Abstract
Calorie restriction (CR) has been shown to decrease H2O2 production in liver mitochondria, although it is not known if this is due to uniform changes in all mitochondria or changes in particular mitochondrial subpopulations. To address this issue, liver mitochondria from control and CR mice were fractionated using differential centrifugation at 1,000 g, 3,000 g and 10,000 g into distinct populations labeled as M1, M3 and M10, respectively. Mitochondrial protein levels, respiration and H2O2 production were measured in each fraction. CR resulted in a decrease in total protein (mg) in each fraction, although this difference disappeared when adjusted for liver weight (mg protein/g liver weight). No differences in respiration (State 3 or 4) were observed between control and CR mice in any of the mitochondrial fractions. CR decreased H2O2 production in all fractions when mitochondria respired on succinate (Succ), succ+antimycin A (Succ+AA) or pyruvate/ malate+rotenone (P/M+ROT). Thus, CR decreased reactive oxygen species (ROS) production under conditions which stimulate mitochondrial complex I ROS production under both forward (P/M+ROT) and backward (Succ & Succ+AA) electron flow. The results indicate that CR decreases H2O2 production in all liver mitochondrial fractions due to a decrease in capacity for ROS production by complex I of the electron transport chain.
Keywords: Mitochondria, Caloric restriction, Reactive oxygen species, Hydrogen peroxide, Respiration, Mouse liver
Introduction
Calorie restriction (CR), without malnutrition, has been shown to increase maximum life span and prevent or delay the onset of age-associated pathophysiological changes in multiple species (Sohal and Weindruch 1996). However, the mechanism responsible for the retardation of aging with CR is still not entirely known. The free radical theory of aging (Harman 1956) is one of the most popular theories to explain the biochemical basis for aging, and the demonstration that CR decreases both oxidative damage and mitochondrial reactive oxygen species (ROS) production (Merry 2004; Page et al. 2010; Pamplona and Barja 2006; Sohal and Weindruch 1996) has led to speculation that a decrease in oxidative stress is the mechanism for life span extension with CR. Although decreases in H2O2 production (an indicator of ROS production) have been reported in multiple tissues with CR (Page et al. 2010; Pamplona and Barja 2006), it is not clear if this is due to uniform changes in all mitochondria or if specific subpopulations of mitochondria within a tissue are driving these changes.
The heterogeneity of mitochondrial populations and their fractionation into various sub-populations has been demonstrated previously in a variety of tissues. One strategy for sorting mitochondria into subpopulations is to separate by size based on the gravitational force at which each mitochondrial fraction is obtained by differential centrifugation. Using this strategy, rat liver mitochondria have been separated into three distinct fractions, with the heaviest showing the highest respiration rates (Lanni et al. 1996). It has been suggested that an association exists between mitochondrial biogenesis and mitochondrial fractions, with a growth cycle existing where lighter mitochondria serve as precursors of the heavy mitochondria (Gianotti et al. 1998; Justo et al. 2005; Koekemoer and Oelofsen 2001; Lombardi et al. 2000). In support of this idea, it has been shown in brown adipose tissue (BAT) that acute cold exposure or fasting influence primarily the lighter mitochondria (Gianotti et al. 1998; Moreno et al. 1994) while chronic fasting, overfeeding or cold acclimation also affects the heavy mitochondria (Gianotti et al. 1998; Matamala et al. 1996; Moreno et al. 1994).
In addition to the morphological differences between heavy and light mitochondria, there are also biochemical differences between these subpopulations. Studies in liver demonstrate that antioxidant capacity is lower and ROS production and oxygen consumption rates are higher in heavy compared to light mitochondrial fractions (Venditti et al. 1996, 2002). Similarly, the activities of several enzymes from pathways of intermediary metabolism have been shown to be increased in heavy compared to light mitochondria in both liver and white adipose tissue (Koekemoer and Oelofsen 2001). In particular, studies have shown that cytochrome c oxidase activity is increased in heavy versus light mitochondria from liver (Koekemoer and Oelofsen 2001; Lanni et al. 1996) white adipose tissue (Koekemoer and Oelofsen 2001) and brown adipose tissue (Moreno et al. 1994). Thus, there are major biochemical differences between heavy and light mitochondria, and the net impact of any intervention on mitochondrial function will depend on which specific mitochondrial subpopulations are altered.
Although it has frequently been reported that CR decreases liver mitochondrial H2O2 production (Gredilla et al. 2001a; Hagopian et al. 2005; Lambert and Merry 2004; Lopez-Torres et al. 2002), there is no information about which mitochondrial subpopulations are responsible for this change. The aim of the current study was to determine the influence of CR on H2O2 production in liver mitochondrial subpopulations separated by size through collection of fractions at 1,000 g (M1), 3,000 g (M3) and 10,000 g (M10).
Materials and methods
Animals and diets
Male C57BL/6J mice were purchased from Jackson Laboratories (West Sacramento, CA) at 3 months of age, housed singly and maintained according to the institutional and federal guidelines for the ethical treatment of animals. Animals were kept on a 12 h dark 12 h light cycle with lights on from 0600 to 1800 h, at 22 °C, and with free access to water. The mice were fed a non-purified diet, 7012 Teklad (Harlan Laboratories, Madison, WI), ad libitum for 1 month until they were 4 months old. At 4 months of age, the mice were assigned to either a control or calorie restricted group and fed a semi-purified diet (AIN93G) with the CR group being fed 40% less calories. All mice were on this diet for 2 months, which made them 6 months old when used for the experiments.
Chemicals
All chemicals were purchased from Sigma Chemical Company (St Louis, MO). Bio-Rad protein assay kit was from Bio-Rad laboratories (Hercules, CA).
Tissue harvesting
Mice were sacrificed by cervical dislocation and body weights recorded. Liver was removed immediately, weighed and processed for mitochondrial isolation. All other organs and fat pads were removed and their weights recorded. These organs included heart, lungs, spleen, brain and kidneys, while the fat pads included perirenal, epidydimal, mesenteric, subcutaneous and inter-scapular fat pads.
