Abstract
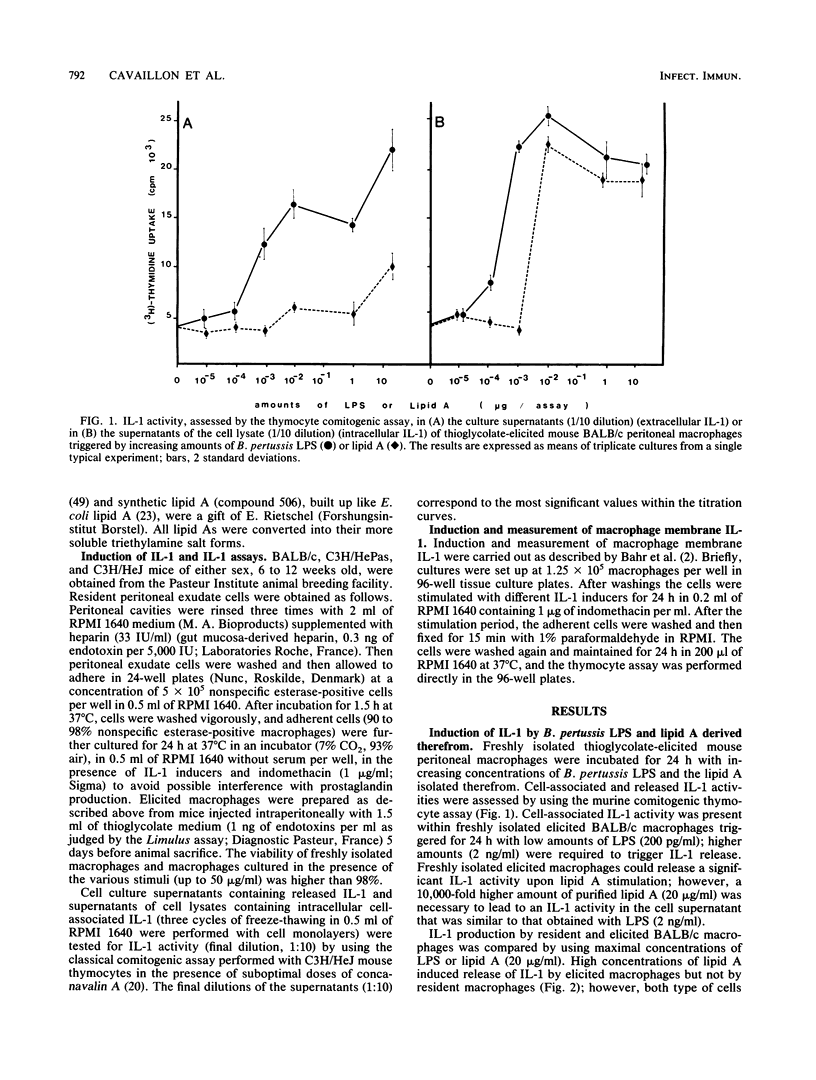
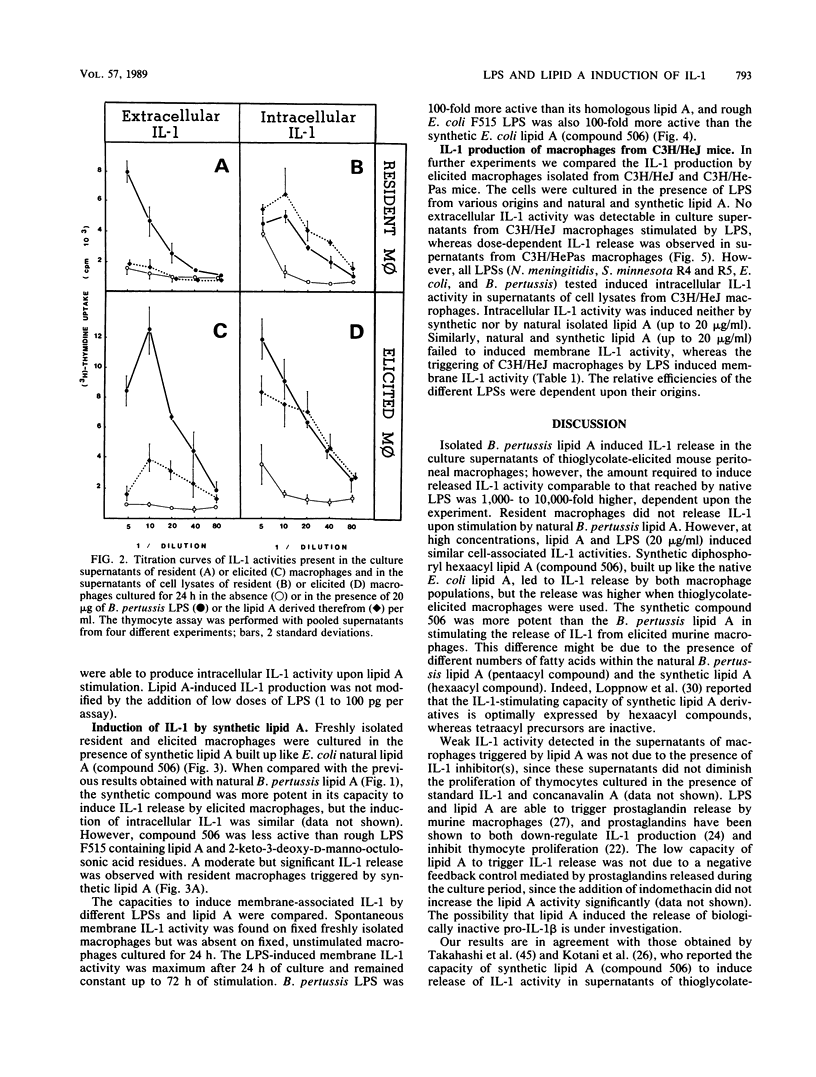
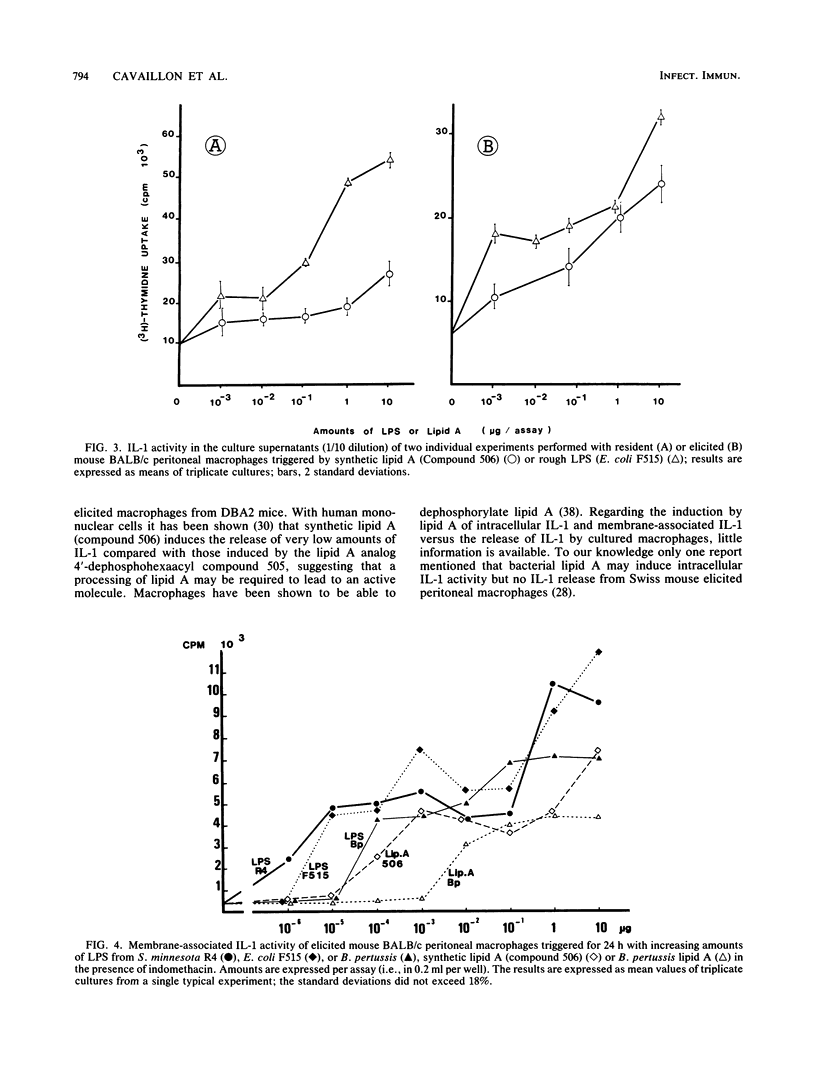
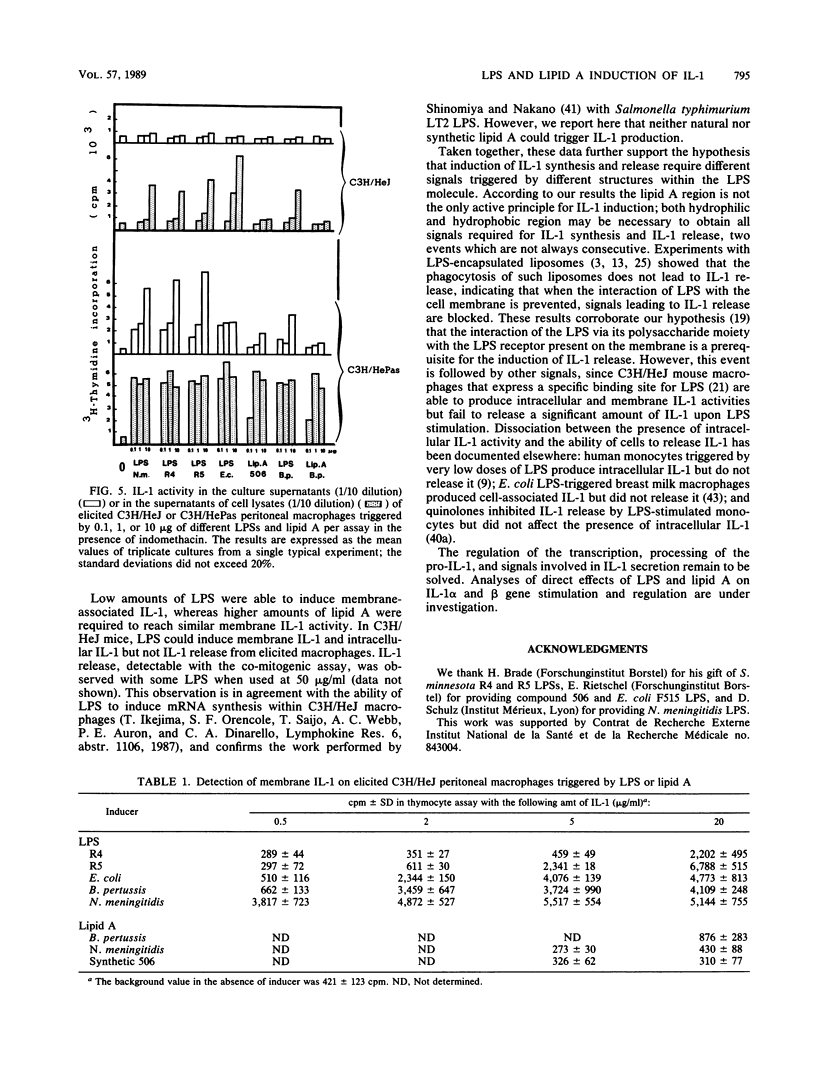
The capacities of lipopolysaccharide (LPS) and lipid A to trigger mouse BALB/c peritoneal macrophages and to induce the production of cell-associated interleukin-1 (IL-1) and membrane-associated IL-1 and IL-1 release have been compared. Bordetella pertussis lipid A was 1,000 to 10,000 times less efficient than the native LPS to induce IL-1 release by freshly isolated elicited macrophages. When resident macrophages were studied, lipid A, at high concentrations (greater than 2 micrograms/ml), induced significant levels of cell-associated IL-1 but little or no IL-1 release. With synthetic lipid A built up with the Escherichia coli lipid A structure (compound 506), IL-1 activity was present in the supernatants of elicited peritoneal macrophages and to a lesser extent in those of resident macrophages. However, the release of IL-1 induced by synthetic lipid A 506 remained much lower than those induced by rough LPS. Membrane-associated IL-1 could be induced on BALB/c macrophages with LPS and natural or synthetic lipid A, the LPS being the most active. In C3H/HeJ mice, neither natural nor synthetic lipid A could induce detectable cell-associated IL-1, whereas LPS could induce cell-associated and membrane IL-1 activity but no IL-1 release. Our results indicate that fragments of endotoxins may induce the production of IL-1 but the entire structure of the LPS molecule is the most effective to induce intracellular IL-1 production, expression of membrane IL-1, and release of IL-1.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arenzana-Seisdedos F., Virelizier J. L., Fiers W. Interferons as macrophage-activating factors. III. Preferential effects of interferon-gamma on the interleukin 1 secretory potential of fresh or aged human monocytes. J Immunol. 1985 Apr;134(4):2444–2448. [PubMed] [Google Scholar]
