Abstract
BACKGROUND:
Bronchiectasis continues to be one of the major causes of morbidity and mortality in developing countries, with a probably underestimated higher prevalence than in developed countries.
OBJECTIVE:
To assess the clinical profile of adult patients with bronchiectasis.
METHODS:
We retrospectively reviewed the clinical, radiologic, and physiologic findings of 304 patients with bronchiectasis confirmed by high-resolution computed tomography.
RESULTS:
Mean age of participants (45.7% males, 54.3% females) was 56 ± 25 years and 65.8% of them were lifetime non-smokers. Most common identified causes of bronchiectasis were childhood disease (22.7%), tuberculosis (15.5%), and pneumonia (11.5%). The predominant symptoms were productive cough (83.6%), dyspnea (72%), and hemoptysis (21.1%). The most common findings on chest examination were crackles (71.1%) and rhonchi (28.3%). Types of bronchiectasis were cylindrical in 47%, varicose in 9.9%, cystic in 45.1%, and multiple types in 24.3%. Involvement was multilobar in 75.3% and bilateral in 62.5%. Of 274 patients, 20.8% displayed normal pulmonary function test results, whereas 47.4%, 8% and 23.7% showed obstructive, restrictive, and mixed pattern, respectively. Patients with cystic disease had a higher frequency of hemoptysis (42%) and a greater degree of functional impairment, compared to other types.
CONCLUSION:
In patients with bronchiectasis from southern Turkey, generally presenting with recurrent productive cough, hemoptysis, dyspnea, and persistent bibasilar rales, the etiology remains mainly idiopathic. Post-infectious bronchial destruction is one of the major identified underlying pathological processes. The clinical picture and the deterioration of the pulmonary function test might be more severe in patients with cystic type bronchiectasis.
Keywords: Asthma, bronchiectasis, chest X-ray, chronic obstructive pulmonary disease, computed tomography, respiratory function test
Bronchiectasis is defined as an irreversible dilatation and destruction of one or more bronchi, with a reduction in clearance of secretions and in the expiratory airflow. This disease can lead to recurrent lower respiratory tract infections, worsening pulmonary functions, respiratory failure and pulmonary hypertension, resulting in deterioration in quality of life, with increased morbidity and premature mortality.[1–4]
The incidence and prevalance of bronchiectasis are generally not well known and are underestimated in developing countries.[5] Although the prevalence once declined over the past years in societies with high socioeconomic status, probably due to the development of preventive medicine, especially childhood immunizations, and improvement of the living conditions and widespread use of antibiotics, nowadays bronchiectasis has been recognized more, mainly due to the frequent use of high-resolution computerized tomography (HRCT).[6,7] Compared to developed countries, its prevalence was suggested to be higher in developing countries, especially in patients with less access to healthcare; however, it is probably underestimated[5,8] when based on healthcare claims or physician-reported cases. In a retrospective analysis, chest computerized tomography findings in health screening examinees revealed a very high prevalence of bronchiectasis (9.1%) in Korean adults, including asymptomatic and mild cases.[8] Bronchiectasis has still been considered as an “orphan” disease because of low clinical suspicion, commercial interest and research activity.[9] As a consequence, scientific concern in non-cystic fibrosis bronchiectasis diminished, with limited literature about this issue compared to other “obstructive lung diseases” and “pneumonia”.[10]
The etiology of bronchiectasis varies between different populations. Immune deficiency syndromes, metabolic and ultrastructural defects are the predominant etiologic factors in developed countries, while bacterial and viral infections continue to be major causes of the disease in developing countries.[11] On the other hand, despite using advanced immunological and genetic diagnostic techniques, up to 40% of patients′ etiology remains undetermined.[12]
Bronchiectasis seems to be a neglected disorder, with no adequate diagnosis and treatment. In this study, we reviewed the underlying etiologic factors, and the clinical, radiologic, and physiologic findings in 304 patients with bronchiectasis to attract clinician's attention.
