Abstract
Migraine is one of the commonest neurological disorders. Despite intensive research, its exact pathomechanism is still not fully understood and effective therapy is not always available. One of the key molecules involved in migraine is glutamate, whose receptors are found on the first-, second- and third-order trigeminal neurones and are also present in the migraine generators, including the dorsal raphe nucleus, nucleus raphe magnus, locus coeruleus and periaqueductal grey matter. Glutamate receptors are important in cortical spreading depression, which may be the electrophysiological correlate of migraine aura.
The kynurenine metabolites, endogenous tryptophan metabolites, include kynurenic acid (KYNA), which exerts a blocking effect on ionotropic glutamate and α7-nicotinic acetylcholine receptors. Thus, KYNA and its derivatives may act as modulators at various levels of the pathomechanism of migraine. They can give rise to antinociceptive effects at the periphery, in the trigeminal nucleus caudalis, and may also act on migraine generators and cortical spreading depression. The experimental data suggest that KYNA or its derivatives might offer a novel approach to migraine therapy.
Keywords: Cortical spreading depression, glutamate, kynurenic acid, kynurenine metabolites, migraine, migraine generators, trigeminal system.
MIGRAINE
Migraine is one of the idiopathic headache syndromes [1], and one of the commonest neurological disorders [2]. Despite intensive research, the exact pathomechanism of migraine is still not fully understood and complete preventive and attack therapy can not always be achieved. Activation of the peripheral and central arms of the trigeminal system (TS) are known to be crucial in the attack [3]. This activation may be related to cortical spreading depression (CSD) or to the activity of distinct areas of the brain stem, known as migraine generators [4, 5].
The fundamental mechanism of the migraine attack involves activation of the trigeminovascular system. Through a trigger mechanism, vasodilatation of the dural and pial blood vessels occurs, which can stimulate the perivascular trigeminal primary nerve endings. The activated nociceptors release neuropeptides at the periphery, including calcitonin gene-related peptide (CGRP), substance P and neurokinin A [6]; the levels of CGRP and substance P are elevated during migraine attacks in humans and in animal migraine models [7]. The released neuropeptides cause sterile neurogenic inflammation in the dura mater, in the course of which the blood vessels further dilate, plasma protein extravasation occurs, the mast cells degranulate and release histamine, and polymorphonuclear leukocytes appear [8]. These reactions can be observed in animal models of migraine too [9, 10]. The released inflammatory substances stimulate the trigeminal first-order neurones, leading to peripheral sensitization [11]. This usually evolves within 30 minutes, and gives rise to a throbbing head pain that is aggravated by activities that increase the intracranial pressure, including physical exercise, bending down, coughing and sneezing [12].
The cell bodies of the trigeminal pseudounipolar first-order neurones are located in the trigeminal ganglion (TG). The peripheral projections of these neurones partially innervate the intracranial pain structures, including the dural and pial blood vessels, the large blood vessels of the brain, the dural sinuses and the dura and pia mater, while the central projections end on the second-order neurones of the trigeminal nucleus caudalis (TNC), located in the medulla and the upper portion of the spinal cord. The activation of these first-order neurones leads to an increase in the glutamate level in the TNC [13] and, presumably via the N-methyl-D-asparate (NMDA) glutamate receptors [14], to activation of the second-order neurones [15]. Besides the NMDA receptors, all the other glutamate receptors are present in the TNC [16], and therefore they can also contribute to this process, which is confirmed by the fact that their antagonists are able to inhibit the increase in the number of c-Fos-immunoreactive (IR) neurones [17] and the evoked potential responses [18] in the TNC. Furthermore, the activation of second-order neurones can be modulated through α7-nicotinic acetylcholine (nACh) receptors, which act presynaptically on the transmission of nociceptive information to the central nervous system [19, 20].
Besides peripheral sensitization, the persistent activation of second-order trigeminal neurones evolves to central sensitization in migraineurs, with the appearance of cutaneous allodynia of the scalp and face [15, 21], when non-nociceptive stimuli produce pain. The central sensitization comprises an exaggerated sensory drive, mediated in part by glutamate receptor activation, since increases in extracellular glutamate are correlated with changes in sensory thresholds on the face of the rat [13]. Moreover, the activated second-order trigeminal neurones have functional connections to other important brain stem centres, such as the nucleus tractus solitarius, which can result in nausea and vomiting. Further activation and sensitization of the TS can provoke the sensitization of the third-order neurones from the thalamus to the cortex, which leads to other symptoms of migraine, including photophobia, phonophobia, osmophobia and allodynia of the extremities [21].
Migraine attacks are casually linked with the activation of distinct brain stem nuclei, known as migraine generators, which include the dorsal raphe nucleus (DRN), the nucleus raphe magnus (NRM), the locus coeruleus (LC) and the periaqueductal grey matter (PAG) [5, 22, 23], which are components of the ascending and descending pain pathways. The importance of these areas in migraine is underlined by the fact that migraine attacks could be induced in human subjects by stimulation of the PAG with an implanted electrode [24]. A possible explanation is that the above-described areas may be dysfunctional [25] and perhaps lose their natural antinociceptive function, resulting in headache. Glutamate appears at this level too since its antagonist can decrease the activity of the NRM [26], and its level is increased after stimulation of the sciatic nerve and mechanical foot shock in the LC [27] or after neuronal stimulation in the PAG [28].
Another potential trigger mechanism of migraine involves CSD. This is a slow continuous spread of excitation, followed by depression [29], and is accompanied by slowly-spreading cortical hypoperfusion [30]. It is widely accepted that CSD is the basis of migraine aura [31], which includes various transient neurologic symptoms, the most common of which are visual symptoms. In the process of CSD, activation of the neuronal apical dendrites [32] and astrocytes [33] seems to be important. The latter can link neuronal and vascular events [34]. Although it is not fully understood how CSD can trigger migraine attacks, under certain experimental conditions in animal models, CSD is able to activate the trigeminovascular afferents [4], increase the persistent blood flow and cause plasma protein extravasation in the dura mater [35] and hence to initiate the above-described sensitization procedures in the TS. Another connection between CSD and trigeminal activation may be glutamate and its receptors [36], which play important roles in the generation and propagation of CSD [37].
ROLE OF GLUTAMATE IN MIGRAINE
Glutamate is known to play an important role in primary afferent neurotransmission and nociception [38], and numerous human and animal studies suggest that glutamate is additionally crucial in the pathomechanism of migraine [39]. Measurements of the level of glutamate in the plasma and platelets in migraine patients led to conflicting results: there have been reports of elevated basal glutamate levels in the plasma and platelets of migraineurs, which are further enhanced during the attacks [40, 41], while other studies have described lower or similar levels to those in control subjects [41, 42]. Elevated levels of glutamate in the cerebrospinal fluid have been measured during attacks in migraineurs, which favours the hypothesis of persistent neuronal hyperexcitability in the disorder [42]. The glutamate receptor antagonists can abolish the aura in patients with familial hemiplegic migraine [43] and headache [44]. Animal and human localization studies have revealed glutamate receptors in the TS [16, 45, 46, 47]. Irritation of the trigeminal nerve results in an increased glutamate level in the TNC [13]. L-Glutamate and NMDA can excite the trigeminothalamic nociceptive neurones [14, 48], and NMDA receptor activation mediates nociceptive transmission in the TNC [14]. The administration of glutamate receptor antagonists mitigated the activation of second-order neurones, i.e. the increase in the number of c-Fos-IR neurones [17, 49], the local blood flow changes [50] and the evoked potential responses [18] in the TNC and the dural plasma protein extravasation [51]. Furthermore, the NMDA receptors in the thalamus contribute to the development and maintenance of inflammation-induced hyperalgesia [52].
Glutamate and its receptors are present in the migraine generators too, and seem to be important from the aspect of nociception. For example, the broad-spectrum excitatory amino acid (EAA) antagonist kynurenic acid (KYNA) can decrease the effect of low-intensity electrical stimulation of the nucleus cuneiformis in the NRM [26], and can reduce the response of the serotoninergic neurones in the DRN [53, 54, 55, 56]. Moreover, electrical stimulation of the sciatic nerve and mechanical foot shock enhanced the rates of glutamate release from the LC [27]. The excitatory effect on the LC of glutamate released from the terminals of the nucleus paragigantocellularis, the main source of glutamate in the LC [57], was inhibited by glutamate receptor antagonists [57, 58]. Finally, in the PAG, the glutamate level was increased after neuronal stimulation [28]. These results suggest that glutamate and its receptors may well be important in the triggering of migraine attacks too, and not merely during headache.
In the generation of CSD, a number of different ion pumps and channels are involved [59], among which NMDA receptors and therefore glutamate seem to play crucial roles: (i) NMDA receptor antagonists can inhibit CSD [60], (ii) glutamate is released during CSD under both in vivo and in vitro conditions [61, 62] and (iii) the administration of glutamate and NMDA can evoke CSD [36, 37]. One rare autosomally inherited subtype of migraine with aura is familial hemiplegic migraine. In patients with this condition, CSD may be triggered more easily presumably because the mutations involved increase the synaptic glutamate level [63].
Overall, it seems that glutamate is one of the key molecules in migraine at many levels of the nervous system. Its modulation may be an important means of understanding the pathomechanisms underlying the attack and it may be of potential therapeutic value in migraine.
KYNURENINE METABOLITES
The oxidative ring opening of tryptophan (TRP) leads to L-kynurenine (L-KYN) and the kynurenine pathway (KP) (Fig. 1). The class of compounds known as kynurenine metabolites comprises the totality of the metabolites of the KP, the central route [64] responsible for around 95% of the TRP metabolism [65]. It takes place in the macrophages and microglial cells, and in part in the astrocytes [66, 67], and gives rise to the formation of nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) [68].
Fig. (1).