- Bahr G. M., Chedid L. A., Behbehani K. Induction, in vivo and in vitro, of macrophage membrane interleukin-1 by adjuvant-active synthetic muramyl peptides. Cell Immunol. 1987 Jul;107(2):443–454. doi: 10.1016/0008-8749(87)90251-6. [DOI] [PubMed] [Google Scholar]
- Bakouche O., Koff W. C., Brown D. C., Lachman L. B. Interleukin 1 release by human monocytes treated with liposome-encapsulated lipopolysaccharide. J Immunol. 1987 Aug 15;139(4):1120–1126. [PubMed] [Google Scholar]
- Beck G., Habicht G. S., Benach J. L., Miller F. Interleukin 1: a common endogenous mediator of inflammation and the local Shwartzman reaction. J Immunol. 1986 Apr 15;136(8):3025–3031. [PubMed] [Google Scholar]
- Brade H., Brade L., Schade U., Zähringer U., Holst O., Kuhn H. M., Rozalski A., Röhrscheidt E., Rietschel E. T. Structure, endotoxicity, immunogenicity and antigenicity of bacterial lipopolysaccharides (endotoxins, O-antigens). Prog Clin Biol Res. 1988;272:17–45. [PubMed] [Google Scholar]
- Caroff M., Cavaillon J. M., Fitting C., Haeffner-Cavaillon N. Inability of pyrogenic, purified Bordetella pertussis lipid A to induce interleukin-1 release by human monocytes. Infect Immun. 1986 Nov;54(2):465–471. doi: 10.1128/iai.54.2.465-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon J. M., Fitting C., Hauttecoeur B., Haeffner-Cavaillon N. Inhibition by gangliosides of the specific binding of lipopolysaccharide (LPS) to human monocytes prevents LPS-induced interleukin-1 production. Cell Immunol. 1987 May;106(2):293–303. doi: 10.1016/0008-8749(87)90173-0. [DOI] [PubMed] [Google Scholar]
