Abstract
Olfaction represents an ancient, evolutionarily critical physiologic system. In humans, chemosensation mediates safety, nutrition, sensation of pleasure, and general well-being. Factors that affect human olfaction included structural aspects of the nasal cavity that can modulate airflow and therefore odorant access to the olfactory cleft, and inflammatory disease, which can affect both airflow as well as olfactory nerve function. After signals are generated, olfactory information is processed and coded in the olfactory bulb and disseminated to several areas in the brain. The discovery of olfactory receptors by Axel and Buck sparked greater understanding of the molecular basis of olfaction. However, the precise mechanisms used by this system are still under great scrutiny due to the complexity of understanding how an enormous number of chemically diverse odorant molecules are coded into signals understood by the brain. Additionally, it has been challenging to dissect olfactory sensation due to the multiple areas of areas of the brain that receive and modulate this information. Consequently, our knowledge of olfactory dysfunction in humans remains primitive. Aging represents the major cause of loss of smell, although a number of clinical and environmental factors are thought to affect chemosensory function. Treatment options focus on reducing sinonasal inflammation when present, ruling out other treatable causes, and counseling patients on safety measures.
Keywords: olfaction, humans, nose, chemosensation, olfactory dysfunction
One of the most critical functions of the nasal airway is chemosensation. Although human beings are less dependent on chemosensory input than are other mammals, reflecting evolutionary changes in sensory and brain development (1), olfactory function still plays a critical role in human physiology. The detection of hazards in the environment is mediated by the olfactory and trigeminal systems that act as surveillance systems over the air as it traverses the upper airway. A second critical function is the role that the sense of smell plays in pleasure, including nutrition, sexuality, and mood. Last, novel functions for the olfactory system are being elucidated. A growing body of evidence has implicated a role for olfaction in such diverse physiologic processes as kin recognition and mating (2–4), pheromone detection (5, 6), mother–infant bonding (7), food preferences (8), central nervous system physiology (9), and even longevity (10). As with other special senses such as audition, olfactory ability declines with age, a phenomenon with enormous implications on the population level as our society ages and on the individual in terms of a detrimental quality of life. A lack of attention clinically (e.g., compare awareness of blindness and deafness with that of olfactory loss) and significant challenges to scientific inquiry in humans has limited progress in delineating the precise mechanisms of olfaction. A better understanding of the clinical and molecular factors that modulate this special sense may allow for the development of therapies for olfactory dysfunction and a deeper understanding of this interesting aspect of human physiology.
ANATOMY AND PHYSIOLOGY OF OLFACTION
Structure of the Nose
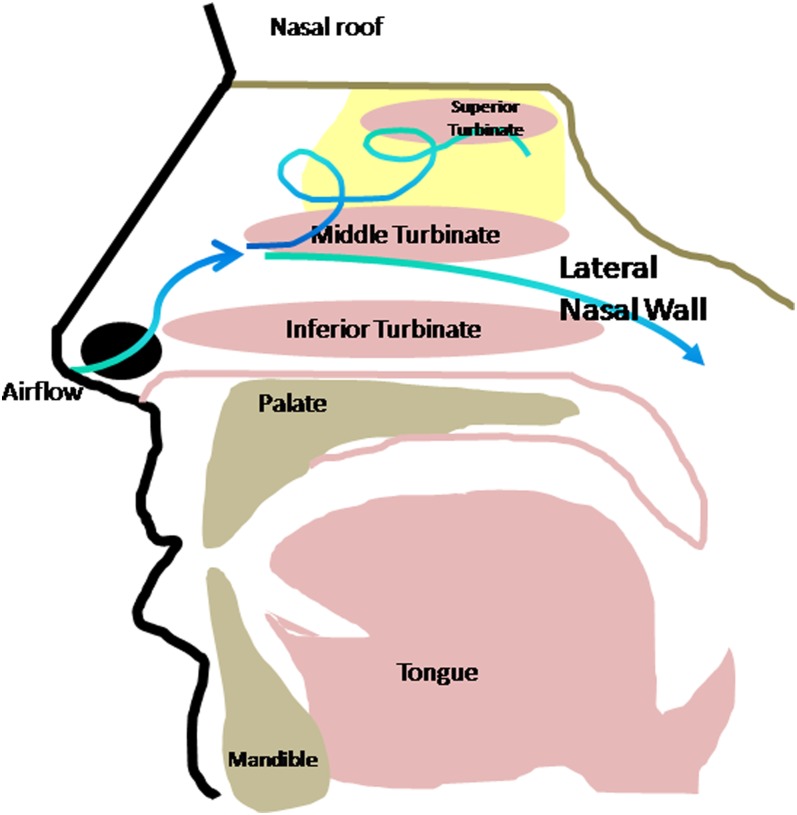
The anatomy of the nose is discussed in detail elsewhere in this issue. Briefly, the nasal passages are divided by the nasal septum in the midline. Each lateral nasal wall is formed by four turbinates (inferior, middle, superior, and supreme). The nasal valve lies anteriorly at the vestibule of the nose and is formed by the lower border of the upper lateral cartilage, the septum, and the anterior portion of the inferior turbinate; this cross-sectional area is the point of highest resistance of the respiratory tract. Airflow patterns in the nose are affected by these anatomic factors. Alteration of the normal laminar airflow through the nose results in turbulence, which not only affects the other functions of the nose (humidification and warming of air before its arrival in the lower airway by the turbinates [11]) but also directs air superiorly toward the olfactory epithelium, thus facilitating olfaction (12) (Figure 1).
