Abstract
Glutamate excitotoxicity contributes to a variety of disorders in the central nervous system, which is triggered primarily by excessive Ca2+ influx arising from overstimulation of glutamate receptors, followed by disintegration of the endoplasmic reticulum (ER) membrane and ER stress, the generation and detoxification of reactive oxygen species as well as mitochondrial dysfunction, leading to neuronal apoptosis and necrosis. Kainic acid (KA), a potent agonist to the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate class of glutamate receptors, is 30-fold more potent in neuro-toxicity than glutamate. In rodents, KA injection resulted in recurrent seizures, behavioral changes and subsequent degeneration of selective populations of neurons in the brain, which has been widely used as a model to study the mechanisms of neurodegenerative pathways induced by excitatory neurotransmitter. Microglial activation and astrocytes proliferation are the other characteristics of KA-induced neurodegeneration. The cytokines and other inflammatory molecules secreted by activated glia cells can modify the outcome of disease progression. Thus, anti-oxidant and anti-inflammatory treatment could attenuate or prevent KA-induced neurodegeneration. In this review, we summarized updated experimental data with regard to the KA-induced neurotoxicity in the brain and emphasized glial responses and glia-oriented cytokines, tumor necrosis factor-α, interleukin (IL)-1, IL-12 and IL-18.
Keywords: Kainic acid, excitotoxicity, microglia, astrocytes, cytokines.
INTRODUCTION
Excitotoxicity mediated by glutamate receptors may underlay the pathology of a number of neurological abnormalities, including Huntington’s chorea, Alzheimer’s disease (AD), and Parkinson’s disease (PD). Excitotoxic cell death is commonly induced experimentally by the administration of kainic acid (KA), a potent agonist to the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate class of glutamate receptors [1-3]. In rodents, injections of KA resulted in recurrent seizures, behavioral changes and subsequent degeneration of selective populations of neurons in the brain [4, 5]. Thus, administration of KA in rodents has been widely used as a model to study the mechanisms of neurodegenerative pathways induced by excitatory neurotransmitters.
1. OVERVIEW OF EXCITOTOXICITY INDUCED BY GLUTAMATE
L-glutamate, the major excitatory transmitter in the brain and spinal cord, is associated with learning, cognition, memory and neuro-endocrine functions [6, 7]. The glutamate receptors can be divided into two broad categories: the ionotropic receptors that mediate fast postsynaptic potentials by activating ion channels directly, and the metabotropic receptors that results in the expression of slow postsynaptic potentials through second messengers [8, 9]. The action of glutamate on the ionotropic receptors is always excitatory [10, 11]. There are three major subtypes of ionotropic glutamate receptors: AMPA, kainate, and N-methyl-D-aspartate (NMDA), named according to the types of synthetic agonists that activate them, respectively [12, 13]. The NMDA glutamate receptor is blocked by specific antagonists such as D(-)-2-amino-5-phosphonovalerate (APV) [14, 15]. Both AMPA and kainate receptors are blocked by 6-cyano-7-nitroquinoxalin-2,3-dione (CNQX) [16, 17]. Thus the AMPA and kainate receptors are sometimes referred to together as non-NMDA receptors. The ion channel of NMDA receptor is a tetrameric structure that results from up to seven genes coding for seven subunits termed GluN1, GluN2A, GluN2B, GluN2C, GluN2D, GluN3A and GluN3B [18, 19]. The AMPA receptor family is composed of four subunits, GluA1–4 [20, 21]. The kainate receptor family comprises five genes, divided into two subfamilies, including GluK4-5 and GluK1–3. GluK4 and GluK5 exhibit higher affinity for kainate than GluK1–3 [22, 23]. Herein, we used the new nomenclature for glutamate receptors recommended by the International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification (NC-IUPHAR) [24]. NC-IUPHAR recommended and previous nomenclatures of ionotropic gluatamate receptor subunits are listed in Table 1.
Table 1.
NC-IUPHAR Recommended and Previous Nomenclatures of Ionotropic Glutamate Receptor Subunits
| Ionotropic Glutamate Family | NC-IUPHAR Subunit Nomenclature | Previous Nomenclatures | Human Gene Name | Human Chromosomal Location |
|---|---|---|---|---|
| NMDA | GluN1 | GLUN1, NMDA-R1, NR1, GluRξ1 | GRIN1 | 9q34.3 |
| GluN2A | GLUN2A, NMDA-R2A, NR2A, GluRε1 | GRIN2A | 16p13.2 | |
| GluN2B | GLUN2B, NMDA-R2B, NR2B, hNR3, GluRε2 | GRIN2B | 12p12 | |
| GluN2C | GLUN2C, NMDA-R2C, NR2C, GluRε3 | GRIN2C | 17q25 | |
| GluN2D | GLUN2D, NMDA-R2D, NR2D, GluRε4 | GRIN2D | 19q13.1 | |
| GluN3A | GLUN3A, NMDA-R3A, NMDAR-L, chi-1 | GRIN3A | 9q31.1 | |
| GluN3B | GLUN3B, NMDA-R3B, | GRIN3B | 19p13.3 | |
| AMPA | GluA1 | GLUA1, GluR1, GluRA, GluR-A, GluR-K1, HBGR1 | GRIA1 | 5q31.1 |
| GluA2 | GLUA2, GluR2, GluRB, GluR-B, GluR-K2, HBGR2 | GRIA2 | 4q32-q33 | |
| GluA3 | GLUA3, GluR3, GluRC, GluR-C, GluR-K3 | GRIA3 | Xq25-q26 | |
| GluA4 | GLUA4, GluR4, GluRD, GluR-D | GRIA4 | 11q22 | |
| Kainate | GluK1 | GLUK5, GluR5, GluR-5, EAA3 | GRIK1 | 21q22.11 |
| GluK2 | GLUK6, GluR6, GluR-6, EAA4 | GRIK2 | 6q16.3-q21 | |
| GluK3 | GLUK7, GluR7, GluR-7, EAA5 | GRIK3 | 1p34-p33 | |
| GluK4 | GLUK1, KA1, KA-1, EAA1 | GRIK4 | 11q22.3 | |
| GluK5 | GLUK2, KA2, KA-2, EAA2 | GRIK5 | 19q13.2 |
Excessive amounts of glutamate are highly toxic to neurons, an action termed glutamate excitotoxicity [25, 26]. Glutamate excitotoxicity is triggered primarily by excessive Ca2+ influx arising from overstimulation of the NMDA subtype of glutamate receptors, followed by disintegration of the endoplasmic reticulum (ER) membrane and ER stress, the generation of reactive oxygen species (ROS) as well as mitochondrial dysfunction, leading to neuronal apoptosis and necrosis [27, 28]. There is increasing realization that the mitochondrial dysfunction occupies the center stage in these processes [28, 29]. In many cell types, glutamate neurotoxicity is induced by NMDA as well as non-NMDA receptors [25, 26, 30].
2. KA ADMINISTRATION TO RODENTS INDUCES SEIZURES, SELECTIVE NEURODEGENERATION AND BEHAVIORAL CHANGES
Kainic acid (KA) is a non-degradable analog of glutamate and 30-fold more potent in neurotoxicity than glutamate [31-33]. Administration of KA to rodents caused a well characterized seizure syndrome, as described by Ben-Ari and other research groups [34, 35]. The seizure activity caused by intravenous, intraperitoneal, intranasal injections or microinjection into amygdala or hippocampus is divided in several distinct phases. During the first 20-30 min, the animals have “staring” spells, followed by head nodding and numerous wet-dog shakes for another 30 min. One hour after KA administration, the animal starts recurrent limbic motor seizures, including masticatory and facial movements, forepaws tremor, rearing and loss of postural control. The seizures then become progressively severer, with a reduction in the intermission. In the following l-2 h, the animal displays a full status epilepticus [34-36].
KA-induced damage seriously impact the hippocampus. The hippocampus is particularly vulnerable to KA-induced neurotoxicity due to the high density of kainate receptors [37]. The hilar neurons are sensitive to KA-induced neurotoxicity, but neuron loss in the other areas of the hippocampus differs between animal species and strains [38-40]. In rats, the systemic injections of KA produced widespread neuronal death, primarily in the hippocampus hilus, CA1 and CA3 areas [30, 41]. Mouse strains vary significantly in their sensitivity to KA-induced neurodegeneration [39, 40, 42, 43]. In general, the C57BL/6, C57BL/10, and (C57BL/6 x CBA/J) F1 strains are resistant to KA-induced neurodegeneration, while the FVB/N, ICR and DBA/2 J strains are vulnerable [40]. C57BL/6, the “relatively” resistant mouse strain, reveals significant neuronal damage in CA1 and CA3, and to a lesser extent, in the polymorphic layer of the dentate gyrus 12 h post-treatment of KA systemically detected by cupric-silver and Fluoro-Jade B staining [44, 45]. CA3 region has the highest abundance of kainate receptors, the activation of which can elevate the concentration of ROS and impair the normal function of mitochondria [46-48]. CA3 neurons are directly excited by stimulation of their KA receptors and indirectly, by increased glutamate efflux secondary to KA stimulation of mossy fibers [49, 50]. CA3 synchronization produces spreading epileptiform activity that extends to CA1 and other limbic structures [51, 52] (Fig. 1).
