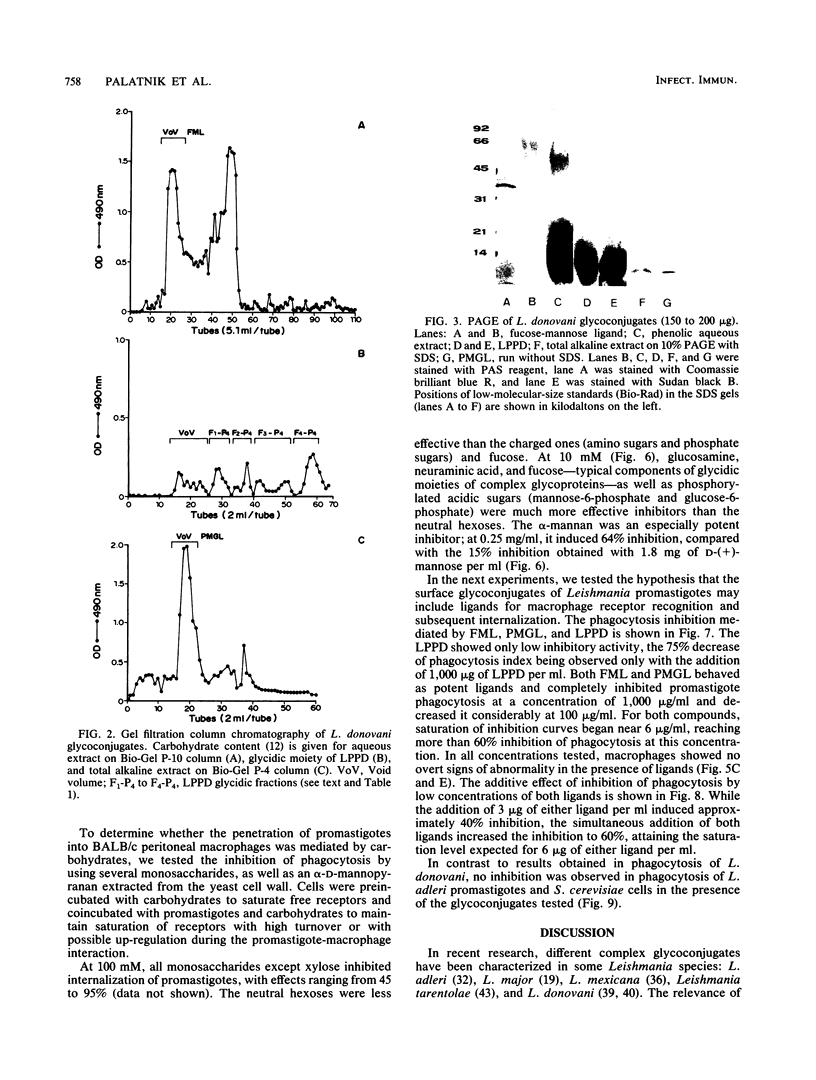
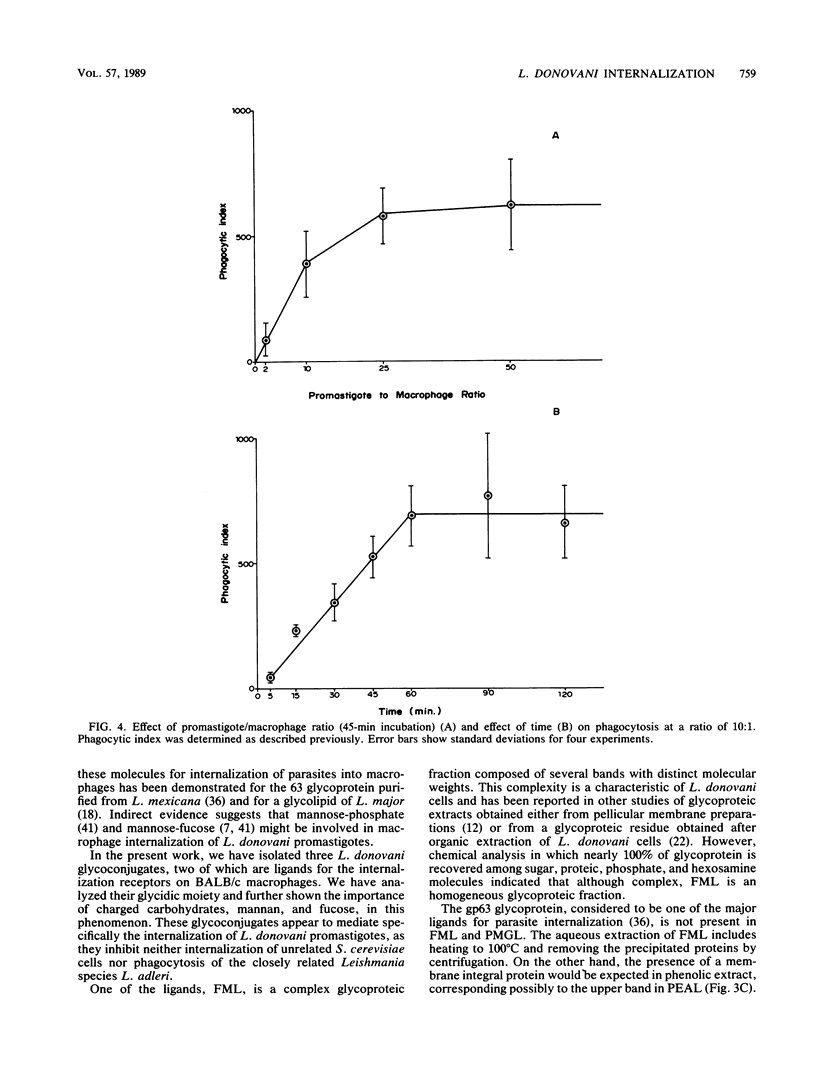
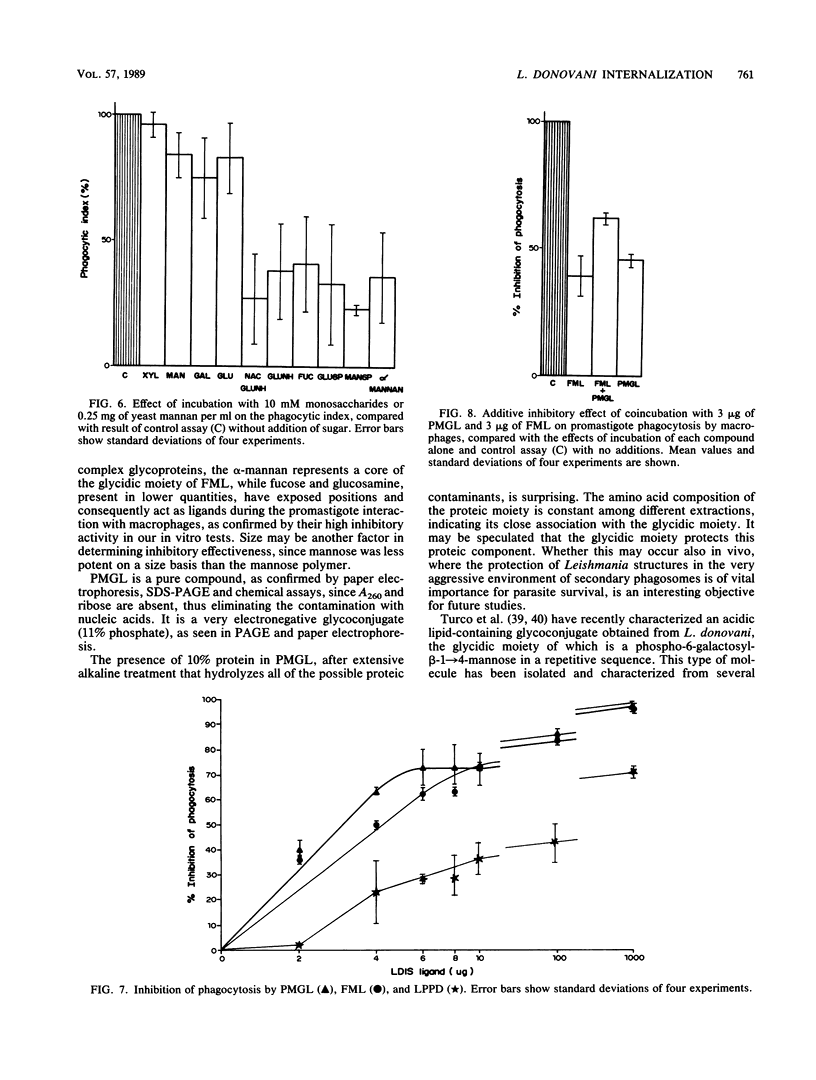
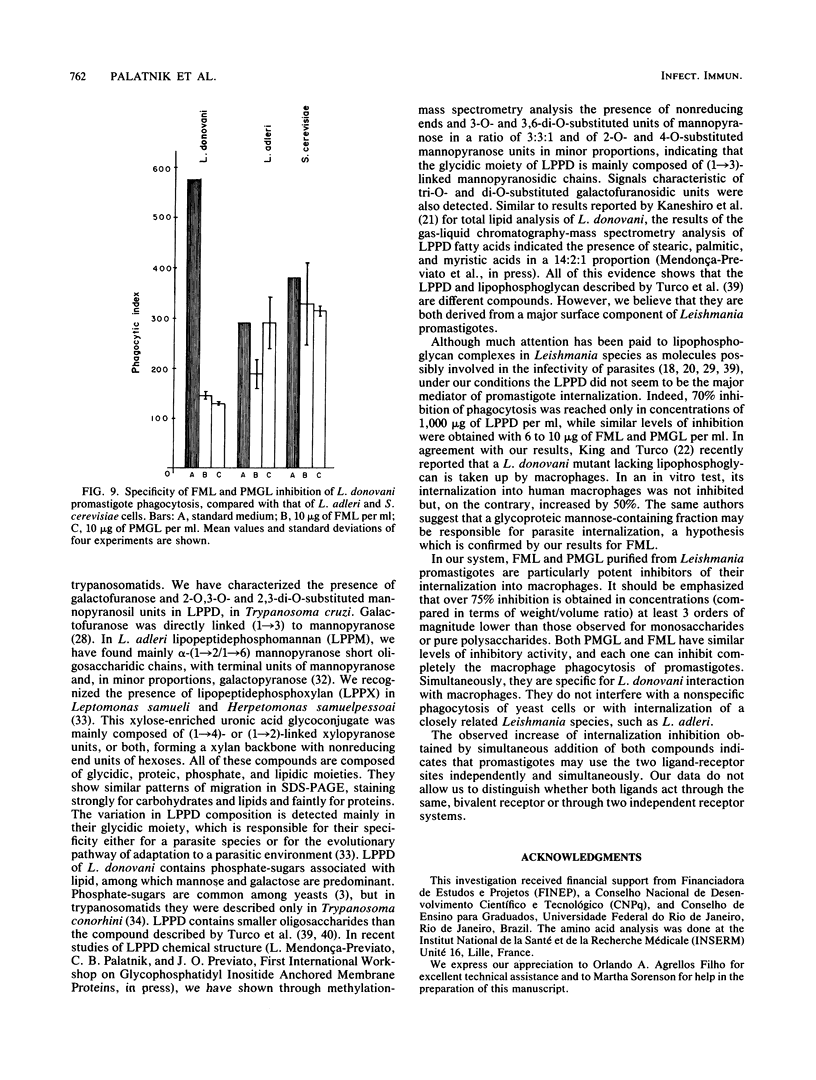
Abstract
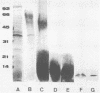
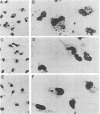
Leishmania donovani, the agent of human visceral leishmaniasis, is an intracellular parasite that must be recognized and internalized by host macrophages to complete its biological cycle. In a search for possible ligands for macrophage surface receptors, glycoconjugates were obtained from Leishmania promastigotes by aqueous, phenol-aqueous, and alkaline extraction. A fucose-mannose glycoproteic ligand, a lipopeptidephosphoglycan, and a phosphate mannogalactan ligand were purified from promastigotes and analyzed for their chemical contents, with special attention to their glycidic moieties. Sugars that were identified as components of these glycoconjugates were tested for their capacity to inhibit promastigote internalization by BALB/c peritoneal macrophages in vitro. Neutral hexoses showed little inhibitory activity; fucose, charged monosaccharides, and a mannose polymer showed the highest activity. Two of the glycoconjugates (fucose-mannose glycoproteic ligand and phosphate mannogalactan ligand) purified from promastigotes were potent inhibitors of internalization, 75% inhibition being obtained at concentrations of 6 to 10 micrograms/ml. The simultaneous presence of both ligands in low concentrations yielded an increase in inhibitory activity above