Methods
Study subjects and study design
This study was conducted in Baskent University Hospital, Adana, Turkey. The medical records of 483 adult patients (age > 16 years) admitted between 2004 and 2009 with suspicion of bronchiectasis were reviewed retrospectively. The patients were enrolled in the study if the diagnosis was confirmed with HRCT of the thorax. Patients with history of cystic fibrosis, connective tissue disease, interstitial lung disease and radiation therapy were excluded from the study.
Data collection
The demographic data, predisposing factors for bronchiectasis, initial symptoms at admission, duration of symptoms, smoking history and physical examination findings were retrieved from the medical records. The symptoms at presentation were analyzed, even if the diagnosis of bronchiectasis was not evident initially.
Radiologic diagnosis
The plain chest X-rays (CXRs) at the time of diagnosis were reviewed by using the criteria outlined by Gudbjerg[13] for the diagnosis of bronchiectasis; these criteria included the presence of increased pulmonary markings, honeycomb-like structures, atelectasis (loss of lung volume), and pleural changes. A radiologist, unaware of the patients’ clinical data, evaluated the HRCT images. The predominant type (i.e. cylindrical, varicose, or cystic according to the Reid classification[14]) of bronchiectasis was noted.
Spirometry
Pulmonary function testing was done as routine follow-up at outpatient settings. Subjects had spirometry (Vmax22; SensorMedics, Yorba Linda, CA, USA) performed for forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC), according to American Thoracic Society guidelines.[15] Patients were divided into four groups on the basis of findings on spirometry. Normal spirometry was defined as FVC and FEV1 greater than 80% predicted as well as FEV1/FVC greater than 70% predicted. Obstructive pattern was defined as the presence of FEV1/FVC less than 70% predicted. Patients with restrictive pattern had FEV1 and FVC values of less than 80% predicted and a preserved FEV/FVC ratio (>70%), whereas patients with mixed disease had FEV1 and FVC values of less than 80% predicted and an FEV/FVC ratio of less than 70%.
Statistical analysis
Statistical analysis was performed using the SPSS package (SPSS Inc., Chicago, IL, USA). Data were expressed as frequency, mean ± standard deviation or median (interquartile range), as appropriate. Clinical and functional features of cystic and non-cystic (cylindrical and varicose) type bronchiectasis were compared. Unpaired Student's t-test was performed for parametric comparisons between two groups, and Mann–Whitney test was applied for nonparametric comparisons between two groups. c2 test was used to analyze categorical variables. A P value < 0.05 was considered to be statistically significant.
Results
A total of 483 patients with a diagnosis of bronchiectasis were screened based on ICD-10 coding. Fifty charts (10.3%) could not be reviewed. The diagnosis was inaccurate due to miscoding, or insufficient clinical data were available for 38 (7.8%) of the patients. Five patients were excluded as HRCT was taken during active infection. In 86 (17.8%) of the patients, the diagnosis was not proved by HRCT. The remaining 304 (62.9%) patients were enrolled into the study.
Demographic data and smoking history of the patients are shown in Table 1. The mean age of the patients was 56 ± 25 years (range 17–92 years). Patients were mostly in the age range between 50 and 80 years as presented in Figure 1. Females were predominant (54.3%). Forty-three out of 104 patients with a history of smoking (14.1%) were current smokers at the time of presentation. Rate of presence of smoking history was 60.4% for males and 12.1% for females (P < 0.001).
Table 1.
Demographic data of patients

Figure 1.
