Abstract
Ayurveda refers to bronchial asthma as Tamaka Swasa and it is well explained in Charaka Samhita. It contributes several modalities of the treatment for Swasa roga(asthma). Among all modalities of treatment, polyherbal combinations are said to be well-accepted, safe and effective in asthma. A study was carried out in 40 patients of either sex in between the age of 15-65 years to assure the clinical response of Padmapatradi yoga in bronchial asthma (Tamaka Swasa) at P.G. department of Kayachikitsa, D.G.M. Ayurvedic Medical College, Gadag, Karnataka. The sum total properties of Padmapatradi yoga is tikta katu rasa, laghu and tikna guna (light and penetrating properties), ushna virya (hot potency) and vatakaphagna (decrease vata and kapha dosa) Padmapatradi yoga is effective in increased peak expiratory flow rate, breath holding time, and reduces the absolute eosinophil count of studied cases and also found statistically highly significant at p<0.001 level. The drug is quite safe and acts as a bronchodilator, antihistaminic and anti-inflammatory.
Keywords: Breath holding time, bronchial asthma, dysponea, forced expiratory volume, Padmapatradi yoga, peak expiratory flow rate, Tamaka Swasa, vital capacity
INTRODUCTION
Bronchial asthma is a chronic inflammatory disorder of the airway and the most common distressing disease affecting 3-5% of the total population.[1] In 1998, the national asthma campaign estimated the prevalence of diagnosed asthma cases in India to be 3.4 million.[2] In the field of allergic respiratory diseases, several indigenous drugs have been successfully tested and used as conservative therapy in asthma. Ayurveda, the great indigenous system of medicine of India is a complete healthcare system and deals with the preventive and curative aspect of many diseases. Ayurveda refers to bronchial asthma as Tamaka Swasa and contributes several modalities of treatment for the same.[3] Among all the treatment modalities polyherbal combinations are said to be well-accepted, safe and effective in asthma.[4] The reason for the therapeutic efficacy of herbal combinations in asthma is due to multiple blocking and homeostasis of very complex and interdependent cellular and mediator networks supporting and involved in the inflammatory process of asthma, whereas modern synthetic drug therapy aimed at blocking one mediator alone would be unlikely to have any significant effect on the disease process. None of the available treatments are found to be effective to provide a complete cure of this disease.[5]
The goal of asthma treatment has shifted from symptom relief to disease control and it also ensures the patient's wellbeing.[6] Herbal preparations have been cited as the third most popular complementary treatment modality.[7] Sometimes herbal remedies increase the morbidity and adherence to inhaled corticosteroids.[8] But Padmapatradi yoga.[9] is an experience-based polyherbal compound having five herbs i.e. Padmapatra (Inula recemosa), Bhargi (Clerodenum serratum),[6] Malaya Vacha (Alpinia galanga), Shati (Hedychium Spicatum), and Pippali (Piper longum). All the drugs have ushana virya (hot potency) and Vata kapha hara properties, which is the main dosa in asthma. There are many evidences of these herbal constituents of Padmapatradi yoga.[10] Inula recemosa can produce relaxation of bronchioles like adrenalin, but action was less powerful and took a longer time to develop and also persisted for a longer period. Hedychium spicatum and Clerodenum serradrum are said to possess anti-inflammatory and antihistaminic properties.[11–15] Piper longum is a known immunomodulator drug. Alpinia galanga has a bronchodilatory and anti-inflammatory effect on bronchioles.[16,17]
In this study, we propose to observe the effect of Padmapatradi yoga on bronchial asthma with the fixed subjective and objective parameters
MATERIALS AND METHODS
Patients
The study involved a total of 40 patients of either sex of bronchial asthma aged between 15-65 years. The study was carried out in the P.G. department of Kayachikitsa, D.G.M. Ayurvedic Medical College (DGAMC), Gadag, Karnataka.
Inclusion criteria
Patients above the age of 15 years and below the age of 65 years were included, irrespective of their sex, on the basis of clinical signs and symptoms. Bronchial asthma with a history for at least one year, nonsmokers and absence of long-term remissions of asthma (lasting more than one month) are included in the study.