that found for each ligand alone, indicating that promastigotes may use at least two receptor sites for penetration into macrophages. These ligands are specific inhibitors of L. donovani promastigote phagocytosis, since 10 micrograms of each ligand per ml interfered neither with internalization of yeast cells nor with phagocytosis of Leishmania adleri promastigotes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P. Leishmania donovani-macrophage binding mediated by surface glycoproteins/antigens: characterization in vitro by a radioisotopic assay. Mol Biochem Parasitol. 1981 Nov;4(1-2):67–76. doi: 10.1016/0166-6851(81)90030-x. [DOI] [PubMed] [Google Scholar]
- Channon J. Y., Roberts M. B., Blackwell J. M. A study of the differential respiratory burst activity elicited by promastigotes and amastigotes of Leishmania donovani in murine resident peritoneal macrophages. Immunology. 1984 Oct;53(2):345–355. [PMC free article] [PubMed] [Google Scholar]
- De Lederkremer R. M., Tanaka C. T., Alves M. J., Colli W. Lipopeptidophosphoglycan from Trypanosoma cruzi. Amide and ester-linked fatty acids. Eur J Biochem. 1977 Apr 1;74(2):263–267. doi: 10.1111/j.1432-1033.1977.tb11389.x. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M. Isolation and partial characterization of surface membranes from Leishmania donovani promastigotes. J Protozool. 1980 May;27(2):176–182. doi: 10.1111/j.1550-7408.1980.tb04676.x. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M., Langreth S. G., Dwyer N. K. Evidence for a polysaccharide surface coat in the developmental stages of Leishmania donovani: a fine structure-cytochemical study. Z Parasitenkd. 1974 Jun 21;43(4):227–249. doi: 10.1007/BF00328879. [DOI] [PubMed] [Google Scholar]