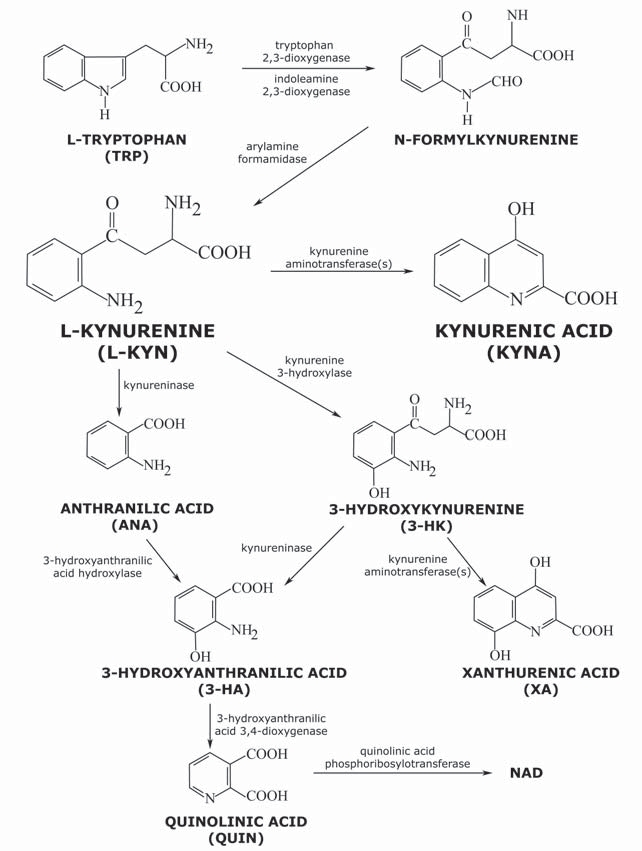
The kynurenine pathway.
The basal compound of the KP is L-KYN, which can cross the blood–brain barrier with the aid of a neutral amino acid carrier [69]. The metabolites of the KP include 3-hydroxykynurenine (3-HK), anthranilic acid (ANA), 3-hydroxyanthranilic acid (3-HA), xanthurenic acid (XA), quinolinic acid (QUIN) and KYNA, all with neuroactive properties [70].
3-HK and 3-HA, generated from L-KYN, can cause neuronal damage, because they can elevate the oxidative stress level by production of free radicals [71, 72] or can provoke primary or secondary excitotoxicity [73, 74]. 3-HK is present in nanomolar concentrations in the mammalian brain, though its level can rise to the micromolar range in several pathological conditions [75]. The content of 3-HA, synthetized from 3-HK and/or ANA, likewise increases in various neurological disorders [76]. 3-HK and 3-HA have been demonstrated to cause the death of cultured neuronal cells [77, 78], the cortical and striatal neurones proving the most vulnerable to the toxic effects of 3-HK [78]. Consequently, these compounds have neurotoxic effects [74].
Transamination of 3-HK leads to XA, this generally being considered part of a detoxification process that reduces the concentration of 3-HK [79]. The role of XA in mammals is not well defined. Under physiological conditions, XA is present in the rat brain at a concentration of about 1 µM; an increase is observed in its level in the urine in an animal model of depression [80]. Administration of high doses of XA to rats seems to induce a degree of sedation and analgesia [81]. XA undergoes vesicular accumulation, is transported by neuronal cells, is present in neuronal circuits and is released via a calcium-dependent process in response to stimulation, these features strongly indicating a physiological role for XA in synaptic signalling [79].
QUIN, from which NAD and NADP are formed [68], resides in the cerebrospinal fluid in nanomolar or low micromolar concentrations [82]. When administered intrastriatally, it causes a significant destruction of neurones [73]; its excitotoxic effect is presumably exerted through agonism of the NMDA receptor [83] or stimulation of the release and inhibition of the uptake of endogenous glutamate [84]. It also induces lipid peroxidation [85, 86] and the production of reactive oxygen species [86]. Changes in the absolute or relative concentration of QUIN play an important role in certain neurodegenerative disorders [75, 87, 88].
In contrast with QUIN, KYNA (4-hydroxyquinoline-2-carboxylic acid) exerts a neuroprotective effect: it is able to prevent the neuronal loss in excitotoxic, ischaemia-induced and neuronal injuries [89, 90]. It is synthesized directly from L-KYN in the astrocytes and neurones [67, 91] enzymatically by the action of kynurenine aminotransferases (KATs) [92, 93], mitochondrial aspartate aminotransferase [94] and hemoperoxidases, or non-enzymatically by reactive oxygen species (ROS) [95]. Beyond this route KYNA can be produced from TRP on an additional pathway by tryptophan aminotransferase and ROS [96, 97]. Similarly to that of QUIN, the concentration of KYNA in the human brain is in the nanomolar range [98], which changes in pathological circumstances, including neurological disorders. The level of KYNA can either decrease or increase in various neurological disorders [75, 87, 99]. KYNA is one of the few known endogenous inhibitors of the EAA receptors, including the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), NMDA and kainate (KA) receptor types at higher concentrations [100, 101, 102]. At around 7.9 μM it can block the NMDA receptor by attaching to its glycine-binding site [101]. As a consequence of its binding to the glutamate-binding site, KYNA may influence the receptors via two mechanisms: in nanomolar to micromolar concentrations, it facilitates the AMPA receptors, whereas at high concentrations, it inhibits the glutamate receptors [103]. It was demonstrated by Rozsa et al. [104] that KYNA in micromolar concentrations exerts a neuroinhibitory effect, while in nanomolar concentrations it behaves as a facilitator in the rat hippocampus. KYNA may therefore play an important role in the regulation (inhibition/excitation) in the neuronal network. The normal concentration of KYNA is too low to influence the EAA receptors, and the published data indicate that, even under pathological conditions, the concentration elevation will not necessarily allow KYNA to influence the co-agonist site of the NMDA receptor [105]. It has also been reported to act as a non-competitive blocker of the α7-nACh receptor [106]. This action, which may play a part in the ability of KYNA to generate a deficit in the sensory system [107, 108], has been suggested to be mediated by its binding to sites located in the N-terminal domain of the α7-nACh receptor subunit [109]. Recent results support the view that the KYNA-sensitive presynaptic α7-nACh receptors inhibit glutamate release at low concentration (30–100 nM) [105, 110]. Thus, the nACh receptors may take part in the inhibitory effects of KYNA at low concentration. KYNA could potentially have therapeutic effects in neurological disorders [75, 111, 112] via the above-described receptor inhibitory effects, but its use as a neuroprotective agent is rather restricted because it has only a very limited ability to cross the blood–brain barrier [69]. The experimental data suggest that peripheral treatment with L-KYN dose-dependently increases the concentration of the neuroprotective KYNA in the brain, offering an opportunity for the treatment of stroke and neurodegenerative disorders [88, 113, 114, 115].
Various studies have identified nACh receptors and subunits in the nociceptors of the TG at the messenger ribonucleic acid (mRNA) and protein levels [116]. The α3ß4 and α4ß2 subtypes of the nACh receptor can presumably be found on the trigeminal free nerve endings [117]. Other studies have reported that the α7-nACh receptor is likely to be present in the TG [116]. These receptors can play a role in the tonic inhibition of spinal pain, which can modulate spinal pain perception [118] and probably reduce neurogenic facial vasodilatation, presumably as a result of the decreased release of CGRP from the trigeminal afferent neurones [119].
KYNURENINE METABOLITES AND MIGRAINE
1. Effects of Kynurenine Metabolites on First-Order Neurones
It is presumably due in part to the existence of various peripheral mechanism that TRP and some of its metabolites, including KYN, KYNA, QUIN, ANA and XA, administered intraperitoneally, can induce analgesia in both the tail-flick and the hot-plate tests, the degree and duration of analgesia varying, depending on the drug, the dose and the test [81] (Fig. 2). The derivatives of ANA, including N-(3,4-dimethoxycinnamoyl)anthranilic acid (tranilast), N-(2,3-xylyl)anthranilic acid (CI-473, mefenamic acid) and the sodium salt of N-(2,6-dichloro-m-tolyl)anthranilic acid (sodium meclofenamate), probably act at the periphery [120], exerting both anti-inflammatory and analgesic properties, with several mechanisms of action [121, 122, 123]. 3-HA also has anti-inflammatory effects [124].
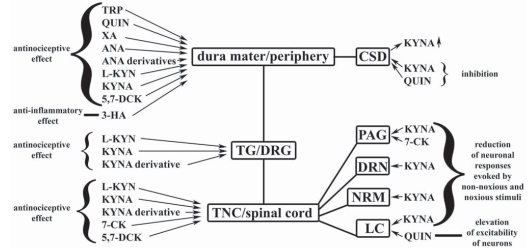
Fig. (2).
Effects of kynurenine metabolites on the structures of nervous system, which are important in the pathomechanism of migraine and pain. 3-HA: 3-hydroxyanthranilic acid, 5,7-DCK: 5,7-dichlorokynurenic acid, 7-CK: 7-chlorokynurenic acid, ANA: anthranilic acid, CSD: cortical spreading depression, DRG: dorsal root ganglion, DRN: dorsal raphe nucleus, KYNA: kynurenic acid, LC: locus coeruleus, L-KYN: L-kynurenine, NRM: nucleus raphe magnus, PAG: periaqueductal grey matter, QUIN: quinolinic acid, TG: trigeminal ganglion, TNC: trigeminal nucleus caudalis, TRP: tryptophan, XA: xanthurenic acid; ↑: increased concentration.
Numerous data are available in connection with the antinociceptive peripheral effect of KYNA. The intraperitoneal injection of rats with KYNA decreased the pain sensitivity in both the tail-flick and the hot-plate tests [125]. Topical intra-articularly administered KYNA, without signs of systemic side-effects, dose-dependently decreased mechanical allodynia, which manifested 30 min after the injection and the highest dose (400 μg) produced prolonged antinociception and almost total relief of allodynia [126]. A KYNA derivative, the 5,7-dichlorokynurenic acid (5,7-DCK), dose-dependently inhibited the development of the nocifensive behaviour evoked by formalin-induced tissue injury and inflammation, and reversed cold allodynia in the chronic constriction injury model, and tactile allodynia in animals subjected to spinal nerve ligation [127]. In one animal model of trigeminovascular activation after electrical stimulation of the TG, the KAT expression of the dural Schwann cells, mast cells and macrophages was decreased, presumably as a result of release from these cells; at the same time, the content of nitric oxide synthase (NOS)-IR nerve fibres in the dura mater increased, suggesting the release of nitric oxide (NO) at the periphery [128]. In another animal model of trigeminal activation, administration of the NO donor nitroglycerine (NTG), the decrease in the area covered by CGRP-IR fibres was prevented by L-KYN in combination with probenecid (PROB) and a KYNA derivative [129], the most likely explanation being that these compounds blocked the activation of first-order neurones and the consecutive release of CGRP from the nerve endings (Fig. 3). These peripheral effects of KYNA can materialize on glutamate receptors localized at the periphery, including the dorsal root and trigeminal ganglion [130, 131], primary sensory afferents [132, 133], postganglionic sympathetic efferents [134], the temporomandibular joint [135] and Schwann cells [136] or on α7-nACh receptors located at the periphery, e.g. the trigeminal ganglion [116]. The G-protein-coupled receptor-35 (GPR35), recently identified as a receptor for KYNA [137], is expressed within nociceptive pathways, including the DRG and spinal cord, at the mRNA and protein levels [138, 139] and is negatively coupled to adenylate cyclase - cyclic adenosine monophosphate (cAMP) signalling in the DRG neurons, which can modulate nociceptive signalling [139]. KYNA proved able to inhibit the forskolin-stimulated formation of cAMP from cultured rat DRG sensory neurones via the GPR35 receptors and can therefore also modulate nociceptive signalling at the periphery [139].