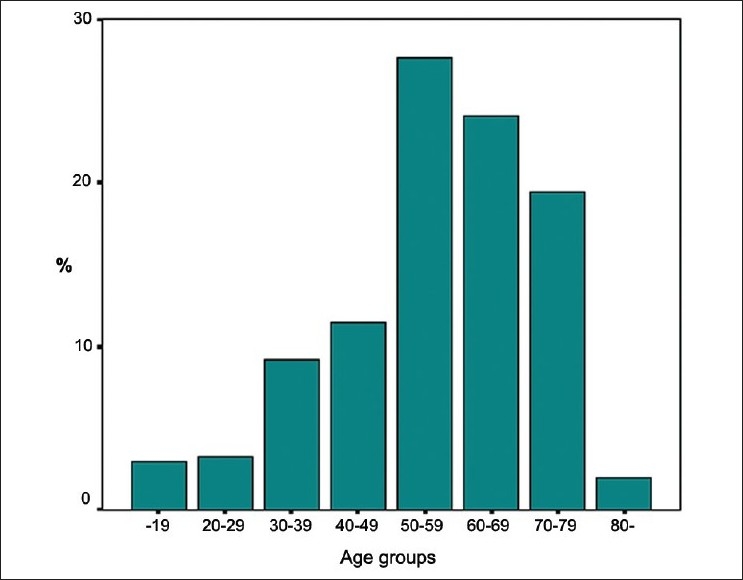
Age distribution in 10-year increments of the 304 patients with bronchiectasis
An underlying etiology was identified in 52.6% of the patients [Table 2]. Most commonly, bronchiectasis was post-infectious (49.7%) due to childhood infections (22.7%), tuberculosis (15.5%) and severe pneumonia (11.5%). There were five patients with immotile cilia syndrome, two patients with immunoglobulin deficiency and two single cases with allergic bronchopulmonary aspergillosis and congenital bronchoesophageal fistula. At the time of assessment, some patients were previously diagnosed with chronic obstructive pulmonary disease (COPD) (18.4%) or asthma (17.8%).
Table 2.
Etiology of bronchiectasis

The mean duration of symptoms was 17.4 ± 15.3 years. Cough (83.6%) and sputum (83.6%) were the most frequent complaints [Table 3]. Of 254 patients producing sputum, 139 (54.7%) had daily sputum, whereas 115 (45.3%) produced phlegm significantly during respiratory tract infections. Patients with cystic type bronchiectasis usually complained of daily sputum production, while patients with non-cystic types (cylindrical or varicose types) often complained of significant quantities of sputum only during respiratory tract infections (P < 0.05). Dyspnea was present in approximately two-thirds of the patients. Hemoptysis, mainly as blood streaked, was present in 103 patients; 19 of these were admitted with massive hemoptysis requiring medical treatment (9 patients), bronchial embolization (8 patients) or surgery (2 patients) in the follow-up. Twenty-six of 143 patients (18.2%) with cystic bronchiectasis and 14 of 161 cases (8.6%) with non-cystic (cylindrical or varicose) bronchiectasis had chronic respiratory failure (CRF), receiving long-term oxygen treatment (LTOT); so, it was detected significantly more in the cystic type (P < 0.05). Nineteen of the patients with CRF had neither COPD nor asthma.
Table 3.
Symptoms and sign of bronchiectasis*
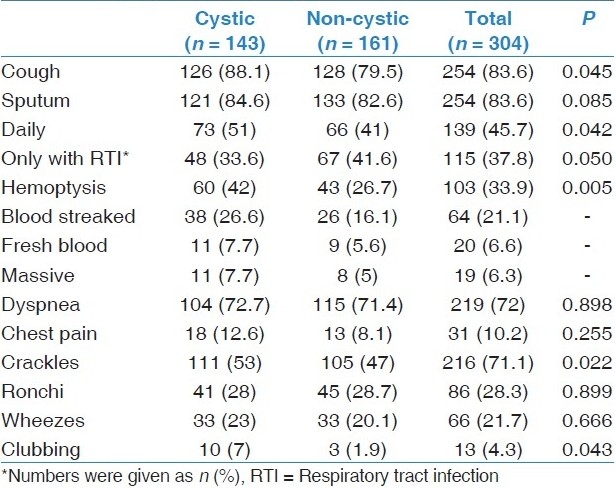
As shown in Table 3, the most common finding of the physical examination was crackles (71.1%); rhonchi (28.3%) and wheezes (21.7%) were the other common examination findings. Only 13 patients had finger clubbing. Crackles as auscultation findings and finger clubbing were more common in cystic type of the disease (P < 0.05).