Exclusion criteria
Patients with bronchial asthma with accompanying diagnosis of heart disease, other infectious disease, arterial hypertension and patients with chronic asthmatic bronchitis along with bronchiectasis were excluded from the study. Patients who had long-term history of smoking, abnormal baseline hematology, blood chemistry or urinalysis, and lactating mothers and pregnant ladies, patients on other therapies (allopathic, homeopathic or other than prescribed Ayurveda drugs) along with our trial drug and not willing for this herbal treatment were excluded. Written informed consent was obtained from each patient and the institutional ethical committee approved clinical protocol.
Study design and duration
The study design was open clinical trial of over 40 cases of bronchial asthma. The study was a preliminary attempt to know the efficacy of this formulation, therefore a control group was not taken. Moreover, the trial was in an Ayurveda hospital and administration of modern control was an ethical problem. The duration of treatment was one month.
Trial drug
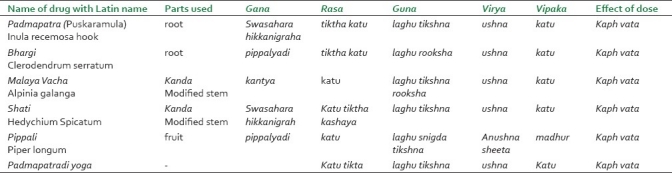
All the ingredients of trial drug (Padmapatradi yoga) were well-identified by taxonomist [Table 1]. All the drug samples were preserved and kept in the museum of DGAMC, Gadag. The drugs were dried and processed into powder form. No binding substance was added. The drug was prepared by Balagi Ayurveda Pharma, Bagalore. The High Pressure liquid chromatography (HPLC) of the tablets was done randomly to ensure that quality and good manufacture practice was followed. Total number of bacteria, pathogens, and aflatoxins are measured and found below the admissible limit.
Table 1.
The Ayurvedic pharmacological properties of individual ingredients and formula in Toto of Padmapatradi yoga[21]
Matra (Dose), Ausadha sevan kala (Drug administration time) and Anupana (vehicle)
The trial drug was delivered, in tablet form, each containing 500 mg. Every patient received four tablets or 2 g per day in two divided doses. However, the dosages were flexible according to rogibala (stamina of patient), rogabala (severity of diseases) and vaya (age) of the patient. The drug administration time was prabhata prag bhukta (morning before food, 7 am) and sandhya prag bhukta (evening before food, 7 pm). The anupana (vehicle) was usna jala (lukewarm water).
Assessment
All the patients were evaluated once a week for their states/ frequency of asthmatic attacks, status of expiratory dyspnoea, severity of cough, period of shortness of breath and reduced tolerance to the physical activities.[18]
Forced expiratory volume in 1 second (FEV1), peak expiratory flow rate (PEFR ), breath holding time (BHT ), and vital capacity were measured at the start and end of treatment.[19] ECG and chest X-ray were done in all the patients. Absolute eosinophil count was also measured before and after treatment.
Method /Procedure to measure peak expiratory flow rate
The Wright peak flow meter was used to measure the PEFR. The patient was asked to take a deep breath and then to blow hard into the mouthpiece of the flow meter with a sharp blast. The movement of the needle on the dial indicates the PEFR in liters per minute. Six readings were taken at 1-min intervals, and the average of four higher readings was recorded. The needle was brought back to zero by pressing the button located near the mouthpiece. Normal range is 350-500 liters /min.
Procedure to measure breath holding time
The patient was asked to sit quietly for a few minutes, breathing normally, before the BHT exercises were started. The patient was asked to pinch his nostrils with the thumb and forefinger and hold his breath after a normal inspiration. The time for which the breath could be held was noted with stop watch. Observations were made at intervals of 2 min. The highest figure for each determination in the BHT for that exercise.
Criteria for overall assessment of results
The overall assessment of results was made with the help of the below mentioned subjective and objective parameters.[20]
Complete remission
Total disappearance of sign and symptoms.
No attack.
No night awakening.
PEFR≥350 l/m, BHT≥40 sec.
Major improvement
Nature of attack from severe to mild.
