mg protein and represent mean ± S.E.M. for three independent experiments with samples assayed in triplicate (* p < 0.05, Student’s t-test). (B) Saturation binding analysis of cortical membrane for A1AR with [3H]-DPCPX in the absence (total binding) or presence (nonspecific binding) of 0.5 mM theophylline (n = 5 per strain). Curves were fitted to a one-site model using GraphPad Prism. Modified from Jhaveri et al., 2007, Neuroscience 146, 415-426, with permission from Elsevier.
5.2. Sleep
Adenosine and AR have been extensively studied for their involvement in the regulation of sleep and wakefulness. Previous studies established adenosine as a sleep-inducing factor that increases in the basal forebrain during prolonged wakefulness [64]. Similarly, reduced expression of A1AR in the magnocellular cholinergic region of the basal forebrain by antisense oligonucleotides alters sleep [65]. However, recent studies indicate a complex role for A1AR in mediating sleep and arousal. For example, evaluation of A1AR and A2AAR KO mice showed that caffeine promotes arousal through its action at the A2AAR, but not the A1AR [66]. However, a subsequent study by the same group found that activation of the A1AR in the tuberomammillary nucleus could mediate non-rapid eye movement (non-REM) sleep through suppression of histamine release [67].
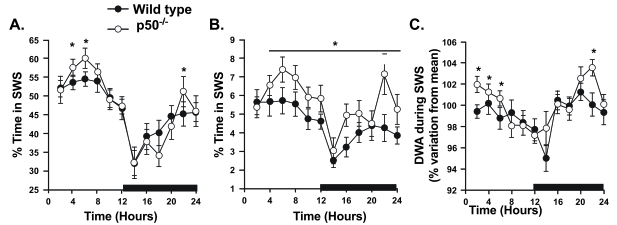
We evaluated p50 KO mice to determine whether their deficit in the A1AR would affect sleep (Fig. 2). The p50 KO mice had more slow wave and rapid eye movement (REM) sleep under normal conditions than did wild type mice (B6129PF2/J strain) [68]. This finding was surprising, because based on the purported role of the A1AR in regulating sleep in rats [65], less A1AR expression should be associated with reduced sleep duration. Dysregulation of another AR (such as the A2AAR) or other factors (such as increased inflammatory cytokine or prostaglandin D2 production) in the p50 KO mice could account for this finding. In addition, p50 KO mice showed an accelerated return to normal sleep after sleep deprivation. Finally, p50 KO mice showed a greater suppression of SWS after administration of bacterial lipopolysaccharide (LPS) challenge. These data support a role of NF-κB in regulating normal sleep and mediating the responses to sleep deprivation and immune challenge. However, the involvement of AR in mediating the sleep profiles of p50 KO mice remains undetermined.
Fig. (2).
Patterns of sleep in B6129PF2/J (wild type) and p50 knockout (KO) mice. The amount of time spent in slow-wave sleep (SWS; A) and rapid-eye-movement sleep (REMS; B) are expressed as percentage of recording time spent in each state. Delta wave amplitude during SWS (C) is expressed as a percentage of mean values measured over the entire 24 h recording period. The black bar along the x-axis denotes the dark phase of the diurnal cycle. Solid circles, F2 (wild-type) mice (n = 16); open circles, p50 KO mice (n =14). Each datum point denotes a 2-h average ± S.E.M. *Significance at the specified time point (p < 0.05 by ANOVA and independent Student’s t-test). Modified from Jhaveri et al. (2006), Am J Physiol Regul Integr Comp Physiol 291, R1516-1526, with permission from the American Physiological Society.
In addition to differences in sleep, the p50 KO mice generally exhibited lower core body temperatures than did the wild-type mice. LPS produced a transient hyperthermia in wild type mice, but persistent hypothermia in p50 KO mice [68]. This finding suggests that the p50 KO mice either lacked a normal homeostatic response to hypothermia or developed a more prolonged and severe response to LPS, in either case implicating NF-κB in the normal reaction. This finding is also interesting in light of a reported temperature-dependent activation of NF-κB in mouse liver [69]. LPS-stimulated TNF-( levels were higher in p50 KO versus the wild type mice, which could also contribute to the persistent hypothermia observed in the p50 KO mice.