- Cavaillon J. M., Haeffner-Cavaillon N. The role of serum in interleukin 1 production by human monocytes activated by endotoxins and their polysaccharide moieties. Immunol Lett. 1985;10(1):35–41. doi: 10.1016/0165-2478(85)90047-1. [DOI] [PubMed] [Google Scholar]
- Cavender D. E., Haskard D. O., Joseph B., Ziff M. Interleukin 1 increases the binding of human B and T lymphocytes to endothelial cell monolayers. J Immunol. 1986 Jan;136(1):203–207. [PubMed] [Google Scholar]
- Cybulsky M. I., Movat H. Z., Dinarello C. A. Role of interleukin-1 and tumour necrosis factor-alpha in acute inflammation. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):505–512. doi: 10.1016/s0769-2625(87)80068-5. [DOI] [PubMed] [Google Scholar]
- Dijkstra J., Mellors J. W., Ryan J. L., Szoka F. C. Modulation of the biological activity of bacterial endotoxin by incorporation into liposomes. J Immunol. 1987 Apr 15;138(8):2663–2670. [PubMed] [Google Scholar]
- Dinarello C. A., Bodel P. T., Atkins E. The role of the liver in the production of fever and in pyrogenic tolerance. Trans Assoc Am Physicians. 1968;81:334–344. [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Matsushima K., Oppenheim J. J. Mechanism of in vitro antitumor effects of interleukin 1 (IL 1). Immunobiology. 1986 Sep;172(3-5):316–322. doi: 10.1016/S0171-2985(86)80113-9. [DOI] [PubMed] [Google Scholar]
- Feinman R., Henriksen-DeStefano D., Tsujimoto M., Vilcek J. Tumor necrosis factor is an important mediator of tumor cell killing by human monocytes. J Immunol. 1987 Jan 15;138(2):635–640. [PubMed] [Google Scholar]