Figure 1.
Structure of the nasal cavity. Shown is a lateral view in the sagittal plane. Distribution of the olfactory neuroepithelium is depicted in yellow along the medial surface of the middle and superior turbinates, the nasal roof/cribriform plate (depicted in beige), and along the superior portion of the nasal septum (not shown). Inspired air passes into the nose above the inferior turbinate and is carried upward toward this epithelium by turbulent airflow. Normal airflow travels above the inferior turbinate toward the nasopharynx. Bone of the palate, skull base, and mandible are depicted in beige, mucosal surfaces in pink.
Neural Pathways for Chemosensation
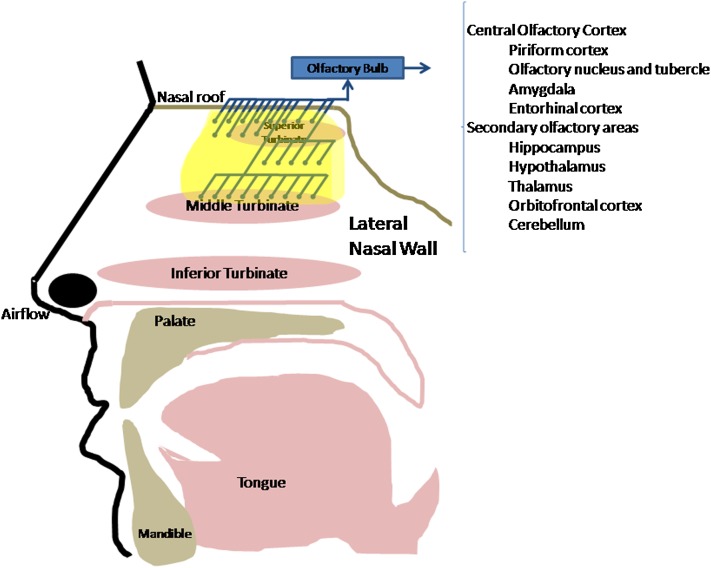
Chemosensation in the nose is mediated by the olfactory nerve (cranial nerve I) and trigeminal nerve (cranial nerve V). The olfactory neuroepithelium is characterized by the presence of olfactory neurons whose axons project across the cribriform plate at the roof of the nasal cavity, where they synapse with neurons in the central olfactory nervous system (see below). Classically, the distribution of olfactory epithelium has been thought to be along the cribriform plate at the superiormost portion of the nose, medial to the superior turbinate and along this turbinate itself. However, more recent studies have revealed a more extensive distribution that extends farther down the nose as far as the anterolateral middle turbinate and also inferiorly from the cribriform plate down the posterior and middle nasal septum (13) (Figure 2). The location of the olfactory epithelium is variable among people and is thought to change with time, resulting in conversion to or ingrowth of respiratory epithelium and loss of olfactory neurons with age and also, potentially, from environmental insult (toxins, volatile chemicals, tobacco smoke, industrial or occupational or airborne pollutants) or pathophysiologic processes such as infection or inflammation (14).
Figure 2.
Neuroanatomic connections of the olfactory nerve. Shown is a lateral view in the sagittal plane. Olfactory neurons are depicted with blue knobs; their axons form filia of the olfactory nerve, cross the cribriform plate, synapse in the olfactory bulb, and then proceed across the central nervous system.
The nervous system in the nose is complex and, besides olfactory innervation, is composed of both general sensory as well as sympathetic and parasympathetic innervation that controls a variety of functions in the nose including nasal blood flow, glandular secretion, and reflexes such as sneezing and sinonasal (15), nasonasal (16), and nasoocular responses (17). These functions are under strict physiological control and depend on local mucosal feedback systems for the sensory and autonomic reflexes (reviewed in Reference 18).
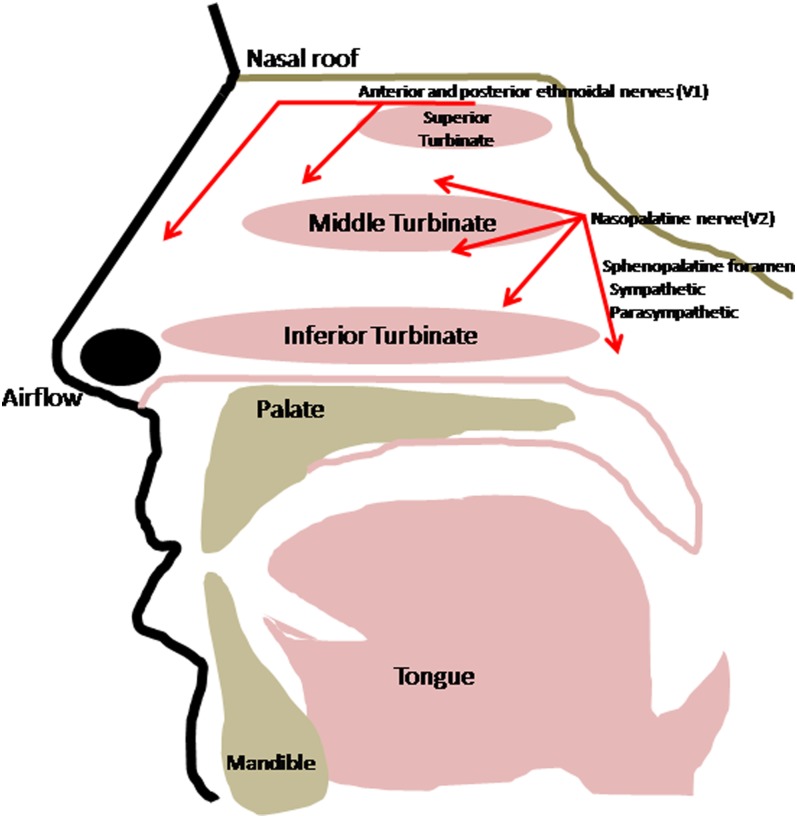
In addition to its function in olfaction, the other major chemosensory component of the nose is the trigeminal system. Trigeminal chemosensory nerve endings in the nose are the airway's first defense against noxious stimuli. Branches of the trigeminal nerve (cranial nerve V) innervate the mucosa of the nose and sinuses and mediate irritant responses (Figure 3). These afferent axons synapse in the trigeminal nucleus, which relays signals to the ventral posterior medial nucleus of the thalamus and then cortical areas that process facial irritation and pain. Nociceptive neurons of the trigeminal nerve are activated by chemicals classified as irritants, including air pollutants, ammonia, ethanol and other alcohols, acetic acid, carbon dioxide, menthol, capsaicin, and others. Many substances also elicit olfactory signals in addition to trigeminal responses, although the threshold concentrations for trigeminal chemoreception seem to be much higher than those for olfaction.