Fig. (3).
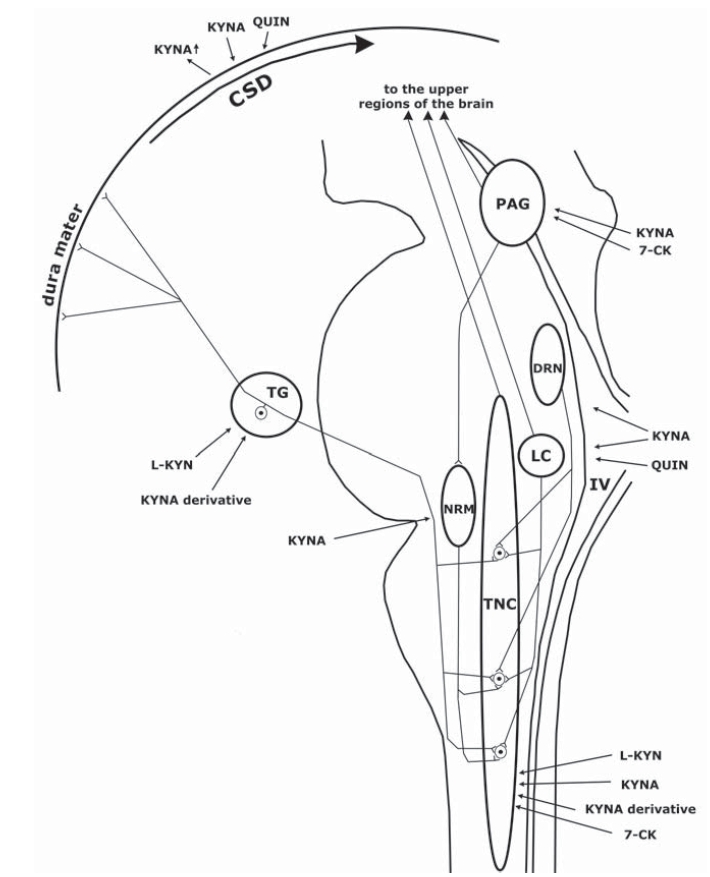
Effects of kynurenine metabolites on the nervous structures involved in the pathogenesis of migraine. 7-CK: 7-chlorokynurenic acid, CSD: cortical spreading depression, DRN: dorsal raphe nucleus, IV: fourth ventricle, KYNA: kynurenic acid, LC: locus coeruleus, L-KYN: L-kynurenine, NRM: nucleus raphe magnus, PAG: periaqueductal grey matter, QUIN: quinolinic acid, TG: trigeminal ganglion, TNC: trigeminal nucleus caudalis; ↑: increased concentration.
2. Effects of Kynurenine Metabolites on Second-Order Neurones
Besides the peripheral effects of the kynurenine metabolites, several studies have confirmed that they can also act on the second-order neurones.
In behavioural examinations the intrathecal (i.t.) injection of KYNA and 7-chlorokynurenic acid (7-CK) produced dose-dependent and reversible analgesic effects in the hot-plate, tail-flick and formalin tests of nociception in mice [140] and in rats [141, 142]. Moreover, the i.t. administration of KYNA and 7-CK suppressed hyperalgesia dose-dependently in rats injected with carrageenan [125, 143], treated with i.t. strychnine [144] or after unilateral partial ligation of the sciatic nerve [145]. In mice treated i.t. with an NMDA receptor agonist, the i.t. co-administered 7-CK inhibited the nociceptive behaviour dose-dependently [146]. Finally, i.t. administration of 5,7-DCK dose-dependently reversed the hyperalgesia in hyperalgesic Mg-deficient rats [147]. However, the injection of 7-CK into the rostral anterior cingulate cortex did not affect formalin-induced acute nociceptive behaviour or electric foot shock-induced conditioned place avoidance [148] and the i.t. infusion of 5,7-DCK failed to block the glycine-induced increased pain response in neuropathic rats made by unilateral partial ligation of the sciatic nerve [149]. These results suggest that the central action of kynurenine metabolites in modulating pain perception does not extend to all brain areas that participate in nociception and is dependent on the receptors that take part in pain transmission.
There is also evidence concerning the antinociceptive effects of kynurenine metabolites at the spinal cord level (Fig. 2). The iontophoric administration of KYNA into the spinal cord of cats, for example, markedly reduced both the cutaneous and the muscular nociceptive responses of a wide dynamic range neurones [150] and the nociceptive responses, irregular spontaneous discharges and C-afferent-induced responses of dorsal horn neurones facilitated by the iontophoretic injection of EAA receptor agonists [151]. Further, the i.t. administration of 7-CK reduced the frequency-dependent potentiation (wind-up) to repeated C-fibre stimulation and the related post-discharges [152], but not the initial responses [153] in the nociceptive neurones located in the dorsal horn of rats. Additionally, single-unit recordings of the responses of dorsal horn neurones to C-, Aδ- and Aβ-fibre stimulation and the wind-up and post-discharge responses of the same cells facilitated by bicuculline were inhibited by 7-CK in intact anaesthetized rats [154]. KYNA pre-administered i.t. significantly reduced the total number of c-Fos-IR neurones increased by carrageenan injection into the rat paw, with a more apparent reduction in laminae I-II and IV-V [125]. In vitro experiments on spinal cord also suggest the antinociceptive effect of KYNA. For example, it blocked the excitation of high-threshold mechanoreceptive units by either cutaneous nerve volleys or mechanical stimulation of the skin, suppressed peripherally evoked responses to innocuous mechanical stimuli in the hamster [155] and blocked the responses to non-nociceptive and nociceptive stimulation of the skin of the leg modulated by motoneurone depolarizations and changes in extracellular potassium concentration in the frog [156].
The second-order nociceptive neurones of the TNC play an important role in the pathomechanism of migraine: the i.t. administration of 7-CK significantly reduced the neuronal mechanoreceptive field size and spontaneous activity increased by neonatal capsaicin treatment in adult rats [157], and intracisternally administered KYNA effectively blocked capsaicin-induced eye wipings [158] (Fig. 3.). After systemic treatment with NTG, a well-known activator of the second-order trigeminal neurones [159], L-KYN combined with PROB attenuated the increase in the number of c-Fos-IR neurones in the TNC [160]. Similarly, at the same location, in the same experimental model, pretreatment with the L-KYN+PROB combination and a KYNA derivative, 2-(2-N,N-dimethylaminoethylamine-1-carbonyl)-1H-quinolin-4-one hydrochloride, mitigated the increase in the number of neuronal NOS- and calmodulin-dependent protein kinase II alpha-IR cells [129, 161]. Since both enzymes may play important roles in trigeminal central sensitization [162, 163], KYNA and its derivatives may exert modulatory effects on this phenomenon. KYNA alone failed to modulate c-Fos activation in the TNC in the same model [164], probably because of its poor ability to cross the blood–brain barrier, in marked contrast with its precursor L-KYN and its derivatives, which cross with ease. In another model of migraine, after electrical stimulation of the trigeminal ganglion, pretreatment with i.p. L-KYN combined with PROB mitigated the increase in the content of c-Fos-IR neurones in the rat TNC [165]. Thus, KYNA and its analogues are able to modulate second-order nociceptors in the TS. The above-described results suggest that kynurenine metabolites may have novel perspectives in the treatment of pain and migraine.
3. Effects of Kynurenine Metabolites on Migraine Generators
There is abundant evidence to indicate that the kynurenine metabolites are able to influence the functioning of migraine generators located at the brain stem level (Fig. 3).
KYNA reduced the responses of serotoninergic neurones of the DRN that were evoked by phasic auditory stimuli [54], by stimulation of the lateral habenula [53], by local electrical stimulation of afferent terminals [55] and by substance P microinfusion [56]. KYNA can also abolish the activation of neurones in the NRM excited by glutamate administration [166] and by low-intensity electrical stimulation of the mesencephalic nucleus cuneiformis [26], and its injection into the PAG can modulate the excitatory and inhibitory effects of electrical and chemical stimulation of the medial preoptic nucleus of the hypothalamus on the NRM [167]. The kynurenine metabolites can modulate the LC too: for example, intracerebroventricular administration of QUIN increased the unit discharge of LC neurones [168]. However, KYNA was able to inhibit the activation of central noradrenergic neurones in the LC evoked by noxious stimulation such as electrical stimulation of the rat hindpaw [57], non-noxious and noxious cutaneous sensory stimuli [158], electrical stimulation of a rear footpad [169] and sciatic nerve stimulation [58]; noxious effect, i.e. sciatic nerve stimulation provokes activation of the catecholamine metabolism within the LC cells, which is decreased by KYNA [170]. The robust activation of the LC neurones by the direct application of KA, NMDA, AMPA or quisqualate was reduced or completely antagonized by KYNA [58, 171, 172]. KYNA was also able to inhibit the activation of the LC neurones evoked by stimulation of nucleus paragigantocellularis [57], which causes increased levels of EAAs in the LC [57, 58]. Furthermore, 7-CK prevented nociceptive behaviour (tail-flick) and pain-related changes in neuronal activity induced in the rostral ventromedial medulla by glycine or D-serine administration into the ventrolateral PAG [173]; the co-administration of KYNA with morphine in the same area enhanced the acute antinociceptive effects of morphine [174]. These results demonstrate that the kynurenine metabolites, are particularly KYNA and its derivatives, can give rise to antinociceptive effects through their influence on higher brain areas.