6. A2AAR EXPRESSION AND FUNCTION IN P50-/- MICE
6.1. Biochemical Characterization
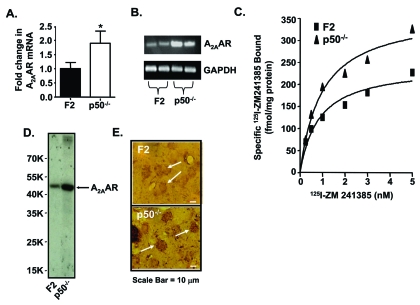
A2AAR expression in brain is localized largely in the striatum. Striatal A2AAR, together with dopamine D2 receptor (D2R), participates in the control of locomotor activity. These receptors demonstrate mutual antagonistic actions by physically interacting with each other and differentially modulating post-receptor signal transduction pathways. As such, inhibition of the A2AAR by caffeine promotes hyperactivity by reducing adenosine inhibition of D2R function. Recent in vitro evidence indicates that expression of the A2AAR [44] and the D2R [70] are differentially regulated by NF-κB, highlighting another potential difference between these two receptors. To extend these observations, we evaluated the in vivo regulation of these receptors using p50 KO mice, with the wild type B6129PF2/J (F2) mice serving as controls (Fig. 3). Quantification of adenosine receptor (AR) subtypes in striata from p50 KO mice using PCR, radioligand binding assays and immunocytochemistry showed more A2AAR mRNA and protein, but less A1AR mRNA and protein as compared with F2 mice. Striata from p50 KO mice also showed less D2R mRNA and [3H]-methylspiperone binding [71]. These studies suggest that absence of the NF-κB p50 subunit leads to dysregulation of ARs and D2R in the striatum, as observed for the A1AR in other brain regions. Overall, these studies suggest a role of the NF-κB p50 subunit and/or the composition of the heterodimers in regulation of striatal A1AR, A2AAR and D2R.
Fig. (3).
A2AAR in striata of p50 KO versus F2 mice. (A) Real time PCR (A; n = 10 per group). (B) Traditional PCR (n = 2 per group). (C) Saturation curves with inset. Scatchard plot for 125I-ZM241385 binding to A2AAR in mouse crude striatal membranes. The plots shown represent pooled striata from 4 mice, with samples at each concentration assayed in triplicate. This analysis was repeated on 3 different pooled samples with similar results. (D) Affinity purification of the A2AAR from F2 and p50 KO striata (n = 4 per strain). E) Immunohistochemistry of striatal A2AAR from F2 and p50 KO mice (n=4 per strain). Arrowheads depict specific A2AAR-immunolabeled neurons. The sections represent a 400-fold magnification of the image. Scale bar represents 10 µm. Reprinted from Xie et al., 2007, Life Sci 81, 1031-1041, with permission from Elsevier.
6.2. Caffeine-Induced Locomotor Activation
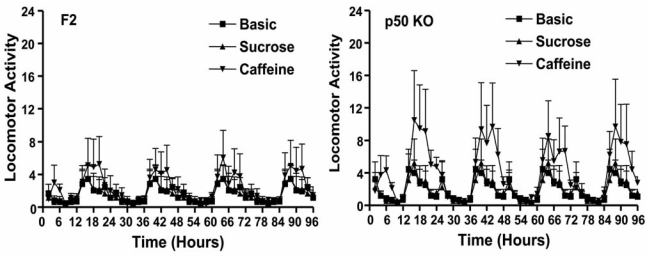
Alterations in the balance of A2AAR and D2R function in striatum could alter locomotor activity. Although F2 and p50 KO mice did not differ in terms of basal locomotor activity, the p50 KO mice demonstrated hypersensitivity to caffeine-induced behavioral activation during the dark phase (when mice are normally active) but not during the light phase (when mice are normally asleep) (Fig. 4) [72]. Similarly, p50 KO mice showed behavioral hypersensitivity to intraperitoneal injections of SCH58261, an A2AAR antagonist [72]. In contrast, intraperitoneal injections of the selective A1AR antagonist, DPCPX, increased locomotor activity in both F2 and p50 KO mice, indicating that the increased sensitivity of p50 KO mice to caffeine likely did not involve the A1AR. The intraperitoneal administration of a combination of SCH 58261 and DPCPX produced an apparent synergistic increase in locomotor activity in both strains of mice. Administration of the D2R antagonist, raclopride, reduced the stimulatory effect of SCH58261 on locomotor activity. This latter finding suggests an involvement of D2R activation in mediating locomotor activation after A2AAR blockade. The locomotor activity data support several conclusions: 1) the p50 KO mice are more sensitive to A2AAR antagonists than are wild type F2 mice, 2) the higher striatal A2AAR in KO mice could provide a larger striatal target for inhibition by caffeine, thus accounting for the heightened behavioral sensitivity and 3) the increased sensitivity to A2AAR antagonists results, at least in part, from increased D2R activity.