Frequency of attack one to two times for 15 days.
No night awakening.
Improvement in PEFR≥250l/m, BHT≥25 sec.
Minor improvement
Nature of attack severe to moderate.
Frequency more than two times for 15 days.
Night awakening present.
Slight improvement in PEFR≥150 l/m, BHT≥15 sec.
No responce
No response in the nature of attack or increased.
Frequency continues.
Night awakening present.
No response/ decrease in PEFR and BHT.
Statistical analysis
Data was analyzed using the computer software of medical statistics “Epilmfo 6.02 issued by the WHO and CDC, USA. Levels of significance were calculated using student ‘t’ test and P value less than 0.05 was considered significant.
OBSERVATION AND RESULTS
It was observed that maximum patients (14, 35%) were from the age group of 45-55 years and male patients (26, 65%) were dominant in this study [Figure 1]. Before treatment, patients had attacks, expiratory dyspnoea, cough, and period of shortness of breath and reduced tolerance to physical activities. Ten (25%) patients had attacks of expiratory dysponea (Shwashastvata) every day, 20 (50%) once in two days and the remaining patients had symptoms two to three times per 15 days [Table 2]. Twenty-two (55%) patients had mucoid sputum and seven (17.5%) cases had purulent sputum; 11 (27.5%) had dry cough without sputum [Table 3]. The tolerance to physical activities was normal in 15 (37.5%) cases, reduced in 20(50%) cases and severely reduced in five (12.5%) cases [Table 4]. It was observed that 11 (27.5%) patients had severe attack, 19 (47.5%) patients had moderate and 10 (25%) patients had mild attack before treatment. Twenty-six patients (65%) had no attack or were free from attack during our study period, six (15%) patients had moderate asthma, six (15%) patients had mild asthma after treatment and two (5%) patients could not respond to our treatment [Table 5]. The maximum number of patients i.e. 22 (55%) had minimum PEFR range (50-150) and three (7.5%) patients had high PEFR range (>300) before treatment. It is interesting to note that 12 patients (30%) had normal PEFR value (>350) and another 20 patients (50%) improved in the said value and two (5%) patients had no change in terms of PEFR value [Table 6]. It was observed that 24 patients (60%) had BHT 0-15 sec, 13 patients (32.5%) had BHT 0-15 sec and three patients (7.5%) had BHT range 21-30 sec before treatment. After treatment 18 patients (47.5%) had normal BHT (>30 sec) and another 10 (25%) improved in BHT and ot patients (5%) had no change in their BHT [Table 7]. The absolute eosinophil counts rose in 28 patients (70%) before treatment which was normalized in 24 cases (85.71) after treatment [Table 8]. Maximum patients (30) were vata kapha predominant.
Figure 1.
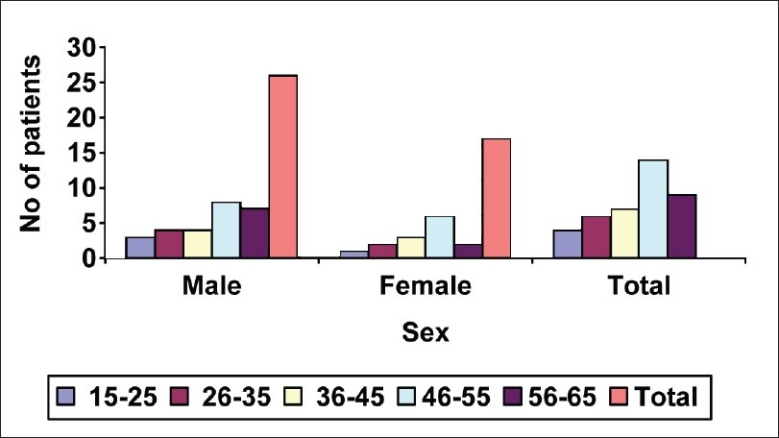
Age and sex distribution of the 40 asthma cases studied
Table 2.
The duration of expiratory dyspnoea (Shwashastvata)
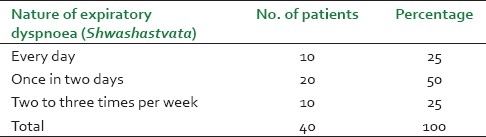
Table 3.