Liver mitochondrial isolation
Livers were removed as quickly as possible and placed in ice-cold isolation medium (220 mM mannitol, 70 mM sucrose, 20 mM Tris–HCl, 1 mM EDTA and 0.1% (w/v) fatty acid-free BSA, pH 7.4 at 4 °C) and minced with scissors into small pieces. The buffer was decanted and fresh buffer added to rinse the liver pieces and help in the removal of blood. This was repeated three times and the liver pieces were placed into a glass/Teflon homogenizer containing isolation medium (1:10 w/v) and homogenized with 4–6 strokes of the Teflon pestle at 500 rpm. The homogenizer is kept in an ice-packed beaker during the homogenization process. The homogenate is centrifuged at 500 g for 10 min at 4 °C and the resulting supernatant is kept for further analysis while the pellet containing cell debris is discarded. The crude supernatant from above is centrifuged at 1,000 g for 10 min at 4 °C and the resulting supernatant (S1) is retained while the pellet is washed twice in suspension medium (same as the isolation medium but without BSA) and re-suspended in a minimal volume of suspension medium, labeled M1 and kept on ice. The supernatant S1 was centrifuged at 3,000 g for 10 min at 4 °C and the resulting supernatant S3 was retained while the pellet was washed twice in suspension medium and re-suspended in a minimal volume of suspension medium, labeled M3 and kept on ice. The supernatant S3 was centrifuged at 10,000 g for 10 min at 4 °C and the resulting S10 supernatant was kept for assaying marker enzymes while the pellet was washed twice in suspension medium and re-suspended in a minimal volume of suspension medium, labeled M10 and kept on ice.
Marker enzyme assays
Enzyme activities were measured from the mitochondrial fractions as well as the supernatant. The activities were measured according to the published procedures for citrate synthase (Shephard and Garland 1968), lactate dehydrogenase (Bergmeyer and Bernt 1974), glucose-6-phosphatase (Gierow and Jergil 1982), acid phosphatase (Moss 1984), and urate oxidase (Bergmeyer et al. 1974), as indicated in the references. Citrate synthase, lactate dehydrogenase, glucose-6-phosphatase, acid phosphatase and urate oxidase were used as mitochondrial, cytosolic, microsomal, lysosomal and peroxisomal markers, respectively. Activities were means ± SEM for six independent measurements and were expressed as µmol/min/mg protein.
Mitochondrial H2O2 production
Mitochondrial H2O2 production was determined fluorimetrically, using a previously described method (Hyslop and Sklar 1984). Briefly, the assay reaction mix contained (final concentrations) 10 mM potassium phosphate, pH 7.4, and containing 154 mM KCl, 0.1 mM EGTA, 3 mM MgCl2, 500 µg p-hydroxyphenylacetate (PHPA), 4 units horseradish peroxidase, using 10 mM succinate or 10 mM Pyruvate/5 mM malate as substrates. The assay was started by adding 190–210 µg mitochondria and was performed in the absence or presence of rotenone (5 µM) or antimycin A (5 µM). The oxidation of p-hydroxyphenylacetate (PHPA) coupled to the enzymatic reduction of H2O2 by horseradish peroxidase, in the presence of the substrates, was monitored as an increase in fluorescence at 37 °C, using a Perkin-Elmer LS 55 luminescence spectrometer equipped with a water peltier heating system and a magnetic stirring sample compartment. The excitation and emission wavelengths were 320 nm and 400 nm, respectively, and the final assay volume was 3 ml. Levels of H2O2 were expressed as pmol H2O2/min/mg protein. Concentrations were calculated from a standard curve generated by using different concentrations of H2O2.
Measurement of mitochondrial oxygen consumption
Mitochondrial oxygen consumption (nmol O/min/mg protein) was measured using a previously described method (Venditti et al. 2006) with modifications. Briefly, mitochondrial respiration was monitored at 30 °C by a Clark-type oxygen electrode (Hansatech, Norfolk, UK) incubated with air-saturated assay medium (120 mM KCl, 5 mM KH2PO4, 5 mM HEPES, 1 mM EGTA, and 5 mM MgCl2 , pH 7.4). All measurements were completed in duplicate using 0.5 mg of mitochondrial protein/ml. Respiration was initiated by the addition of 5 mM succinate (plus 5 µM rotenone) in the absence (State 4) and in the presence (State 3) of 500 µM ADP. Respiratory control ratios (RCR) were calculated as state 3 divided by state 4 respiration rates.
Other methods
Protein concentrations were determined using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA). All data were expressed as means ± SEM and statistical analysis to determine differences between groups was performed using Student’s t-test.
Results
Body, organ and fat pad weights
Body and organ weights are summarized in Table 1. The results showed a 31% decrease in body weight (P<0.001) in CR mice which was accompanied by a 25% decrease (P<0.003) in CR liver weights. When liver tissue mass was normalized to body weight (g liver/kg body weight), a significant increase (P<0.02) was observed in the CR group when compared with controls. Significant (P<0.001) decreases in the kidney, heart and spleen weights were also observed while no changes were detected in the weights of the brain and lungs. All fat pads isolated from CR mice showed significant decreases of 55–78% (P<0.001) in their weight when compared with controls, and total fat pad weights show a 71% decrease in the CR mice when compared with controls (Table 2).
Table 1.
Body and organ weights from control and CR mice
| Body/organ weights (g) | Control | CR | % Change |
|---|---|---|---|
| Body weight | 35.87±1.38 | 24.77±0.45* | −30.9 |
| Liver | 1.36±0.088 | 1.02±0.037* | −25.0 |
| Kidneys | 0.36±0.008 | 0.30±0.007* | −16.7 |
| Brain | 0.42±0.007 | 0.41±0.008 | −2.4 |
| Heart | 0.14±0.005 | 0.11±0.002* | −21.4 |
| Spleen | 0.08±0.003 | 0.06±0.003* | −25.0 |
| Lungs | 0.16±0.010 | 0.15±0.004 | −6.3 |
| Tissue mass (g/kg body weight) | 37.04±1.18 | 41.32±1.14* | +11.6 |
Values represent mean ± SEM for n=6 animals per group.
P<0.05 for control versus CR
Table 2.