7. CONCLUSION
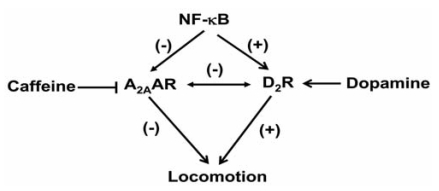
NF-κB plays a significant role in the regulation of genes involved in response to immune activation and oxidative stress. To date, only a few studies have detailed the involvement of this transcription factor in regulation of G protein coupled receptors. In this review, we describe differential regulation of the A1AR and A2AAR by NF-κB and show that these receptors are differentially expressed in mice deficient in the p50 subunit of NF-κB. Behavioral studies performed on these mice suggest a role for the p50 subunit and the A1AR in the regulation of normal sleep, the response to sleep deprivation, and the sleep patterns that develop after an immune challenge. The p50 KO mice also show behavioral sensitivity to caffeine and A2AAR antagonists, which may be related to the greater expression of the A2AAR in the striatum and possibly to increased dopamine release and D2R activity in the KO mice (Fig. 5). However, the potential for various A1AR, A2AAR and D2R heterodimer combinations [73] with different functions in the striatum of the p50 KO mice complicates interpretation of their behavioral hypersensitivity to caffeine. Nevertheless, the p50 KO mice could provide a useful model for studying the expression and integration of multiple NF-κB targets in the regulation of behavior.
Fig. (5).
Proposed model of NF-κB interactions with different A2AAR and D2R in the striatum. Activation of NF-κB decreases A2AAR expression but up regulates D2R expression and thereby influences the contribution of these receptors to locomotion. Such a model could contribute to differing efficacies of drugs such as caffeine (an antagonist of the A2AAR) and D2R agonists (such as dopamine).
Fig. (4).
Locomotor activity of p50 KO and F2 mice in response to caffeine ingestion. Mice of both strains were randomly assigned into caffeine treatment or control groups (n=14 per group). Caffeine was administered in drinking water (300 mg/L) in combination with sucrose (20 g/L). Control groups received only sucrose (20 g/L) in drinking water. Mice received sequentially regular drinking water for 48 h (black circles), sucrose only in drinking water for the next 48 h (red circles), and caffeine with sucrose for the final 96 h (blue circles). Reprinted from Xie et al., 2009, Life Sci 85, 226-234, with permission from Elsevier.
ACKNOWLEDGEMENTS
This work was supported in part from NIH grants to VR (CA135494) and to LT (K26-RR17543 and AR052819) and by the Excellence in Academic Medicine program of the Southern Illinois University School of Medicine (LT).
