Abstract
OBJECTIVE:
The prevalence of obstructive sleep apnea syndrome (OSAS) and metabolic syndrome is increasing worldwide, in part linked to epidemic of obesity. The purposes of this study were to establish the rate of metabolic syndrome and to compare fibrinogen, homocysteine, high-sensitivity C-reactive protein (hsCRP), leptin levels, and homeostasis model assessment insulin resistance (HOMA-IR) in the obese patients with and without OSAS.
METHODS:
The study population included 36 consecutive obese patients with OSAS (23 males; mean age, 50.0 ±19.7 years), and 34 obese patients without OSAS (17 males; mean age, 49.7±11.1 years) were enrolled as control group. Metabolic syndrome was investigated; fibrinogen, homocysteine, CRP, and leptin levels were measured, and IR was assessed.
RESULTS:
Metabolic syndrome was found in 17 (47.2%) obese OSAS patients, whereas only 29.4% of obese subjects had metabolic syndrome (P > 0.05). Obese patients with OSAS had significantly higher mean levels of triglyceride (P < 0.001), total-cholesterol (P = 0.003), low-density lipoprotein-cholesterol (P = 0.001), fasting glucose (P = 0.01), HOMA-IR (P <0.001), thyroid-stimulating hormone (P = 0.03), fibrinogen (P < 0.003), hsCRP (P <0.001), and leptin (P = 0.03) than control group . Besides, leptin level was positively correlated with waist (r = 0.512, P = 0.03) and neck circumferences (r = 0.547, P = 0.03), and fasting glucose (r = 0.471, P = 0.04) in OSAS patients, but not in obese subjects.
CONCLUSION:
This study demonstrated that obese OSAS patients may have an increased rate of metabolic syndrome and higher levels of serum lipids, fasting glucose, IR, leptin, fibrinogen, and hsCRP than obese subjects without sleep apnea. Thus, clinicians should be encouraged to systematically evaluate the presence of metabolic abnormalities in OSAS and vice versa.
Keywords: C-reactive protein, fibrinogen, homocysteine, insulin resistance, leptin, metabolic syndrome, obesity, obstructive sleep apnea syndrome
Obstructive sleep apnea syndrome (OSAS) is a common disorder that has a major impact on public health.[1–4] Patients with obstructive sleep apnea (OSA) have abnormalities in each of the components of the metabolic syndrome—high blood pressure, high fasting glucose, increased waist circumference, low high-density lipoprotein (HDL) cholesterol, and high triglycerides—as well as in many of its other features, including sympathetic activation, endothelial dysfunction, systemic inflammation, hypercoagulability, and insulin resistance (IR).[1] In a previous study, Coughlin et al.[5] reported that OSA was independently associated with an increase in the cardiovascular risk factors that comprise the metabolic syndrome and its overall prevalence. The underlying pathophysiology linking these disorders with OSAS has not been fully elucidated. Several proposed mechanisms to explain these links have been investigated, including metabolic disturbances and inflammation.[3,5,6] Greenberg et al.[7] reported that sustained hypoxia leads to the activation of inflammatory response with an increased production of proinflammatory cytokines including tumor necrosis factor a and interleukin 6. However, studies on the association of IR, leptin, and high-sensitivity C-reactive protein (hsCRP) with OSAS show conflicting results. Some studies have reported significant relationship,[8–12] whereas others have not.[13–15] In one study, the very severe OSAS group had significantly higher homeostasis model assessment of IR (HOMA-IR) and HOMA beta-cell function than other OSAS groups and the control group.[16] The relationship of OSAS with IR may be the pathway that leads to increased risk for the development of cardiovascular disease in these patients. The mechanisms involved in the association between OSAS and cardiovascular diseases are actually complex and very diverse. These also include episodic hypoxia-reoxygenation, tonic elevation of sympathetic neural activity, and endothelial dysfunction as well as hypercoagulability, oxidative stress, and inflammation. Hypercoagulability resulting from imbalance between coagulation and fibrinolysis is associated with an increased risk for cardiovascular disease. A variety of findings support the existence of a relation between