Pathological findings were not detected on CXR only in 18 patients. Increased pulmonary marking was the most common radiologic finding (71.1%). These findings are usually described as chronic fibrotic changes. Honeycombing (34.9%), loss of lung volume (30.6%), and pleural abnormalities (8.5%) were the other CXR findings.
According to radiologic examination with HRCT, the predominant type of bronchiectasis was cystic in 143 cases (47%) and non-cystic in 161 cases (53%). More than one type of bronchiectasis was observed in 74 subjects (24.3%).
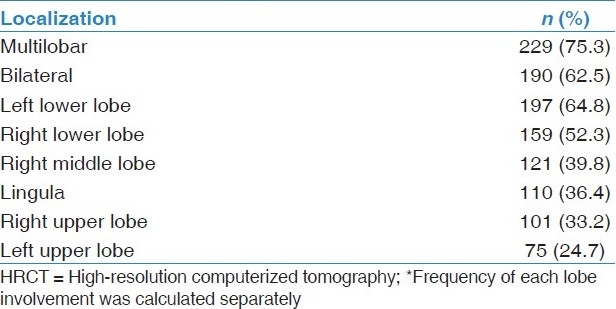
The radiologic findings of bronchiectasis determined by HRCT were quite extensive as shown in Table 4. A median of two lobes (interquartile range 2-3) was involved. A single lobe was affected in 75 cases (24.7%), as with all six lobes in 8 cases (2.6%). It was often distributed to both the lower lobes.
Table 4.
Radiologic distribution of bronchiectasis based on HRCT findings*
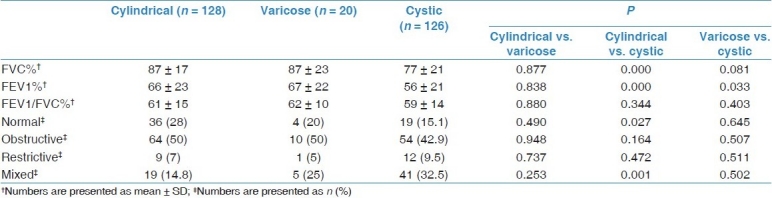
Spirometric measurements of 274 patients were analyzed [Table 5]. The test was normal in 59 patients (21.5%), obstructive in 128 (46.7%), restrictive in 22 (8%), and mixed in 65 (23.7%) patients. FEV1 and FVC values of patients with cystic type were lower than the values of those with non-cystic type bronchiectasis. Additionally, a mixed pattern was detected more commonly in the cystic group.
Table 5.
Pulmonary function test results according to predominant bronchiectasis type of 274 patients
Discussion
In our study with the highest number of adult patients stated in the literature recently, post-infectious bronchial destruction was detected to be the most common reason of non-cystic fibrosis bronchiectasis. The disease was typically characterized by chronic productive cough, dyspnea, bi-basilar crackles and obstructive type spirometric abnormality in our patients. In patients with cystic type bronchiectasis, the clinical picture and the deterioration of the pulmonary function test abnormality were observed to be more severe.
Bronchiectasis is generally not a primary disease, but an anatomical abnormality as a result of many different factors. The primary etiologic factor could not be determined in 30–74% of the patients according to the literature.[16–18] Similarly, the etiologic factors could be identified in 52.6% of the cases in our study. Pneumonia including tuberculosis infections in younger ages or childhood infections such as measles or pertussis were determined to be the reason of bronchial distortion in most of our patients. In a prospective study searching for the etiology of bronchiectasis, childhood infections were detected in 43% of the cases; however, the disease was attributed primarily to infectious reasons in only 29% of the cases.[18] The bronchial or immunologic abnormalities predisposing to those infections were claimed to be the main reasons in the same study. Because of the retrospective nature of our study, all the underlying anatomic and systemic diseases could not be investigated; therefore, they might be underdetected and understated in this article. In spite of this, our findings support the thesis of infectious agents, preserving their importance in the etiology of bronchiectasis.[16–19]
Most of our patients were detected to be symptomatic in middle age. They often complained of productive cough and dyspnea at the initial evaluation. While eight cases were admitted because of first-time hemoptysis, there was a history of hemoptysis in approximately one-third of the patients. Hemoptysis was established sometimes as the only reason of assessment and sometimes as a life-threatening complication. In 10 out of 19 cases with massive hemoptysis, bleeding could be controlled by either bronchial artery embolization or surgery.