4. Effects of Kynurenine Metabolites on CSD
There are a number of experimental data which suggest that glutamate plays an important role in the phenomenon of CSD. The glutamate level was found to be elevated during CSD [62, 61], glutamate or NMDA was able to trigger CSD [36, 37], and the NMDA, AMPA and KA receptor binding sites were increased 1 hour after the induction of CSD in rat neocortical tissues, which may be responsible for the delayed excitatory phase after it [175]. On the other hand, NMDA receptor antagonists, including the non-competitive channel blocking antagonists and competitive glutamate-recognition site antagonists, can inhibit the initiation, propagation, amplitude, frequency and susceptibility of CSD, whereas the non-NMDA receptor antagonists can not [60]. Those of the NMDA receptor antagonists that act on the NR2-B subunit may selectively inhibit the initiation and propagation of CSD [176]. These data strongly suggest that only the NMDA receptors play a role in CSD. This is further supported by the results of studies, which examined the effects of Mg2+ (an NMDA receptor channel blocker), and found that it can selectively inhibit glutamate-induced spreading depression (SD) [177], and that the Mg2+ depletion, which releases the voltage-dependent block of the NMDA receptor channel, induces CSD [178].
Few studies have been made of the link between CSD and the kynurenine metabolites, and the available results are conflicting (Fig. 3). It has been established that unilateral, consecutive CSDs result in ipsilateral increases in KYNA levels in the frontal, parietal and occipital cortices [179]. Some studies have indicated that KYNA can inhibit SD under certain conditions in the turtle cerebellum [37] and in the adult rat neocortex [180], while others were not able to detect such an effect in the CA1 neurones of the rat hippocampus [181] or in neocortical brain slices [182]. Interestingly, QUIN concentration-dependently suppressed the elicitation of CSD in the cerebral cortex of the rat, presumably because of NMDA receptor desensitization [183, 184]. Since a wide range of NMDA receptor antagonists are able to inhibit electrical CSD, it is highly likely that KYNA can also do this. In experiments where KYNA was ineffective, ischaemic SD was elicited by O2/glucose deprivation, in which glutamate probably does not play a role, whereas it seems to be crucial in potassium-triggered SD. Consequently, KYNA and its derivatives may be of promise in the therapy against migraine aura, where important parts are played by the ion pumps and hence the ion currents.
CONCLUSIONS
Overall, the involvement of KP metabolites (particularly KYNA and its derivatives) at various sites of nociception and in migraine is of appreciable importance. The evidence points to the ability of these compounds to modulate migraine at several levels of the related neuronal areas, including the primary nociceptive afferents, the neurones in the TNC, and the migraine generators, and presumably at the CSD level too. KYNA and its derivatives may therefore offer new opportunities in the therapy of migraine and other diseases related to trigeminal nociception.
ACKNOWLEDGEMENTS
This work was supported by Teller Ede funding (NAP-BIO-06-BAYBIOSZ), ETT (026-04), TÁMOP-4 (2.2-08/1/2008-0002) and cNEUPRO (LSHM-CT-2007-037950) grants.
Thanks are due to David Durham for linguistic correction of the manuscript.
REFERENCES
- 1.The International Classification of Headache Disorders: 2nd ed. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 2.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41(7):646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 3.Moskowitz MA. Defining a pathway to discovery from bench to bedside: the trigeminovascular system and sensitization. Headache. 2008;48(5):688–690. doi: 10.1111/j.1526-4610.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- 4.Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J. Neurosci. 1993;13(3):1167–1177. doi: 10.1523/JNEUROSCI.13-03-01167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, Coenen HH, Diener HC. Brain stem activation in spontaneous human migraine attacks. Nat. Med. 1995;1(7):658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- 6.Ebersberger A, Averbeck B, Messlinger K, Reeh PW. Release of substance P, calcitonin gene-related peptide and prostaglandin E2 from rat dura mater encephali following electrical and chemical stimulation in vitro. Neuroscience. 1999;89(3):901–907. doi: 10.1016/s0306-4522(98)00366-2. [DOI] [PubMed] [Google Scholar]
- 7.Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988;23(2):193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- 8.Williamson DJ, Hargreaves RJ. Neurogenic inflammation in the context of migraine. Microsc. Res. Tech. 2001;53(3):167–178. doi: 10.1002/jemt.1081. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated plasma extravasation in dura mater: effect of ergot alkaloids. A possible mechanism of action in vascular headache. Cephalalgia. 1988;8(2):83–91. doi: 10.1046/j.1468-2982.1988.0802083.x. [DOI] [PubMed] [Google Scholar]
- 10.Williamson DJ, Hargreaves RJ, Hill RG, Shepheard SL. Intravital microscope studies on the effects of neurokinin agonists and calcitonin gene-related peptide on dural vessel diameter in the anaesthetized rat. Cephalalgia. 1997;17(4):518–524. doi: 10.1046/j.1468-2982.1997.1704518.x. [DOI] [PubMed] [Google Scholar]
- 11.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384(6609):560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 12.Blau JN, Dexter SL. The site of pain origin during migraine attacks. Cephalalgia. 1981;1(3):143–147. doi: 10.1046/j.1468-2982.1981.0103143.x. [DOI] [PubMed] [Google Scholar]
- 13.Oshinsky ML, Luo J. Neurochemistry of trigeminal activation in an animal model of migraine. Headache. 2006;46(Suppl 1):S39–S44. doi: 10.1111/j.1526-4610.2006.00489.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang XM, Mokha SS. Opioids modulate N-methyl-D-aspartic acid (NMDA)-evoked responses of trigeminothalamic neurons. J. Neurophysiol. 1996;76(3):2093–2096. doi: 10.1152/jn.1996.76.3.2093. [DOI] [PubMed] [Google Scholar]
- 15.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J. Neurophysiol. 1998;79(2):964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 16.Tallaksen-Greene SJ, Young AB, Penney JB, Beitz AJ. Excitatory amino acid binding sites in the trigeminal principal sensory and spinal trigeminal nuclei of the rat. Neurosci. Lett. 1992;141(1):79–83. doi: 10.1016/0304-3940(92)90339-9. [DOI] [PubMed] [Google Scholar]
- 17.Mitsikostas DD, Sanchez del RM, Waeber C, Huang Z, Cutrer FM, Moskowitz MA. Non-NMDA glutamate receptors modulate capsaicin induced c-fos expression within trigeminal nucleus caudalis. Br. J. Pharmacol. 1999;127(3):623–630. doi: 10.1038/sj.bjp.0702584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storer RJ, Goadsby PJ. Trigeminovascular nociceptive transmission involves N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptors. Neuroscience. 1999;90(4):1371–1376. doi: 10.1016/s0306-4522(98)00536-3. [DOI] [PubMed] [Google Scholar]
- 19.McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269(5231):1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 20.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383(6602):713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 21.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann. Neurol. 2000;47(5):614–624. [PubMed] [Google Scholar]
- 22.Lance JW, Lambert GA, Goadsby PJ, Duckworth JW. Brainstem influences on the cephalic circulation: experimental data from cat and monkey of relevance to the mechanism of migraine. Headache. 1983;23(6):258–265. doi: 10.1111/j.1526-4610.1983.hed2306258.x. [DOI] [PubMed] [Google Scholar]
- 23.Diener HC. Positron emission tomography studies in headache. Headache. 1997;37(10):622–625. doi: 10.1046/j.1526-4610.1997.3710622.x. [DOI] [PubMed] [Google Scholar]
- 24.Raskin NH, Hosobuchi Y, Lamb S. Headache may arise from perturbation of brain. Headache. 1987;27(8):416–420. doi: 10.1111/j.1526-4610.1987.hed2708416.x. [DOI] [PubMed] [Google Scholar]
- 25.Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001;41(7):629–637. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]
- 26.Richter RC, Behbehani MM. Evidence for glutamic acid as a possible neurotransmitter between the mesencephalic nucleus cuneiformis and the medullary nucleus raphe magnus in the lightly anesthetized rat. Brain Res. 1991;544(2):279–286. doi: 10.1016/0006-8993(91)90065-4. [DOI] [PubMed] [Google Scholar]
- 27.Singewald N, Schneider C, Philippu A. Effects of neuroactive compounds, noxious and cardiovascular stimuli on the release of amino acids in the rat locus coeruleus. Neurosci. Lett. 1994;180(1):55–58. doi: 10.1016/0304-3940(94)90912-1. [DOI] [PubMed] [Google Scholar]
- 28.Renno WM, Alkhalaf M, Mousa A, Kanaan RA. A comparative study of excitatory and inhibitory amino acids in three different brainstem nuclei. Neurochem. Res. 2008;33(1):150–159. doi: 10.1007/s11064-007-9427-5. [DOI] [PubMed] [Google Scholar]
- 29.Leao AAP. Spreading depression of activity in cerebral cortex. J. Neurophysiol. 1944;7(6):359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- 30.Lauritzen M, Jorgensen MB, Diemer NH, Gjedde A, Hansen AJ. Persistent oligemia of rat cerebral cortex in the wake of spreading depression. Ann. Neurol. 1982;12(5):469–474. doi: 10.1002/ana.410120510. [DOI] [PubMed] [Google Scholar]