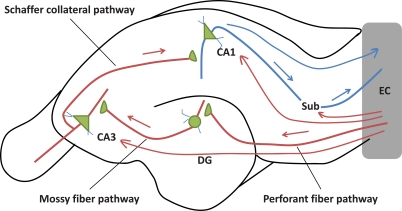
Fig. (1).
The Input and Output Pathways of Hippocampal Formation. Entorhinal cortex (EC) is the main input to the hippocampus. EC projects to the dentate gyrus (DG) via perforant fiber pathway and provides the critical input to CA3 via mossy fiber pathway, then to CA1 by means of the Schaffer collateral pathway. Additionally, EC can also project directly to CA3, CA1 and subiculum (Sub). Meantime, EC is the major output of the hippocampus. Arrows denote the direction of impulse flow.
CA1 pyramidal neurons receive two distinct excitatory inputs that are capable of influencing hippocampal output and involving in spatial memory and memory consolidation [53, 54]. Damage in CA1/CA3 regions of hippocampus induced by KA mainly results in the spatial learning deficits [55, 56]. KA-treated Wistar rats are impaired in the water maze and object exploration tasks, and hyperactive in the open field test, which can be improved by physical exercise and the selective cyclooxygenase (COX)-2 inhibitor [57, 58]. Intraperitoneal injections of KA into the developing rat brains induce the impaired short-term spatial memory in the radial-arm maze, deficient long-term spatial learning and retrieval in the water maze, and a greater degree of anxiety in the elevated plus maze [59, 60]. Mice with a single unilateral injection of KA into the dorsal hippocampus exhibit a decrease in depression-like behavior in the forced swimming test and retarded acquisition as well as impaired retention of visual-spatial information in the Morris water maze test [61].
3. KA MEDIATES GENERATION OF OXIDATIVE STRESS
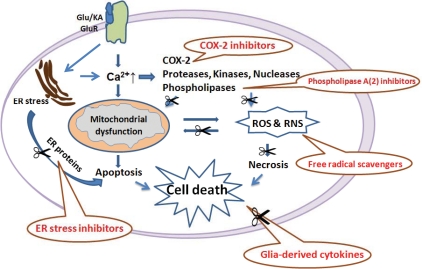
KA receptors have both presynaptic modulatory and direct postsynaptic excitatory actions [62, 63]. The activation of KA receptors produces membrane depolarization and results in alteration in intracellular calcium concentrations, which is required to trigger the neuronal death cascade (Fig. 2) [64]. KA can also induce the release of lactate dehydrogenase (LDH), and a decrease in 3-(4, 5-dimethylthiazole-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT), which result in damage of mitochondrial function [2]. KA administration increases the generation of ROS and reactive nitrogen species (RNS). There is growing evidence that free radical generation plays a key role in the neuronal damage [65]. KA has been shown to immediately induce COX-2 expression that might be involved in hippocampal neuronal death [66]. Early induced COX-2 facilitates the recurrence of hippocampal seizures, and late synthesized COX-2 stimulates hippocampal neuron loss after KA administration [67]. COX catalyzes the first step in the synthesis of prostanoids, including prostaglandins (PGs), prostacyclin, and thromboxanes. PGE(2) is pathologically increased in the brain after KA treatment, and has been proven to be closely associated with neuronal death [68]. In addition, lipid peroxides play critical roles in the initiation and modulation of inflammation and oxidative stress upon KA insult. Seizures can induce the products of lipid peroxidation, such as F(2)-isoprostanes and Isofurans, which have been thought to be the reliable indices of oxidative stress in vivo [69]. Moreover, KA causes the disintegration of the ER membrane in hippocampal neurons and ER stress with the activation of the ER proteins Bip, Chop, and caspase-12 [70]. ER stress appears to act at an early stage of the cell death process prior to disruption of calcium homeostasis, excessive accumulation of ROS, and mitochondrial dysfunction [71]. Old astrocyte specifically induced substance (OASIS) is involved in the endoplasmic reticulum stress response [72]. A recent study showed that OASIS expressed in astrocytes plays an important role in protection against neuronal damage induced by KA [1].
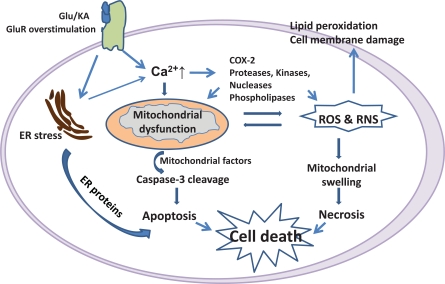
Fig. (2).
Schematic Overview of KA-Mediated Neuronal Death. (1) By stimulating glutamate receptors (GluR), kainic acid (KA) elicits the increase of intracellular Ca2+, activation of Ca2+-dependent enzyme and production of free radicals; (2) Excessive Ca2+ and free radicals cause mitochondrial dysfunction, release of mitochondrial factors, activation of caspase-3, leading to neuronal apoptosis; (3) KA causes the disintegration of the endoplasmic reticulum (ER) and ER stress with the activation of the ER proteins Bip, Chop, and caspase-12, involved in neuronal apoptosis; (4) Ca2+ overload and excessive free radicals cause directly mitochondrial swelling, leading to neuronal necrosis. COX: cyclooxygenase; ROS: reactive oxygen species; RNS: reactive nitrogen species.
4. GLIAL CELLS ARE ACTIVATED UPON KA ADMINISTRATION
KA-induced neuronal death is accompanied by increased activation of microglia and astrocytes [73-75]. Additionally, the activated glial cells cluster at the hippocampal lesions and the immunostaining reactivity is particularly strong around areas of debris (Fig. 3).
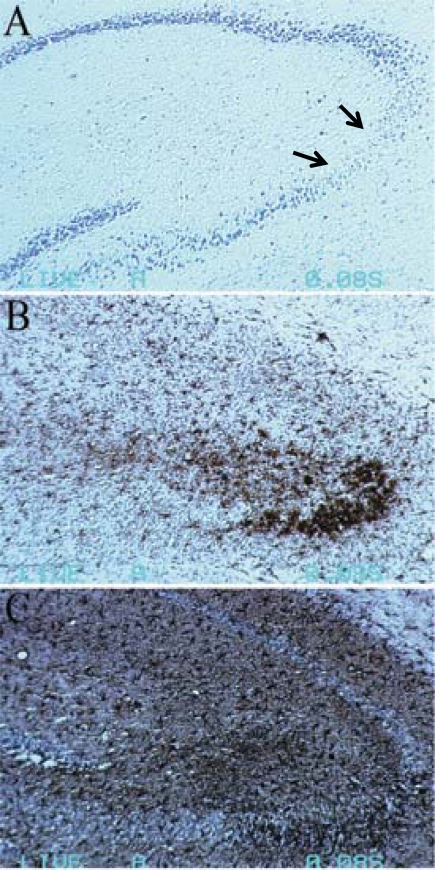
Fig. (3).
Glial cells activation accompanied the neuronal death 7 days after KA (45 mg/kg body weight) treatment to C57BL/6 mice. (A) Obvious neuronal loss was showed in CA3 area of hippocampus by Nissl’s staining. (B) CD11b positive cells (microglia) accumulated in the lesioned CA3 area. (C) GFAP positive cells (astrocytes) spread the whole hippocampus, especially in CA3 area. Arrows in A indicate the areas of neuronal loss.
4.1. Microglia
Microglia account for approximately 20% of the total glial population in the central nervous system (CNS). Microglia are the main effector cell type of the immune and inflammatory responses in the CNS, as earlier reviewed by Streit and his colleagues [76]. The normal role of microglia could be partly connected to neuroprotection, whereas in pathological conditions microglia may become disease-promoting cells. Upon neuronal injury, microglia rapidly acquire changes in morphology and secrete a variety of soluble mediators [77, 78] (Fig. 4). Some studies suggested that the activated microglia might exert a neuroprotective function, especially in multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE) by creating a microenvironment for reparative and regenerative processes [79]. Evidence is also accumulating that activated microglia induce and/or exacerbate neuropathological changes in several CNS diseases such as AD and PD through secreting proinflammatory and neurotoxic factors [80, 81]. In KA-induced hippocampal injury, microglial activation is generally believed to contribute to neuroinflammation and neurodegeneration [74, 82, 83]. A recent study showed that IκB kinase/nuclear factor kappa B (NF-κB) dependent microglial activation participated in KA-mediated injury in vivo through induction of inflammatory mediators [82]. However, whether microglial activation initiates the disease progression or merely responds to neuronal death is still unclear.