Figure 3.
Sensory and autonomic nervous supply of the nose. Lateral view in the sagittal plane. General sensation is provided by ophthalmic (V1) and maxillary (V2) branches of the trigeminal nerve. Autonomic supply comes via the sphenopalatine foramen. These nerves also supply the nasal septum (medial sagittal view, not depicted).
Responses to trigeminal chemosensory stimuli include pain, irritation, sneezing, salivation, vasodilation with resultant nasal obstruction, tearing, nasal secretion, sweating, a decreased respiratory rate, and bronchoconstriction. Many of these responses are stimulated by neuropeptides released from stimulated nerve endings. If irritants reach the lower airways, analogous responses in the lower airway can trigger sensory activity with resultant bronchoconstriction, bronchospasm, mucus secretion and neurogenic inflammation.
Although much less is known about irritant receptors than olfactory receptors, emerging data demonstrate a model of the transient receptor potential vanilloid receptor (TRPV1; also known as the receptor for capsaicin). This protein is a member of a family of ion channels originally found to transmit specific pain sensations (19) but now known to be broadly expressed in a variety of mucosal and epithelial surfaces and other tissues. These channels initiate neuron depolarization and mediate sustained depolarization, repolarization, and maintenance of resting membrane potential. Some of these proteins respond to various stimuli including temperature, mechanical stimulation, specific food substances (e.g., garlic or mustard oil), compounds such as menthol and tetrahydrocannabinol, and changes in osmotic cell swelling, all of which may be generated from foods, or from particulate, gaseous, or other irritants (reviewed in References 18 and 20).
The Olfactory Epithelium and Central Connections
Approximately 10 to 20 million olfactory neurons within the olfactory epithelium are located among a variety of supportive cells. This pseudostratified columnar epithelium includes basal cells that have been shown (in animals, but not conclusively in humans) to function as stem cells that can give rise to all components of the epithelium, Bowman's glands, microvillar cells, and sustentacular cells, which are thought to support olfactory neuron function. Bowman's acini are exocrine and produce substances that are essential for olfaction. Key components of olfactory mucus are chaperone proteins called odorant-binding proteins that act to facilitate odorant–receptor interaction. The precise function of the other cell types aiding in neuronal function via other, less well-defined mechanisms are not well known, although perhaps this occurs through providing an appropriate local environment for optimal signal transduction. Indeed, it is possible to obtain putative stem cells from humans through endoscopic biopsies for growth and differentiation in culture, with potential therapeutic effects (21).
The regenerative power of the olfactory neuron perhaps represents an evolutionary response to the continual physical challenge of this cranial nerve's unique direct exposure to the environment and allows for a reparative function on damage. In addition, ensheathing shells that support these olfactory axons may have therapeutic uses (22), potentially in nerve injury or neurodegeneration models.
Olfactory neurons are bipolar cells that project a single dendrite with a thickened ending (the olfactory knob) that extends to the epithelial surface and contains nonmotile, sensory cilia where odor molecules bind to their receptor (see below), and an axon that transmits signals to the brain. Axons from these olfactory neurons form nerve bundles (fila olfactoria) that cross the cribriform plate to synapse with other neurons in the glomeruli of the olfactory bulb. The crossing of the olfactory nerve across the skull base through about 20 foramina in the bone makes this region particularly sensitive to traumatic injury, especially through frontal or occipital trauma, a frequent etiology of olfactory loss (23). In addition, this also provides a potential route of access to the central nervous system for toxins and pathogens.
A complex process of signal transduction and coding of complex signals occurs in the olfactory bulb before information is processed and sent to other areas of the central nervous system (24, 25). Subsequent connections as defined by human functional magnetic resonance imaging studies to the “primary olfactory region” (piriform cortex, olfactory nucleus and tubercle, amygdala, entorhinal cortex) and secondary olfactory areas (hippocampus, hypothalamus, thalamus, orbitofrontal cortex, and the cerebellum) may account for the role of olfaction in mood and emotion, pleasure sensation, memory, and many other processes of the central nervous system (26, 27). Hence, both peripheral and central components of the olfactory system can affect the perception and function of this chemical sense.