Fat pad weights from control and CR mice
| Fat pad weights (g) | Control | CR | % Change |
|---|---|---|---|
| Total fat pads | 6.37±0.070 | 1.87±0.137* | −70.6 |
| Epidydimal | 2.01±0.155 | 0.62±0.050* | −69.2 |
| Subcutaneous | 2.46±0.356 | 0.56±0.072* | −77.2 |
| Perirenal | 0.72±0.072 | 0.16±0.020* | −77.8 |
| Mesenteric | 0.89±0.123 | 0.35±0.029* | −60.7 |
| Interscapular | 0.29±0.048 | 0.13±0.026* | −55.2 |
Values represent mean ± SEM for n=6 animals per group.
P <0.05 for control versus CR
Mitochondrial isolation and sub-population fractionation
Mitochondria were isolated and separated into three major fractions, labeled as M1 (1,000 g), M3 (3,000 g) and M10 (10,000 g). To determine the purity of the mitochondria, citrate synthase and lactate dehydrogenase assays were performed and results showed that in both control and CR mice the overwhelming majority of citrate synthase activity was in the mitochondrial fractions. The control cytosolic fraction contained 1.2%, 2.5% and 12% of the activity and the CR cytosolic fractions contained 1.2%, 2.6% and 15% of the activity when compared with their corresponding M1, M3 and M10 mitochondrial fractions, respectively (Table 3). Citrate synthase activity was significantly lower in CR M1 (P<0.03) and M3 (P<0.05) fractions when compared with control M1 and M3 fractions, while no differences were observed between the M10 fractions from control and CR mice. In the case of lactate dehydrogenase, the opposite was observed (Table 3), with a low percent of total LDH activity in the M1 (1.5% control & 1.8% CR), M3 (3% control & 6% CR) and M10 (5% control & 5.5% CR) mitochondrial fractions. Lactate dehydrogenase activity from control M1, M3 and M10 was not different from the corresponding CR fractions, but the activity in the CR cytosol was significantly lower (P<0.03) than controls.
Table 3.
Marker enzymes for various organelles of 6 month old control and CR mice
| Enzymes | M1 | M3 | M10 | Cytosol |
|---|---|---|---|---|
| CS | ||||
| Control | 0.706±0.020 | 0.332±0.016 | 0.064±0.008 | 0.008±0.0003 |
| CR | 0.610±0.025* | 0.290±0.009* | 0.049±0.006 | 0.008±0.0002 |
| LDH | ||||
| Control | 0.063±0.010 | 0.129±0.024 | 0.216±0.041 | 4.202±0.318 |
| CR | 0.058±0.007 | 0.190±0.037 | 0.172±0.015 | 3.126±0.253* |
| AP | ||||
| Control | 0.164±0.023a | 0.265±0.018b | 1.099±0.066c | – |
| CR | 0.178±0.020a | 0.252±0.021b | 0.990±0.059c | – |
| UO | ||||
| Control | 0.026±0.004a | 0.030±0.004a | 0.304±0.029b | – |
| CR | 0.022±0.006a | 0.033±0.005a | 0.299±0.026b | – |
| G6Pase | ||||
| Control | 0.010±0.003a | 0.013±0.001a | 0.124±0.009b | – |
| CR | 0.011±0.002a | 0.013±0.001a | 0.133±0.006a | – |
CS citrate synthase, LDH lactate dehydrogenase, AP acid phosphatase, UO urate oxidase, G6Pase glucose-6-phosphatase
Values represent mean±SEM for n=6 animals per group
P<0.05 for control versus CR within a mitochondrial or cytosolic fraction. Values within a row which do not share a common letter differ significantly (P<0.05)
Marker enzyme activities
The activities of acid phosphatase, urate oxidase and glucose-6-phosphatase were measured as markers for lysosomal, peroxisomal and microsomal contamination, respectively. For all three enzymes (Table 3), the lowest levels of activity were found in the M1 and M3 fractions while the highest activities were observed in M10 fraction (P<0.001 for M10 vs. M1 or M3). Acid phosphatase showed slightly higher activity (P<0.05) in M3 in both control and CR groups when compared with their corresponding M1. No differences were observed in acid phosphatase activity when all three fractions from control were compared with those from CR mice. Urate oxidase and glucose-6-phosphatase showed no differences in their activities between M1 and M3 fractions in control and CR mice and no differences in all three fractions were observed between control and CR mice.
Protein content of mitochondrial fractions
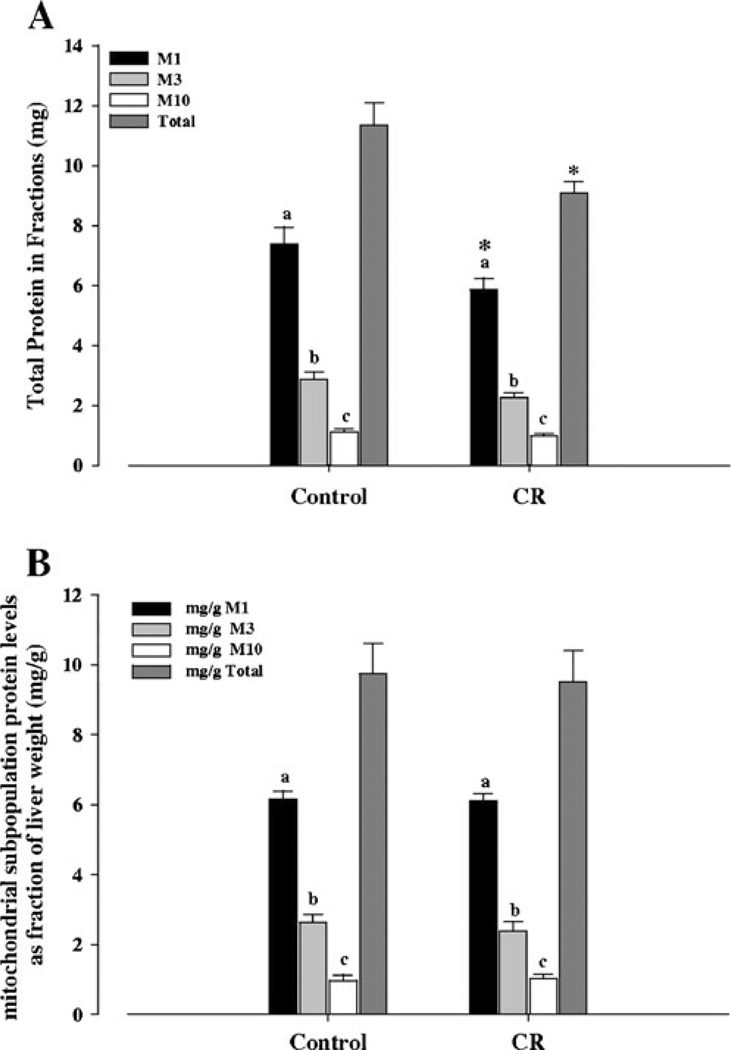
Protein levels were determined for all three mitochondrial fractions in both control and CR mice and the highest levels were observed in M1, followed by M3 and M10 fractions, respectively (Fig. 1A). In both control and CR groups, the differences in protein levels between M1, M3 and M10 fractions were significant (P<0.01). The difference in the M1 fraction between control and CR groups was significant (P<0.05), while the difference in M3 between control and CR showed a trend (P=0.07) toward a decrease in CR (Fig. 1A). However, no differences were observed in M10 fraction values between control and CR groups. Total mitochondrial protein levels were significantly lower (P<0.03) in CR when compared with controls (Fig. 1A).