The types of cough in 40 treated cases of asthma (Kapha Sthivana)

Table 4.
The tolerance of the 40 treated cases to physical activities
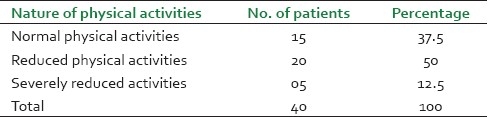
Table 5.
The nature of attack before and after treatment
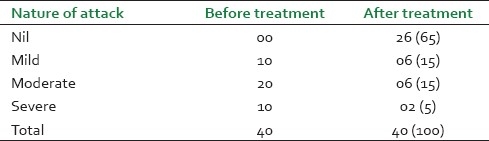
Table 6.
Peak expiratory flow rate of 40 treated cases of asthma before and after treatment
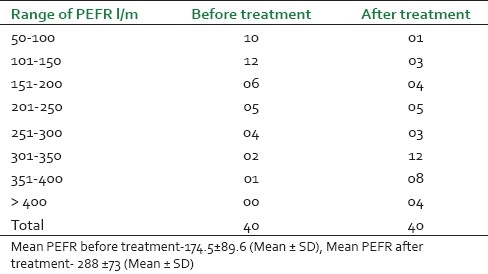
Table 7.
Breath holding time of 40 treated cases at different intervals before and treatment
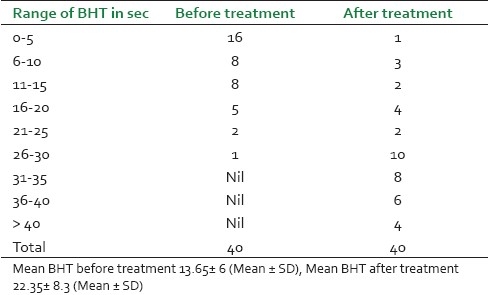
Table 8.
Absolute Eosinophil Count of 40 treated cases before and after treatment

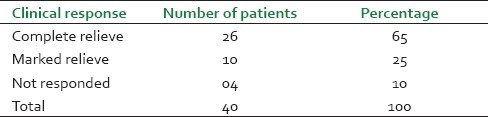
A total number of 26 (65%) patients were completely relived from the attack, reduction of severity was observed in 10 (25%) cases of asthma and four (10%) patients did not respond to the treatment [Table 9]. This lack of response may be due to the chronicity of the patients. The improved PEFR values in 36 (90%) cases suggest a bronchodilatation effect which increased the vital capacity of lungs. The PEFR values did not increase in the remaining four (10%) cases, which may be due to the development of respiratory infection and other causes of increased airway obstruction. Breath holding time was normal (>30 sec) in 18 patients (47.5%) and another 10 (25%) improved in BHT. Two patients (5%) had no change in BHT, maybe due to the unstable asthma and reduced oxygen saturation capacity in the alveoli of lungs.
Table 9.
Overall clinical response of 40 treated cases of asthma (Tamaka Swasa)
There was a reduction in the absolute eosinophil count in 24 out of 28 cases (85.71%). It clearly indicates that the drug has action over absolute eosinophil count, their mediators and other effector cells responsible in the asthma reaction.
Side-effect
One patient complained of constipation, dryness of mouth and burning sensation in stomach during the treatment. The cause of the side-effect was the vata prakuti and old age of the patient.