The detection of odorants on a physical basis starts with a sniff, resulting in turbulent airflow that carries odorants to the olfactory epithelium superiorly in the nose. The odorants then diffuse into the mucus and are transported to the olfactory receptor by chaperones called odorant-binding proteins, which are thought to speed up the transport of the odorants to their receptors on the surface and also to help remove them to clear the signal. Binding of the odorant to the specific olfactory receptor(s) then induces signaling. A second method of perception of odorants comes posteriorly through the nose via retronasal olfaction, where odorants arise through the nasopharynx, ascend through the choanae of the nose posteriorly, and rise to the epithelium via this route. Retronasal olfaction is thought to play a key role in the sensation of flavor during consumption of food and liquids (28).
Molecular Basis for Chemosensation in the Nose
In 1991, Linda Buck and Richard Axel discovered both the family of transmembrane proteins that were believed to be the odor receptors and some of the genes that encode them, a seminal breakthrough in our understanding of the olfactory system culminating in a Nobel Prize awarded in 2004 (29). The superfamily of olfactory receptor (OR) genes, the largest in the genome, includes approximately 900 genes (although about half are nonfunctional) from 18 gene families that reside across the genome, emphasizing their ancient nature, and comprises nearly 3% of the approximately 30,000 genes of the human genome, highlighting their critical role in mammalian physiology and evolution (30). Perhaps most interesting are more recent discoveries of expression of subsets of these genes in nonolfactory tissues including sperm and gut, implicating functions for these genes outside their traditional role in olfaction.
In mice, each olfactory neuron expresses only one OR gene (a process regulated by mechanisms that are not completely clear) and precise spatial patterns of expression of certain classes of ORs and chemosensory receptors (see below) exist in animals and probably in humans. Odorants or mixtures of odorants bind a pattern of olfactory receptors, resulting in activation of the G proteins. This results in cyclic AMP–mediated opening of cyclic nucleotide–gated ion channels as well as calcium and sodium ion influx, depolarizing the olfactory receptor neuron and beginning an action potential that carries the information to the brain. Odorants are thought to bind a number of ORs of varying affinities, resulting in complex signals that the brain is able to interpret through a complex system that is now under active investigation.
Because most odors in the environment are mixtures of many components, the complexity of studying the precise signaling responses is enormous and such research relies on animal systems. In addition, the methods used by the olfactory system to distinguish among odors are not clear. Specific anosmias, the inability to detect certain odors, may reflect a lack of particular OR genes or downstream signaling pathways rendering the subject unable to smell particular scents (31). In other situations, this may manifest as hyperosmia to certain odors (32).
Adding further complexity to odor detection are the discoveries of additional chemoreceptor genes. For example, trace amine–associated receptors (TAARs) have been found in mice to respond to biogenic amines and mouse urine, potentially modulating species-specific olfactory signals (33). There are six TAAR genes in humans, but there are few data to show how they might function in chemosensation in our species. Another family of genes involved in chemosensation in animals are the vomeronasal type 1 receptors, a multigene family (34, 35) that is expressed in an accessory olfactory region in the nose called the vomeronasal organ, which is vestigial in humans (36). These genes are thought to have degenerated through evolution; for example, there are only five of these genes that retain an intact open reading frame in the human genome (37). Last, formyl peptide receptors are candidate chemosensory receptors that might be involved in the detection of normal bacterial flora or mitochondrial proteins in lower animals (38).
Overall, the combinatorial diversity of signals allows for the detection and discrimination of perhaps an unlimited diversity of odorants. Models of olfactory information processing from insects and lower animals are providing insights for application to human physiology (39).
Variability in Olfactory Function
The etiology of the wide variability in olfactory performance is one of the most fundamental questions in olfactory biology. This may reflect different expression patterns of sets of OR genes, central processing effects, or genetic variability in the OR genes themselves, as has been shown in a proof-of-principle study (40).
Studies have implicated genetic variation as a factor in the interindividual variation in human olfaction. Surveys of more recently identified forms of genetic variation in the human genome have demonstrated the evolutionary importance of olfaction. For example, a high percentage (∼68%) of the regions containing segmental copy number variations, which are associated with developmental disorders and susceptibility to diseases, overlap with genes involved in sensation, including olfaction (41). Similarly, common deletion variants were found to be present in genes involved in olfaction (42).
Two examples show the ability to tie genetic variation with specific variation in human olfactory function. Keller and colleagues demonstrated the first link between the function of a human odorant receptor both in vitro and in odor perception, highlighting a mechanistic basis of variation in olfactory ability between individuals (40). Similarly, Menashe and colleagues identified variation in an olfactory receptor gene and related it to sensitivity to a specific odor, that of isovaleric acid (32). Last, two linkage studies for olfactory phenotypes have been performed (43), including our study in which the largest linkage signal for hyposmia (P = 0.0013) was on chromosome 4q, suggesting a role for genetic variation in olfactory performance in humans (44).
Interestingly, the identification of olfactory loss as one component of Bardet-Biedl syndrome (BBS) has led to an improved understanding of the role of sensory cilia in olfaction and in human physiology in general (45), because the genes underlying this complex disease seem to be involved in the structure and/or function of cilia. Several BBS genes are located within the linkage signal on chromosome 4.