- Etges R. J., Bouvier J., Hoffman R., Bordier C. Evidence that the major surface proteins of three Leishmania species are structurally related. Mol Biochem Parasitol. 1985 Feb;14(2):141–149. doi: 10.1016/0166-6851(85)90033-7. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fauconnet M., Rochemont J. A single-column amino acid analysis method which resolves hexosamines and several cysteine derivatives. Anal Biochem. 1978 Dec;91(2):403–409. doi: 10.1016/0003-2697(78)90525-0. [DOI] [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Greenblatt C. L., Goding J. W. An amphipathic sulphated glycoconjugate of Leishmania: characterization with monoclonal antibodies. EMBO J. 1984 Oct;3(10):2301–2306. doi: 10.1002/j.1460-2075.1984.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshiro E. S., Jayasimhulu K., Lester R. L. Characterization of inositol lipids from Leishmania donovani promastigotes: identification of an inositol sphingophospholipid. J Lipid Res. 1986 Dec;27(12):1294–1303. [PubMed] [Google Scholar]
- King D. L., Turco S. J. A ricin agglutinin-resistant clone of Leishmania donovani deficient in lipophosphoglycan. Mol Biochem Parasitol. 1988 Apr;28(3):285–293. doi: 10.1016/0166-6851(88)90013-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Scocca J. R. A common structural unit in asparagine-oligosaccharides of several glycoproteins from different sources. J Biol Chem. 1972 Sep 25;247(18):5753–5758. [PubMed] [Google Scholar]