DISCUSSION
This study is an attempt to examine the clinical response of Padmapatradi yoga according to the clinical guidelines of Acharya Charaka and global initiation for asthma (1998). The sum total properties of Padmapatradi yoga are tikta katu rasa, laghu and tikna guna (light and penetrating properties), ushna virya (hot potency) and vatakaphagna (decrease vata and kapha dosa). The gunas of the drug are laghu, tikshna which are antagonistic to the gunas of kapha dosa, thereby normalizing kapha dosa. The virya (potency) of this drug is ushna (hot), whereas that of vata is sheeta guna (cold in Character). All the ingredients of Padmapatradi yoga have the quality to normalise or suppress the prakupitha vata dosha (vitiated vata dosa) by ushna virya (hot potency). Agni mandya (diminished digestion power) is corrected by pippali. Srotas vitiated are pranavaha srotas, which are corrected by all the drugs as they are Swasa hara (reduce expiratory dysponea) and kasahara (decrease cough). Srotodusty (The mechanism of manifestation of diseases) is sanga (occlusion), which is relieved by the ushna (hot)properties of the drug and swasahara property. Adhistana (site of disease) is amasaya (upper part of stomach), which is seat of kapha, as the ingredient of Padmapatradi yoga are katu, tiktha rasapradhana, acting over kapha dosha and amasaya restoring the normal function of amasaya (upper part of Stomach).[22] By these properties breaking of the pathogenesis (samprapti vighatana) takes place.
The cause of the side-effect may be due to the vata pitta prakuti and old age of the patient, where katu, tiktha rasa may have further increased the vata and manifest constipation. But it is a case of individual response to the treatment.
Bronchial asthma is a chronic inflammatory disorder and mast cell, eosinphils and T-lymphocytes plays an important role.[23] The ingredients may be collectively effective on airflow obstruction and airway hyper-responsiveness by bronchodilatory, anti-inflammatory and antihistaminic properties.[24,25] Inula recemosa and Clerodendron serratum have anti-allergic activities in experimental models. Hedychium spicatum have an anti-inflammatory effect. The mechanism of action of Padmapatradi yoga is not clear but ingredients of Padmapatradi yoga have bronchodilatory, anti-inflammatory and antihistamine activities.
The therapeutic efficacy of this drug is due to multiple blocking and homeostasis of very complex and interdependent cellular and mediator networks supporting and involved in the inflammatory process of asthma. The pharmacological properties like bronchodilatory, anti-inflammatory and antihistaminic may activate the action of Swasahara and kasahara as described in Ayurveda.
CONCLUSION
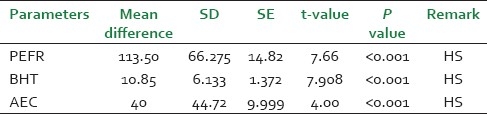
Padmapatradi is effective in reducing the severity of attack, and helps in increased PEFR and BHT, Of studied cases and also found statistically highly significant at P<0.001 level [Table 10]. The therapeutic effect of the drug is observed same response irrespective of age and sex in the studied patients. The drug is quite safe and acting as broncho- dilation, anti-inflammatory and anti histaminic.
Table 10.
Statistical analysis of different parameters before and after treatment
It is a new therapeutic option in asthma control. Further double-blind placebo-control study in a higher population is recommended.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Kumar S, Mohan A, Sharma SK. Recent concepts in the pathogenesis of bronchial asthma. Indian J Chest Allied Sci. 1997;39:27–45. [PubMed] [Google Scholar]
- 2.Udwadia ZF. Pulmonary disorders in 21st Century. Indian Pract. 2000;2:38–42. [Google Scholar]
- 3.Sharma PV. 1st ed. Varanasi: Chaukhamba Orientalia; 1982. Charaka Samhita-Shvasa Chikitsa- English translation. Chapter 17 Sloka 45-76. [Google Scholar]