- 31.Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain. 1994;117(Pt 1):199–210. doi: 10.1093/brain/117.1.199. [DOI] [PubMed] [Google Scholar]
- 32.Henning EC, Meng X, Fisher M, Sotak CH. Visualization of cortical spreading depression using manganese-enhanced magnetic resonance imaging. Magn Reson. Med. 2005;53(4):851–857. doi: 10.1002/mrm.20438. [DOI] [PubMed] [Google Scholar]
- 33.Peters O, Schipke CG, Hashimoto Y, Kettenmann H. Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex. J. Neurosci. 2003;23(30):9888–9896. doi: 10.1523/JNEUROSCI.23-30-09888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chuquet J, Hollender L, Nimchinsky EA. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J. Neurosci. 2007;27(15):4036–4044. doi: 10.1523/JNEUROSCI.0721-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat. Med. 2002;8(2):136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 36.Ayata C, Moskowitz MA. Cortical spreading depression confounds concentration-dependent pial arteriolar dilation during N-methyl-D-aspartate superfusion. Am. J. Physiol Heart Circ. Physiol. 2006;290(5):H1837–H1841. doi: 10.1152/ajpheart.01102.2005. [DOI] [PubMed] [Google Scholar]
- 37.Lauritzen M, Rice ME, Okada Y, Nicholson C. Quisqualate, kainate and NMDA can initiate spreading depression in the turtle cerebellum. Brain Res. 1988;475(2):317–327. doi: 10.1016/0006-8993(88)90620-8. [DOI] [PubMed] [Google Scholar]
- 38.Dickenson AH, Chapman V, Green GM. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen. Pharmacol. 1997;28(5):633–638. doi: 10.1016/s0306-3623(96)00359-x. [DOI] [PubMed] [Google Scholar]
- 39.Vikelis M, Mitsikostas DD. The role of glutamate and its receptors in migraine. CNS. Neurol. Disord. Drug Targets. 2007;6(4):251–257. doi: 10.2174/187152707781387279. [DOI] [PubMed] [Google Scholar]
- 40.Ferrari MD, Odink J, Bos KD, Malessy MJ, Bruyn GW. Neuroexcitatory plasma amino acids are elevated in migraine. Neurology. 1990;40(10):1582–1586. doi: 10.1212/wnl.40.10.1582. [DOI] [PubMed] [Google Scholar]
- 41.Cananzi AR, D'Andrea G, Perini F, Zamberlan F, Welch KM. Platelet and plasma levels of glutamate and glutamine in migraine with and without aura. Cephalalgia. 1995;15(2):132–135. doi: 10.1046/j.1468-2982.1995.015002132.x. [DOI] [PubMed] [Google Scholar]
- 42.Martinez F, Castillo J, Rodriguez JR, Leira R, Noya M. Neuroexcitatory amino acid levels in plasma and cerebrospinal fluid during migraine attacks. Cephalalgia. 1993;13(2):89–93. doi: 10.1046/j.1468-2982.1993.1302089.x. [DOI] [PubMed] [Google Scholar]
- 43.Kaube H, Herzog J, Kaufer T, Dichgans M, Diener HC. Aura in some patients with familial hemiplegic migraine can be stopped by intranasal ketamine. Neurology. 2000;55(1):139–141. doi: 10.1212/wnl.55.1.139. [DOI] [PubMed] [Google Scholar]
- 44.Sang CN, Ramadan NM, Wallihan RG, Chappell AS, Freitag FG, Smith TR, Silberstein SD, Johnson KW, Phebus LA, Bleakman D, Ornstein PL, Arnold B, Tepper SJ, Vandenhende F. LY293558, a novel AMPA/GluR5 an-tagonist, is efficacious and well-tolerated in acute migraine. Cephalalgia. 2004;24(7):596–602. doi: 10.1111/j.1468-2982.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe M, Mishina M, Inoue Y. Distinct gene expression of the N-methyl-D-aspartate receptor channel subunit in peripheral neurons of the mouse sensory ganglia and adrenal gland. Neurosci. Lett. 1994;165(1-2):183–186. doi: 10.1016/0304-3940(94)90740-4. [DOI] [PubMed] [Google Scholar]
- 46.Sahara Y, Noro N, Iida Y, Soma K, Nakamura Y. Glutamate receptor subunits GluR5 and KA-2 are coexpressed in rat trigeminal ganglion neurons. J. Neurosci. 1997;17(17):6611–6620. doi: 10.1523/JNEUROSCI.17-17-06611.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quartu M, Serra MP, Ambu R, Lai ML, Del FM. AMPA-type glutamate receptor subunits 2/3 in the human trigeminal sensory ganglion and subnucleus caudalis from prenatal ages to adulthood. Mech. Ageing Dev. 2002;123(5):463–471. doi: 10.1016/s0047-6374(01)00358-x. [DOI] [PubMed] [Google Scholar]
- 48.Hill RG, Salt TE. An ionophoretic study of the responses of rat caudal trigeminal nucleus neurones to non-noxious mechanical sensory stimuli. J. Physiol. 1982;327:65–78. doi: 10.1113/jphysiol.1982.sp014220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitsikostas DD, Sanchez del RM, Waeber C, Moskowitz MA, Cutrer FM. The NMDA receptor antagonist MK-801 reduces capsaicin-induced c-fos expression within rat trigeminal nucleus caudalis. Pain. 1998;76(1-2):239–248. doi: 10.1016/s0304-3959(98)00051-7. [DOI] [PubMed] [Google Scholar]
- 50.Goadsby PJ, Classey JD. Glutamatergic transmission in the trigeminal nucleus assessed with local blood flow. Brain Res. 2000;875(1-2):119–124. doi: 10.1016/s0006-8993(00)02630-5. [DOI] [PubMed] [Google Scholar]
- 51.Filla SA, Winter MA, Johnson KW, Bleakman D, Bell MG, Bleisch TJ, Castano AM, Clemens-Smith A, del PM, Dieckman DK, Dominguez E, Escribano A, Ho KH, Hudziak KJ, Katofiasc MA, Martinez-Perez JA, Mateo A, Mathes BM, Mattiuz EL, Ogden AM, Phebus LA, Stack DR, Stratford RE, Ornstein PL. Ethyl (3S,4aR,6S,8aR)-6-(4-ethoxycar- bonylimidazol-1-ylmethyl)decahydroiso-quinoline-3-carboxylic ester: a prodrug of a GluR5 kainate receptor antagonist active in two animal models of acute migraine. J. Med. Chem. 2002;45(20):4383–4386. doi: 10.1021/jm025548q. [DOI] [PubMed] [Google Scholar]
- 52.Kolhekar R, Murphy S, Gebhart GF. Thalamic NMDA receptors modulate inflammation-produced hyperalgesia in the rat. Pain. 1997;71(1):31–40. doi: 10.1016/s0304-3959(97)03334-4. [DOI] [PubMed] [Google Scholar]
- 53.Kalen P, Strecker RE, Rosengren E, Bjorklund A. Regulation of striatal serotonin release by the lateral habenula-dorsal raphe pathway in the rat as demonstrated by in vivo microdialysis: role of excitatory amino acids and GABA. Brain Res. 1989;492(1-2):187–202. doi: 10.1016/0006-8993(89)90901-3. [DOI] [PubMed] [Google Scholar]
- 54.Levine ES, Jacobs BL. Neurochemical afferents controlling the activity of serotonergic neurons in the dorsal raphe nucleus: microiontophoretic studies in the awake cat. J. Neurosci. 1992;12(10):4037–4044. doi: 10.1523/JNEUROSCI.12-10-04037.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinnock RD. Activation of kappa-opioid receptors depresses electrically evoked excitatory postsynaptic potentials on 5-HT-sensitive neurones in the rat dorsal raphe nucleus in vitro. Brain Res. 1992;583(1-2):237–246. doi: 10.1016/s0006-8993(10)80029-0. [DOI] [PubMed] [Google Scholar]
- 56.Valentino RJ, Bey V, Pernar L, Commons KG. Substance P Acts through local circuits within the rat dorsal raphe nucleus to alter serotonergic neuronal activity. J. Neurosci. 2003;23(18):7155–7159. doi: 10.1523/JNEUROSCI.23-18-07155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ennis M, Aston-Jones G. Activation of locus coeruleus from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain. J. Neurosci. 1988;8(10):3644–3657. doi: 10.1523/JNEUROSCI.08-10-03644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ennis M, Aston-Jones G, Shiekhattar R. Activation of locus coeruleus neurons by nucleus paragigantocellularis or noxious sensory stimulation is mediated by intracoerulear excitatory amino acid neurotransmission. Brain Res. 1992;598(1-2):185–195. doi: 10.1016/0006-8993(92)90182-9. [DOI] [PubMed] [Google Scholar]
- 59.Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol. Rev. 2001;81(3):1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- 60.Nellgard B, Wieloch T. NMDA-receptor blockers but not NBQX, an AMPA-receptor antagonist, inhibit spreading depression in the rat brain. Acta Physiol. Scand. 1992;146(4):497–503. doi: 10.1111/j.1748-1716.1992.tb09451.x. [DOI] [PubMed] [Google Scholar]
- 61.Fabricius M, Jensen LH, Lauritzen M. Microdialysis of interstitial amino acids during spreading depression and anoxic depolarization in rat neocortex. Brain Res. 1993;612(1-2):61–69. doi: 10.1016/0006-8993(93)91644-8. [DOI] [PubMed] [Google Scholar]
- 62.Basarsky TA, Feighan D, MacVicar BA. Glutamate release through volume-activated channels during spreading depression. J. Neurosci. 1999;19(15):6439–6445. doi: 10.1523/JNEUROSCI.19-15-06439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pietrobon D. Familial hemiplegic migraine. Neurotherapeutics. 2007;4(2):274–284. doi: 10.1016/j.nurt.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 64.Wolf H. The effect of hormones and vitamin B6 on urinary excretion of metabolites of the kynurenine pathway. Scand. J. Clin. Lab. Invest. 1974;136:1–186. [PubMed] [Google Scholar]
- 65.Tyce GM. Origin and metabolism of serotonin. J. Cardiovasc. Pharmacol. 1990;16(Suppl 3):S1–S7. [PubMed] [Google Scholar]
- 66.Guillemin GJ, Kerr SJ, Pemberton LA, Smith DG, Smythe GA, Armati PJ, Brew BJ. IFN-beta1b induces kynurenine pathway metabolism in human macrophages: potential implications for multiple sclerosis treatment. J. Interferon Cytokine Res. 2001;21(12):1097–1101. doi: 10.1089/107999001317205231. [DOI] [PubMed] [Google Scholar]
- 67.Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv. Exp. Med. Biol. 2003;527:105–112. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- 68.Beadle GW, Mitchell HK, Nyc JF. Kynurenine as an Intermediate in the Formation of Nicotinic Acid from Tryptophane by Neurospora. Proc. Natl. Acad. Sci. USA. 1947;33(6):155–158. doi: 10.1073/pnas.33.6.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J. Neurochem. 1991;56(6):2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 70.Lapin IP. Stimulant and convulsive effects of kynurenines injected into brain ventricles in mice. J. Neural Transm. 1978;42(1):37–43. doi: 10.1007/BF01262727. [DOI] [PubMed] [Google Scholar]