- Gery I., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. II. The cellular source of potentiating mediator(s). J Exp Med. 1972 Jul 1;136(1):143–155. doi: 10.1084/jem.136.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Cavaillon J. M. Involvement of the LPS receptor in the induction of interleukin-1 in human monocytes stimulated with endotoxins. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):473–477. doi: 10.1016/s0769-2625(87)80060-0. [DOI] [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Cavaillon J. M., Moreau M., Szabó L. Interleukin 1 secretion by human monocytes stimulated by the isolated polysaccharide region of the Bordetella pertussis endotoxin. Mol Immunol. 1984 May;21(5):389–395. doi: 10.1016/0161-5890(84)90036-1. [DOI] [PubMed] [Google Scholar]
- Hayari Y., Kukulansky T., Globerson A. Regulation of thymocyte proliferative response by macrophage-derived prostaglandin E2 and interleukin 1. Eur J Immunol. 1985 Jan;15(1):43–47. doi: 10.1002/eji.1830150109. [DOI] [PubMed] [Google Scholar]
- Knudsen P. J., Dinarello C. A., Strom T. B. Prostaglandins posttranscriptionally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J Immunol. 1986 Nov 15;137(10):3189–3194. [PubMed] [Google Scholar]
- Kotani S., Takada H., Takahashi I., Ogawa T., Tsujimoto M., Shimauchi H., Ikeda T., Okamura H., Tamura T., Harada K. Immunobiological activities of synthetic lipid A analogs with low endotoxicity. Infect Immun. 1986 Dec;54(3):673–682. doi: 10.1128/iai.54.3.673-682.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasfargues A., Ledur A., Charon D., Szabo L., Chaby R. Induction by lipopolysaccharide of intracellular and extracellular interleukin 1 production: analysis with synthetic models. J Immunol. 1987 Jul 15;139(2):429–436. [PubMed] [Google Scholar]