Fig. 1.
Fractional and total liver protein levels A and fractional and total mitochondrial protein density B of 6 month old control and CR mice. Protein levels of the different fractions and total protein, in mg, were measured in control and CR mice and protein densities as mg protein/g tissue weight calculated, from 6 independent experiments. A Fractional total protein levels. B Fractional and total mitochondrial protein density (mg/g). Bars within a group that do not share the same letter are significantly different (P<0.05). * P<0.05 for the indicated control versus CR groups
Tissue mass and mitochondrial protein yield data showed that fractional mitochondrial protein density (F-mg/g) (mg protein of fraction normalized to g liver weight) was the highest in M1, followed by M3 and M10 fractions, respectively, in both control and CR groups (Fig. 1B). In both control and CR groups, the differences between M1, M3 and M10 protein densities were significant (P<0.01). However, no differences were observed when fractional densities from the control group were compared with the CR group. Moreover, no differences were observed between control and CR total mitochondrial protein densities (T-mg/g) (Fig. 1B).
Hydrogen peroxide production by mitochondrial fractions
The levels of H2O2 production by the mitochondrial fractions were also determined, using succinate and pyruvate plus malate (P/M) as substrates, in the presence and absence of the inhibitors rotenone and antimycin A.
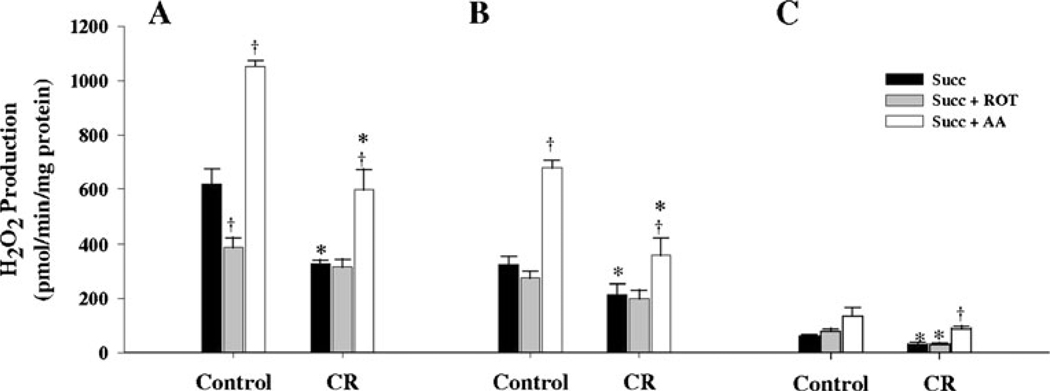
In both control and CR groups, with succinate alone as substrate, the highest levels of H2O2 production were observed in the M1 followed by M3 and M10 fractions (Fig. 2), respectively, and all three fractions were significantly different (P<0.001) from each other.
Fig. 2.
Hydrogen peroxide production by mitochondrial sub-populations respiring on succinate as substrate from 6 month old control and CR mice. Hydrogen peroxide generation was measured with succinate alone or with succinate plus rotenone or succinate plus antimycin A, as described in the text, and results expressed as mean ± SEM for 6 independent experiments. A M1 sub-population. B M3 sub-population. C M10 subpopulation. † P<0.05 when compared to succinate alone within a group. * P<0.05 when CR is compared to its corresponding control
When succinate was used with rotenone, a significant decrease (P<0.004) in H2O2 production compared to succinate alone was observed in the M1 fraction from controls, but no changes were observed in the M1 fraction from CR group (Fig. 2A). However, when succinate was used with antimycin A, a significant increase in the H2O2 production was observed in the M1 fraction from both control (P<0.001) and CR (P<0.004) groups (Fig. 2A) when compared with succinate alone. In M3 fractions, when succinate was used with rotenone, no significant differences were observed when compared with succinate alone in both control and CR groups (Fig. 2B). When succinate was used with antimycin A, again a significant increase in the H2O2 production was observed when compared with succinate alone (Fig. 2B) in both control (P<0.001) and CR (P<0.04) groups. As for the M10 fraction, no significant differences in the H2O2 production were observed between succinate alone and succinate with rotenone in both control and CR groups (Fig. 2C), but when antimycin A was used with succinate, a trend (P=0.06) towards an increase was observed in the controls while a significant increase (P<0.01) was observed in the CR group (Fig. 2C) when compared with succinate alone. Comparing M1, M3 and M10 fractions from the control group with their corresponding CR fractions showed a significant decrease in M1 (P<0.002), M3 (P<0.05) and M10 (P<0.02) in the CR group when succinate was used alone. When succinate was used with antimycin A, a significant decrease in M1 (P<0.002) and M3 (P<0.01) in the CR group was observed, while no significant differences were observed for the M10 fractions. When succinate was used with rotenone, only the M10 fraction from the CR group showed a decrease (P<0.01) when compared with M10 controls.