Chronic respiratory failure is another important complication leading to increased morbidity and worsening of quality of life. In a study of 67 patients with more extended bronchiectasis, admitted to ICU because of acute respiratory failure, 25% of the cases had used LTOT previously. The mortality rate in 1 year was 40% and a previous history of LTOT was identified as a predictor for mortality.[20] In our study including patients with less extended as well as advanced disease, CRF was present in 40 of our patients with the necessity of LTOT at home, and nearly half of them did not have history of COPD or asthma. CRF was observed more in the cystic type of disease.
Bronchiectasis, known as “other obstructive airway disease”,[21] may not be always easily differentiated clinically from COPD or asthma. However, in the presence of clinical suspicion, it can be detected and diagnosed by HRCT even in patients with COPD and asthma. It was stated to be present in 29–50% of COPD patients[22,23] and 9–40% of asthma patients.[24–27] In this study, COPD and asthma were frequently observed in patients with bronchiectasis. Asthma or COPD were the co-morbidities in 17.8 and 18.4% of our patients, respectively. These rates may give rise to the thought of non-cystic fibrosis bronchiectasis being more prevalent than expected as a co-morbidity to COPD or asthma in our population. Bronchiectasis can unfavorably affect the clinical course of both these diseases by increasing the airway inflammation and bacterial colonization, by increasing the attack severity in COPD,[23] and by contributing to severe and difficult-to-control asthma with pulmonary complications.[27] Therefore, to improve the control of these disorders, bronchiectasis should be considered and investigated as co-morbidity in certain patients.
According to the evaluation of CXR findings, the most common finding was nonspecific chronic fibrotic changes. It was completely normal in 18 cases. In the presence of clinical picture as described above, even though the X-ray findings were normal or nonspecific chronic infiltrations, bronchiectasis should be confirmed with HRCT. On the other hand, a temporary bronchial dilatation and thickening of the bronchial wall named as “pseudo-bronchiectasis” may arise secondary to infection or inflammation at the acute phase. But it becomes permanent when destruction of muscle and elastic tissue in bronchi develops with scarring. Accordingly, it is recommended that HRCT be ordered at least 4 months after an acute infection for diagnosing post-infectious bronchiectasis.[28]
HRCT scanning has been the gold standard diagnostic method in bronchiectasis.[29] The extent and type of the disease can also be determined with HRCT. In nearly half of our patients (47%), cystic type was detected predominantly. It was observed that patients with cystic type had a worse clinical picture than the others, with daily sputum rather than having sputum only during respiratory tract infections, more frequent life-threatening hemoptysis, more significant auscultation findings and more frequent finger clubbing and chronic respiratory failure as a complication. As compared to other types, cystic type bronchiectasis was stated to increase the bacterial colonization in airways (especially Pseudomonas), leading to more significant worsening of pulmonary functions and more frequent development of pulmonary hypertension.[30,31] Lynch et al. pointed out that type of bronchiectasis might be a predictor of the severity of the disease.[32] Probably, the distortion and inflammation of the airways can be more serious in patients with cystic component, and parallel to this, the clinical picture becomes more severe, as shown in our patients.
Obstructive type spirometric abnormality was detected in most of our patients. Spirometric findings were similar in cylindrical and varicose bronchiectasis, as FEV1 and FVC values were lower in the cystic type than in others. As stated in earlier studies, mixed type pulmonary function abnormality was observed more frequently in the cystic type.[32,33]
There were some limitations of our study. First, as with any retrospective study, our data were dependent on the availability and accuracy of data in medical records. As such, assessment of rather less frequent predisposing factors, such as immunoglobulinopathies or immotile cilia syndrome, was possible only in some patients, based on the clinical suspicion of the primary evaluating physician. This might have led to overestimation of idiopathic cases, with underestimation of other rare causes. Second, this study is subject to selection bias, since patients were selected from the inpatients of a single tertiary hospital. Therefore, the results might not represent the general population, probably including less severe or asymptomatic cases.