CLINICAL APPROACHES TO OLFACTORY DISEASE
Epidemiology
Olfactory decline is an important public health problem in the United States (46, 47), with a reported prevalence, based on clinical testing in a population sample, of 24.5% in persons over age 53 years (48) and rising to 62.5% in those over age 80 years. Similar findings were noted abroad (49, 50). Approximately 14 million Americans are estimated to have chronic olfactory impairment (48). The impact of this special sensory loss leads to more than 200,000 physician visits each year for chemosensory complaints (51, 52). Because olfactory function declines with age (53, 54), the clinical impact of olfactory dysfunction will increase as our population ages. General population-based prevalence studies of olfactory dysfunction are few, although associations between olfactory decline and increased age and male sex are common findings (55–57). Perhaps the best characterized study, that of 2,491 subjects from Beaver Dam, Wisconsin, showed that current smoking, stroke, epilepsy, and sinonasal disease were associated with decreased olfaction (48). European studies showed similar, but not identical, findings (49, 58).
Classification of Olfactory Impairments
Olfactory impairments can be classified into three broad categories of etiology: conductive losses stemming from obstruction of the nasal passages, sensorineural causes from damage to the olfactory neuroepithelium, or central dysfunction related to central nervous system disease. These categories are not mutually exclusive. Olfactory disease can also be categorized on the basis of perceptual symptoms: difficulty with odor identification (dysosmia); sensation of an odor different that the typical for that substance (parosmia); and perception of an odor when none is present (phantosmia).
Etiology of Olfactory Dysfunction
Olfactory dysfunction has been reported to arise from multiple etiologies, but the data are clouded by anecdotal reports or small series (reviewed in Reference 59). Most importantly, aging is the predominant cause of olfactory decline. In specialized olfactory clinics, the other common causes of hyposmia/anosmia include prior upper respiratory tract infections, head trauma, and sinonasal disease; these etiologies account for up to two-thirds of patients with olfactory complaints seen in such clinics (59). However, aging is thought to represent the most important influence on olfactory decline in the general population (24), and this burden is predominantly a geriatric condition. Indeed, this has been confirmed by biopsy studies that show degeneration of the olfactory epithelium with age.
The etiology of age-related olfactory loss is unclear. Animal studies demonstrate effects of aging on olfaction independent of confounding environmental factors and suggest a genetic basis for a variety of related phenotypes. For example, mutations of olfactory genes in Drosophila affect longevity, highlighting the critical importance of this special sense (60). However, evolutionary differences in the olfactory system preclude translatable studies, and to date there are no appropriate animal models of age-related olfactory decline. There have been few comprehensive studies of nongenetic factors associated with olfactory impairment as measured by testing. Other than age and sex, which have long been noted to affect olfactory function, many of the demographic influences on olfactory decline in aging have not been well studied. At present, the precise factors that modulate age-related loss of smell are unknown.
There are a wide number of diseases that can cause olfactory dysfunction. Inflammatory diseases such as rhinitis or sinusitis are common causes of conductive olfactory loss as the edema from these diseases can impair airflow in the nasal airway, impeding odorant movement to the olfactory epithelium. It should also be noted that biopsy studies have implicated damage to the olfactory epithelium itself, lending credence to the hypothesis that they could cause sensorineural and/or permanent impairment as well. Similarly, autoimmune disease such as sarcoidosis, Wegener's granulomatosis, or Sjögren's syndrome, and also viral infection of the nose could lead to olfactory dysfunction. Trauma to the nose such as skull base fractures, or surgery of the nose, sinuses, brain, or airway (laryngectomy) can all decrease olfaction either physically through airflow changes or via severance of olfactory fila. Congenital disorders such as choanal stenosis or nasal cysts can block nasal airflow and syndromes such as Kallmann's syndrome or agenesis of the olfactory bulb, or BBS can result in a generalized lack of olfaction itself.
Olfactory decline has been associated with several neurodegenerative diseases, including Alzheimer's disease and Parkinson's disease, perhaps leading to central olfactory dysfunction; although olfactory decline may precede the more severe manifestations of these diseases, its role a predictor or marker of disease onset has not been clearly established. Although carefully controlled data are not available, endocrine changes including pregnancy, diabetes, Addison's disease, vitamin deficiency (primarily vitamins A and B and thiamine) (61), as well as renal and liver disease are associated with altered olfactory function. Chemicals such as benzene, menthol, sulfur dioxide, carbon disulfide, heavy metals, and dust have also been associated with olfactory loss. Medications such as steroids, cancer chemotherapy, antibiotics (aminoglycosides, macrolides, tetracycline), antithyroid medication, opiates, sympathomimetics, antacids, and l-dopa can all affect olfaction. The precise etiology of how these diseases, chemicals, and medications affect olfaction are not known in humans.
Assessment of Olfactory Loss
Studies have shown that many people are unaware of olfactory loss; thus, objective testing is critical. Testing allows objective characterization of the nature and degree of the olfactory loss, rules out malingerers by establishing the validity of disease, and allows the ability to monitor temporal or post intervention changes. A variety of psychophysical methods have been described (62), including threshold testing, where increasing concentrations of odorants are presented and detection recorded; neurophysiologic techniques that include electroencephalogram, electroolfactogram, and imaging protocols (primarily in the research setting); and odor discrimination or recognition techniques that present the subject with similar or different odorants and assess how well they can be distinguished. The most commonly used is the odor identification test, which assesses how well patients can identify specific odors. In the United States, the most commonly employed test is the University of Pennsylvania Smell Identification Test, a 40-item scratch-and-sniff smell test that has been validated in cross-cultural populations, has a high test–retest reliability, is easy to administer and inexpensive, and for which age and sex norms are available (63).