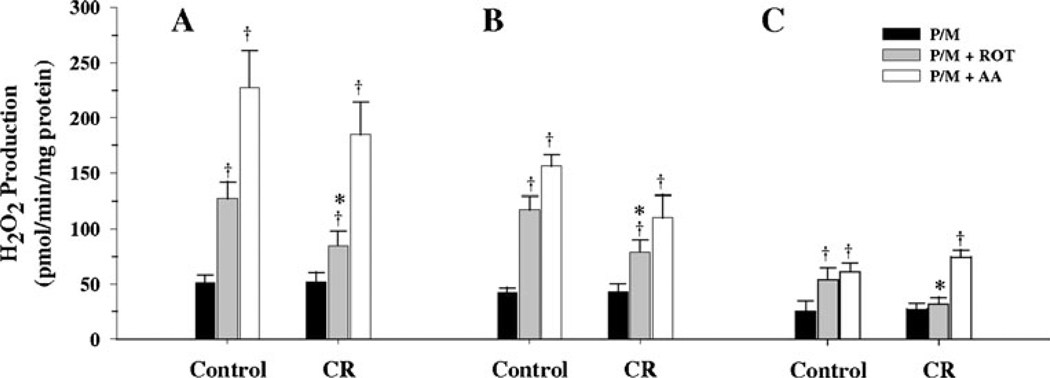
When P/M was used alone as substrate, no significant differences in the H2O2 production in controls were observed between M1 and M3 fractions, however, both of these were significantly different (P<0.01) from the M10 fraction (Fig. 3). In CR group, no significant differences were observed between M1 and M3 fractions or between M3 and M10 fractions, however a trend (P=0.08) towards a decrease in M10 was observed when compared with M1 (Fig. 3). When P/M was used with rotenone, a significant increase in H2O2 production was observed in the M1 fraction from control (P<0.001) and CR (P<0.05) groups, when compared to P/M alone, with the magnitude of the increase greater in the controls (Fig. 3A). When P/M with antimycin A was used, a significant increase (P<0.001) in the H2O2 production was observed in M1 fraction from both control and CR groups (Fig. 3A), similar to the pattern observed for rotenone. In M3 fractions, when P/M was used with rotenone, again a significant increase in H2O2 production was observed in the control (P<0.001) and CR (P<0.03) groups when compared with P/M alone (Fig. 3B). When P/M was used with antimycin A, again a significant increase in the H2O2 production was observed in control (P<0.001) and CR (P<0.02) groups when compared with P/M alone (Fig. 3B). As for the M10 fraction, when P/M was used with rotenone (Fig. 3C), a trend towards an increase (P=0.075) was observed in controls when compared with P/M alone, but no differences were observed in the CR group. When P/M was used with antimycin A, a significant increase was observed in both control (P<0.01) and CR (P<0.02) groups when compared with P/M alone (Fig. 3C). Comparing M1, M3 and M10 fractions from the control group with their corresponding fractions from the CR group, no significant differences were observed when P/M was used alone. The same pattern was true when P/M was used with antimycin A. When succinate was used with rotenone, a significant decrease in M1 (P<0.05), M3 (P<0.05) and a trend (P=0.08) toward a decrease in M10 was observed in fractions from the CR group when compared with the corresponding controls.
Fig. 3.
Hydrogen peroxide production by mitochondrial sub-populations respiring on pyruvate plus malate as substrate from 6 month old control and CR mice. Hydrogen peroxide generation was measured with pyruvate/malate (P/M) alone or with P/M plus rotenone or P/M plus antimycin A, as described in the text, and results expressed as mean ± SEM of 6 independent experiments. A M1 sub-population. B M3 sub-population. C M10 sub-population. † P<0.05 when compared to P/M alone within a group. * P<0.05 when CR is compared to its corresponding control
Mitochondrial state 4, state 3 respiration and respiratory control ratio
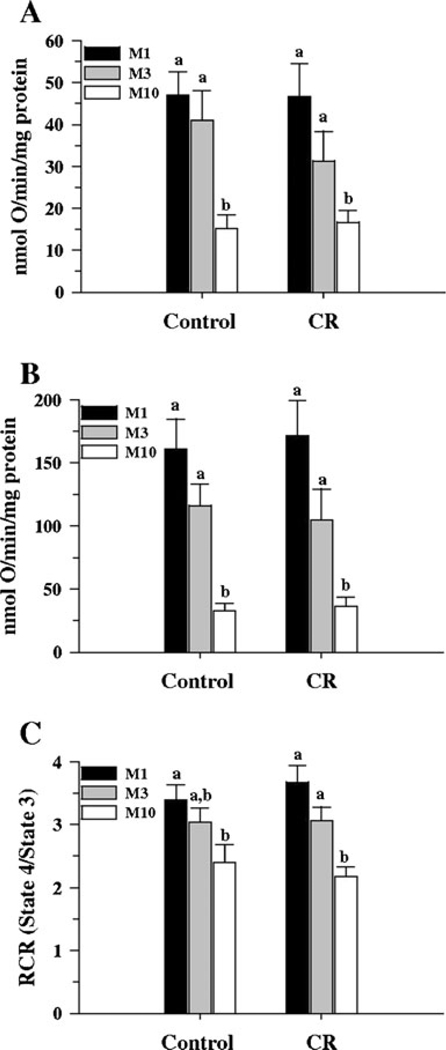
Mitochondrial respiration measurements were performed with succinate as substrate. No significant differences in state 4 respiration were observed when the three fractions from controls were compared with their corresponding fractions from the CR group (Fig. 4A). In the control group, no differences were observed between M1 and M3 fractions, however, a significant difference was observed between M1 and M10 (P<0.001) and between M3 and M10 (P<0.001). In the CR group, M1 and M3 were not significantly different while M1 and M10 were significantly different (P<0.002), however, when M3 and M10 were compared, M10 showed a trend towards a decrease (P=0.065).
Fig. 4.
Mitochondrial respiration from 6 month old control and CR mice. State 4 and 3 respiration, and RCR values, were measured as described in the text, using succinate as substrate, from 6 independent experiments. A State 4 respiration. B State 3 respiration. C RCR values. Bars within a group that do not share the same letter are significantly different (P<0.05). No differences between CR and their corresponding controls were observed in all three measurements
For state 3 respiration (Fig. 4B), no significant differences were observed when the three fractions from the controls and CR groups were compared. In both the control and CR groups, no differences were observed between M1 and M3 fractions, while the differences between M1 and M10, and M3 and M10, were significantly different (P<0.001).