hypercoagulability, OSAS, and cardiovascular disease. Patients with OSAS have elevated plasma fibrinogen levels, exaggerated platelet activity, and reduced fibrinolytic capacity. Although not consistently shown, severity of OSA and plasma epinephrine were independent predictors of platelet activity, and average minimal oxygen saturation was an independent predictor of fibrinogen. These findings suggest that severity of intermittent nocturnal hypoxemia may contribute to procoagulant disturbances in OSA. In some studies, treatment with continuous positive airway pressure decreased platelet activity, plasma fibrinogen levels, and activity of clotting factor VII.[17,18] Conversely, Steiropoulos et al.[19] failed to demonstrate a difference in fibrinogen levels between obese individuals with or without OSAS, suggesting that the presence of OSAS probably does not play an important role in the upregulation of fibrinogen levels. Homocysteine levels are also associated with an increased cardiovascular risk and may be a predictor of long-term prognosis following premature myocardial infarction. Homocysteine levels have previously been reported to be raised in patients with OSA, but only in patients with associated ischemic heart disease and/or hypertension, and severe OSAS.[20–22] Besides, it has been observed that OSAS patients have increased leptin and CRP levels and IR,[23] indicating a possible role in the pathogenesis of cardiovascular morbidity. There were no much studies related to the incidence of metabolic syndrome and levels of fibrinogen, homocysteine, hsCRP, leptin, and IR in obese patients with OSAS and obese patients without sleep-disordered breathing. However, identifying possible risk factors involved in cardiovascular morbidity is of great clinical importance in OSA. The measurement of circulating cardiovascular risk factors (including several new phenotypic markers of cardiovascular disease) enables a more accurate prediction of cardiovascular risk to be made, as there are clearly established relationships between levels of various circulating haemostatic risk factors and a subsequent cardiovascular event. Therefore, the purposes of this study were to assess the rate of metabolic syndrome and its related components, and to compare the fibrinogen, homocysteine, hsCRP, and leptin levels and IR in obese patients with and without OSAS.
Methods
The study population consisted of 36 consecutive obese patients with OSAS (23 males; mean age, 50.0 ± 19.7 years [45-70 years]). Thirty-four obese patients without OSAS (17 males; mean age, 49.7 ± 11.1 years [44-69 years]) were enrolled as a control group. Body mass index (BMI) of the patients in the study and control groups were similar (33.5 ± 5.7 kg/m2 and 34.5 ± 2.9 kg/m2, respectively, P = 0.30). The study and control groups were matched for age, gender, and BMI. The subjects of the control group were selected among patients who were admitted to The Internal Medicine Outpatient Clinic for various reasons and matched randomly to OSAS patients using a computed technique. They were all questioned in details by the same doctor who was experienced in sleep medicine (OKB), and none of them had symptoms related to OSAS. Obese patients with any symptoms of OSAS were excluded from the control group of the study. The local ethics committee approved the study and all subjects gave informed consent. Metabolic syndrome was defined as in ATP III.[24]
Anthropometric measurements were evaluated in all groups. Habitual alcohol consumption for each subject was ascertained based on the following two questions: “Do you drink alcohol at least once a month? Yes/No.” Smoking was evaluated based on the following two questions. “Do you smoke? Yes/No.” If Yes, smokers were classified as ex-smoker and nonsmoker.[25]
Spirometry (Sensor Medics 2400, USA) and arterial blood gas sampling (Ciba Corning, 238 pH-Blood gas analyzer, UK) were performed before nocturnal sleep study in the OSAS patients. Subjective daytime sleepiness was assessed by using the Turkish version of the Epworth Sleepiness Scale (ESS) and ≥11 was considered as sleepiness.[26] A two-point bioelectrical impedance apparatus calibrated for adults (Tanita TBF 300, TANITA Corp.) was used to measure the percentage body fat and fat mass in all patients.