In assessment of olfactory function, a careful history recording the onset, rate of decline, and associated factors is critical. Because inflammation accounts for the most easily treatable cause of olfactory decline, determining the presence of nasal symptoms including sneezing, rhinorrhea, pain, obstruction, and epistaxis is useful. Exclusion of central nervous system disease focuses on any pertinent neurologic or psychiatric symptoms. One should also ascertain a history of autoimmunity, viral infection, occupational exposure, and prior history of nasal or neurologic surgery. A quick dietary history may be helpful, although most Western patients are not malnourished. A family history of genetic diseases with olfactory loss as one component, such as Kallmann's syndrome (64) or BBS (65), is usually obvious. Physical examination focuses on a thorough nasal examination including nasal endoscopy and a complete neurologic examination. Imaging with sinus computed tomography scan with fine cuts through the cribriform plate or magnetic resonance imaging are not useful as screening measures, but remain indicated if the history or physical findings warrant it.
Therapy and Prognosis
Although medical and surgical treatments are available for some causes, they are limited in scope and success (66). Therapeutic measures center on that with the best outcome, conductive disease. In most cases, this will consist of intranasal or systemic antiinflammatory medication and sinus surgery as indicated for medical treatment failures of those patients who have chronic rhinosinusitis. There are few controlled trials that would accurately predict recovery with these treatments, but improvement likely depends on the severity of the sinonasal inflammatory burden. Patients may recover from traumatic and postviral loss, with return of some smell function at 1 year being a positive prognostic sign. Sensorineural and central dysfunction are very difficult to treat. The treating physician can address associated diseases and provide supportive care. In particular, because olfactory decline manifests as taste loss, an emphasis on other characteristics of food (such as texture, temperature, visual appeal) is critical to helping the patient maintain pleasure in eating and nutrition overall. Counseling regarding detection of spoiled food, installation of smoke detectors, and monitoring for gas leaks is also paramount for safety.
CONCLUSION
Olfaction is a critical physiologic process of the nasal airway. The sense of smell plays an important role in pleasure, including the palatability and flavor of food, and can even modify dietary behavior and nutrition, a key component of health. Decline in olfaction in older people is associated with impaired ability to discriminate among flavors, altered food preferences, and reduced appetite and food intake. Patients with impaired olfaction have a decreased ability to detect hazards in the environment, including smoke, spoiled food, toxins, and gas leaks. Olfaction influences mood, cognition, and behavior. Not surprisingly, patients with olfactory impairment have a markedly decreased quality of life. Importantly, decline in olfaction has been linked with several neurodegenerative conditions (Alzheimer's disease, Parkinson's, and cognitive decline). Thus, olfaction is related to a number of health and social factors that are critical to human quality of life. Unfortunately, physicians have little prognostic information and few diagnostic tests or therapeutic options for the vast majority of patients who have olfactory dysfunction.
Acknowledgments
The author gratefully thanks Ms. Jamie Phillips for logistical assistance.
Supported by the McHugh Otolaryngology Research Fund, a Dennis W. Jahnigen Career Development Award from the American Geriatrics Society, and the K12 Scholars Program (KL2RR025000) from the Institute for Translational Medicine at the University of Chicago (UL1 RR024999) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the author and do not necessarily represent the official view of NCRR or NIH.
Author Disclosure: J.M.P. received grant support from Schering Plough ($10,001–$50,000), the American Geriatrics Society (more than $100,001), and the American Rhinologic Society ($10,001–$50,000). He owns a patent on Imaging Protocols for Chronic Rhinosinusitis.
References
- 1.Niimura Y. Evolutionary dynamics of olfactory receptor genes in chordates: interaction between environments and genomic contents. Hum Genomics 2009;4:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havlicek J, Roberts SC. MHC-correlated mate choice in humans: a review. Psychoneuroendocrinology 2009;34:497–512. [DOI] [PubMed] [Google Scholar]