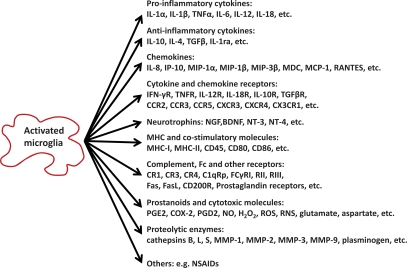
Fig. (4).
The Categories of Molecules Produced by Activated Microglia. IL: interleukin; TNF: tumor necrosis factor; TGF: transforming growth factor; IL-1ra: IL-1 receptor antagonist; MIP: macrophage inflammatory protein; MDC: macrophage-derived chemokine; MCP: monocyte-chemoattractant protein; RANTES: regulated on activation normal T cell expressed and secreted; IFN: interferon; IP: IFN-inducible protein; R: receptor; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; NT: neurotrophin; MHC: major histocompatibility complex; CR: complement receptor; C1qRp: C1q receptor for phagocytosis enhancement; FasL: Fas ligand; PG: prostaglandin; COX: cyclooxygenase; NO: nitric oxide; ROS: reactive oxygen species; RNS: reactive nitrogen species; MMP: matrix metalloproteinase; NSAIDs: nonsteroidal antiinflammatory drugs.
4.2. Astrocytes
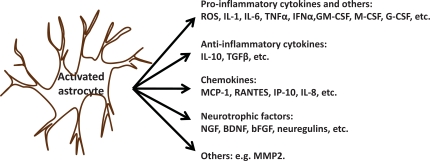
Astrocytes, the most numerous glia cells, have been regarded as passive supporters of neurons in CNS for decades. Studies of the last 20 years, however, challenged this assumption by demonstrating that astrocytes possess functional neurotransmitter receptors [84, 85]. These findings have led to a new concept of neuron-glia intercommunication where astrocytes play an undoubted dynamic role by integrating neuronal inputs and modulating synaptic activity, and so contribute to disease development [86]. Astrocytes have functional receptors for the excitatory neurotransmitter glutamate and respond to physiological concentrations of this substance with oscillations in intracellular Ca2+ concentrations and spatially propagating Ca2+ signals [87-89]. In vitro studies provided evidence that astrocytes can take up glutamate at synapses and release glutamate in a calcium-dependent manner [90]. A proliferative response of astrocytes at two days after KA treatment has been reported already in 1981 [91]. The expression of glial fibrillary acidic protein (GFAP) has been shown to steadily increase from one/three days up to one month after intra-hippocampal or intraperitoneal injection of KA [92, 93]. Astrogliosis induced by excitotoxicity has been considered as a marker for neurotoxicity [73, 94]. Activated astrocytes produce pro- and anti-inflammatory cytokines, chemokines, neurotrophic factors and other modulators to be involved in neuron-glia communication (Fig. 5). It is believed that astrocytes produce growth factors to prevent neurons from death and to promote proliferation and differentiation of precursor cells [95-97]. Activation of transcription factors, including nuclear factor erythroid-2-related factor 2 (Nrf2) and NF-κB, in astrocytes induces the neuroprotective molecule expression and confers protection to neighboring neurons [98-100].
Fig. (5).
The Categories of Molecules Produced by Activated Astrocytes. ROS: reactive oxygen species; IL: interleukin; TNF: tumor necrosis factor; IFN: interferon; CSF: colony-stimulating factor; GM-CSF: granulocyte-macrophage CSF; TGF: transforming growth factor; MCP: monocyte-chemoattractant protein; RANTES: regulated on activation normal T cell expressed and secreted; IP: IFN-inducible protein; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; bFGF: basic fibroblast growth factor; MMP: matrix metalloproteinase.
5. ALTERED CYTOKINE EXPRESSION AFFECTS KA-INDUCED INJURY
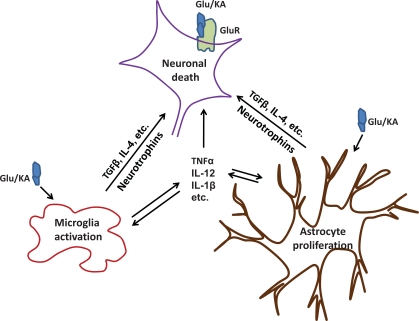
Altered expression of cytokines in response to brain injury has diverse actions that can exacerbate, mediate, reduce or inhibit neuronal damage and influence the disease development in a variety of CNS disorders, such as AD, MS, viral or bacterial infections, ischemia, stroke, and various forms of encephalopathies [101-105]. Cytokines can be divided into pro-inflammatory and anti-inflammatory cytokines, which play the neurodestructive and neuroprotective roles, respectively. It is the balance between destructive and protective factors that ultimately determines the net result of the neuro-immune and neuro-inflammation interaction, as reviewed by Kerschensteiner et al. [106]. Results from studies using KA model also indicated that cytokines are involved in neuron-glia intercommunication and manipulation of pro- and anti-inflammatory cytokines can modify the outcome with regard to the seizure activity, behavioral changes as well as the neuropathological consequences [75, 107-109] (Fig. 6). The roles of several important cytokines in the CNS disorders are reviewed here, including tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-12 and IL-18.
Fig. (6).
Cytokines Involved in Neuron-glia Intercommunication. KA administration enhances further release of endogenous excitatory amino acids, activates microglia and astrocytes. Activated glial cells secrete inflammatory molecules, e.g. cytokines, chemokines, and neurotrophins to influence the outcome of neuronal damage. Glu/KA: glutamate/kainic acid; GluR: glutamate receptors; TGF: transforming growth factor; IL: interleukin; TNF: tumor necrosis factor.
5.1. TNF-α
Tumor necrosis factor-α (TNF-α) is mainly produced by microglia and astrocytes in the CNS. Its functions are mediated through two receptors, TNF receptor (TNFR) 1 (p55) and TNFR2 (p75), both of which are expressed on various cells types [110]. TNF-α over-expression participates in the pathogenesis of several CNS disorders, such as AD [111], bacterial meningitis [112], MS [113] and cerebral malaria [114]. TNF-α potentiates excitotoxic injury to human fetal brain cells [115]. In contrast to its well known deleterious roles, multiple lines of evidence suggested that TNF-α also exhibit neuroprotective properties. This implies an intricate biological function of TNF-α in modulating immune and inflammatory responses. TNF-α knockout worsens Listeria infection in the CNS [116] and TNF-α receptor knockout enhances the neuronal damage after excitotoxic [108, 117], ischemic [118] or traumatic injury [119]. Our study showed that mice lacking TNFR1 exhibited a more severe seizure activity, hippocampal neurodegeneration and increased microglial activation, suggesting that TNF-α plays its protective role through TNFR1 signaling [108], which is in agreement with a previous report [120]. Another study also proved that the protective roles of TNF-α in KA-induced neurodegeneration are via TNFR2 signaling [117]. Several neuroprotective molecules were identified as TNFR1 targets, including members of the Bcl-2 family, DNA repair machinery and cell cycle, developmental, and differentiation factors, neurotransmitters and growth factors, as well as their receptors [121]. The mechanisms by which TNF reduced neuron loss after brain injury may involve the up-regulation of proteins, such as neuronal apoptosis inhibitor protein (NAIP), which maintain calcium homeostasis and reduce free radical generation [122].
5.2. IL-1
IL-1 plays a pivotal role in the neuroinflammation that has been well addressed in KA induced neurodegenerative model [109, 123]. Systemic KA administration induced the expression of IL-1β, IL-1 receptor antagonist and IL-1β converting enzyme as early as 3 h, 12 h, and 24 h post-treatment, respectively, which is localized in the areas known to display neuronal and tissue damage upon excitotoxic lesions [124, 125]. Recombinant IL-1 receptor antagonist has dose- and region-dependent effects on neuronal survival after KA treatment and prevents damage-induced changes in amyloid precursor protein and GFAP mRNAs [126]. It has also been shown that IL-1β is activated in the cerebellum with systemic administration of KA, and its type I receptor (IL-1R) is expressed at a soma of cerebellar Purkinje cells [127]. The proconvulsive actions of IL-1β in the hippocampus may depend on the activation of a sphingomyelinase- and Src-family of kinases-dependent pathway which leads to the phosphorylation of the GluN2B subunit [128].