REFERENCES
- 1.Cunha RA. Adenosine acts as a neuromodulator and as a homeostatic regulator in the central nervous system: Different roles, different sources and different receptors. Neurochem. Int. 2001;38:107–125. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 2.Olsson RA, Pearson JD. Cardiovascular purinoceptors. Physiol. Rev. 1990;70:761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- 3.Narimatsu E, Aoki M. Transient depression of excitatory synaptic transmission induced by adenosine uptake inhibition in rat hippocampal slices. Brain Res. 2000;862:284–287. doi: 10.1016/s0006-8993(00)02123-5. [DOI] [PubMed] [Google Scholar]
- 4.Fredholm B. Adenosine receptors as drug targets. Exp. Cell. Res. 2010;318:1284–1288. doi: 10.1016/j.yexcr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson's disease. Trends Pharmacol. Sci. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Zhou FQ, Olah ME, Li C, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of a novel adenosine receptor: the A3 adenosine receptor. Proc. Natl. Acad. Sci. U.S.A. 1991;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pugliese AM, Latini S, Corradetti R, Pedata F. Brief, repeated, oxygen-glucose deprivation episodes protect neurotransmission from a longer ischemic episode in the in vitro hippocampus: role of adenosine receptors. Br. J. Pharmacol. 2003;140:305–314. doi: 10.1038/sj.bjp.0705442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivkees SA, Price SL, Zhou FC. Immunohistochemical detection of A1 adenosine receptor in rat brain with emphasis on localization in the hippocampal formation, cerebral cortex, cerebellum and basal ganglia. Brain Res. 1995;677:193–203. doi: 10.1016/0006-8993(95)00062-u. [DOI] [PubMed] [Google Scholar]
- 9.Matsumato K, Graf R, Rosner G, Schimada N, Weiss WD. Flow thresholds for extracellular purine catabolite elevation in rat focal ischemia. Brain Res. 1992;579:309–314. doi: 10.1016/0006-8993(92)90066-i. [DOI] [PubMed] [Google Scholar]
- 10.Evans MC, Swan JH, Meldrum BS. An adenosine analogue, 2-chloroadenosine, protects against long term development of ischemic cell loss in the rat hippocampus. Neurosci. Lett. 1987;83:287–292. doi: 10.1016/0304-3940(87)90101-7. [DOI] [PubMed] [Google Scholar]
- 11.Januszewicz von Lubitz DK, Dambrosia JM, Redmond DJ. Neuroprotective effect of cyclohexyl adenosine in treatment of cerebral ischemia in gerbils. Neuroscience. 1989;30:451–462. doi: 10.1016/0306-4522(89)90265-0. [DOI] [PubMed] [Google Scholar]
- 12.Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB. Neuroprotective role of adenosine in cerebral ischaemia. Trends Pharmacol. Sci. 1992;13:439–445. doi: 10.1016/0165-6147(92)90141-r. [DOI] [PubMed] [Google Scholar]
- 13.Rudolphi KA, Keil M, Fastbom J, Fredholm BB. Ischemic damage in gerbil hippocampus is reduced following up-regulation of adenosine (A1) receptors by caffeine treatment. Neurosci. Lett. 1989;103:275–280. doi: 10.1016/0304-3940(89)90112-2. [DOI] [PubMed] [Google Scholar]
- 14.von Lubitz DK, Lin RC, Melman N, Ji XD, Carter MF, Jacobson KA. Chronic administration of selective adenosine A1 receptor agonist or antagonist in cerebral ischemia. Eur. J. Pharmacol. 1994;256:161–167. doi: 10.1016/0014-2999(94)90241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolphin AC, Archer ER. An adenosine agonist inhibits and cyclic AMP analogue enhances the release of glutamate but not GABA from slices of rat dentate gyrus. Neurosci. Lett. 1983;43:49–54. doi: 10.1016/0304-3940(83)90127-1. [DOI] [PubMed] [Google Scholar]
- 16.Coradetti R, Lo Conte G, Moroni F, Passani MB, Pepeu G. Adenosine decreases aspartate and glutamate release from rat hippocampal slices. Eur. J. Pharmacol. 1984;104:19–26. doi: 10.1016/0014-2999(84)90364-9. [DOI] [PubMed] [Google Scholar]
- 17.Fastbom J, Fredholm BB. Inhibition of [3H]-glutamate release from rat hippocampal slices by L-isopropyladenosine. Acta Physiol. Scand. 1985;125:121–123. doi: 10.1111/j.1748-1716.1985.tb07698.x. [DOI] [PubMed] [Google Scholar]