To conclude, our findings state that bronchiectasis might be one of the ongoing important reasons of mortality and morbidity, with worsening quality of life in our region. The disorder is generally presented with recurrent productive cough, hemoptysis, dypnea, and persistent secretory bibasilar rales. It should be considered as co-morbidity in severe and complicated forms of asthma or COPD. Clinicians should be aware of the clinical and radiologic presentation of bronchiectasis for accurate diagnosis and appropriate multimodality treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Martinez-Garcia MA, Soler-Cataluna JJ, Perpina-Tordera M, Roman-Sanchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest. 2007;132:1565–72. doi: 10.1378/chest.07-0490. [DOI] [PubMed] [Google Scholar]
- 2.Alzeer AH, Al-Mobeirek AF, Al-Otair HA, Elzamzamy UA, Joherjy IA, Shaffi AS. Right and left ventricular function and pulmonary artery pressure in patients with bronchiectasis. Chest. 2008;133:468–73. doi: 10.1378/chest.07-1639. [DOI] [PubMed] [Google Scholar]
- 3.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Gallagher M, Holmes PW. Outcome in adult bronchiectasis. COPD. 2005;2:27–34. doi: 10.1081/copd-200050685. [DOI] [PubMed] [Google Scholar]
- 4.Alzeer AH, Masood M, Basha SJ, Shaik SA. Survival of bronchiectatic patients with respiratory failure in ICU. BMC Pulm Med. 2007;7:17. doi: 10.1186/1471-2466-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang KW, Tipoe GL. Bronchiectasis: Not an orphan disease in the East. Int J Tuberc Lung Dis. 2004;8:691–702. [PubMed] [Google Scholar]
- 6.O’Donnel AE. Bronchiectasis. Chest. 2008;134:815–23. doi: 10.1378/chest.08-0776. [DOI] [PubMed] [Google Scholar]
- 7.Cohen M, Sahn SA. Bronchiectasis in systemic diseases. Chest. 1999;116:1063–74. doi: 10.1378/chest.116.4.1063. [DOI] [PubMed] [Google Scholar]
- 8.Kwak HJ, Moon JY, Choi YW, Kim TH, Sohn JW, Yoon HJ, et al. High prevalence of bronchiectasis in adults: Analysis of CT findings in a health screening program. Tohoku J Exp Med. 2010;222:237–42. doi: 10.1620/tjem.222.237. [DOI] [PubMed] [Google Scholar]
- 9.Barker AF, Bardana EJ., Jr Bronchiectasis: Update of an orphan disease. Am Rev Respir Dis. 1988;137:969–78. doi: 10.1164/ajrccm/137.4.969. [DOI] [PubMed] [Google Scholar]
- 10.Martínez García MA. Bronchiectasis: Still an orphan disease? Arch Bronconeumol. 2005;41:407–9. doi: 10.1016/s1579-2129(06)60253-x. [DOI] [PubMed] [Google Scholar]
- 11.Cobanoglu U, Yalcinkaya I, Er M, Isik AF, Sayir F, Mergan D. Surgery for bronchiectasis: The effect of morphological types to prognosis. Ann Thorac Med. 2011;6:25–32. doi: 10.4103/1817-1737.74273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasteur MC, Bilton D, Hill AT. British Thoracic Society Bronchiectasis non-CF Guideline Group.British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65:i1–58. doi: 10.1136/thx.2010.142778. [DOI] [PubMed] [Google Scholar]
- 13.Gudbjerg CE. Roentgenologic diagnosis of bronchiectasis: An analysis of 112 cases. Acta Radiol. 1955;43:210–26. [PubMed] [Google Scholar]
- 14.Swartz MN. Bronchiectasis. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Kaiser LR, Senior RM, editors. Fishman's pulmonary disease and disorders. 3rd ed. New York: McGraw-Hill; 1998. pp. 2045–70. [Google Scholar]
- 15.Celli BR, MacNee W. ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 16.Nicotra MB, Rivera M, Dale AM, Shepherd R, Carter R. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest. 1995;108:955–61. doi: 10.1378/chest.108.4.955. [DOI] [PubMed] [Google Scholar]