5.3. IL-12
IL-12 is a heterodimeric cytokine that consists of a heavy chain, p40, and a light chain, p35 [129]. In the CNS, microglia and astrocytes are the main source of producing IL-12 [130]. Human CNS-derived microglia produce IL-12 in vitro after activation with LPS and IFN-γ [131]. Murine microglia can be induced to express mRNA encoding the IL-12 receptor (IL-12R) [132], indicating an autocrine regulation pathway of IL-12 in these cells. IL-12 plays a critical role in several CNS diseases, such as MS and Borna disease. IL-12p40 is increased in cerebrospinal fluid and serum of MS patients [133]. Mice lacking IL-12p40 or administered with anti-IL-12p40 monoclonal antibodies are resistant to the development of EAE [134, 135]. In contrast, IL-12p35 deficient mice are susceptible to EAE [114]. Borna disease virus, which does not trigger disease in most strains, is harmful after infecting the CNS of mice that overexpress IL-12, suggesting an important role of IL-12 in viral infection of the CNS [136]. IL-12 is an active participant in excitotoxic brain injury as suggested by our previous observation that IL-12p35 deficiency alleviates KA-induced hippocampal neurodegeneration [107] and by another finding that IL-12 expression is reduced in hippocampi of transgenic mice protected against KA-induced excitotoxicity by metallothionein overexpression [137].
5.4. IL-18
Interleukin (IL)-18 is most closely related to IL-1β. The similarities between both cytokines comprise structure, receptor complex, and pro-inflammatory properties [138]. IL-18 serves as a link between innate and adaptive immune responses, such as stimulating the expression of adhesion molecules, inducing the production of chemokines (IL-8) and cytokines (TNF-α and IL-6), stimulating the activity of NK cells, and promoting T helper 1 (Th1) cells responses in combination with IL-12, Th2 responses in combination with IL-4, and Th17 responses in combination with IL-23 [139]. IL-18 and IL-18 receptor (IL-18R) mRNA have been found in brain tissue and in cultured astrocytes and microglia [140]. IL-18 enhanced postsynaptic AMPA receptor responses in CA1 pyramidal neurons via the release of glutamate, thereby facilitating basal hippocampal synaptic transmission [141]. IL-18 deficient mice showed a diminished microglial activation and reduced dopaminergic neuron loss after acute 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine treatment [142]. The roles of IL-18 in KA-induced model are controversial. Levels of IL-18 and IL-18R in hippocampus increase progressively from day 1 and peaked at day 3 post-KA treatment [143]. Interestingly, intracerebellar coinjection of IL-18 counteracts the effect of IL-1β in KA-induced ataxia in mice [144]. We showed that exogenous IL-18 administration aggravated the KA-induced injury in normal C57BL/6 mice, while in the condition of IL-18 deficiency, IL-12 could overcompensate the function of IL-18 and worsen the seizure activity as well as hippocampal neurodegeneration [75].
6. THERAPEUTIC STRATEGIES
Considering that oxidative stress is central to KA-induced excitotoxic damage, anti-oxidant and anti-inflammatory treatments may attenuate or prevent the KA-mediated neurodegeneration (Fig. 7). The potential role of COX-2 inhibitors as a new therapeutic drug for the neuron loss after KA treatment has been studied. The selective COX-2 inhibitors, celecoxib, NS398, rofecoxib and SC58125, can suppress an elevation of PGE (2) and block hippocampal cell death [57, 145]. The other drugs tested experimentally include curcumin, fluoxetine, ethyl pyruvate and statins, whose neuroprotective effects are associated with their anti-inflammatory and anti-oxidant effects [146]. Free radical scavengers are well known to prevent neuron loss induced by exposure to excitotoxins. Edaravone (Ed), a newly developed free radical scavenger, could inhibit lipid peroxidation and prevent neuron loss when administered after the onset of seizures in a KA-induced neurodegenerative animal model [147]. The pineal secretory product, melatonin, has free-radical-scavenger and antioxidant properties, which attenuates KA-induced neuronal death, lipid peroxidation, and microglial activation [148]. Several phospholipase A (2) inhibitors, quinacrine and chloroquine, arachidonyl trifluoromethyl ketone, bromoenol lactone, cytidine 5-diphosphoamines, and vitamin E, have been shown to prevent the neurodegeneration in KA-mediated neurotoxicity [149]. Moreover, inhibition of ER stress by small molecular compounds, such as salubrinal, may benefit for treatment of KA-mediated neurotoxicity [70]. Furthermore, the microtubule interacting drug candidate NAP has been shown to protect against KA toxicity via regulating the expression of key genes involved in the epileptogenic pathway [150]. A novel approach to engineer patient derived adult stem cells for therapeutic adenosine delivery in autologous cell transplantation has been proved to suppress seizure activity and protect hippocampal neurons from KA-induced damage [151]. Additionally, targeting the pro-inflammatory cytokines, by blocking the unique signal transduction of the specific cytokine is another potential therapeutic strategy.
Fig. (7).
Schematic Illustration of Anti-Oxidant and Anti-inflammatory Treatments. By inhibiting one of the major components of the neuroinflammatory response after KA treatment, there could be less inflammation and neuronal loss. The potential treatments include (1) cyclooxygenase (COX)-2 inhibitors and other antioxidants; (2) Phospholipase A2 inhibitors; (3) Free radical scavengers; (4) endoplasmic reticulum (ER) stress inhibitors; and (5) Glia-derived cytokines. Glu/KA: glutamate/kainic acid; GluR: glutamate receptors; ROS: reactive oxygen species; RNS: reactive nitrogen species.
7. CONCLUDING REMARKS
Glutamate excitotoxicity contributes to a variety of CNS diseases, which is involved in overstimulation of glutamate receptors, excessive Ca2+ influx, ER stress, generation of ROS and mitochondrial dysfunction, leading to neuronal damage. KA is a potent agonist to the AMPA/kainate class of glutamate receptors, which can result in seizures, behavioral changes and neurodegenration in susceptible brain regions like the hippocampus in rodents.
The activated glia cells and subsequent secretion of inflammatory molecules can modify the outcome of disease progression. By inhibiting one of the major components of the neuroinflammatory response after KA treatment, there could be less inflammation and neuronal loss, therefore an improvement of cognitive function.
ACKNOWLEDGEMENTS
The work was supported by grants from SADF (Insamlingsstiftelsen för Alzheimer- och Demensforskning) foundation, Swedish Medicine Association, Gamla Tjänarinnor foundation, Gun och Bertil Stohnes foundation and Swedish National Board of Health and Welfare.