- 3.Jacob S, McClintock MK, Zelano B, Ober C. Paternally inherited HLA alleles are associated with women's choice of male odor. Nat Genet 2002;30:175–179. [DOI] [PubMed] [Google Scholar]
- 4.Horth L. Sensory genes and mate choice: evidence that duplications, mutations, and adaptive evolution alter variation in mating cue genes and their receptors. Genomics 2007;90:159–175. [DOI] [PubMed] [Google Scholar]
- 5.Jacob S, Garcia S, Hayreh D, McClintock MK. Psychological effects of musky compounds: comparison of androstadienone with androstenol and muscone. Horm Behav 2002;42:274–283. [DOI] [PubMed] [Google Scholar]
- 6.Wyart C, Webster WW, Chen JH, Wilson SR, McClary A, Khan RM, Sobel N. Smelling a single component of male sweat alters levels of cortisol in women. J Neurosci 2007;27:1261–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doucet S, Soussignan R, Sagot P, Schaal B. The secretion of areolar (Montgomery's) glands from lactating women elicits selective, unconditional responses in neonates. PLoS ONE 2009;4:e7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mennella JA, Jagnow CP, Beauchamp GK. Prenatal and postnatal flavor learning by human infants. Pediatrics 2001;107:E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welge-Lussen A. Ageing, neurodegeneration, and olfactory and gustatory loss. B-ENT 2009;5:129–132. [PubMed] [Google Scholar]
- 10.Murphy C. Symposium overview: chemical senses and longevity. Ann N Y Acad Sci 2009;1170:680–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingelstedt S. Studies on the conditioning of air in the respiratory tract. Acta Otolaryngol 1956;131:3–81. [PubMed] [Google Scholar]
- 12.Zhao K, Scherer PW, Hajiloo SA, Dalton P. Effect of anatomy on human nasal air flow and odorant transport patterns: implications for olfaction. Chem Senses 2004;29:365–379. [DOI] [PubMed] [Google Scholar]
- 13.Leopold DA, Hummel T, Schwob JE, Hong SC, Knecht M, Kobal G. Anterior distribution of human olfactory epithelium. Laryngoscope 2000;110:417–421. [DOI] [PubMed] [Google Scholar]
- 14.Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope 2000;110:1071–1077. [DOI] [PubMed] [Google Scholar]
- 15.Baroody FM, Gungor A, deTineo M, Haney L, Blair C, Naclerio RM. Comparison of the response to histamine challenge of the nose and the maxillary sinus: effect of loratadine. J Appl Physiol 1999;87:1038–1047. [DOI] [PubMed] [Google Scholar]
- 16.Wagenmann M, Baroody FM, Kagey-Sobotka A, Lichtenstein LM, Naclerio RM. The effect of terfenadine on unilateral nasal challenge with allergen. J Allergy Clin Immunol 1994;93:594–605. [DOI] [PubMed] [Google Scholar]
- 17.Baroody FM, Shenaq D, DeTineo M, Wang J, Naclerio RM. Fluticasone furoate nasal spray reduces the nasal–ocular reflex: a mechanism for the efficacy of topical steroids in controlling allergic eye symptoms. J Allergy Clin Immunol 2009;123:1342–1348. [DOI] [PubMed] [Google Scholar]
- 18.Baraniuk JN. Neural regulation of mucosal function. Pulm Pharmacol Ther 2008;21:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seki N, Shirasaki H, Kikuchi M, Sakamoto T, Watanabe N, Himi T. Expression and localization of TRPV1 in human nasal mucosa. Rhinology 2006;44:128–134. [PubMed] [Google Scholar]
- 20.Damann N, Voets T, Nilius B. TRPs in our senses. Curr Biol 2008;18:R880–R889. [DOI] [PubMed] [Google Scholar]
- 21.Winstead W, Marshall CT, Lu CL, Klueber KM, Roisen FJ. Endoscopic biopsy of human olfactory epithelium as a source of progenitor cells. Am J Rhinol 2005;19:83–90. [PubMed] [Google Scholar]
- 22.Chiu SC, Hung HS, Lin SZ, Chiang E, Liu DD. Therapeutic potential of olfactory ensheathing cells in neurodegenerative diseases. J Mol Med 2009;87:1179–1189. [DOI] [PubMed] [Google Scholar]
- 23.Collet S, Grulois V, Bertrand B, Rombaux P. Post-traumatic olfactory dysfunction: a cohort study and update. B-ENT 2009;5:97–107. [PubMed] [Google Scholar]
- 24.Rawson NE. Olfactory loss in aging. Sci SAGE KE 2006;2006:pe6. [DOI] [PubMed] [Google Scholar]
- 25.Ma M. Encoding olfactory signals via multiple chemosensory systems. Crit Rev Biochem Mol Biol 2007;42:463–480. [DOI] [PubMed] [Google Scholar]
- 26.Katata K, Sakai N, Doi K, Kawamitsu H, Fujii M, Sugimura K, Nibu K. Functional MRI of regional brain responses to “pleasant” and “unpleasant” odors. Acta Otolaryngol Suppl 2009;562:85–90. [DOI] [PubMed] [Google Scholar]
- 27.Sobel N, Johnson BN, Mainland J. Functional neuroimaging of human olfaction. In: Doty RL, editor. Handbook of olfaction and gustation. New York: Marcel Dekker; 2003. pp. 251–274.