Polysomnography
Polysomnographic evaluation was performed solely on the OSAS group, since none of the control subjects had OSAS symptoms. All the patients in the study population underwent full overnight in-laboratory polysomnography with a 44-channel recording system (Compumedics E series, Melbourne, Australia). Electroencephalography electrodes were positioned according to the international 10–20 system. Polysomnographic monitoring consisted of monitoring of sleep by electroencephalography, electrooculography, electromyography, airflow, and respiratory muscle effort, and included measures of electrocardiographic rhythm and blood oxygen saturation. Thoracoabdominal plethysmograph, oronasal temperature thermistor and nasal-cannula-pressure transducer system were used to identify apneas and hypopneas. Transcutaneous finger pulse oximeter was used to measure oxygen saturation. Sleep was recorded and scored according to the standard method.[27] An apnea was defined as a total cessation of airflow for ≥10 seconds. Hypopnea was defined as a reduction in airflow with a 50% from baseline for at least 10 seconds, a 3% drop in oxygen saturation from the preceding stable saturation, and/or arousal. Apnea-hypopnea index (AHI) was the sum of the number of apneas and hypopneas per hour of sleep. OSAS was defined as an AHI of 5 events/h and the presence of clinical symptoms, e.g., excessive daytime sleepiness, loud snoring, witnessed apneas, and nocturnal choking or AHI of 15 events/h without any OSAS symptoms.[28] Besides, an AHI of <5 events/h considered within normal limits, and numbers of 5-15, 15-30, and >30 representing mild, moderate, and severe OSAS, respectively.
Biochemical analysis
Serum concentrations of glucose, triglyceride, total and HDL-cholesterol were determined by enzymatic procedures. Serum insulin was measured by chemiluminescence, hsCRP by immunoturbidimetric assay, and fibrinogen by coagulation method. Serum leptin level was measured by enzyme-linked immunosorbent assays (BIOSOURCE, Leptin EASIA). The mean intra and inter-assay coefficients of variation were 3.6 to 5.2%. Liver and thyroid function tests were measured by spectrophotometric method and chemiluminescence (IMMULITE 2000, Diagnostic Products Corporation, Los Angeles, CA, USA). Homocysteine level was evaluated with immunoassay (Immulate 2000 (reference range, 5.0-12 μmol/l).
IR was estimated using the homeostasis model assessment (HOMA) from fasting glucose and insulin concentrations using the following formula:[29]

Statistical analysis
Statistical analysis was performed with SPSS for Windows (13.0) packaged software. Numerical variables were summarized with mean ± STD deviation. Baseline data were compared using one-way analysis of variance (ANOVA). Variables with skewed distribution were transformed into logarithms before all analyses. Pearson correlation coefficients were calculated to identify associations of clinical variables. The correlations among the groups were examined using the stepwise regression analysis. A value of P < 0.05 was considered significant for all statistical analysis.
Results
The characteristics and clinical findings of the study and control groups are shown in Table 1. Obese patients with OSAS had higher mean systolic and diastolic blood pressure, and neck circumference compared with obese without OSAS (P < 0.001, P = 0.001, P = 0.03, respectively).
Table 1.
Characteristics and clinical findings of obese patients with and without OSAS
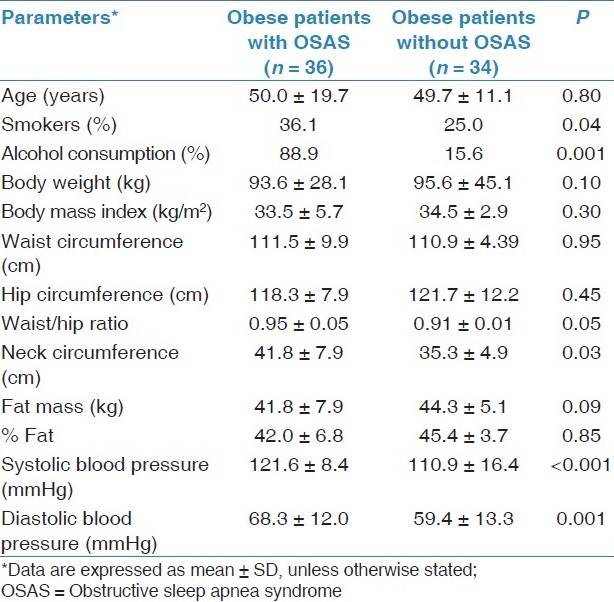
Among OSAS patients, 36.1% were smokers and 88.9% informed alcohol consumption, whereas 25.0% of obese patients without OSAS were smokers and only 15.6% of them consumed alcohol (P = 0.04, P = 0.001). Of 36 OSAS patients, 5 (13.9%) had a history of cardiovascular disease, 18 (50.0%) had dyslipidemia, 2 (5.5%) had stroke, 14 (38.9%) had hypertension, and 7 (19.4%) had diabetes mellitus. In control group, 3 (8.8%) had family history of cardiovascular disease, 7 (20.6%) had dyslipidemia, 9 (26.5%) had hypertension, and 3 (8.8%) had glucose metabolism disorders.