REFERENCES
- 1.Chihara K, Saito A, Murakami T, Hino S, Aoki Y, Sekiya H, Aikawa Y, Wanaka A, Imaizumi K. Increased vulnerability of hippocampal pyramidal neurons to the toxicity of kainic acid in OASIS-deficient mice. J. Neurochem. 2009;110(3):956–965. doi: 10.1111/j.1471-4159.2009.06188.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang Q, Yu S, Simonyi A, Sun GY, Sun AY. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol. Neurobiol. 2005;31(1-3):3–16. doi: 10.1385/MN:31:1-3:003. [DOI] [PubMed] [Google Scholar]
- 3.Yang DD, Kuan CY, Whitmarsh AJ, Rincon M, Zheng TS, Davis RJ, Rakic P, Flavell RA. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389(6653):865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 4.McKhann GM, 2nd, Wenzel HJ, Robbins CA, Sosunov AA, Schwartzkroin PA. Mouse strain differences in kainic acid sensitivity, seizure behavior, mortality, and hippocampal pathology. Neuroscience. 2003;122(2):551–561. doi: 10.1016/s0306-4522(03)00562-1. [DOI] [PubMed] [Google Scholar]
- 5.Tripathi PP, Sgado P, Scali M, Viaggi C, Casarosa S, Simon HH, Vaglini F, Corsini GU, Bozzi Y. Increased susceptibility to kainic acid-induced seizures in Engrailed-2 knockout mice. Neuroscience. 2009;159(2):842–849. doi: 10.1016/j.neuroscience.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Herron CE, Lester RA, Coan EJ, Collingridge GL. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986;322(6076):265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- 7.Zahr NM, Mayer D, Pfefferbaum A, Sullivan EV. Low striatal glutamate levels underlie cognitive decline in the elderly: evidence from in vivo molecular spectroscopy. Cereb. Cortex. 2008;18(10):2241–2250. doi: 10.1093/cercor/bhm250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363(6427):347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- 9.Rainnie DG, Holmes KH, Shinnick-Gallagher P. Activation of postsynaptic metabotropic glutamate receptors by trans-ACPD hyperpolarizes neurons of the basolateral amygdala. J. Neurosci. 1994;14(11 Pt 2):7208–7220. doi: 10.1523/JNEUROSCI.14-11-07208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephens GJ, Djamgoz MB, Wilkin GP. A patch clamp study of excitatory amino acid effects on cortical astrocyte subtypes in culture. Receptors Channels. 1993;1(1):39–52. [PubMed] [Google Scholar]
- 11.Sugiyama H, Ito I, Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- 12.Loscher W, Lehmann H, Behl B, Seemann D, Teschendorf HJ, Hofmann HP, Lubisch W, Hoger T, Lemaire HG, Gross G. A new pyrrolyl-quinoxalinedione series of non-NMDA glutamate receptor antagonists: pharmacological characterization and comparison with NBQX and valproate in the kindling model of epilepsy. Eur. J. Neurosci. 1999;11(1):250–262. doi: 10.1046/j.1460-9568.1999.00432.x. [DOI] [PubMed] [Google Scholar]
- 13.Trist DG. Excitatory amino acid agonists and antagonists: pharmacology and therapeutic applications. Pharm. Acta. Helv. 2000;74(2-3):221–229. doi: 10.1016/s0031-6865(99)00053-9. [DOI] [PubMed] [Google Scholar]
- 14.Choi DW, Koh JY, Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J. Neurosci. 1988;8(1):185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies J, Evans RH, Jones AW, Smith DA, Watkins JC. Differential activation and blockade of excitatory amino acid receptors in the mammalian and amphibian central nervous systems. Comp. Biochem. Physiol. C. 1982;72(2):211–224. doi: 10.1016/0306-4492(82)90086-7. [DOI] [PubMed] [Google Scholar]
- 16.Evstratova AA, Mironova EV, Dvoretskova EA, Antonov SM. Apoptosis and the receptor specificity of its mechanisms during the neurotoxic action of glutamate. Neurosci. Behav. Physiol. 2009;39(4):353–362. doi: 10.1007/s11055-009-9141-7. [DOI] [PubMed] [Google Scholar]
- 17.Koh JY, Goldberg MP, Hartley DM, Choi DW. Non-NMDA receptor-mediated neurotoxicity in cortical culture. J. Neurosci. 1990;10(2):693–705. doi: 10.1523/JNEUROSCI.10-02-00693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J. Neurosci. 1998;18(8):2954–2961. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438(7071):1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 20.Bochet P, Audinat E, Lambolez B, Crepel F, Rossier J. Analysis of AMPA receptor subunits expressed by single Purkinje cells using RNA polymerase chain reaction. Biochem. Soc. Trans. 1993;21(1):93–97. doi: 10.1042/bst0210093. [DOI] [PubMed] [Google Scholar]
- 21.Kwok KH, Tse YC, Wong RN, Yung KK. Cellular localization of GluR1, GluR2/3 and GluR4 glutamate receptor subunits in neurons of the rat neostriatum. Brain Res. 1997;778(1):43–55. doi: 10.1016/s0006-8993(97)00950-5. [DOI] [PubMed] [Google Scholar]
- 22.Niedzielski AS, Wenthold RJ. Expression of AMPA, kainate, and NMDA receptor subunits in cochlear and vestibular ganglia. J. Neurosci. 1995;15(3 (Pt 2)):2338–2353. doi: 10.1523/JNEUROSCI.15-03-02338.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raymond LA, Blackstone CD, Huganir RL. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature. 1993;361(6413):637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- 24.Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56(1):2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi DW. Ionic dependence of glutamate neurotoxicity. J. Neurosci. 1987;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frandsen A, Drejer J, Schousboe A. Direct evidence that excitotoxicity in cultured neurons is mediated via N-methyl-D-aspartate (NMDA) as well as non-NMDA receptors. J. Neurochem. 1989;53(1):297–299. doi: 10.1111/j.1471-4159.1989.tb07327.x. [DOI] [PubMed] [Google Scholar]
- 27.Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr. Mol. Med. 2004;4(2):149–177. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- 28.Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J. Neurosci. 1996;16(19):6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunet N, Tarabal O, Esquerda JE, Caldero J. Excitotoxic motoneuron degeneration induced by glutamate receptor agonists and mitochondrial toxins in organotypic cultures of chick embryo spinal cord. J. Comp. Neurol. 2009;516(4):277–290. doi: 10.1002/cne.22118. [DOI] [PubMed] [Google Scholar]
- 30.Morales-Garcia JA, Luna-Medina R, Martinez A, Santos A, Perez-Castillo A. Anticonvulsant and neuroprotective effects of the novel calcium antagonist NP04634 on kainic acid-induced seizures in rats. J. Neurosci. Res. 2009;87:3687–3697. doi: 10.1002/jnr.22165. [DOI] [PubMed] [Google Scholar]
- 31.Lee JK, Won JS, Singh AK, Singh I. Statin inhibits kainic acid-induced seizure and associated inflammation and hippocampal cell death. Neurosci. Lett. 2008;440(3):260–264. doi: 10.1016/j.neulet.2008.05.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGeer EG, McGeer PL. Some factors influencing the neuro-toxicity of intrastriatal injections of kainic acid. Neurochem. Res. 1978;3(4):501–517. doi: 10.1007/BF00966331. [DOI] [PubMed] [Google Scholar]
- 33.Olney JW, Rhee V, Ho OL. Kainic acid: a powerful neurotoxic analogue of glutamate. Brain Res. 1974;77(3):507–512. doi: 10.1016/0006-8993(74)90640-4. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14(2):375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 35.Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392(6676):601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- 36.Chuang YC, Chang AY, Lin JW, Hsu SP, Chan SH. Mitochondrial dysfunction and ultrastructural damage in the hippocampus during kainic acid-induced status epilepticus in the rat. Epilepsia. 2004;45(10):1202–1209. doi: 10.1111/j.0013-9580.2004.18204.x. [DOI] [PubMed] [Google Scholar]
- 37.Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF. Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J. Neurosci. 2003;23(22):8013–8019. doi: 10.1523/JNEUROSCI.23-22-08013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantallops I, Routtenberg A. Kainic acid induction of mossy fiber sprouting: dependence on mouse strain. Hippocampus. 2000;10(3):269–273. doi: 10.1002/1098-1063(2000)10:3<269::AID-HIPO7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 39.Kasugai M, Akaike K, Imamura S, Matsukubo H, Tojo H, Nakamura M, Tanaka S, Sano A. Differences in two mice strains on kainic acid-induced amygdalar seizures. Biochem. Biophys. Res. Commun. 2007;357(4):1078–1083. doi: 10.1016/j.bbrc.2007.04.067. [DOI] [PubMed] [Google Scholar]
- 40.McLin JP, Steward O. Comparison of seizure phenotype and neurodegeneration induced by systemic kainic acid in inbred, out-bred, and hybrid mouse strains. Eur. J. Neurosci. 2006;24(8):2191–2202. doi: 10.1111/j.1460-9568.2006.05111.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee HN, Jeon GS, Kim DW, Cho IH, Cho SS. Expression of adenomatous polyposis coli protein in reactive astrocytes in hippocampus of kainic acid-induced rat. Neurochem. Res. 2010;35(1):114–121. doi: 10.1007/s11064-009-0036-3. [DOI] [PubMed] [Google Scholar]