- 71.Dykens JA, Sullivan SG, Stern A. Oxidative reactivity of the tryptophan metabolites 3-hydroxyanthranilate, cinnabarinate, quinolinate and picolinate. Biochem. Pharmacol. 1987;36(2):211–217. doi: 10.1016/0006-2952(87)90691-5. [DOI] [PubMed] [Google Scholar]
- 72.Eastman CL, Guilarte TR. The role of hydrogen peroxide in the in vitro cytotoxicity of 3-hydroxykynurenine. Neurochem. Res. 1990;15(11):1101–1107. doi: 10.1007/BF01101711. [DOI] [PubMed] [Google Scholar]
- 73.Guidetti P, Schwarcz R. 3-Hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Eur. J. Neurosci. 1999;11(11):3857–3863. doi: 10.1046/j.1460-9568.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 74.Jhamandas K, Boegman RJ, Beninger RJ, Bialik M. Quinolinate-induced cortical cholinergic damage: modulation by tryptophan metabolites. Brain Res. 1990;529(1-2):185–191. doi: 10.1016/0006-8993(90)90826-w. [DOI] [PubMed] [Google Scholar]
- 75.Stone TW, Mackay GM, Forrest CM, Clark CJ, Darlington LG. Tryptophan metabolites and brain disorders. Clin. Chem. Lab Med. 2003;41(7):852–859. doi: 10.1515/CCLM.2003.129. [DOI] [PubMed] [Google Scholar]
- 76.Pearson SJ, Reynolds GP. Determination of 3-hydroxykynurenine in human brain and plasma by high-performance liquid chromatography with electrochemical detection. Increased concentrations in hepatic encephalopathy. J. Chromatogr. 1991;565(1-2):436–440. doi: 10.1016/0378-4347(91)80406-3. [DOI] [PubMed] [Google Scholar]
- 77.Okuda S, Nishiyama N, Saito H, Katsuki H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proc. Natl. Acad. Sci. USA. 1996;93(22):12553–12558. doi: 10.1073/pnas.93.22.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxy-kynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 1998;70(1):299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- 79.Gobaille S, Kemmel V, Brumaru D, Dugave C, Aunis D, Maitre M. Xanthurenic acid distribution, transport, accumulation and release in the rat brain. J. Neurochem. 2008;105(3):982–993. doi: 10.1111/j.1471-4159.2008.05219.x. [DOI] [PubMed] [Google Scholar]
- 80.Zheng S, Yu M, Lu X, Huo T, Ge L, Yang J, Wu C, Li F. Urinary metabonomic study on biochemical changes in chronic unpredictable mild stress model of depression. Clin. Chim. Acta. 2010;411(3-4):204–209. doi: 10.1016/j.cca.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 81.Heyliger SO, Goodman CB, Ngong JM, Soliman KF. The analgesic effects of tryptophan and its metabolites in the rat. Pharmacol. Res. 1998;38(4):243–250. doi: 10.1006/phrs.1998.0362. [DOI] [PubMed] [Google Scholar]
- 82.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J. Pharmacol. Exp. Ther. 2002;303(1):1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 83.de Carvalho LP, Bochet P, Rossier J. The endogenous agonist quinolinic acid and the non endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunits. Neurochem. Int. 1996;28(4):445–452. doi: 10.1016/0197-0186(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 84.Tavares RG, Tasca CI, Santos CE, Alves LB, Porciuncula LO, Emanuelli T, Souza DO. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem. Int. 2002;40(7):621–627. doi: 10.1016/s0197-0186(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 85.Rios C, Santamaria A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem. Res. 1991;16(10):1139–1143. doi: 10.1007/BF00966592. [DOI] [PubMed] [Google Scholar]
- 86.Behan WM, McDonald M, Darlington LG, Stone TW. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: protection by melatonin and deprenyl. Br. J. Pharmacol. 1999;128(8):1754–1760. doi: 10.1038/sj.bjp.0702940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vecsei L, Schwab F. [Kynurenine and its metabolites in nervous system diseases] Orv. Hetil. 1992;133(29):1803–1807. [PubMed] [Google Scholar]
- 88.Vecsei L, Beal MF. Huntington's disease, behavioral disturbances, and kynurenines: preclinical findings and therapeutic perspectives. Biol. Psychiatry. 1996;39(12):1061–1063. doi: 10.1016/0006-3223(95)00377-0. [DOI] [PubMed] [Google Scholar]
- 89.Smith DH, Okiyama K, Thomas MJ, McIntosh TK. Effects of the excitatory amino acid receptor antagonists kynurenate and indole-2-carboxylic acid on behavioral and neurochemical outcome following experimental brain injury. J. Neurosci. 1993;13(12):5383–5392. doi: 10.1523/JNEUROSCI.13-12-05383.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gigler G, Szenasi G, Simo A, Levay G, Harsing LG, Jr., Sas K, Vecsei L, Toldi J. Neuroprotective effect of L-kynurenine sulfate administered before focal cerebral ischemia in mice and global cerebral ischemia in gerbils. Eur. J. Pharmacol. 2007;564(1-3):116–122. doi: 10.1016/j.ejphar.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 91.Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, Takikawa O, Brew BJ. Characterization of the kynurenine pathway in human neurons. J. Neurosci. 2007;27(47):12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guidetti P, Okuno E, Schwarcz R. Characterization of rat brain kynurenine aminotransferases I and II. J. Neurosci. Res. 1997;50(3):457–465. doi: 10.1002/(SICI)1097-4547(19971101)50:3<457::AID-JNR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 93.Yu P, Li Z, Zhang L, Tagle DA, Cai T. Characterization of kynurenine aminotransferase III, a novel member of a phylogenetically conserved KAT family. Gene. 2006;365:111–118. doi: 10.1016/j.gene.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 94.Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J. Neurochem. 2007;102(1):103–111. doi: 10.1111/j.1471-4159.2007.04556.x. [DOI] [PubMed] [Google Scholar]
- 95.Zsizsik BK, Hardeland R. Formation of kynurenic and xanthurenic acids from kynurenine and 3-hydroxykynurenine in the dinoflagellate Lingulodinium polyedrum: role of a novel, oxidative pathway. Comp. Biochem. Physiol C. Toxicol. Pharmacol. 2002;133(3):383–392. doi: 10.1016/s1532-0456(02)00126-6. [DOI] [PubMed] [Google Scholar]
- 96.Politi V, Lavaggi MV, Di SG, Margonelli A. Indole-3-pyruvic acid as a direct precursor of kynurenic acid. Adv. Exp. Med. Biol. 1991;294:515–518. doi: 10.1007/978-1-4684-5952-4_57. [DOI] [PubMed] [Google Scholar]
- 97.Politi V, D'Alessio S, Di SG, De LG. Antioxidant properties of indole-3-pyruvic acid. Adv. Exp. Med. Biol. 1996;398:291–298. doi: 10.1007/978-1-4613-0381-7_46. [DOI] [PubMed] [Google Scholar]
- 98.Turski WA, Nakamura M, Todd WP, Carpenter BK, Whetsell WO Jr, Schwarcz R. Identification and quantification of kynurenic acid in human brain tissue. Brain Res. 1988;454(1-2):164–169. doi: 10.1016/0006-8993(88)90815-3. [DOI] [PubMed] [Google Scholar]
- 99.Hartai Z, Klivenyi P, Janaky T, Penke B, Dux L, Vecsei L. Kynurenine metabolism in multiple sclerosis. Acta Neurol. Scand. 2005;112(2):93–96. doi: 10.1111/j.1600-0404.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 100.Birch PJ, Grossman CJ, Hayes AG. Kynurenate and FG9041 have both competitive and non-competitive antagonist actions at excitatory amino acid receptors. Eur. J. Pharmacol. 1988;151(2):313–315. doi: 10.1016/0014-2999(88)90814-x. [DOI] [PubMed] [Google Scholar]
- 101.Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J. Neurochem. 1989;52(4):1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 102.Bertolino M, Vicini S, Costa E. Kynurenic acid inhibits the activation of kainic and N-methyl-D-aspartic acid-sensitive ionotropic receptors by a different mechanism. Neuropharmacology. 1989;28(5):453–457. doi: 10.1016/0028-3908(89)90078-6. [DOI] [PubMed] [Google Scholar]
- 103.Prescott C, Weeks AM, Staley KJ, Partin KM. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci. Lett. 2006;402(1-2):108–112. doi: 10.1016/j.neulet.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 104.Rozsa E, Robotka H, Vecsei L, Toldi J. The Janus-face kynurenic acid. J. Neural Transm. 2008;115(8):1087–1091. doi: 10.1007/s00702-008-0052-5. [DOI] [PubMed] [Google Scholar]
- 105.Carpenedo R, Pittaluga A, Cozzi A, Attucci S, Galli A, Raiteri M, Moroni F. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur. J. Neurosci. 2001;13(11):2141–2147. doi: 10.1046/j.0953-816x.2001.01592.x. [DOI] [PubMed] [Google Scholar]
- 106.Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J. Neurosci. 2001;21(19):7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shepard PD, Joy B, Clerkin L, Schwarcz R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology. 2003;28(8):1454–1462. doi: 10.1038/sj.npp.1300188. [DOI] [PubMed] [Google Scholar]
- 108.Chess AC, Bucci DJ. Increased concentration of cerebral kynurenic acid alters stimulus processing and conditioned responding. Behav. Brain Res. 2006;170(2):326–332. doi: 10.1016/j.bbr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 109.Pereira EF, Hilmas C, Santos MD, Alkondon M, Maelicke A, Albuquerque EX. Unconventional ligands and modulators of nicotinic receptors. J. Neurobiol. 2002;53(4):479–500. doi: 10.1002/neu.10146. [DOI] [PubMed] [Google Scholar]
- 110.Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J. Neurochem. 2002;80(6):1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- 111.Nemeth H, Toldi J, Vecsei L. Role of kynurenines in the central and peripheral nervous systems. Curr. Neurovasc. Res. 2005;2(3):249–260. doi: 10.2174/1567202054368326. [DOI] [PubMed] [Google Scholar]