- 18.Fredholm BB, Dunwiddie TV. How does adenosine inhibit neurotransmitter release? Trends Pharmacol. Sci. 1988;9:130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- 19.Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine A1 receptor, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc. Natl. Acad. Sci. U. S. A. 1995;92:4666–4670. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostopoulos GK, Phillis JW. Purinergic depression of neurons in different areas of the brain. Exp. Neurol. 1977;55:719–924. doi: 10.1016/0014-4886(77)90296-5. [DOI] [PubMed] [Google Scholar]
- 21.Mager R, Ferroni S, Schubert P. Adenosine modulates a voltage-dependent chloride conductance in cultured neurons. Brain Res. 1990;532:58–62. doi: 10.1016/0006-8993(90)91741-x. [DOI] [PubMed] [Google Scholar]
- 22.Hosli L, Hosli E, Della Briotta G. Electrophysiological evidence for adenosine receptors on astrocytes of cultured rat central nervous system. Neurosci. Lett. 1987;79:108–112. doi: 10.1016/0304-3940(87)90680-x. [DOI] [PubMed] [Google Scholar]
- 23.Phillis JW. Adenosine in the control of the cerebral circulation. Cerebrovasc. Brain Metabol. Rev. 1989;1:26–54. [PubMed] [Google Scholar]
- 24.Fischer C, Sharma HS, Karliczek GF, Schaper W. Expression of vascular permeability factor/vascular endothelial growth factor in pig cerebral microvascular endothelial cells and its upregulation by adenosine. Brain Res. Mol. Brain Res. 1995;28:141–148. doi: 10.1016/0169-328x(94)00193-i. [DOI] [PubMed] [Google Scholar]
- 25.Gao Y, Phillis JW. CGS 15943, an adenosine A2 receptor antagonist, reduces cerebral ischemic injury in the Mongolian gerbil. Life Sci. 1994;55:PL61–65. doi: 10.1016/0024-3205(94)00889-2. [DOI] [PubMed] [Google Scholar]
- 26.Phillis JW. The effects of selective A1 and A2a adenosine receptor antagonists on cerebral ischemic injury in the gerbil. Brain Res. 1995;705:79–84. doi: 10.1016/0006-8993(95)01153-6. [DOI] [PubMed] [Google Scholar]
- 27.von Lubitz DK, Lin RC, Jacobson KA. Cerebral ischemia in gerbils: effects of acute and chronic treatment with adenosine A2A receptor agonist and antagonist. Eur. J. Pharmacol. 1995;287:295–302. doi: 10.1016/0014-2999(95)00498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monopoli A, Lozza G, Forlani A, Mattavelli A, Ongini E. Blockade of adenosine A2A receptors by SCH 58261 results in neuroprotective effects in cerebral ischaemia in rats. Neuroreport. 1998;9:3955–3959. doi: 10.1097/00001756-199812010-00034. [DOI] [PubMed] [Google Scholar]
- 29.Cunha RA. Neuroprotection by adenosine in the brain: From A1 receptor activation to A2A receptor blockade. Purinergic Signal. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J-F, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pintor A, Galluzzo M, Grieco R, Pexxola A, Reggio R, Popoli P. Adenosine A2A receptor antagonists prevent the increase in striatal glutamate levels induced by glutamate uptake inhibitors. J. Neurochem. 2004;89:152–156. doi: 10.1111/j.1471-4159.2003.02306.x. [DOI] [PubMed] [Google Scholar]
- 32.Li XX, Nomura T, Aihara H, Nishizaki T. Adenosine enhances glial glutamate efflux via A2a adenosine receptors. Life Sci. 1990;68:1343–1350. doi: 10.1016/s0024-3205(00)01036-5. [DOI] [PubMed] [Google Scholar]
- 33.Pugliese AM, Coppi E, Volpini R, Cristalli G, Corradetti R, Jeong LS, Jacobson KA, Pedata F. Role of adenosine A3 receptors on CA1 hippocampal neurotransmission during oxygen-glucose deprivation episodes of different duration. Biochem. Pharmacol. 2007;74:768–79. doi: 10.1016/j.bcp.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen GJ, Harvey BK, Shen H, Chou J, Victor A, Wang Y. Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci. Res. 2006;84:1848–1855. doi: 10.1002/jnr.21071. [DOI] [PubMed] [Google Scholar]