- 42.Schauwecker PE. Genetic basis of kainate-induced excitotoxicity in mice: phenotypic modulation of seizure-induced cell death. Epilepsy Res. 2003;55(3):201–210. doi: 10.1016/s0920-1211(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Houk B, Shah J, Hauser KF, Luo Y, Smith G, Schauwecker E, Barnes GN. Genetic background regulates semaphorin gene expression and epileptogenesis in mouse brain after kainic acid status epilepticus. Neuroscience. 2005;131(4):853–869. doi: 10.1016/j.neuroscience.2004.09.064. [DOI] [PubMed] [Google Scholar]
- 44.Benkovic SA, O'Callaghan JP, Miller DB. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res. 2004;1024(1-2):59–76. doi: 10.1016/j.brainres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 45.Benkovic SA, O'Callaghan JP, Miller DB. Regional neuropathology following kainic acid intoxication in adult and aged C57BL/6J mice. Brain Res. 2006;1070(1):215–231. doi: 10.1016/j.brainres.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 46.Carriedo SG, Sensi SL, Yin HZ, Weiss JH. AMPA exposures induce mitochondrial Ca(2+) overload and ROS generation in spinal motor neurons in vitro. J. Neurosci. 2000;20(1):240–250. doi: 10.1523/JNEUROSCI.20-01-00240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A critical role of a facilitatory presynaptic kainate receptor in mossy fiber LTP. Neuron. 2001;32(4):697–709. doi: 10.1016/s0896-6273(01)00511-6. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J. Neurosci. 1995;15(5 Pt 1):3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2-/- mice. J. Neurosci. 2003;23(2):422–429. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Moreno A, Sihra TS. Presynaptic kainate receptor facilitation of glutamate release involves protein kinase A in the rat hippocampus. J. Physiol. 2004;557(Pt 3):733–745. doi: 10.1113/jphysiol.2004.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bausch SB, McNamara JO. Contributions of mossy fiber and CA1 pyramidal cell sprouting to dentate granule cell hyperexcitability in kainic acid-treated hippocampal slice cultures. J. Neurophysiol. 2004;92(6):3582–3595. doi: 10.1152/jn.01028.2003. [DOI] [PubMed] [Google Scholar]
- 52.Ding R, Asada H, Obata K. Changes in extracellular glutamate and GABA levels in the hippocampal CA3 and CA1 areas and the induction of glutamic acid decarboxylase-67 in dentate granule cells of rats treated with kainic acid. Brain Res. 1998;800(1):105–113. doi: 10.1016/s0006-8993(98)00507-1. [DOI] [PubMed] [Google Scholar]
- 53.Iijima T, Witter MP, Ichikawa M, Tominaga T, Kajiwara R, Matsumoto G. Entorhinal-hippocampal interactions revealed by real-time imaging. Science. 1996;272(5265):1176–1179. doi: 10.1126/science.272.5265.1176. [DOI] [PubMed] [Google Scholar]
- 54.Speed HE, Dobrunz LE. Developmental changes in short-term facilitation are opposite at temporoammonic synapses compared to Schaffer collateral synapses onto CA1 pyramidal cells. Hippocampus. 2009;19(2):187–204. doi: 10.1002/hipo.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gayoso MJ, Primo C, al-Majdalawi A, Fernandez JM, Garrosa M, Iniguez C. Brain lesions and water-maze learning deficits after systemic administration of kainic acid to adult rats. Brain Res. 1994;653(1-2):92–100. doi: 10.1016/0006-8993(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 56.Milgram NW, Isen DA, Mandel D, Palantzas H, Pepkowski MJ. Deficits in spontaneous behavior and cognitive function following systemic administration of kainic acid. Neurotoxicology. 1988;9(4):611–624. [PubMed] [Google Scholar]
- 57.Gobbo OL, O'Mara SM. Post-treatment, but not pre-treatment, with the selective cyclooxygenase-2 inhibitor celecoxib markedly enhances functional recovery from kainic acid-induced neurodegeneration. Neuroscience. 2004;125(2):317–327. doi: 10.1016/j.neuroscience.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 58.Gobbo OL, O'Mara SM. Exercise, but not environmental enrichment, improves learning after kainic acid-induced hippocampal neurodegeneration in association with an increase in brain-derived neurotrophic factor. Behav. Brain Res. 2005;159(1):21–26. doi: 10.1016/j.bbr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 59.Sarkisian MR, Tandon P, Liu Z, Yang Y, Hori A, Holmes GL, Stafstrom CE. Multiple kainic acid seizures in the immature and adult brain: ictal manifestations and long-term effects on learning and memory. Epilepsia. 1997;38(11):1157–1166. doi: 10.1111/j.1528-1157.1997.tb01211.x. [DOI] [PubMed] [Google Scholar]
- 60.Sayin U, Sutula TP, Stafstrom CE. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia. 2004;45(12):1539–1548. doi: 10.1111/j.0013-9580.2004.54903.x. [DOI] [PubMed] [Google Scholar]
- 61.Groticke I, Hoffmann K, Loscher W. Behavioral alterations in a mouse model of temporal lobe epilepsy induced by intrahippocampal injection of kainate. Exp. Neurol. 2008;213(1):71–83. doi: 10.1016/j.expneurol.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 62.Campbell SL, Mathew SS, Hablitz JJ. Pre- and postsynaptic effects of kainate on layer II/III pyramidal cells in rat neocortex. Neuropharmacology. 2007;53(1):37–47. doi: 10.1016/j.neuropharm.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Youn DH, Randic M. Modulation of excitatory synaptic transmission in the spinal substantia gelatinosa of mice deficient in the kainate receptor GluR5 and/or GluR6 subunit. J. Physiol. 2004;555(Pt 3):683–698. doi: 10.1113/jphysiol.2003.057570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brorson JR, Manzolillo PA, Miller RJ. Ca2+ entry via AMPA/KA receptors and excitotoxicity in cultured cerebellar Purkinje cells. J. Neurosci. 1994;14(1):187–197. doi: 10.1523/JNEUROSCI.14-01-00187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ueda Y, Yokoyama H, Nakajima A, Tokumaru J, Doi T, Mitsuyama Y. Glutamate excess and free radical formation during and following kainic acid-induced status epilepticus. Exp. Brain Res. 2002;147(2):219–226. doi: 10.1007/s00221-002-1224-4. [DOI] [PubMed] [Google Scholar]
- 66.Kim EJ, Lee JE, Kwon KJ, Lee SH, Moon CH, Baik EJ. Differential roles of cyclooxygenase isoforms after kainic acid-induced prostaglandin E(2) production and neurodegeneration in cortical and hippocampal cell cultures. Brain Res. 2001;908(1):1–9. doi: 10.1016/s0006-8993(01)02432-5. [DOI] [PubMed] [Google Scholar]
- 67.Takemiya T, Matsumura K, Yamagata K. Roles of prostaglandin synthesis in excitotoxic brain diseases. Neurochem. Int. 2007;51(2-4):112–120. doi: 10.1016/j.neuint.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Kawaguchi K, Hickey RW, Rose ME, Zhu L, Chen J, Graham SH. Cyclooxygenase-2 expression is induced in rat brain after kainate-induced seizures and promotes neuronal death in CA3 hippocampus. Brain Res. 2005;1050(1-2):130–137. doi: 10.1016/j.brainres.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 69.Patel M, Liang LP, Hou H, Williams BB, Kmiec M, Swartz HM, Fessel JP, Roberts LJ., 2nd Seizure-induced formation of isofurans: novel products of lipid peroxidation whose formation is positively modulated by oxygen tension. J. Neurochem. 2008;104(1):264–270. doi: 10.1111/j.1471-4159.2007.04974.x. [DOI] [PubMed] [Google Scholar]
- 70.Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J. Neurosci. 2007;27(4):901–908. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp. Neurol. 1999;155(2):302–314. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- 72.Saito A, Hino S, Murakami T, Kondo S, Imaizumi K. A novel ER stress transducer, OASIS, expressed in astrocytes. Antioxid. Redox Signal. 2007;9(5):563–571. doi: 10.1089/ars.2006.1520. [DOI] [PubMed] [Google Scholar]
- 73.Chen Z, Duan RS, Quezada HC, Mix E, Nennesmo I, Adem A, Winblad B, Zhu J. Increased microglial activation and astrogliosis after intranasal administration of kainic acid in C57BL/6 mice. J. Neurobiol. 2005;62(2):207–218. doi: 10.1002/neu.20099. [DOI] [PubMed] [Google Scholar]
- 74.Ravizza T, Rizzi M, Perego C, Richichi C, Veliskova J, Moshe SL, De Simoni MG, Vezzani A. Inflammatory response and glia activation in developing rat hippocampus after status epilepticus. Epilepsia. 2005;46(Suppl 5):113–117. doi: 10.1111/j.1528-1167.2005.01006.x. [DOI] [PubMed] [Google Scholar]
- 75.Zhang XM, Duan RS, Chen Z, Quezada HC, Mix E, Winblad B, Zhu J. IL-18 deficiency aggravates kainic acid-induced hippocampal neurodegeneration in C57BL/6 mice due to an overcompensation by IL-12. Exp. Neurol. 2007;205(1):64–73. doi: 10.1016/j.expneurol.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 76.Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1(5):301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- 77.Kato H, Walz W. The initiation of the microglial response. Brain Pathol. 2000;10(1):137–143. doi: 10.1111/j.1750-3639.2000.tb00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 79.Napoli I, Neumann H. Protective effects of microglia in multiple sclerosis. Exp. Neurol. 2010;225(1):24–28. doi: 10.1016/j.expneurol.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 80.Marinova-Mutafchieva L, Sadeghian M, Broom L, Davis JB, Medhurst AD, Dexter DT. Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: a time course study in a 6-hydroxydopamine model of Parkinson's disease. J. Neurochem. 2009;110(3):966–975. doi: 10.1111/j.1471-4159.2009.06189.x. [DOI] [PubMed] [Google Scholar]