- 112.Vamos E, Pardutz A, Klivenyi P, Toldi J, Vecsei L. The role of kynurenines in disorders of the central nervous system: possibilities for neuroprotection. J. Neurol. Sci. 2009;283(1-2):21–27. doi: 10.1016/j.jns.2009.02.326. [DOI] [PubMed] [Google Scholar]
- 113.Swartz KJ, During MJ, Freese A, Beal MF. Cerebral synthesis and release of kynurenic acid: an endogenous antagonist of excitatory amino acid receptors. J. Neurosci. 1990;10(9):2965–2973. doi: 10.1523/JNEUROSCI.10-09-02965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vecsei L, Miller J, MacGarvey U, Beal MF. Kynurenine and probenecid inhibit pentylenetetrazol- and NMDLA-induced seizures and increase kynurenic acid concentrations in the brain. Brain Res. Bull. 1992;28(2):233–238. doi: 10.1016/0361-9230(92)90184-y. [DOI] [PubMed] [Google Scholar]
- 115.Zadori D, Klivenyi P, Vamos E, Fulop F, Toldi J, Vecsei L. Kynurenines in chronic neurodegenerative disorders: future therapeutic strategies. J. Neural Transm. 2009;116(11):1403–1409. doi: 10.1007/s00702-009-0263-4. [DOI] [PubMed] [Google Scholar]
- 116.Liu L, Chang GQ, Jiao YQ, Simon SA. Neuronal nicotinic acetylcholine receptors in rat trigeminal ganglia. Brain Res. 1998;809(2):238–245. doi: 10.1016/s0006-8993(98)00862-2. [DOI] [PubMed] [Google Scholar]
- 117.Alimohammadi H, Silver WL. Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem. Senses. 2000;25(1):61–66. doi: 10.1093/chemse/25.1.61. [DOI] [PubMed] [Google Scholar]
- 118.Matsumoto M, Xie W, Inoue M, Ueda H. Evidence for the tonic inhibition of spinal pain by nicotinic cholinergic transmission through primary afferents. Mol. Pain. 2007;3:41. doi: 10.1186/1744-8069-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Just S, Arndt K, Doods H. The role of CGRP and nicotinic receptors in centrally evoked facial blood flow changes. Neurosci. Lett. 2005;381(1-2):120–124. doi: 10.1016/j.neulet.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 120.Brogden RN. Non-steroidal anti-inflammatory analgesics other than salicylates. Drugs. 1986;32(Suppl 4):27–45. doi: 10.2165/00003495-198600324-00004. [DOI] [PubMed] [Google Scholar]
- 121.Winder CV, Wax J, Scotti L, Scherrer RA, Jones EM, Short FW. Anti-inflammatory, antipyretic and antinociceptive properties of N-(2,3-xylyl)anthranilic acid (mefenamic acid) J. Pharmacol. Exp. Ther. 1962;133:405–418. [PubMed] [Google Scholar]
- 122.Honorato PJ, Marti MR, Imizcoz Barriola JL. Meclofenamate sodium in the treatment of post-traumatic edema. Report of a controlled double-blind study. Arzneimittelforschung. 1983;33(4A):663–667. [PubMed] [Google Scholar]
- 123.Inglis JJ, Criado G, Andrews M, Feldmann M, Williams RO, Selley ML. The anti-allergic drug, N-(3',4'-dimethoxy-cinnamonyl) anthranilic acid, exhibits potent anti-inflammatory and analgesic properties in arthritis. Rheumatology (Oxford) 2007;46(9):1428–1432. doi: 10.1093/rheumatology/kem160. [DOI] [PubMed] [Google Scholar]
- 124.Pae HO, Oh GS, Lee BS, Rim JS, Kim YM, Chung HT. 3-Hydroxyanthranilic acid, one of L-tryptophan metabolites, inhibits monocyte chemoattractant protein-1 secretion and vascular cell adhesion molecule-1 expression via heme oxygenase-1 induction in human umbilical vein endothelial cells. Atherosclerosis. 2006;187(2):274–284. doi: 10.1016/j.atherosclerosis.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 125.Zhang YQ, Ji GC, Wu GC, Zhao ZQ. Kynurenic acid enhances electroacupuncture analgesia in normal and carrageenan-injected rats. Brain Res. 2003;966(2):300–307. doi: 10.1016/s0006-8993(02)04228-2. [DOI] [PubMed] [Google Scholar]
- 126.Mecs L, Tuboly G, Nagy E, Benedek G, Horvath G. The peripheral antinociceptive effects of endomorphin-1 and kynurenic acid in the rat inflamed joint model. Anesth. Analg. 2009;109(4):1297–1304. doi: 10.1213/ane.0b013e3181b21c5e. [DOI] [PubMed] [Google Scholar]
- 127.Christoph T, Reissmuller E, Schiene K, Englberger W, Chizh BA. Antiallodynic effects of NMDA glycine(B) antagonists in neuropathic pain: possible peripheral mechanisms. Brain Res. 2005;1048(1-2):218–227. doi: 10.1016/j.brainres.2005.04.081. [DOI] [PubMed] [Google Scholar]
- 128.Knyihar-Csillik E, Chadaide Z, Okuno E, Krisztin-Peva B, Toldi J, Varga C, Molnar A, Csillik B, Vecsei L. Kynurenine aminotransferase in the supratentorial dura mater of the rat: effect of stimulation of the trigeminal ganglion. Exp. Neurol. 2004;186(2):242–247. doi: 10.1016/j.expneurol.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 129.Vamos E, Fejes A, Koch J, Tajti J, Fulop F, Toldi J, Pardutz A, Vecsei L. Kynurenate derivative attenuates the nitroglycerin-induced camkiialpha and CGRP expression changes. Headache. 2009;50(5):834–43. doi: 10.1111/j.1526-4610.2009.01574.x. [DOI] [PubMed] [Google Scholar]
- 130.Shigemoto R, Ohishi H, Nakanishi S, Mizuno N. Expression of the mRNA for the rat NMDA receptor (NMDAR1) in the sensory and autonomic ganglion neurons. Neurosci. Lett. 1992;144(1-2):229–232. doi: 10.1016/0304-3940(92)90756-w. [DOI] [PubMed] [Google Scholar]
- 131.Sato K, Kiyama H, Park HT, Tohyama M, AMPA KA. NMDA receptors are expressed in the rat DRG neurones. Neuroreport. 1993;4(11):1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 132.Liu H, Wang H, Sheng M, Jan LY, Jan YN, Basbaum AI. Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. Proc. Natl. Acad. Sci. USA. 1994;91(18):8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci. Lett. 1995;197(1):25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- 134.Coggeshall RE, Carlton SM. Evidence for an inflammation-induced change in the local glutamatergic regulation of postganglionic sympathetic efferents. Pain. 1999;83(2):163–168. doi: 10.1016/s0304-3959(99)00098-6. [DOI] [PubMed] [Google Scholar]
- 135.Cairns BE, Sessle BJ, Hu JW. Evidence that excitatory amino acid receptors within the temporomandibular joint region are involved in the reflex activation of the jaw muscles. J. Neurosci. 1998;18(19):8056–8064. doi: 10.1523/JNEUROSCI.18-19-08056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kinkelin I, Brocker EB, Koltzenburg M, Carlton SM. Localization of ionotropic glutamate receptors in peripheral axons of human skin. Neurosci. Lett. 2000;283(2):149–152. doi: 10.1016/s0304-3940(00)00944-7. [DOI] [PubMed] [Google Scholar]
- 137.Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006;281(31):22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 138.Taniguchi Y, Tonai-Kachi H, Shinjo K. Zaprinast, a well-known cyclic guanosine monophosphate-specific phosphodiesterase inhibitor, is an agonist for GPR35. FEBS Lett. 2006;580(21):5003–5008. doi: 10.1016/j.febslet.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 139.Ohshiro H, Tonai-Kachi H, Ichikawa K. GPR35 is a functional receptor in rat dorsal root ganglion neurons. Biochem. Biophys. Res. Commun. 2008;365(2):344–348. doi: 10.1016/j.bbrc.2007.10.197. [DOI] [PubMed] [Google Scholar]
- 140.Nasstrom J, Karlsson U, Post C. Antinociceptive actions of different classes of excitatory amino acid receptor antagonists in mice. Eur. J. Pharmacol. 1992;212(1):21–29. doi: 10.1016/0014-2999(92)90067-e. [DOI] [PubMed] [Google Scholar]
- 141.Kristensen JD, Post C, Gordh T Jr, Svensson BA. Spinal cord morphology and antinociception after chronic intrathecal administration of excitatory amino acid antagonists in the rat. Pain. 1993;54(3):309–316. doi: 10.1016/0304-3959(93)90030-S. [DOI] [PubMed] [Google Scholar]
- 142.Chapman V, Dickenson AH. Time-related roles of excitatory amino acid receptors during persistent noxiously evoked responses of rat dorsal horn neurones. Brain Res. 1995;703(1-2):45–50. doi: 10.1016/0006-8993(95)01063-7. [DOI] [PubMed] [Google Scholar]
- 143.Ren K, Williams GM, Hylden JL, Ruda MA, Dubner R. The intrathecal administration of excitatory amino acid receptor antagonists selectively attenuated carrageenan-induced behavioral hyperalgesia in rats. Eur. J. Pharmacol. 1992;219(2):235–243. doi: 10.1016/0014-2999(92)90301-j. [DOI] [PubMed] [Google Scholar]
- 144.Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37(1):111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- 145.Yamamoto T, Yaksh TL. Spinal pharmacology of thermal hyperesthesia induced by constriction injury of sciatic nerve Excitatory amino acid antagonists. Pain. 1992;49(1):121–128. doi: 10.1016/0304-3959(92)90198-K. [DOI] [PubMed] [Google Scholar]
- 146.Tan-No K, Esashi A, Nakagawasai O, Niijima F, Furuta S, Sato T, Satoh S, Yasuhara H, Tadano T. Intrathecally administered D-cycloserine produces nociceptive behavior through the activation of N-methyl-D-aspartate receptor ion-channel complex acting on the glycine recognition site. J. Pharmacol. Sci. 2007;104(1):39–45. doi: 10.1254/jphs.fp0070203. [DOI] [PubMed] [Google Scholar]
- 147.Begon S, Pickering G, Eschalier A, Mazur A, Rayssiguier Y, Dubray C. Role of spinal NMDA receptors, protein kinase C and nitric oxide synthase in the hyperalgesia induced by magnesium deficiency in rats. Br. J. Pharmacol. 2001;134(6):1227–1236. doi: 10.1038/sj.bjp.0704354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ren WH, Guo JD, Cao H, Wang H, Wang PF, Sha H, Ji RR, Zhao ZQ, Zhang YQ. Is endogenous D-serine in the rostral anterior cingulate cortex necessary for pain-related negative affect? J. Neurochem. 2006;96(6):1636–1647. doi: 10.1111/j.1471-4159.2006.03677.x. [DOI] [PubMed] [Google Scholar]