- 17.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med. 2006;100:2183–9. doi: 10.1016/j.rmed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162:1277–84. doi: 10.1164/ajrccm.162.4.9906120. [DOI] [PubMed] [Google Scholar]
- 19.Shoemark A, Ozerovitch L, Wilson R. Aetiology in adult patients with bronchiectasis. Respir Med. 2007;101:1163–70. doi: 10.1016/j.rmed.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Dupont M, Gacouin A, Lena H, Lavoue S, Brinchault G, Delaval P, et al. Survival of patients with bronchiectasis after the first ICU stay for respiratory failure. Chest. 2004;125:1815–20. doi: 10.1378/chest.125.5.1815. [DOI] [PubMed] [Google Scholar]
- 21.Mysliwiec V, Pina JS. Bronchiectasis: the ‘other’ obstructive lung disease. Postgrad Med. 1999;106:123–6. doi: 10.3810/pgm.1999.07.607. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55:635–42. doi: 10.1136/thorax.55.8.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel IS, Vlahos I, Wilkinson TM, Lloyd-Owen SJ, Donaldson GC, Wilks M, et al. Bronchiectasis, exacerbations indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:400–7. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- 24.Harmanci E, Kebapci M, Metintas M, Ozkan R. High-resolution computed tomography findings are correlated with disease severity in asthma. Respiration. 2002;69:420–6. doi: 10.1159/000064018. [DOI] [PubMed] [Google Scholar]
- 25.Grenier P, Mourey-Gerosa I, Benali K, Brauner MW, Leung AN, Lenoir S, et al. Abnormalities of the airways and lung parenchyma in asthmatics: CT observations in 50 patients and inter- and intra-observer variability. Eur Radiol. 1996;6:199–206. doi: 10.1007/BF00181147. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Siddiqui S, Haldar P, Raj JV, Entwisle JJ, Wardlaw AJ. Qualitative analysis of high-resolution CT scans in severe asthma. Chest. 2009;136:1521–8. doi: 10.1378/chest.09-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oguzulgen IK, Kervan F, Ozis T, Turktas H. The impact of bronchiectasis in clinical presentation of asthma. South Med J. 2007;100:468–71. doi: 10.1097/SMJ.0b013e31802fa16f. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal R. Bronchiectasis in acute pneumonia… pseudobronchiectasis. Chest. 2007;132:2054–5. doi: 10.1378/chest.07-1529. [DOI] [PubMed] [Google Scholar]
- 29.Webb WR, Muller NL, Naidich DP. Highresolution CT of the lung. Philadelphia: Lippincott Williams and Wilkins; 2001. Airway diseases; pp. 467–546. [Google Scholar]
- 30.Angrill J, Agusti C, de Celis R, Filella X, Rañó A, Elena M, et al. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am J Respir Crit Care Med. 2001;164:1628–32. doi: 10.1164/ajrccm.164.9.2105083. [DOI] [PubMed] [Google Scholar]
- 31.Alzeer AH. HRCT score in bronchiectasis: Correlation with pulmonary function tests and pulmonary artery pressure. Ann Thorac Med. 2008;3:82–6. doi: 10.4103/1817-1737.39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch DA, Newell J, Hale V, Dyer D, Corkery K, Fox NL, et al. Correlation of CT findings with clinical evaluations in 261 patients with symptomatic bronchiectasis. AJR Am J Roentgenol. 1999;173:53–8. doi: 10.2214/ajr.173.1.10397099. [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, Kim YK, Kwang HJ, Chang JH. Relationship between high-resolution computed tomography, lung functionand bacteriology in stable bronchiectasis. J Korean Med Sci. 2004;19:62–8. doi: 10.3346/jkms.2004.19.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]