- 4.Ng TP, Wong ML, Hong CY, Koh KT, Goh LG. The Use of complimentary and alternative medicine by asthma patients. QJM. 2003;96:747–54. doi: 10.1093/qjmed/hcg121. [DOI] [PubMed] [Google Scholar]
- 5.Gabrielian ES, Narimanian MZ, Asianian G. A Placebo controlled double blind study with an Ayurvedic drug Pulmoflex in brochial asthma. Phytomedica. 2004;5:45–9. [Google Scholar]
- 6.Behera D. New Delhi: Jaypee Brothers; 2000. Bronchial asthma; pp. 23–7. [Google Scholar]
- 7.Bielory L, Lupoli K. Herbal intervention in asthma and allergy. J Asthma. 1999;36:1–65. doi: 10.3109/02770909909065150. [DOI] [PubMed] [Google Scholar]
- 8.Roy A, Lurslurchachai L, Halm EA, Li XM, Leventhal H, Wisnivesky JP. Use of herbal remedies and adherence to inhaled corticosteroids among the inner city asthmatic patients. Ann Allergy Asthma Immunol. 2010;104:132–8. doi: 10.1016/j.anai.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaidy Ram Shankar Joshi. Hubli: Sevaji Nath Publication; 1935. Ousadhi Sara Samgraha (in Kanada) pp. 62–3. [Google Scholar]
- 10.Shankar A. A clinical trial of Bhargi in the cases of Tamaka swasa (Brochial asthma) J Ayurveda Siddha. 1980;15:34–8. [Google Scholar]
- 11.Huntley A, Ernst E. Herbal medicine for asthma: A systemic review. Thorax. 2000;55:925–9. doi: 10.1136/thorax.55.11.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta SS, Gupta NK. Effect of Solanum xanthocarpum and Clerodendron serratum on histamine release. Indian J Med Sci. 1967;21:795–9. [PubMed] [Google Scholar]
- 13.Gupta SS, Rai M, Gupta NK. Histamine Releasing Effects of a Few Indian Medicinal Plants Used in Bronchial Asthma Guinea-Pig Clerodendron-Serratum-D Metab Solanum-Xanthocarpum-D Metab. Curr Sci (Bangalore) 1967;36:42–3. [Google Scholar]
- 14.Bhujbal S, Nanda RK, Ganu GP, Jadav S, Dongre W, Pakale D, et al. Proctive effect of Icosahydropicenic acid isolated from the root of Clerodendron-Serratum(L) Moon on experimental allergic asthma. J Com Int Med. 2010;7:32. [Google Scholar]
- 15.Srivastava S, Gupta PP, Prasad R, Dixit KS, Palit G, Ali B, et al. Evaluation of antiallergic activity (type I hypersensitivity) of Inula racemosa in rats. Indian J Pharmacol. 1999;43:235–41. [PubMed] [Google Scholar]
- 16.Khare CP. 1st ed. New york: Spinger Science; 2007. Indian medicinal plants -an illustrated disctinary; pp. 15–9. [Google Scholar]
- 17.Kasirajan B, Maruthamuthu R, Gopalakrishnan V, Arumugam K, Asirvatham H, Murali V, et al. A database for medicinal plant use in treatment of asthma. Bioinformation. 2007;2:105–6. doi: 10.6026/97320630002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spector SL, Nicklas RA. Practice parameters for the diagnosis and treatment of asthma. J Allergy Clin Immunol. 1995;96:729–31. doi: 10.1016/s0091-6749(95)70026-9. [DOI] [PubMed] [Google Scholar]
- 19.Quackenboss JJ, Lebowitz MD, Krzyzanowski M. The normal range of diurnal changes in peak expiratory flow rates. Relationship to symptoms and respiratory disease. Am Rev Respir Dis. 1991;143:323–30. doi: 10.1164/ajrccm/143.2.323. [DOI] [PubMed] [Google Scholar]
- 20.Kumar C, Robbins C. Noida: Thomson Press India limited; 1999. Pathological Basis of disease; pp. 367–80. [Google Scholar]
- 21.Sharma PV. 8th ed. Vol. 2. Varanasi: Chaukhamba Bharati Academy; 1986. Dravya guna Vigyana; pp. 13–121. [Google Scholar]
- 22.Sharma AK. “All you need to know about Swasa roga”. J Ayurveda Vikas. 2000;4:20–3. [Google Scholar]
- 23.Tripathy KD. 5th ed. New Delhi: Jaypee Brothers; 2003. Essentials of Medical Pharmacology; pp. 198–209. [Google Scholar]
- 24.Srimal RC, Sharma SC, Tendon JS. Anti inflammatory and other pharmacological effect of Hedychium spicatum. Indian J Pharmacol. 1994;16:143–7. [Google Scholar]
- 25.Mahammad Y, Mahamad I. Anti histaminic herbal drugs: A review. Int J Pharm Pharm Sci. 2010;2:28–9. [Google Scholar]