The results also showed that the RCR values for the three fractions were not different between the two groups (Fig. 4C). In the control group, comparisons between M1 and M3 and between M3 and M10 showed no significant differences, while M1 and M10 were significantly different (P<0.01). In the CR group, M1 and M3 were not significantly different from each other, while M1 and M10 (P<0.001) and M3 and M10 (P<0.005) were different.
Discussion
The purpose of this study was to investigate the influence of CR on H2O2 production in liver mitochondrial subpopulations. Changes in ROS production or other biochemical characteristics of specific mitochondrial subpopulations may be an important mechanism by which CR exerts its beneficial effects and retards aging. To our knowledge, no studies have been undertaken previously whereby liver mitochondria have been fractionated into sub-populations and their biochemical characteristics studied under control and CR conditions.
As expected, CR produces a rapid and sustained decrease in body weight (Table 1). While there is considerable information about the influence of CR on organ and tissue weights in rats, there is less information about the impact of feeding a 40% CR diet on the weights of internal organs and fat pads in mice. The magnitude of the decreases in organ weights observed in the present study (Table 1) are similar to those reported for rats (Ramsey and Hagopian 2006; Ramsey et al. 2004; Weindruch and Sohal 1997). CR also produced dramatic decreases in the weights of all the fat pad (Table 2), and the magnitude of these changes are consistent with the known effects of CR in rats (Ramsey and Hagopian 2006). The results indicate that the magnitude of changes in organ and fat pad weights in response to CR are similar in rats and mice.
In this study, we separated the mitochondria into three sub-populations using 1,000 g, 3,000 g and 10,000 g and the resulting sub-populations were labeled as M1, M3 and M10, respectively. The results of the current study show the effects of CR on some of the biochemical parameters of liver mitochondrial sub-populations. It was important to show initially that the mitochondrial fractions were relatively free from contamination by other organelles and cytoplasmic proteins, and our results have shown this and are consistent with previous findings from rat liver (Lanni et al. 1996). However, there was evidence of some contamination of the M10 fraction with other cellular organelles, therefore, all M10 measurements were at least slightly influenced by non-mitochondrial protein and future studies may require gradient centrifugation to purify this fraction.
The pattern of protein distribution across the mitochondrial fractions was the same in both the control and CR groups (Fig. 1A), and similar to results reported for rat liver mitochondria (Lanni et al. 1996; Venditti et al. 2004). Total mitochondrial protein levels were also significantly lower in the CR group, as were the M1 and M3 fractions when compared with their corresponding controls (Fig. 1A). This could reflect a CR-induced decrease in mitochondrial number or simply a decrease in liver weight. The latter appears to be the case. There has been considerable interest in the idea that CR induces mitochondrial biogenesis (Civitarese et al. 2007; Lopez-Lluch et al. 2006; Nisoli et al. 2005), and this would be expected to increase mitochondrial protein, at least in some fractions, when expressed per gram of tissue weight. We determined the fractional mitochondrial protein density, which were calculated as protein amounts normalized to gram liver weight (Fig. 1B) and although the results showed different densities between the fractions, no differences were observed between CR and control for any of the fractions, and no difference was observed between control and CR for the total mitochondrial protein densities. These results indicate that decreases in mitochondrial protein levels with CR primarily reflect a decrease in liver weights. These results are consistent with a recent study which reported that CR does not induce mitochondrial biogenesis or increase the level of specific mitochondrial proteins (Hancock et al. 2011).
Several studies in rats have shown that CR decreases liver mitochondrial H2O2 production (Gredilla et al. 2001a; Hagopian et al. 2005; Lambert and Merry 2004; Lopez-Torres et al. 2002), and it has been reported that this is primarily due to a decrease in ROS production from complex I of the electron transport chain (Hagopian et al. 2005; Lopez-Torres et al. 2002). The results of the present study indicate that liver mitochondrial H2O2 production is also decreased in mice with CR. Furthermore, three primary conclusions can be drawn from the results of the current study. First, CR produces uniform changes in H2O2 production across each of the mitochondrial subpopulations. Second, CR-induced decreases in H2O2 production are primarily due to decreases in complex I-linked ROS production which occur in each of the mitochondrial subpopulations. Third, the M1 fraction is responsible for the majority of the H2O2 produced by mouse liver mitochondria. In the present study, the rate of H2O2 production was very low in all fractions of mitochondria from control and CR groups when respiring on pyruvate/malate. To determine the capacity for complex I to produce ROS, rotenone was added to mitochondria respiring on pyruvate/malate. Rotenone (complex I inhibitor) maintains complex I in a reduced state by blocking the flow of electrons to coenzyme Q, and thus creates a state which produces maximum ROS production from complex I. Under these conditions, there was a decrease in H2O2 production in the CR compared to control mitochondria in all fractions, indicating decreased ROS production from complex I. In contrast, there was no difference in H2O2 production between control and CR groups when mitochondria were respiring on P/M plus antimycin A (complex III inhibitor). Antimycin A blocks electron flow from complex III and maintains both complexes I and III in a reduced state. Thus, this creates a condition which promotes ROS production from both complexes I and III. Under these conditions, H2O2 production was not decreased with CR (in any mitochondrial fraction) indicating that the capacity for complex III to produce ROS is not diminished with CR.
When mitochondria were respiring on succinate there was a decrease in H2O2 production in all mitochondrial fractions from CR compared to control mice. Succinate stimulates a high rate of ROS production due to backflow of electrons from complex II to complex I (Murphy 2009). This CR-induced decrease in H2O2 production disappears when mitochondria are respiring in the presence of rotenone, indicating that CR decreases ROS production due to backflow into complex I. Furthermore, CR decreases H2O2 production in mitochondria respiring on succinate in the presence of antimycin A. Under this condition, antimycin A not only stimulates ROS production from complex III, but it also promotes backflow into complex I. The studies using P/M showed that CR does not decrease complex III capacity to produce ROS, and thus, the decrease in H2O2 production observed in CR mitochondria with succinate and antimycin A must be primarily due to decreased ROS production from backflow into complex I. Overall, the results of these studies indicate that CR reduces ROS production by complex I under conditions of both forward and reverse electron flow in all mitochondrial fractions. These results in mice are consistent with studies in rats which have shown that decreases in ROS production from complex I are primarily responsible for the low rates of H2O2 production in tissues from CR animals (Gredilla et al. 2001a, b; Hagopian et al. 2005; Lopez-Torres et al. 2002; Sanz et al. 2005).