- Lebbar S., Cavaillon J. M., Caroff M., Ledur A., Brade H., Sarfati R., Haeffner-Cavaillon N. Molecular requirement for interleukin 1 induction by lipopolysaccharide-stimulated human monocytes: involvement of the heptosyl-2-keto-3-deoxyoctulosonate region. Eur J Immunol. 1986 Jan;16(1):87–91. doi: 10.1002/eji.1830160117. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Brade L., Brade H., Rietschel E. T., Kusumoto S., Shiba T., Flad H. D. Induction of human interleukin 1 by bacterial and synthetic lipid A. Eur J Immunol. 1986 Oct;16(10):1263–1267. doi: 10.1002/eji.1830161013. [DOI] [PubMed] [Google Scholar]
- Movat H. Z., Cybulsky M. I., Colditz I. G., Chan M. K., Dinarello C. A. Acute inflammation in gram-negative infection: endotoxin, interleukin 1, tumor necrosis factor, and neutrophils. Fed Proc. 1987 Jan;46(1):97–104. [PubMed] [Google Scholar]
- Murphy P. A., Simon P. L., Willoughby W. F. Endogenous pyrogens made by rabbit peritoneal exudate cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol. 1980 May;124(5):2498–2501. [PubMed] [Google Scholar]
- Neta R., Douches S., Oppenheim J. J. Interleukin 1 is a radioprotector. J Immunol. 1986 Apr 1;136(7):2483–2485. [PubMed] [Google Scholar]
- Peterson A. A., Munford R. S. Dephosphorylation of the lipid A moiety of Escherichia coli lipopolysaccharide by mouse macrophages. Infect Immun. 1987 Apr;55(4):974–978. doi: 10.1128/iai.55.4.974-978.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche Y., Fay M., Gougerot-Pocidalo M. A. Interleukin-1 production by antibiotic-treated human monocytes. J Antimicrob Chemother. 1988 May;21(5):597–607. doi: 10.1093/jac/21.5.597. [DOI] [PubMed] [Google Scholar]