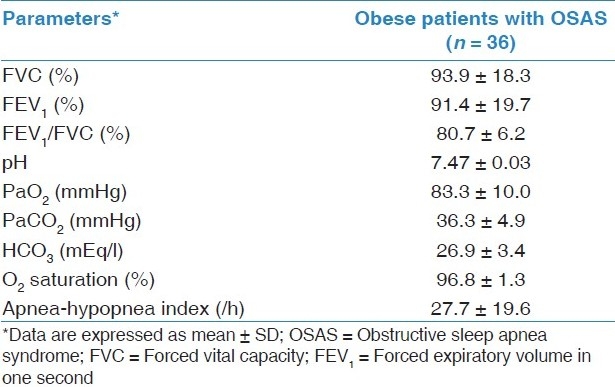
Pulmonary function tests, arterial blood gas analysis, and polysomnography were evaluated in OSAS patients and are shown in Table 2. The mean ESS was significantly higher in OSAS patients than the control group (11.9 ± 6.6 vs 4.0 ± 0.9, P = 0.01).
Table 2.
Spirometry, blood gas analysis, and polysomnography results of OSAS patients
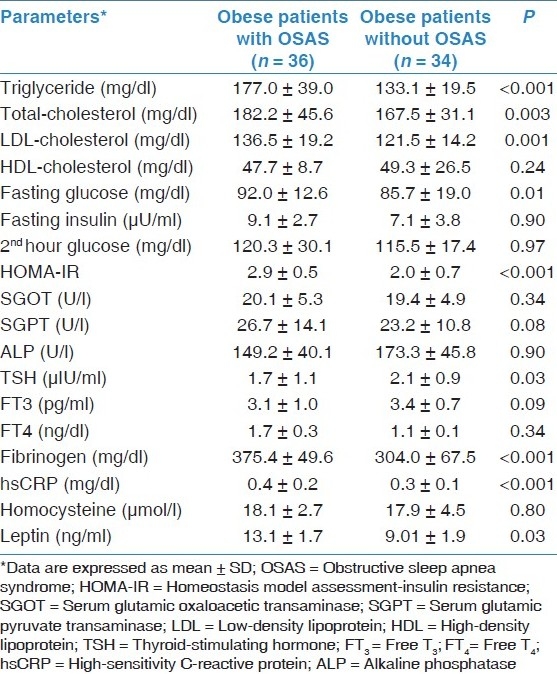
Metabolic syndrome was found in 17 (47.2%) obese patients with OSAS, whereas only 10 (29.4%) obese subjects had metabolic syndrome. However, the difference between groups was not statistically significant. The biochemical analyses of two groups were compared with each other. Obese patients with OSAS had significantly higher mean levels of triglyceride (P < 0.001), total-cholesterol (P = 0.003), low-density lipoprotein (LDL)-cholesterol (P = 0.001), fasting glucose (P = 0.01), HOMA-IR (P < 0.001), thyroid-stimulating hormone (TSH) (P = 0.03), fibrinogen (P < 0.003), hsCRP (P < 0.001), and leptin (P = 0.03) than obese subjects without OSAS [Table 3]. Besides, hypertensive patients with OSAS had significantly higher insulin (12.8 ± 2.9 vs 8.1 ± 1.9 U/ml, P = 0.02) and HOMA-IR (3.0 ± 1.1 vs 2.1 ± 0.8, P = 0.02) values than normotensive OSAS patients. In multivariate regression analysis, it was observed that leptin levels were not higher in OSAS patients than control group but the levels were affected from fasting glucose (r = 0 .436, P < 0.0001) and alkaline phosphatase levels (r = –0.488, P < 0.0001).
Table 3.