- Lepay D. A., Nogueira N., Cohn Z. Surface antigens of Leishmania donovani promastigotes. J Exp Med. 1983 May 1;157(5):1562–1572. doi: 10.1084/jem.157.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça-Previato L., Gorin P. A., Braga A. F., Scharfstein J., Previato J. O. Chemical structure and antigenic aspects of complexes obtained from epimastigotes of Trypanosoma cruzi. Biochemistry. 1983 Oct 11;22(21):4980–4987. doi: 10.1021/bi00290a016. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E. The glycoconjugate derived from a Leishmania major receptor for macrophages is a suppressogenic, disease-promoting antigen in murine cutaneous leishmaniasis. Parasite Immunol. 1986 May;8(3):255–263. doi: 10.1111/j.1365-3024.1986.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Palatnik C. B., Previato J. O., Gorin P. A., Mendonça Previato L. Partial chemical characterization of the carbohydrate moieties in Leishmania adleri glycoconjugates. Mol Biochem Parasitol. 1985 Jan;14(1):41–54. doi: 10.1016/0166-6851(85)90104-5. [DOI] [PubMed] [Google Scholar]
- Palatnik C. B., Previato J. O., Gorin P. A., Mendonça-Previato L. Leptomonas samueli glycoconjugates. Comparison with Herpetomonas samuelpessoai. Comp Biochem Physiol B. 1987;86(3):593–599. doi: 10.1016/0305-0491(87)90454-8. [DOI] [PubMed] [Google Scholar]