- Shinomiya H., Nakano M. Calcium ionophore A23187 does not stimulate lipopolysaccharide nonresponsive C3H/HeJ peritoneal macrophages to produce interleukin 1. J Immunol. 1987 Oct 15;139(8):2730–2736. [PubMed] [Google Scholar]
- Staruch M. J., Wood D. D. The adjuvanticity of interleukin 1 in vivo. J Immunol. 1983 May;130(5):2191–2194. [PubMed] [Google Scholar]
- Subiza J. L., Rodriguez C., Figueredo A., Mateos P., Alvarez R., de la Concha E. G. Impaired production and lack of secretion of interleukin 1 by human breast milk macrophages. Clin Exp Immunol. 1988 Mar;71(3):493–496. [PMC free article] [PubMed] [Google Scholar]
- Tacken A., Rietschel E. T., Brade H. Methylation analysis of the heptose/3-deoxy-D-manno-2-octulosonic acid region (inner core) of the lipopolysaccharide from Salmonella minnesota rough mutants. Carbohydr Res. 1986 Jul 1;149(2):279–291. doi: 10.1016/s0008-6215(00)90051-x. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Kotani S., Takada H., Tsujimoto M., Ogawa T., Shiba T., Kusumoto S., Yamamoto M., Hasegawa A., Kiso M. Requirement of a properly acylated beta(1-6)-D-glucosamine disaccharide bisphosphate structure for efficient manifestation of full endotoxic and associated bioactivities of lipid A. Infect Immun. 1987 Jan;55(1):57–68. doi: 10.1128/iai.55.1.57-68.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaschek R., Urbaschek B. Induction of nonspecific resistance and stimulation of granulopoiesis by endotoxins and nontoxic bacterial cell wall components and their passive transfer. Ann N Y Acad Sci. 1985;459:97–110. doi: 10.1111/j.1749-6632.1985.tb20819.x. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Haskard D., Cavender D., Ziff M. Effects of bacterial lipopolysaccharide on the binding of lymphocytes to endothelial cell monolayers. J Immunol. 1986 Jan;136(2):569–573. [PubMed] [Google Scholar]