Taking into consideration that the M1 fraction accounts for greater than 50% of total mitochondrial protein, it is evident that the M1 fraction is responsible for the greatest amount of liver mitochondrial ROS production. These results in mice are in agreement with a study in rats which also showed an increase in H2O2 production from M1 to M3 to M10 (Venditti et al. 2004). It is likely that the differences in the H2O2 production between the three fractions is due to decreased content of the respiratory chain in the light fractions (Lanni et al. 1996; Venditti et al. 1996), since the rate of mitochondrial ROS production is associated with the content and level of reduction of the autoxidizable electron carriers (Balaban et al. 2005; Chance et al. 1979). However, it is not known if further decreases in electron transport chain content also contribute to the decreases in H2O2 production with CR. The difference in H2O2 production between mitochondria may also be influenced by increased age of the M1 fraction. It has been proposed that mitochondria undergo a growth and maturation cycle, with the lightest fraction representing nascent mitochondria and the heaviest fraction representing mature mitochondria (Lanni et al. 1996). According to this theory, the M1 fraction would contain the oldest mitochondria. Older mitochondria can suffer from damage to their membrane lipids and proteins (specifically ETC proteins) due to oxidative stress, resulting in altered or inefficient transfer of electrons through the chain and increased production of ROS (Shigenaga et al. 1994). While this is possible, it is important to note that all of the mice used in this study were young adults (6 months of age) and it is not clear if sufficient oxidative damage was occurring at this time.
State 4 and state 3 respiration remained unchanged in all three fractions when control and CR groups were compared (Fig. 4), which is in agreement with previous studies investigating the influence of CR on mitochondrial respiration in rat liver (Gredilla et al. 2001a; Lambert and Merry 2005; Lambert et al. 2004; Ramsey et al. 2004). This lack of CR effect is not age-dependent since long-term CR had been reported to show the same effect (Lambert et al. 2004). Furthermore, CR was found to have no effect on state 4 respiration rate in mitochondria from adult or old mice (Weindruch et al. 1980), and the duration of CR (short- or long-term) similarly had no effect on state 4 respiration rates from rat liver mitochondria (Gredilla et al. 2001a, b; Lopez-Torres et al. 2002). In the present study both control and CR groups showed the highest respiration rates in the M1 fraction and the lowest respiration rates in the M10 fraction. This is in agreement with studies using rat mitochondria (Lanni et al. 1996; Venditti et al. 2004). Overall, the results of the current study indicate that CR had no influence on respiration in any of the mitochondrial fractions.
In this study, we have shown that mouse liver mitochondria can be resolved into three fractions, namely M1 (1,000 g), M3 (3,000 g) and M10 (10,000 g), by differential centrifugation. The results of the present study indicate that CR decreases H2O2 production in all liver mitochondrial factions due to a decrease in capacity for ROS production by complex I of the electron transport chain. CR was also shown to produce uniform changes in the relative amounts (mg protein/g tissue) of each mitochondrial fraction. Thus, the actions of CR are not limited to a particular mitochondrial fraction but rather influence all fractions.
Acknowledgements
The work was funded by National Institutes of Health Grants AG028125 and AG025532
Abbreviations
- CR
calorie restriction
- ROS
reactive oxygen species
- Succ
succinate
- P/M
pyruvate/malate
- LDH
lactate dehydrogenase
- Rot
rotenone
- AA
antimycin A
Contributor Information
Kevork Hagopian, Email: khagopian@ucdavis.edu, VM Molecular Biosciences, School of Veterinary Medicine, University of California, 1 Shields Avenue, Davis, CA 95616, USA.
Yana Chen, VM Molecular Biosciences, School of Veterinary Medicine, University of California, 1 Shields Avenue, Davis, CA 95616, USA.
Keira Simmons Domer, VM Molecular Biosciences, School of Veterinary Medicine, University of California, 1 Shields Avenue, Davis, CA 95616, USA.
Robert Soo Hoo, VM Molecular Biosciences, School of Veterinary Medicine, University of California, 1 Shields Avenue, Davis, CA 95616, USA.
Trevor Bentley, VM Molecular Biosciences, School of Veterinary Medicine, University of California, 1 Shields Avenue, Davis, CA 95616, USA.
Roger B. McDonald, Department of Nutrition, University of California, Davis, CA 95616, USA
Jon J. Ramsey, VM Molecular Biosciences, School of Veterinary Medicine, University of California, 1 Shields Avenue, Davis, CA 95616, USA
References
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bergmeyer H, Bernt E. Lactate dehydrogenase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. 2nd edn. New York: Academic; 1974. pp. 590–593. [Google Scholar]
- Bergmeyer HU, Gawehn K, Grassl M. Uricase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. 2nd edn. New York: Academic; 1974. p. 518. [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianotti M, Clapes J, Llado I, Palou A. Effect of 12, 24 and 72 h fasting in thermogenic parameters of rat brown adipose tissue mitochondrial subpopulations. Life Sci. 1998;62:1889–1899. doi: 10.1016/s0024-3205(98)00153-2. [DOI] [PubMed] [Google Scholar]
- Gierow P, Jergil B. Spectrophotometric method for glucose-6-phosphate phosphatase. Meth Enzymol. 1982;89:44–47. doi: 10.1016/s0076-6879(82)89010-1. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Barja G, Lopez-Torres M. Effect of short-term caloric restriction on H2O2 production and oxidative DNA damage in rat liver mitochondria and location of the free radical source. J Bioenerg Biomembr. 2001a;33:279–287. doi: 10.1023/a:1010603206190. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001b;15:1589–1591. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- Hagopian K, Harper ME, Ram JJ, Humble SJ, Weindruch R, Ramsey JJ. Long-term calorie restriction reduces proton leak and hydrogen peroxide production in liver mitochondria. Am J Physiol. 2005;288:E674–E684. doi: 10.1152/ajpendo.00382.2004. [DOI] [PubMed] [Google Scholar]