Blood profiles of obese patients with and without OSAS
In obese patients with OSAS, waist circumference was positively correlated with mean levels of HOMA (r = 0.873, P = 0.01), and neck circumference was negatively correlated with HDL-cholesterol (r = –0.349, P = 0.02). Besides, mean level of leptin was positively correlated with waist (r = 0.512, P = 0.03) and neck circumferences (r = 0.547, P = 0.03) and fasting glucose (r = 0.471, P = 0.04), and leptin level was negatively correlated with triglyceride (r = –0.336, P = 0.04) in OSAS patients. There was a negative correlation between leptin and LDL-cholesterol levels (r = –0.876, P = 0.01) in the obese without OSAS.
Discussion
The present study has demonstrated an increased rate of metabolic syndrome in obese OSAS patients. Metabolic syndrome was observed in 47.2% obese patients with OSAS, whereas only 29.4% obese subjects had metabolic syndrome. Obese patients with OSAS had significantly higher levels of triglyceride, total-cholesterol, LDL-cholesterol, fasting glucose, IR, TSH, fibrinogen, hsCRP, and leptin than obese subjects without OSAS. Besides, mean level of leptin was positively correlated with fasting glucose, waist and neck circumferences in OSAS patients.
OSAS is associated with increased cardiovascular morbidity. It is also known that many subjects with OSAS have features of the metabolic syndrome, which is most widely accepted as being comprised of hypertension, central obesity, dyslipidemia, hyperinsulinemia, and glucose intolerance.[30] The mechanisms which contribute to the development of cardiovascular disease in the metabolic syndrome and OSAS are similar. The pathogenesis of cardiovascular disease in OSAS is not completely understood but likely to be multifactorial, involving a diverse range of mechanisms including sympathetic nervous system overactivity, oxidative stress, selective activation of inflammatory molecular pathways, endothelial dysfunction, abnormal coagulation, and metabolic dysregulation, the latter particularly involving IR and disordered lipid metabolism. The factors that may contribute to metabolic dysregulation in OSAS also include sleep fragmentation, increased sympathetic activity, and intermittent hypoxia.[31] In addition, dyslipidemia and adipose tissue dysfunction caused by IR and obesity also contribute to the development of vascular risk factors and cardiovascular disease in metabolic syndrome.[32] The patients with OSAS appear to suffer from the disorders which characterize the metabolic syndrome, and have hypertension, high fasting blood glucose levels, increased waist circumference, low HDL-cholesterol, high triglyceride levels, and many systemic inflammations. It was found that the prevalence of the metabolic syndrome according to the ATP-III criteria is almost 40% greater in patients with OSA.[5] Likewise, it has been suggested that OSA, an increasingly prevalent condition, may contribute to the development of metabolic syndrome and diabetes.[33] In the present study, we observed metabolic syndrome in 47.2% of obese patients with OSAS, whereas it was found in 29.4% of obese subjects without OSAS. However, the difference was not statistically significant, maybe because of our sample size.
Obesity is strongly associated with OSAS, IR, leptin and CRP levels.[23] It has been observed that OSAS patients have increased leptin and CRP levels. In the present study, as both groups were obese and BMI-matched, the affect of obesity on leptin and CRP and HOMA levels were similar. Davies et al.[34] found no difference in fasting blood insulin levels when OSAS patients were compared with carefully selected controls matched for age, gender, and BMI. Otherwise, Stoohs et al.[35] reported that in healthy subjects, the relationship of hypoxic respiratory events with IR was entirely dependent on body mass. Ip et al.[36] showed that in subjects with OSAS, obesity was the primary determinant of IR and noted that sleep apnea had an independent but smaller effect. Zhang et al.[37] found that after adjustment for age, BMI, and waist-to-hip ratio, OSAS group was more insulin resistant, as indicated by the higher levels of AHI and HOMA-IR. In our study, mean level of HOMA-IR in obese OSAS patients was higher than obese subjects without sleep apnea, but AHI was not associated with HOMA. Also, in patients with OSAS, waist circumference was positively correlated with mean levels of HOMA.
Leptin is a multiple-function adipocyte-derived cytokine involved in the pathogenesis of obesity and increased cardiovascular risk. Previously, leptin levels in OSAS were investigated in many studies.[16,22,23] It was shown that leptin was significantly higher in OSAS patients compared with those in non-OSAS control subjects with similar BMI, age, and gender. Serum leptin levels were positively correlated with BMI, skinfold thickness, AHI, and percentage of sleep time with SaO2 < 90%.[38] Ursavas et al.[39] also found a positive correlation between serum leptin levels and BMI in OSAS patients. In the present study, leptin levels were significantly higher in OSAS patients than obese subjects. Besides, leptin was positively correlated with fasting glucose, waist and neck circumferences in OSAS patients, but not in the control group.