- 149.Simpson RK Jr, Gondo M, Robertson CS, Goodman JC. Reduction in the mechanonociceptive response by intrathecal administration of glycine and related compounds. Neurochem. Res. 1996;21(10):1221–1226. doi: 10.1007/BF02532399. [DOI] [PubMed] [Google Scholar]
- 150.Song XJ, Zhao ZQ. Differential effects of NMDA and non-NMDA receptor antagonists on spinal cutaneous vs muscular nociception in the cat. Neuroreport. 1993;4(1):17–20. doi: 10.1097/00001756-199301000-00004. [DOI] [PubMed] [Google Scholar]
- 151.Song XJ, Zhao ZQ. NMDA and non-NMDA receptors mediating nociceptive and non-nociceptive transmission in spinal cord of cat. Zhongguo Yao Li Xue Bao. 1993;14(6):481–485. [PubMed] [Google Scholar]
- 152.Dickenson AH, Aydar E. Antagonism at the glycine site on the NMDA receptor reduces spinal nociception in the rat. Neurosci. Lett. 1991;121(1-2):263–266. doi: 10.1016/0304-3940(91)90700-4. [DOI] [PubMed] [Google Scholar]
- 153.Chapman V, Dickenson AH. The combination of NMDA antagonism and morphine produces profound antinociception in the rat dorsal horn. Brain Res. 1992;573(2):321–323. doi: 10.1016/0006-8993(92)90780-d. [DOI] [PubMed] [Google Scholar]
- 154.Reeve AJ, Dickenson AH, Kerr NC. Spinal effects of bicuculline: modulation of an allodynia-like state by an A1-receptor agonist, morphine, and an NMDA-receptor antagonist. J. Neurophysiol. 1998;79(3):1494–1507. doi: 10.1152/jn.1998.79.3.1494. [DOI] [PubMed] [Google Scholar]
- 155.Schneider SP, Perl ER. Synaptic mediation from cutaneous mechanical nociceptors. J. Neurophysiol. 1994;72(2):612–621. doi: 10.1152/jn.1994.72.2.612. [DOI] [PubMed] [Google Scholar]
- 156.Holohean AM, Vega JL, Hackman JC, Davidoff RA. Excitatory transmitters, ventral root potentials and [K+]o in the isolated frog leg-spinal cord preparation. Neurosci. Lett. 1988;95(1-3):173–178. doi: 10.1016/0304-3940(88)90652-0. [DOI] [PubMed] [Google Scholar]
- 157.Chiang CY, Hu JW, Sessle BJ. NMDA receptor involvement in neuroplastic changes induced by neonatal capsaicin treatment in trigeminal nociceptive neurons. J. Neurophysiol. 1997;78(5):2799–2803. doi: 10.1152/jn.1997.78.5.2799. [DOI] [PubMed] [Google Scholar]
- 158.Hajos M, Engberg G. A role of excitatory amino acids in the activation of locus coeruleus neurons following cutaneous thermal stimuli. Brain Res. 1990;521(1-2):325–328. doi: 10.1016/0006-8993(90)91560-4. [DOI] [PubMed] [Google Scholar]
- 159.Tassorelli C, Joseph SA. Systemic nitroglycerin induces Fos immunoreactivity in brainstem and forebrain structures of the rat. Brain Res. 1995;682(1-2):167–181. doi: 10.1016/0006-8993(95)00348-t. [DOI] [PubMed] [Google Scholar]
- 160.Knyihar-Csillik E, Toldi J, Mihaly A, Krisztin-Peva B, Chadaide Z, Nemeth H, Fenyo R, Vecsei L. Kynurenine in combination with probenecid mitigates the stimulation-induced increase of c-fos immunoreactivity of the rat caudal trigeminal nucleus in an experimental migraine model. J. Neural Transm. 2007;114(4):417–421. doi: 10.1007/s00702-006-0545-z. [DOI] [PubMed] [Google Scholar]
- 161.Vamos E, Pardutz A, Varga H, Bohar Z, Tajti J, Fulop F, Toldi J, Vecsei L. l-kynurenine combined with probenecid and the novel synthetic kynurenic acid derivative attenuate nitroglycerin-induced nNOS in the rat caudal trigeminal nucleus. Neuropharmacology. 2009;57(4):425–429. doi: 10.1016/j.neuropharm.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 162.Pardutz A, Krizbai I, Multon S, Vecsei L, Schoenen J. Systemic nitroglycerin increases nNOS levels in rat trigeminal nucleus caudalis. Neuroreport. 2000;11(14):3071–3075. doi: 10.1097/00001756-200009280-00008. [DOI] [PubMed] [Google Scholar]
- 163.Pardutz A, Hoyk Z, Varga H, Vecsei L, Schoenen J. Oestrogen-modulated increase of calmodulin-dependent protein kinase II (CamKII) in rat spinal trigeminal nucleus after systemic nitroglycerin. Cephalalgia. 2007;27(1):46–53. doi: 10.1111/j.1468-2982.2006.01244.x. [DOI] [PubMed] [Google Scholar]
- 164.Knyihar-Csillik E, Mihaly A, Krisztin-Peva B, Robotka H, Szatmari I, Fulop F, Toldi J, Csillik B, Vecsei L. The kynurenate analog SZR-72 prevents the nitroglycerol-induced increase of c-fos immunoreactivity in the rat caudal trigeminal nucleus: comparative studies of the effects of SZR-72 and kynurenic acid. Neurosci. Res. 2008;61(4):429–432. doi: 10.1016/j.neures.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 165.Knyihar-Csillik E, Toldi J, Krisztin-Peva B, Chadaide Z, Nemeth H, Fenyo R, Vecsei L. Prevention of electrical stimulation-induced increase of c-fos immunoreaction in the caudal trigeminal nucleus by kynurenine combined with probenecid. Neurosci. Lett. 2007;418(2):122–126. doi: 10.1016/j.neulet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 166.Murphy AZ, Behbehani MM. Electrophysiological characterization of the projection from the nucleus raphe magnus to the lateral reticular nucleus: possible role of an excitatory amino acid in synaptic activation. Brain Res. 1993;606(1):68–78. doi: 10.1016/0006-8993(93)91571-9. [DOI] [PubMed] [Google Scholar]
- 167.Jiang M, Behbehani MM. Physiological characteristics of the projection pathway from the medial preoptic to the nucleus raphe magnus of the rat and its modulation by the periaqueductal gray. Pain. 2001;94(2):139–147. doi: 10.1016/S0304-3959(01)00348-7. [DOI] [PubMed] [Google Scholar]
- 168.Ping HX, Wu HQ, Liu GQ. Modulation of neuronal activity of locus coeruleus in rats induced by excitatory amino acids. Zhongguo Yao Li Xue Bao. 1990;11(3):193–195. [PubMed] [Google Scholar]
- 169.Akaoka H, ston-Jones G. Opiate withdrawal-induced hyperactivity of locus coeruleus neurons is substantially mediated by augmented excitatory amino acid input. J. Neurosci. 1991;11(12):3830–3839. doi: 10.1523/JNEUROSCI.11-12-03830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brun P, Suaud-Chagny MF, Lachuer J, Gonon F, Buda M. Catecholamine Metabolism in Locus Coeruleus Neurons: A Study of its Activation by Sciatic Nerve Stimulation in the Rat. Eur. J. Neurosci. 1991;3(5):397–406. doi: 10.1111/j.1460-9568.1991.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 171.Olpe HR, Steinmann MW, Brugger F, Pozza MF. Excitatory amino acid receptors in rat locus coeruleus. An extracellular in vitro study. Naunyn Schmiedebergs Arch. Pharmacol. 1989;339(3):312–314. doi: 10.1007/BF00173584. [DOI] [PubMed] [Google Scholar]
- 172.Nieber K, Poelchen W, Illes P. Role of ATP in fast excitatory synaptic potentials in locus coeruleus neurones of the rat. Br. J. Pharmacol. 1997;122(3):423–430. doi: 10.1038/sj.bjp.0701386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Palazzo E, Guida F, Migliozzi A, Gatta L, Marabese I, Luongo L, Rossi C, de NV, Fernandez-Sanchez E, Soukupova M, Zafra F, Maione S. Intraperiaqueductal gray glycine and D-serine exert dual effects on rostral ventromedial medulla ON- and OFF-cell activity and thermoceptive threshold in the rat. J. Neurophysiol. 2009;102(6):3169–3179. doi: 10.1152/jn.00124.2009. [DOI] [PubMed] [Google Scholar]
- 174.Morgan MM, Bobeck EN, Ingram SL. Glutamate modulation of antinociception, but not tolerance, produced by morphine microinjection into the periaqueductal gray of the rat. Brain Res. 2009;1295:59–66. doi: 10.1016/j.brainres.2009.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Haghir H, Kovac S, Speckmann EJ, Zilles K, Gorji A. Patterns of neurotransmitter receptor distributions following cortical spreading depression. Neuroscience. 2009;163(4):1340–1352. doi: 10.1016/j.neuroscience.2009.07.067. [DOI] [PubMed] [Google Scholar]
- 176.Faria LC, Mody I. Protective effect of ifenprodil against spreading depression in the mouse entorhinal cortex. J. Neurophysiol. 2004;92(4):2610–2614. doi: 10.1152/jn.00466.2004. [DOI] [PubMed] [Google Scholar]
- 177.Van Harreveld A, Fifkova E. Mechanisms involved in spreading depression. J. Neurobiol. 1973;4(4):375–387. doi: 10.1002/neu.480040406. [DOI] [PubMed] [Google Scholar]
- 178.Mody I, Lambert JD, Heinemann U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J. Neurophysiol. 1987;57(3):869–888. doi: 10.1152/jn.1987.57.3.869. [DOI] [PubMed] [Google Scholar]
- 179.Kiss C, Shepard PD, Bari F, Schwarcz R. Cortical spreading depression augments kynurenate levels and reduces malonate toxicity in the rat cortex. Brain Res. 2004;1002(1-2):129–135. doi: 10.1016/j.brainres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 180.Vilagi I, Klapka N, Luhmann HJ. Optical recording of spreading depression in rat neocortical slices. Brain Res. 2001;898(2):288–296. doi: 10.1016/s0006-8993(01)02196-5. [DOI] [PubMed] [Google Scholar]
- 181.Obeidat AS, Jarvis CR, Andrew RD. Glutamate does not mediate acute neuronal damage after spreading depression induced by O2/glucose deprivation in the hippocampal slice. J. Cereb. Blood Flow Metab. 2000;20(2):412–422. doi: 10.1097/00004647-200002000-00024. [DOI] [PubMed] [Google Scholar]
- 182.Jarvis CR, Anderson TR, Andrew RD. Anoxic depolarization mediates acute damage independent of glutamate in neocortical brain slices. Cereb. Cortex. 2001;11(3):249–259. doi: 10.1093/cercor/11.3.249. [DOI] [PubMed] [Google Scholar]
- 183.Obrenovitch TP. Quinolinic acid accumulation during neuroinflammation. Does it imply excitotoxicity? . Ann. N. Y. Acad. Sci. 2001;939:1–10. doi: 10.1111/j.1749-6632.2001.tb03605.x. (neuroprotective agents: fifth international conference) [DOI] [PubMed] [Google Scholar]
- 184.Obrenovitch TP, Urenjak J. Accumulation of quinolinic acid with neuroinflammation: does it mean excitotoxicity? Adv. Exp. Med. Biol. 2003;527:147–154. doi: 10.1007/978-1-4615-0135-0_17. [DOI] [PubMed] [Google Scholar]