- 35.Björklund O, Shang M, Tonazzini I, Elisabetta Daré E, Fredholm BB. Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. Eur. J. Pharmacol. 2008;596:6–13. doi: 10.1016/j.ejphar.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Abbracchio MP, Cattabeni F. Brain adenosine receptor as targets for therapeutic intervention in neurodegenerative diseases. Ann. NY Acad. Sci. 1999;890:79–92. doi: 10.1111/j.1749-6632.1999.tb07983.x. [DOI] [PubMed] [Google Scholar]
- 37.Maggirwar SB, Dhanraj DN, Somani SM, Ramkumar V. Adenosine acts as an endogenous activator of the cellular antioxidant defense system. Biochem. Biophys. Res. Commun. 1994;201:508–515. doi: 10.1006/bbrc.1994.1731. [DOI] [PubMed] [Google Scholar]
- 38.Ford MS, Nie Z, Whitworth C, Rybak LP, Ramkumar V. Up-regulation of adenosine receptors in the cochlea by cisplatin. Hear. Res. 1997;111:143–152. doi: 10.1016/s0378-5955(97)00103-2. [DOI] [PubMed] [Google Scholar]
- 39.Kopke RD. Use of organotypic cultures of Corti’s organ to study the protective effects of antioxidant molecules on cisplatin-induced damage to auditory hair cells. Am. J. Otol. 1997;18:559–571. [PubMed] [Google Scholar]
- 40.Hu BH. R-Phenylisopropyladenosine attenuates noise-induced hearing loss in the chinchilla. Hear. Res. 1997;113:198–206. doi: 10.1016/s0378-5955(97)00143-3. [DOI] [PubMed] [Google Scholar]
- 41.Whitworth CA, Ramkumar V, Jones B, Tsukasaki N, Rybak LP. Protection against cisplatin ototoxicity by adenosine agonists. Biochem. Pharmacol. 2004;67:1801–1807. doi: 10.1016/j.bcp.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Wong AC, Guo CX, Gupta R, Housley GD, Thorne PR, Vlajkovic SM. Post exposure administration of A1 adenosine receptor agonists attenuates noise-induced hearing loss. Hear. Res. 2010;260:81–88. doi: 10.1016/j.heares.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Nie Z, Mei Y, Ford M, Rybak L, Marcuzzi A, Ren H, Stiles GL, Ramkumar V. Oxidative stress increases A1 adenosine receptor expression by activating nuclear factor kappa B. Mol. Pharmacol. 1998;53:663–669. doi: 10.1124/mol.53.4.663. [DOI] [PubMed] [Google Scholar]
- 44.Nie Z, Mei Y, Malek RL, Marcuzzi A, Lee NH, Ramkumar V. A role of p75 in NGF-mediated down-regulation of the A2A adenosine receptors in PC12 cells. Mol. Pharmacol. 1999;56:947–954. doi: 10.1124/mol.56.5.947. [DOI] [PubMed] [Google Scholar]
- 45.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. [PubMed] [Google Scholar]
- 46.Hayden M, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 47.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 48.Plaksin D, Baeuerle PA, Eisenbach L. KBF1 (p50 NF-kappa B homodimer) acts as a repressor of H-2Kb gene expression in metastatic tumor cells. J. Exp. Med. 1993;177:1651–1662. doi: 10.1084/jem.177.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franzoso G, Bours V, Park S, Tomita-Yamaguchi M, Kelly K, Siebenlist U. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-κB mediated inhibition. Nature. 1992;359:339–342. doi: 10.1038/359339a0. [DOI] [PubMed] [Google Scholar]
- 50.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 51.Sun SC, Ley SC. New insights into NF-κB regulation and function. Trends Immunol. 2008;29:469–478. doi: 10.1016/j.it.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren H, Stiles GL. Separate promoters in the human A1 adenosine receptor gene direct the synthesis of distinct messenger RNAs that regulate receptor abundance. Mol. Pharmacol. 1995;48:975–980. [PubMed] [Google Scholar]
- 53.Lai DM, Tu YK, Liu IM, Cheng JT. Increase of adenosine A1 receptor gene expression in cerebral ischemia of Wistar rats. Neurosci. Lett. 2005;387:59–61. doi: 10.1016/j.neulet.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 54.Jhaveri KA, Toth LA, Sekino Y, Ramkumar V. Nitric oxide serves as an endogenous regulator of neuronal adenosine A1 receptor expression. J. Neurochem. 2006a;99:42–53. doi: 10.1111/j.1471-4159.2006.04095.x. [DOI] [PubMed] [Google Scholar]
- 55.Ramkumar V, Whitworth CA, Pingle SC, Hughes LF, Rybak LP. Noise induces A1 adenosine receptor expression in the chinchilla cochlea. Hear. Res. 2004;188:47–56. doi: 10.1016/S0378-5955(03)00344-7. [DOI] [PubMed] [Google Scholar]