- 81.Venneti S, Wiley CA, Kofler J. Imaging microglial activation during neuroinflammation and Alzheimer's disease. J. Neuroimmune Pharmacol. 2009;4(2):227–243. doi: 10.1007/s11481-008-9142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cho IH, Hong J, Suh EC, Kim JH, Lee H, Lee JE, Lee S, Kim CH, Kim DW, Jo EK, Lee KE, Karin M, Lee SJ. Role of microglial IKKbeta in kainic acid-induced hippocampal neuronal cell death. Brain. 2008;131(Pt 11):3019–3033. doi: 10.1093/brain/awn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Penkowa M, Molinero A, Carrasco J, Hidalgo J. Interleukin-6 deficiency reduces the brain inflammatory response and increases oxidative stress and neurodegeneration after kainic acid-induced seizures. Neuroscience. 2001;102(4):805–818. doi: 10.1016/s0306-4522(00)00515-7. [DOI] [PubMed] [Google Scholar]
- 84.Hosli L, Hosli E. Receptors for dopamine and serotonin on astrocytes of cultured rat central nervous system. J. Physiol. (Paris) 1987;82(4):191–195. [PubMed] [Google Scholar]
- 85.Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263(5154):1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 86.Vesce S, Rossi D, Brambilla L, Volterra A. Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int. Rev. Neurobiol. 2007;82:57–71. doi: 10.1016/S0074-7742(07)82003-4. [DOI] [PubMed] [Google Scholar]
- 87.Dani JW, Smith SJ. The triggering of astrocytic calcium waves by NMDA-induced neuronal activation. Ciba Found Symp. 1995;188:195–205. doi: 10.1002/9780470514696.ch11. [DOI] [PubMed] [Google Scholar]
- 88.van den Pol AN, Finkbeiner SM, Cornell-Bell AH. Calcium excitability and oscillations in suprachiasmatic nucleus neurons and glia in vitro. J. Neurosci. 1992;12(7):2648–2664. doi: 10.1523/JNEUROSCI.12-07-02648.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yagodin S, Holtzclaw LA, Russell JT. Subcellular calcium oscillators and calcium influx support agonist-induced calcium waves in cultured astrocytes. Mol. Cell Biochem. 1995;149-150:137–144. doi: 10.1007/BF01076572. [DOI] [PubMed] [Google Scholar]
- 90.Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129(4):877–896. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 91.Murabe Y, Ibata Y, Sano Y. Morphological studies on neuro-glia. IV. Proliferative response of non-neuronal elements in the hippocampus of the rat to kainic acid-induced lesions. Cell Tissue Res. 1982;222(1):223–226. doi: 10.1007/BF00218302. [DOI] [PubMed] [Google Scholar]
- 92.Bendotti C, Guglielmetti F, Tortarolo M, Samanin R, Hirst WD. Differential expression of S100beta and glial fibrillary acidic protein in the hippocampus after kainic acid-induced lesions and mossy fiber sprouting in adult rat. Exp. Neurol. 2000;161(1):317–329. doi: 10.1006/exnr.1999.7262. [DOI] [PubMed] [Google Scholar]
- 93.Ding M, Haglid KG, Hamberger A. Quantitative immunochemistry on neuronal loss, reactive gliosis and BBB damage in cortex/striatum and hippocampus/amygdala after systemic kainic acid administration. Neurochem. Int. 2000;36(4-5):313–318. doi: 10.1016/s0197-0186(99)00139-4. [DOI] [PubMed] [Google Scholar]
- 94.Torre ER, Lothman E, Steward O. Glial response to neuronal activity: GFAP-mRNA and protein levels are transiently increased in the hippocampus after seizures. Brain Res. 1993;631(2):256–264. doi: 10.1016/0006-8993(93)91543-2. [DOI] [PubMed] [Google Scholar]
- 95.Braun A, Dang J, Johann S, Beyer C, Kipp M. Selective regulation of growth factor expression in cultured cortical astrocytes by neuro-pathological toxins. Neurochem. Int. 2009;55(7):610–618. doi: 10.1016/j.neuint.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 96.Dakubo GD, Beug ST, Mazerolle CJ, Thurig S, Wang Y, Wallace VA. Control of glial precursor cell development in the mouse optic nerve by sonic hedgehog from retinal ganglion cells. Brain Res. 2008;1228:27–42. doi: 10.1016/j.brainres.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 97.Sandhu JK, Gardaneh M, Iwasiow R, Lanthier P, Gangaraju S, Ribecco-Lutkiewicz M, Tremblay R, Kiuchi K, Sikorska M. Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol. Dis. 2009;33(3):405–414. doi: 10.1016/j.nbd.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 98.Lerner-Natoli M, Montpied P, Rousset MC, Bockaert J, Rondouin G. Sequential expression of surface antigens and transcription factor NFkappaB by hippocampal cells in excitotoxicity and experimental epilepsy. Epilepsy Res. 2000;41(2):141–154. doi: 10.1016/s0920-1211(00)00132-7. [DOI] [PubMed] [Google Scholar]
- 99.Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J. Neurosci. 2008;28(50):13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev. Mol. Med. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001;2(10):734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 102.Basic Kes V, Simundic AM, Nikolac N, Topic E, Demarin V. Pro-inflammatory and anti-inflammatory cytokines in acute ischemic stroke and their relation to early neurological deficit and stroke outcome. Clin. Biochem. 2008;41(16-17):1330–1334. doi: 10.1016/j.clinbiochem.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 103.Bennett JL, Stuve O. Update on inflammation, neurodegeneration, and immunoregulation in multiple sclerosis: therapeutic implications. Clin. Neuropharmacol. 2009;32(3):121–132. doi: 10.1097/WNF.0b013e3181880359. [DOI] [PubMed] [Google Scholar]
- 104.Rojo LE, Fernandez JA, Maccioni AA, Jimenez JM, Maccioni RB. Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer's disease. Arch. Med. Res. 2008;39(1):1–16. doi: 10.1016/j.arcmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 105.Shiraishi M, Ichiyama T, Matsushige T, Iwaki T, Iyoda K, Fukuda K, Makata H, Matsubara T, Furukawa S. Soluble tumor necrosis factor receptor 1 and tissue inhibitor of metalloproteinase-1 in hemolytic uremic syndrome with encephalopathy. J. Neuroimmunol. 2008;196(1-2):147–152. doi: 10.1016/j.jneuroim.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 106.Kerschensteiner M, Meinl E, Hohlfeld R. Neuro-immune crosstalk in CNS diseases. Results Probl. Cell Differ. 2010;51:197–216. doi: 10.1007/400_2009_6. [DOI] [PubMed] [Google Scholar]
- 107.Chen Z, Duan RS, Q HC, Wu Q, Mix E, Winblad B, Ljunggren HG, Zhu J. IL-12p35 deficiency alleviates kainic acid-induced hippocampal neurodegeneration in C57BL/6 mice. Neurobiol. Dis. 2004;17(2):171–178. doi: 10.1016/j.nbd.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 108.Lu MO, Zhang XM, Mix E, Quezada HC, Jin T, Zhu J, Adem A. TNF-alpha receptor 1 deficiency enhances kainic acid-induced hippocampal injury in mice. J. Neurosci. Res. 2008;86(7):1608–1614. doi: 10.1002/jnr.21600. [DOI] [PubMed] [Google Scholar]
- 109.Oprica M, Eriksson C, Schultzberg M. Inflammatory mechanisms associated with brain damage induced by kainic acid with special reference to the interleukin-1 system. J. Cell Mol. Med. 2003;7(2):127–140. doi: 10.1111/j.1582-4934.2003.tb00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ware CF, Crowe PD, Vanarsdale TL, Andrews JL, Grayson MH, Jerzy R, Smith CA, Goodwin RG. Tumor necrosis factor (TNF) receptor expression in T lymphocytes. Differential regulation of the type I TNF receptor during activation of resting and effector T cells. J. Immunol. 1991;147(12):4229–4238. [PubMed] [Google Scholar]
- 111.Zhao M, Cribbs DH, Anderson AJ, Cummings BJ, Su JH, Wasserman AJ, Cotman CW. The induction of the TNFalpha death domain signaling pathway in Alzheimer's disease brain. Neurochem. Res. 2003;28(2):307–318. doi: 10.1023/a:1022337519035. [DOI] [PubMed] [Google Scholar]
- 112.Leist TP, Frei K, Kam-Hansen S, Zinkernagel RM, Fontana A. Tumor necrosis factor alpha in cerebrospinal fluid during bacterial, but not viral, meningitis. Evaluation in murine model infections and in patients. J. Exp. Med. 1988;167(5):1743–1748. doi: 10.1084/jem.167.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raine CS. Multiple sclerosis: TNF revisited, with promise. Nat. Med. 1995;1(3):211–214. doi: 10.1038/nm0395-211. [DOI] [PubMed] [Google Scholar]
- 114.Grau GE, Piguet PF, Vassalli P, Lambert PH. Tumor-necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol. Rev. 1989;112:49–70. doi: 10.1111/j.1600-065x.1989.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 115.Chao CC, Hu S. Tumor necrosis factor-alpha potentiates glutamate neurotoxicity in human fetal brain cell cultures. Dev. Neurosci. 1994;16(3-4):172–179. doi: 10.1159/000112104. [DOI] [PubMed] [Google Scholar]