- Hancock CR, Han DH, Higashida K, Kim SH, Holloszy JO. Does calorie restriction induce mitochondrial biogenesis? A reevaluation. FASEB J. 2011;25:785–791. doi: 10.1096/fj.10-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hyslop PA, Sklar LA. A quantitative fluorimetric assay for the determination of oxidant production by polymorphonuclear leukocytes: its use in the simultaneous fluorimetric assay of cellular activation processes. Anal Biochem. 1984;141:280–286. doi: 10.1016/0003-2697(84)90457-3. [DOI] [PubMed] [Google Scholar]
- Justo R, Oliver J, Gianotti M. Brown adipose tissue mitochondrial subpopulations show different morphological and thermogenic characteristics. Mitochondrion. 2005;5:45–53. doi: 10.1016/j.mito.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Koekemoer TC, Oelofsen W. Properties of porcine white adipose tissue and liver mitochondrial subpopulations. Int J Biochem Cell Biol. 2001;33:889–901. doi: 10.1016/s1357-2725(01)00064-4. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Merry BJ. Effect of caloric restriction on mitochondrial reactive oxygen species production and bioenergetics: reversal by insulin. Am J Physiol. 2004;286:R71–R79. doi: 10.1152/ajpregu.00341.2003. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Merry BJ. Lack of effect of caloric restriction on bioenergetics and reactive oxygen species production in intact rat hepatocytes. J Gerontol A Biol Sci Med Sci. 2005;60:175–180. doi: 10.1093/gerona/60.2.175. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Wang B, Yardley J, Edwards J, Merry BJ. The effect of aging and caloric restriction on mitochondrial protein density and oxygen consumption. Exp Gerontol. 2004;39:289–295. doi: 10.1016/j.exger.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Lanni A, Moreno M, Lombardi A, Goglia F. Biochemical and functional differences in rat liver mitochondrial subpopulations obtained at different gravitational forces. Int J Biochem Cell Biol. 1996;28:337–343. doi: 10.1016/1357-2725(95)00137-9. [DOI] [PubMed] [Google Scholar]
- Lombardi A, Damon M, Vincent A, Goglia F, Herpin P. Characterisation of oxidative phosphorylation in skeletal muscle mitochondria subpopulations in pig: a study using top-down elasticity analysis. FEBS Lett. 2000;475:84–88. doi: 10.1016/s0014-5793(00)01633-1. [DOI] [PubMed] [Google Scholar]
- Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Torres M, Gredilla R, Sanz A, Barja G. Influence of aging and long-term caloric restriction on oxygen radical generation and oxidative DNA damage in rat liver mitochondria. Free Radic Biol Med. 2002;32:882–889. doi: 10.1016/s0891-5849(02)00773-6. [DOI] [PubMed] [Google Scholar]
- Matamala JC, Gianotti M, Pericas J, Quevedo S, Roca P, Palou A, Garcia-Palmer FJ. Changes induced by fasting and dietetic obesity in thermogenic parameters of rat brown adipose tissue mitochondrial subpopulations. Biochem J. 1996;319:529–534. doi: 10.1042/bj3190529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry BJ. Oxidative stress and mitochondrial function with aging-the effects of calorie restriction. Aging Cell. 2004;3:7–12. doi: 10.1046/j.1474-9728.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- Moreno M, Puigserver P, Llull J, Gianotti M, Lanni A, Goglia F, Palou A. Cold exposure induces different uncoupling-protein thermogenin masking/unmasking processes in brown adipose tissue depending on mitochondrial subtypes. Biochem J. 1994;300:463–468. doi: 10.1042/bj3000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss DW. Verlag Cheme. Weinheim: 1984. Acid phosphatase. [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Page MM, Robb EL, Salway KD, Stuart JA. Mitochondrial redox metabolism: aging, longevity and dietary effects. Mech Ageing Dev. 2010;131:242–252. doi: 10.1016/j.mad.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Barja G. Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochim Biophys Acta. 2006;1757:496–508. doi: 10.1016/j.bbabio.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Hagopian K. Energy expenditure and restriction of energy intake: could energy restriction alter energy expenditure in companion animals? J Nutr. 2006;136:1958S–1966S. doi: 10.1093/jn/136.7.1958S. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Hagopian K, Kenny TM, Koomson EK, Bevilacqua L, Weindruch R, Harper ME. Proton leak and hydrogen peroxide production in liver mitochondria from energy-restricted rats. Am J Physiol Endocrinol Metab. 2004;286:E31–E40. doi: 10.1152/ajpendo.00283.2003. [DOI] [PubMed] [Google Scholar]
- Sanz A, Caro P, Ibanez J, Gomez J, Gredilla R, Barja G. Dietary restriction at old age lowers mitochondrial oxygen radical production and leak at complex I and oxidative DNA damage in rat brain. J Bioenerg Biomembr. 2005;37:83–90. doi: 10.1007/s10863-005-4131-0. [DOI] [PubMed] [Google Scholar]
- Shephard D, Garland PB. Citrate synthase from rat liver. Meth Enzymol. 1968;13:11–19. [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti P, Di Meo S, De Leo T. Effect of thyroid state on characteristics determining the susceptibility to oxidative stress of mitochondrial fractions from rat liver. Cell Physiol Biochem. 1996;6:283–295. [Google Scholar]
- Venditti P, Costagliola IR, Di Meo S. H2O2 production and response to stress conditions by mitochondrial fractions from rat liver. J Bioenerg Biomembr. 2002;34:115–125. doi: 10.1023/a:1015175925756. [DOI] [PubMed] [Google Scholar]
- Venditti P, De Rosa R, Caldarone G, Di Meo S. Functional and biochemical characteristics of mitochondrial fractions from rat liver in cold-induced oxidative stress. Cell Mol Life Sci. 2004;61:3104–3116. doi: 10.1007/s00018-004-4308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti P, Pamplona R, Portero-Otin M, De Rosa R, Di Meo S. Effect of experimental and cold exposure induced hyperthyroidism on H2O2 production and susceptibility to oxidative stress of rat liver mitochondria. Arch Biochem Biophys. 2006;447:11–22. doi: 10.1016/j.abb.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch RH, Cheung MK, Verity MA, Walford RL. Modification of mitochondrial respiration by aging and dietary restriction. Mech Ageing Dev. 1980;12:375–392. doi: 10.1016/0047-6374(80)90070-6. [DOI] [PubMed] [Google Scholar]