- 56.Sundaram U, Hassanain H, Suntres Z, Yu JG, Cooke HJ, Guzman J, Christofi FL. Rabbit chronic ileitis leads to up-regulation of adenosine A1/A3 gene products, oxidative stress, and immune modulation. Biochem. Pharmacol. 2003;65:1529–38. doi: 10.1016/s0006-2952(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 57.Pingle SC, Mishra S, Marcuzzi A, Bhat SG, Sekino Y, Rybak LP, Ramkumar V. Osmotic diuretics induce adenosine A1 receptor expression and protect renal proximal tubular epithelial cells against cisplatin-mediated apoptosis. J. Biol. Chem. 2005;279:43157–43167. doi: 10.1074/jbc.M405666200. [DOI] [PubMed] [Google Scholar]
- 58.Lee NH, Weinstock KG, Kirkness EF, Earle-Hughes JA, Fuldner RA, Marmaros S, Glodek A, Gocayne JD, Adams MD, Kerlavage AR, Fraser CM, Venter JC. Comparative expressed-sequence-tag analysis of differential gene expression profiles in PC-12 cells before and after nerve growth factor treatment. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8303–8307. doi: 10.1073/pnas.92.18.8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu YY, Tu KH, Lee YC, Kuo ZJ, Lai HL, Chern Y. Characterization of the rat A2a adenosine receptor gene. DNA Cell. Biol. 1996;15:329–337. doi: 10.1089/dna.1996.15.329. [DOI] [PubMed] [Google Scholar]
- 60.Malek RL, Nie Z, Ramkumar V, Lee NH. Adenosine A2A receptor mRNA regulation by nerve growth factor is TrkA-, Src-, and Ras-dependent via extracellular regulated kinase and stress-activated protein kinase/c-Jun NH(2)-terminal kinase. J. Biol. Chem. 1999;274:35499–35504. doi: 10.1074/jbc.274.50.35499. [DOI] [PubMed] [Google Scholar]
- 61.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Target disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 62.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 63.Jhaveri KA, Reichensperger J, Toth LA, Sekino Y, Ramkumar V. Reduced basal and lipopolysaccharide-stimulated adenosine A1 receptor expression in the brain of NF-κB p50-/- mice. Neuroscience. 2007;146:415–426. doi: 10.1016/j.neuroscience.2006.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J. Neurosci. 2003;23:4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 67.Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19992–19997. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jhaveri KA, Ramkumar V, Trammell RA, Toth LA. Spontaneous, homeostatic, and inflammation-induced sleep in NF-kappaB p50 knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006b;291:R1516–1526. doi: 10.1152/ajpregu.00262.2006. [DOI] [PubMed] [Google Scholar]
- 69.Kuboki S, Okaya T, Schuster R, Blanchard J, Denenberg A, Wong HR, Lentsch AB. Hepatocyte NF-kappaB activation is hepatoprotective during ischemia-reperfusion injury and is augmented by ischemic hypothermia. Am. J. Physiol. Gastrointest. Liver. Physiol. 2007;292:G201–G207. doi: 10.1152/ajpgi.00186.2006. [DOI] [PubMed] [Google Scholar]
- 70.Fiorentini C, Guerra N, Facchetti M, Finardi A, Tiberio L, Schiaffonati L, Spano P, Missale C. Nerve growth factor regulates dopamine D2 receptor expression in prolactinoma cell lines via p75NGFR-mediated activation of nuclear factor-κB. Mol. Endocrinol. 2002;16:353–366. doi: 10.1210/mend.16.2.0773. [DOI] [PubMed] [Google Scholar]
- 71.Xie X, Jhaveri KA, Ding M, Hughes LF, Toth LA, Ramkumar V. Expression of striatal adenosine and dopamine receptors in mice deficient in the p50 subunit of NF-κB. Life Sci. 2007;81:1031–1041. doi: 10.1016/j.lfs.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie X, Mhaskar Y, Arbogast LA, Trammell RA, Hughes LF, Toth LA. Adenosine receptor antagonists and behavioral activation in NF-κB p50 subunit knockout mice. Life Sci. 2009;85:226–234. doi: 10.1016/j.lfs.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferré S, Ciruela F, Woods AS, Lluis C, Franco R. Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci. 2007;30:440–446. doi: 10.1016/j.tins.2007.07.001. [DOI] [PubMed] [Google Scholar]