Although the exact cause that links OSA with cardiovascular disease is unknown, there is evidence that OSA is associated with a group of proinflammatory and prothrombotic factors that have been identified as important in the development of atherosclerosis. Presence of high CRP and fibrinogen levels were associated with increased risk of cardiovascular and cerebrovascular mortality in OSAS patients. CRP is a sensitive marker for systemic inflammation. An elevated plasma level of this acute-phase reactant indicates heightened activity of inflammation in human beings. Also, it is a surrogate marker of low-grade inflammation linked to obesity. In many studies, obesity is correlated with elevated serum CRP.[40–42] Yokoe et al.[43] found that CRP was significantly higher in 30 patients with newly diagnosed OSA than 14 obese subjects and there was a relationship between OSAS severity and CRP levels. Similarly, Shamsuzzaman et al.[44] showed that CRP levels were higher in 22 OSAS patients when compared with 20 control subjects. The groups were matched for age, and importantly, BMI. Similarly, in our study, mean levels of hsCRP were significantly higher in OSAS patients than the control group. Besides, we found a significant increase in terms of mean levels of fibrinogen in sleep apnea patients, which was compatible with the literature.[18,45]
The role of plasma homocysteine levels in OSAS is unclear, with some studies reporting higher levels only in OSAS patients suffering from pre-existing cardiac disease and other reports identifying homocysteine levels to be independently associated with OSAS.[46–48] Yavuz et al.[22] suggested that homocysteine might be an important factor for the development of cardiovascular disease in patients with OSAS. However, we did not find any difference related to homocysteine levels between obese patients with and without OSAS.
Our study had limitations which warrant discussion. First, the numbers of subjects in the study and control groups were somehow small. Metabolic syndrome was higher in OSAS patients, but the difference was not significant probably because of sample size. Second, obese patients without OSAS did not undergo polysomnography as the waiting list of our sleep laboratory was quite long and it was very difficult to perform polysomnography even to the patients with obvious OSAS symptoms. However, they were all questioned in details and none of them had symptoms related to OSAS and no excessive daytime sleepiness measured by ESS. Finally, it would be better to include obese patients without any comorbidity in the study in order not to confound the effects of these diseases. However, it should be noted that it is really very difficult to find obese subjects without any diseases.
In conclusion, the present study demonstrated an increased rate of metabolic syndrome in obese OSAS patients when compared with obese subjects without OSAS. It was also shown that patients with OSAS had higher levels of serum lipids, fasting glucose, IR, leptin, fibrinogen, and hsCRP than obese without sleep apnea. The prevalence of both OSAS and metabolic syndrome is increasing worldwide, in part linked to the epidemic of obesity. Beyond their epidemiologic relationship, growing evidence suggests that OSAS may be causally related to metabolic syndrome. Thus, clinicians should be encouraged to systematically evaluate the presence of metabolic abnormalities in OSAS and vice versa.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: Implications for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 2.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study, Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 3.Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: A key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. 2009;64:631–6. doi: 10.1136/thx.2008.105577. [DOI] [PubMed] [Google Scholar]