- Pessolani M. C., Mendonça-Previato L., Andrade A. F., Gorin P. A., Previato J. O. Structural features and antigenic properties of carbohydrate-containing components of Trypanosoma conorhini. Mol Biochem Parasitol. 1987 Nov;26(1-2):193–202. doi: 10.1016/0166-6851(87)90143-5. [DOI] [PubMed] [Google Scholar]
- Prat J. P., Lamy J. N., Weill J. D. Coloration des lipoprotéines après électrophorèse en gel de polyacrylamide. Bull Soc Chim Biol (Paris) 1969 Dec 18;51(9):1367–1367. [PubMed] [Google Scholar]
- Russell D. G., Wilhelm H. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J Immunol. 1986 Apr 1;136(7):2613–2620. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Hull S. R., Orlandi P. A., Jr, Shepherd S. D., Homans S. W., Dwek R. A., Rademacher T. W. Structure of the major carbohydrate fragment of the Leishmania donovani lipophosphoglycan. Biochemistry. 1987 Sep 22;26(19):6233–6238. doi: 10.1021/bi00393a042. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Wilkerson M. A., Clawson D. R. Expression of an unusual acidic glycoconjugate in Leishmania donovani. J Biol Chem. 1984 Mar 25;259(6):3883–3889. [PubMed] [Google Scholar]
- Wilson M. E., Pearson R. D. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect Immun. 1988 Feb;56(2):363–369. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier M. T., Previato J. O., Gorin P. A., Mendonça-Previato L. Chemical structures of a galactose-rich glycoprotein of Leishmania tarentolae. Comp Biochem Physiol B. 1987;88(1):101–104. doi: 10.1016/0305-0491(87)90086-1. [DOI] [PubMed] [Google Scholar]