- 116.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364(6440):798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 117.Balosso S, Ravizza T, Perego C, Peschon J, Campbell IL, De Simoni MG, Vezzani A. Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann. Neurol. 2005;57(6):804–812. doi: 10.1002/ana.20480. [DOI] [PubMed] [Google Scholar]
- 118.Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat. Med. 1996;2(7):788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 119.Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J. Neurosci. 1999;19(15):6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gary DS, Bruce-Keller AJ, Kindy MS, Mattson MP. Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor. J. Cereb. Blood Flow Metab. 1998;18(12):1283–1287. doi: 10.1097/00004647-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 121.Taoufik E, Petit E, Divoux D, Tseveleki V, Mengozzi M, Roberts ML, Valable S, Ghezzi P, Quackenbush J, Brines M, Cerami A, Probert L. TNF receptor I sensitizes neurons to erythropoietin- and VEGF-mediated neuroprotection after ischemic and excitotoxic injury. Proc. Natl. Acad. Sci. U S A. 2008;105(16):6185–6190. doi: 10.1073/pnas.0801447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thompson C, Gary D, Mattson M, Mackenzie A, Robertson GS. Kainic acid-induced naip expression in the hippocampus is blocked in mice lacking TNF receptors. Brain Res. Mol. Brain Res. 2004;123(1-2):126–131. doi: 10.1016/j.molbrainres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 123.Simi A, Tsakiri N, Wang P, Rothwell NJ. Interleukin-1 and inflammatory neurodegeneration. Biochem. Soc. Trans. 2007;35(Pt 5):1122–1126. doi: 10.1042/BST0351122. [DOI] [PubMed] [Google Scholar]
- 124.Eriksson C, Van Dam AM, Lucassen PJ, Bol JG, Winblad B, Schultzberg M. Immunohistochemical localization of interleukin-1beta, interleukin-1 receptor antagonist and interleukin-1beta converting enzyme/caspase-1 in the rat brain after peripheral administration of kainic acid. Neuroscience. 1999;93(3):915–930. doi: 10.1016/s0306-4522(99)00178-5. [DOI] [PubMed] [Google Scholar]
- 125.Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, De Simoni MG. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J. Neurosci. 1999;19(12):5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Panegyres PK, Hughes J. The neuroprotective effects of the recombinant interleukin-1 receptor antagonist rhIL-1ra after excitotoxic stimulation with kainic acid and its relationship to the amyloid precursor protein gene. J. Neurol. Sci. 1998;154(2):123–132. doi: 10.1016/s0022-510x(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 127.Motoki K, Kishi H, Hori E, Tajiri K, Nishijo H, Muraguchi A. The direct excitatory effect of IL-1beta on cerebellar Purkinje cell. Biochem. Biophys. Res. Commun. 2009;379(3):665–668. doi: 10.1016/j.bbrc.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 128.Balosso S, Maroso M, Sanchez-Alavez M, Ravizza T, Frasca A, Bartfai T, Vezzani A. A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta. Brain. 2008;131(Pt 12):3256–3265. doi: 10.1093/brain/awn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stalder AK, Pagenstecher A, Yu NC, Kincaid C, Chiang CS, Hobbs MV, Bloom FE, Campbell IL. Lipopolysaccharide-induced IL-12 expression in the central nervous system and cultured astrocytes and microglia. J. Immunol. 1997;159(3):1344–1351. [PubMed] [Google Scholar]
- 131.Becher B, Dodelet V, Fedorowicz V, Antel JP. Soluble tumor necrosis factor receptor inhibits interleukin 12 production by stimulated human adult microglial cells in vitro. J. Clin. Invest. 1996;98(7):1539–1543. doi: 10.1172/JCI118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Suzumura A, Sawada M, Takayanagi T. Production of interleukin-12 and expression of its receptors by murine microglia. Brain Res. 1998;787(1):139–142. doi: 10.1016/s0006-8993(97)01166-9. [DOI] [PubMed] [Google Scholar]
- 133.Fassbender K, Ragoschke A, Rossol S, Schwartz A, Mielke O, Paulig A, Hennerici M. Increased release of interleukin-12p40 in MS: association with intracerebral inflammation. Neurology. 1998;51(3):753–758. doi: 10.1212/wnl.51.3.753. [DOI] [PubMed] [Google Scholar]
- 134.Brok HP, van Meurs M, Blezer E, Schantz A, Peritt D, Treacy G, Laman JD, Bauer J, t Hart BA. Prevention of experimental autoimmune encephalomyelitis in common marmosets using an anti-IL-12p40 monoclonal antibody. J. Immunol. 2002;169(11):6554–6563. doi: 10.4049/jimmunol.169.11.6554. [DOI] [PubMed] [Google Scholar]
- 135.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J. Immunol. 2002;169(12):7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 136.Freude S, Hausmann J, Hofer M, Pham-Mitchell N, Campbell IL, Staeheli P, Pagenstecher A. Borna disease virus accelerates inflammation and disease associated with transgenic expression of interleukin-12 in the central nervous system. J. Virol. 2002;76(23):12223–12232. doi: 10.1128/JVI.76.23.12223-12232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Penkowa M, Florit S, Giralt M, Quintana A, Molinero A, Carrasco J, Hidalgo J. Metallothionein reduces central nervous system inflammation, neurodegeneration, and cell death following kainic acid-induced epileptic seizures. J. Neurosci. Res. 2005;79(4):522–534. doi: 10.1002/jnr.20387. [DOI] [PubMed] [Google Scholar]
- 138.Dinarello CA. IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J. Allergy Clin. Immunol. 1999;103(1(Pt 1)):11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 139.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 140.Conti B, Park LC, Calingasan NY, Kim Y, Kim H, Bae Y, Gibson GE, Joh TH. Cultures of astrocytes and microglia express interleukin 18. Brain Res. Mol. Brain Res. 1999;67(1):46–52. doi: 10.1016/s0169-328x(99)00034-0. [DOI] [PubMed] [Google Scholar]
- 141.Kanno T, Nagata T, Yamamoto S, Okamura H, Nishizaki T. Interleukin-18 stimulates synaptically released glutamate and enhances postsynaptic AMPA receptor responses in the CA1 region of mouse hippocampal slices. Brain Res. 2004;1012(1-2):190–193. doi: 10.1016/j.brainres.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 142.Sugama S, Wirz SA, Barr AM, Conti B, Bartfai T, Shibasaki T. Interleukin-18 null mice show diminished microglial activation and reduced dopaminergic neuron loss following acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment. Neuroscience. 2004;128(2):451–458. doi: 10.1016/j.neuroscience.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 143.Jeon GS, Park SK, Park SW, Kim DW, Chung CK, Cho SS. Glial expression of interleukin-18 and its receptor after excitotoxic damage in the mouse hippocampus. Neurochem. Res. 2008;33(1):179–184. doi: 10.1007/s11064-007-9434-6. [DOI] [PubMed] [Google Scholar]
- 144.Andoh T, Kishi H, Motoki K, Nakanishi K, Kuraishi Y, Muraguchi A. Protective effect of IL-18 on kainate- and IL-1 beta-induced cerebellar ataxia in mice. J. Immunol. 2008;180(4):2322–2328. doi: 10.4049/jimmunol.180.4.2322. [DOI] [PubMed] [Google Scholar]
- 145.Takemiya T, Maehara M, Matsumura K, Yasuda S, Sugiura H, Yamagata K. Prostaglandin E2 produced by late induced COX-2 stimulates hippocampal neuron loss after seizure in the CA3 region. Neurosci. Res. 2006;56(1):103–110. doi: 10.1016/j.neures.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 146.Gupta YK, Briyal S, Sharma M. Protective effect of curcumin against kainic acid induced seizures and oxidative stress in rats. Indian J. Physiol. Pharmacol. 2009;53(1):39–46. [PubMed] [Google Scholar]
- 147.Miyamoto R, Shimakawa S, Suzuki S, Ogihara T, Tamai H. Edaravone prevents kainic acid-induced neuronal death. Brain Res. 2008;1209:85–91. doi: 10.1016/j.brainres.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 148.Chung SY, Han SH. Melatonin attenuates kainic acid-induced hippocampal neurodegeneration and oxidative stress through microglial inhibition. J. Pineal Res. 2003;34(2):95–102. doi: 10.1034/j.1600-079x.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 149.Farooqui AA, Ong WY, Horrocks LA. Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol. Rev. 2006;58(3):591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- 150.Zemlyak I, Manley N, Vulih-Shultzman I, Cutler AB, Graber K, Sapolsky RM, Gozes I. The microtubule interacting drug candidate NAP protects against kainic acid toxicity in a rat model of epilepsy. J. Neurochem. 2009;111(5):1252–1263. doi: 10.1111/j.1471-4159.2009.06415.x. [DOI] [PubMed] [Google Scholar]
- 151.Ren G, Li T, Lan JQ, Wilz A, Simon RP, Boison D. Lentiviral RNAi-induced downregulation of adenosine kinase in human mesenchymal stem cell grafts: a novel perspective for seizure control. Exp. Neurol. 2007;208(1):26–37. doi: 10.1016/j.expneurol.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]