- 4.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: The sleep heart health study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–41. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 6.McNicholas WT. Obstructive sleep apnea and inflammation. Prog Cardiovasc Dis. 2009;51:392–9. doi: 10.1016/j.pcad.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg H, Ye X, Wilson D, Htoo AK, Hendersen T, Liu SF. Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivo. Biochem Biophys Res Commun. 2006;343:591–6. doi: 10.1016/j.bbrc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Bhushan B, Guleria R, Misra A, Pandey RM, Luthra K, Vikram NK. Obstructive sleep apnoea correlates with C-reactive protein in obese Asian Indians. Nutr Metab Cardiovasc Dis. 2009;19:184–9. doi: 10.1016/j.numecd.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J. Snoring and sleep apnoea in men: Association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1998;17:533–40. [PubMed] [Google Scholar]
- 10.Tan KC, Chow WS, Lam JC, Lam B, Wong WK, Tam S, et al. HDL dysfunction in obstructive sleep apnea. Atherosclerosis. 2006;184:377–82. doi: 10.1016/j.atherosclerosis.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Gami AS, Caples SM, Somers VK. Obesity and obstructive sleep apnea. Endocrinol Metab Clin North Am. 2003;32:869–94. doi: 10.1016/s0889-8529(03)00069-0. [DOI] [PubMed] [Google Scholar]
- 12.Punjabi NM, Sorkin JD, Katzel L, Goldberg A, Schwartz A, Smith PL. Sleep-disordered breathing and insulin resistance in middle aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–82. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 13.Gruber A, Horwood F, Sithole J, Ali NJ, Idris I. Obstructive sleep apnoea is independently associated with the metabolic syndrome but not insulin resistance state. Cardiovasc Diabetol. 2006;5:22–7. doi: 10.1186/1475-2840-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma SK, Kumpawat S, Coel A, Banga A, Ramakrishnan L, Chaturvedi P. Obesity, and not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep-disordered breathing. Sleep Med. 2007;8:5–7. doi: 10.1016/j.sleep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Otake K, Sasanabe R, Hasegawa R, Banno K, Hori R, Okura Y, et al. Glucose intolerance in Japanese patients with obstructive sleep apnea. Intern Med. 2009;48:1863–8. doi: 10.2169/internalmedicine.48.2465. [DOI] [PubMed] [Google Scholar]
- 16.Grosfeld A, Zilberfarb V, Turban S, Andre J, Guerre-Millo M, Issad T. Hypoxia increases leptin expression in human PAZ6 adipose cells. Diabetologia. 2002;45:527–30. doi: 10.1007/s00125-002-0804-y. [DOI] [PubMed] [Google Scholar]
- 17.Zamarron C, García Paz V, Riveiro A. Obstructive sleep apnea syndrome is a systemic disease.Current evidence. Eur J Intern Med. 2008;19:390–8. doi: 10.1016/j.ejim.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 18.von Känel R, Dimsdale JE. Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest. 2003;124:1956–67. doi: 10.1378/chest.124.5.1956. [DOI] [PubMed] [Google Scholar]
- 19.Steiropoulos P, Papanas N, Nena E, Antoniadou M, Serasli E, Papoti S, et al. Inflammatory markers in middle-aged obese subjects: Does obstructive sleep apnea syndrome play a role? Mediators Inflamm 2010. 2010:675320. doi: 10.1155/2010/675320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR. Circulating cardiovascular risk factors in obstructive sleep apnoea: Data from randomised controlled trials. Thorax. 2004;59:777–82. doi: 10.1136/thx.2003.018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozkan Y, Firat H, Simşek B, Torun M, Yardim-Akaydin S. Circulating nitric oxide (NO), asymmetric dimethylarginine (ADMA), homocysteine, and oxidative status in obstructive sleep apnea-hypopnea syndrome (OSAHS) Sleep Breath. 2008;12:149–54. doi: 10.1007/s11325-007-0148-4. [DOI] [PubMed] [Google Scholar]
- 22.Yavuz Z, Ursavaş A, Ege E, Ozarda Ilçol Y, Karadağ M, Uzaslan E, et al. Homocysteine levels in patients with obstructive sleep apnea syndrome. Tuberk Toraks. 2008;56:37–42. [PubMed] [Google Scholar]
- 23.Kapsimalis F, Varouchakis G, Manousaki A, Daskas S, Nikita D, Kryger M, et al. Association of sleep apnea severity and obesity with insulin resistance, C-reactive protein, and leptin levels in male patients with obstructive sleep apnea. Lung. 2008;186:209–17. doi: 10.1007/s00408-008-9082-x. [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 25.Akbartabartoori M, Lean ME, Hankey CR. Smoking combined with overweight or obesity markedly elevates cardiovascular risk factors. Eur J Card Preven. 2006;13:938–46. doi: 10.1097/01.hjr.0000214613.29608.f5. [DOI] [PubMed] [Google Scholar]
- 26.Izci B, Ardic S, Firat H, Sahin A, Altinors M, Karacan I. Reliability and validity studies of the Turkish version of the Epworth Sleepiness Scale. Sleep Breath. 2008;12:161–8. doi: 10.1007/s11325-007-0145-7. [DOI] [PubMed] [Google Scholar]
- 27.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring for Sleep Stages of Human Subjects. Los Angeles, Brain Information Service/Brain Research Institute, University of California, 1968 [Google Scholar]
- 28.Diagnostic and coding manual. 2nd edition. Westchester, Illinois: American Academy of Sleep Medicine; 2005. American Academy of Sleep Medicine. International classification of sleep disorders. [Google Scholar]
- 29.Mohn A, Marcovecchio M, Chiarelli F. Validity of HOMA-IR as index of insulin resistance in obesity. J Pediatr. 2006;148:565–6. doi: 10.1016/j.jpeds.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 30.McArdle N, Hillman D, Beilin L, Watts G. Metabolic risk factors in obstructive sleep apnea: A matched controlled study. Am J Respir Crit Care Med. 2007;175:190–5. doi: 10.1164/rccm.200602-270OC. [DOI] [PubMed] [Google Scholar]
- 31.McNicholas WT, Bonsigore MR. Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: Current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 32.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–71. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 33.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: Alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–17. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 34.Davies RJ, Turner R, Crosby J, Stradling JR. Plasma insulin and lipid levels in untreated obstructive sleep apnoea and snoring: Their comparison with matched controls and response to treatment. J Sleep Res. 1994;3:180–5. doi: 10.1111/j.1365-2869.1994.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 35.Stoohs RA, Facchini F, Guilleminault C. Insulin resistance and sleep-disordered breathing in healthy humans. Am J Respir Crit Care Med. 1996;154:170–4. doi: 10.1164/ajrccm.154.1.8680675. [DOI] [PubMed] [Google Scholar]
- 36.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 37.Zhang LQ, Yao WZ, Wang YZ, Ren B, Lin YP. Relationship between obstructive sleep apnea/hypopnea syndrome and insulin resistance. Zhonghua Nei Ke Za Zhi. 2006;45:184–7. [PubMed] [Google Scholar]
- 38.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–6. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 39.Ursavas A, Ilcol YO, Nalci N, Karadag M, Ege E. Ghrelin, leptin, adiponectin, and resistin levels in sleep apnea syndrome: Role of obesity. Ann Thorac Med. 2010;5:161–5. doi: 10.4103/1817-1737.65050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mojiminiyi OA, Al Mulla F, Abdella NA. Which obesity index best explains the link between adipokines, coronary heart disease risk and metabolic abnormalities in type 2 diabetes mellitus? Med Princ Pract. 2009;18:123–9. doi: 10.1159/000189810. [DOI] [PubMed] [Google Scholar]
- 41.Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity (Silver Spring) 2009;17:1062–9. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarac F, Ozgen AG, Yilmaz C, Tuzun M. Cardiovascular risk factors in obese women and their first-degree relatives. Anadolu kardiyol derg. 2007;7:371–7. [PubMed] [Google Scholar]
- 43.Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 44.Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 45.Chin K, Ohi M, Kita H, Noguchi T, Otsuka N, Tsuboi T, et al. Effects of NCPAP therapy on fibrinogen levels in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;153:1972–6. doi: 10.1164/ajrccm.153.6.8665063. [DOI] [PubMed] [Google Scholar]
- 46.Lavie L, Perelman A, Lavie P. Plasma homocysteine levels in obstructive sleep apnea: Association with cardiovascular morbidity. Chest. 2001;120:900–8. doi: 10.1378/chest.120.3.900. [DOI] [PubMed] [Google Scholar]
- 47.Svatikova A, Wolk R, Magera MJ, Shamsuzzaman AS, Phillips BG, Somers VK. Plasma homocysteine in obstructive sleep apnoea. Eur Heart J. 2004;25:1325–9. doi: 10.1016/j.ehj.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Jordan W, Berger C, Cohrs S, Rodenbeck A, Mayer G, Niedmann PD, et al. CPAP-therapy effectively lowers serum homocysteine in obstructive sleep apnea syndrome. J Neural Transm. 2004;111:683–9. doi: 10.1007/s00702-004-0130-2. [DOI] [PubMed] [Google Scholar]