- 28.Hummel T. Retronasal perception of odors. Chem Biodivers 2008;5:853–861. [DOI] [PubMed] [Google Scholar]
- 29.Miller G. 2004 Nobel Prizes: Axel, Buck share award for deciphering how the nose knows. Science 2004;306:207. [DOI] [PubMed] [Google Scholar]
- 30.Olender T, Lancet D, Nebert DW. Update on the olfactory receptor (OR) gene superfamily. Hum Genomics 2008;3:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whissell-Buechy D, Amoore JE. Odour-blindness to musk: simple recessive inheritance. Nature 1973;242:271–273. [DOI] [PubMed] [Google Scholar]
- 32.Menashe I, Abaffy T, Hasin Y, Goshen S, Yahalom V, Luetje CW, Lancet D. Genetic elucidation of human hyperosmia to isovaleric acid. PLoS Biol 2007;5:e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature 2006;442:645–650. [DOI] [PubMed] [Google Scholar]
- 34.Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell 1995;83:195–206. [DOI] [PubMed] [Google Scholar]
- 35.Young JM, Massa HF, Hsu L, Trask BJ. Extreme variability among mammalian V1R gene families. Genome Res 2010;20:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witt M, Hummel T. Vomeronasal versus olfactory epithelium: is there a cellular basis for human vomeronasal perception? Int Rev Cytol 2006;248:209–259. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez I, Mombaerts P. Novel human vomeronasal receptor-like genes reveal species-specific families. Curr Biol 2002;12:R409–R411. [DOI] [PubMed] [Google Scholar]
- 38.Liberles SD, Horowitz LF, Kuang D, Contos JJ, Wilson KL, Siltberg-Liberles J, Liberles DA, Buck LB. Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proc Natl Acad Sci USA 2009;106:9842–9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassenaer S, Laurent G. Hebbian STDP in mushroom bodies facilitates the synchronous flow of olfactory information in locusts. Nature 2007;448:709–713. [DOI] [PubMed] [Google Scholar]
- 40.Keller A, Zhuang H, Chi Q, Vosshall LB, Matsunami H. Genetic variation in a human odorant receptor alters odour perception. Nature 2007;449:468–472. [DOI] [PubMed] [Google Scholar]
- 41.Wong KK, deLeeuw RJ, Dosanjh NS, Kimm LR, Cheng Z, Horsman DE, MacAulay C, Ng RT, Brown CJ, Eichler EE, et al. A comprehensive analysis of common copy-number variations in the human genome. Am J Hum Genet 2007;80:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarroll SA, Hadnott TN, Perry GH, Sabeti PC, Zody MC, Barrett JC, Dallaire S, Gabriel SB, Lee C, Daly MJ, et al. Common deletion polymorphisms in the human genome. Nat Genet 2006;38:86–92. [DOI] [PubMed] [Google Scholar]
- 43.Doty RL, Marcus A, Lee WW. Development of the 12-item cross-cultural smell identification test (CC-SIT). Laryngoscope 1996;106:353–356. [DOI] [PubMed] [Google Scholar]
- 44.Pinto JM, Thanaviratananich S, Hayes MG, Naclerio RM, Ober C. A genome-wide screen for hyposmia susceptibility loci. Chem Senses 2008;33:319–329. [DOI] [PubMed] [Google Scholar]
- 45.Jenkins PM, McEwen DP, Martens JR. Olfactory cilia: linking sensory cilia function and human disease. Chem Senses 2009;34:451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffman HJ, Ishii EK, MacTurk RH. Age-related changes in the prevalence of smell/taste problems among the United States adult population: results of the 1994. Disability Supplement to the National Health Interview Survey (NHIS). Ann N Y Acad Sci 1998;855:716–722. [DOI] [PubMed] [Google Scholar]
- 47.Wysocki CJ, Gilbert AN. National Geographic smell survey: effects of age are heterogenous. Ann N Y Acad Sci 1989;561:12–28. [DOI] [PubMed] [Google Scholar]
- 48.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA 2002;288:2307–2312. [DOI] [PubMed] [Google Scholar]
- 49.Bramerson A, Johansson L, Ek L, Nordin S, Bende M. Prevalence of olfactory dysfunction: the Skövde population-based study. Laryngoscope 2004;114:733–737. [DOI] [PubMed] [Google Scholar]
- 50.Landis BN, Konnerth CG, Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope 2004;114:1764–1769. [DOI] [PubMed] [Google Scholar]
- 51.National Institute on Deafness and Other Communication Disorders (NIDCD). Available at http://www.nidcd.nih.gov/health/statistics/smell.asp.2004 (accessed November 2010).
- 52.National Institute of Neurological and Communicative Disorders and Stroke, National Institutes of Health, Public Health Service, Department of Health, Education, and Welfare.. Report of the Panel on Communicative Disorders to the National Advisory Neurological and Communicative Disorders and Stroke Council. Washington, DC: Public Health Service; 1979. DHEW publication no. (NIH) 79-1914.
- 53.Schemper T, Voss S, Cain WS. Odor identification in young and elderly persons: sensory and cognitive limitations. J Gerontol 1981;36:446–452. [DOI] [PubMed] [Google Scholar]
- 54.Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science 1984;226:1441–1443. [DOI] [PubMed] [Google Scholar]
- 55.Ship JA, Weiffenbach JM. Age, gender, medical treatment, and medication effects on smell identification. J Gerontol 1993;48:M26–M32. [DOI] [PubMed] [Google Scholar]
- 56.Larsson M, Finkel D, Pedersen NL. Odor identification: influences of age, gender, cognition, and personality. J Gerontol B Psychol Sci Soc Sci 2000;55:304–310. [DOI] [PubMed] [Google Scholar]
- 57.Hummel T, Konnerth CG, Rosenheim K, Kobal G. Screening of olfactory function with a four-minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol 2001;110:976–981. [DOI] [PubMed] [Google Scholar]
- 58.Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol 2008;255:1121–1126. [DOI] [PubMed] [Google Scholar]
- 59.Murphy C, Doty R, Duncan H. Clinical disorders of olfaction. In: Doty R, editor. Handbook of olfaction and gustation. New York: Marcel Dekker; 2003.
- 60.Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science 2007;315:1133–1137. [DOI] [PubMed] [Google Scholar]
- 61.Martin B, Maudsley S, White CM, Egan JM. Hormones in the naso-oropharynx: endocrine modulation of taste and smell. Trends Endocrinol Metab 2009;20:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simmen D, Briner HR. Olfaction in rhinology—methods of assessing the sense of smell. Rhinology 2006;44:98–101. [PubMed] [Google Scholar]
- 63.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 1984;94:176–178. [DOI] [PubMed] [Google Scholar]
- 64.Online Mendelian Inheritance in Man (OMIM). Available at http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=308700 (accessed November 2010).
- 65.Online Mendelian Inheritance in Man (OMIM). Available at http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=209900 (accessed November 2010).
- 66.Holbrook EH, Leopold DA. An updated review of clinical olfaction. Curr Opin Otolaryngol Head Neck Surg 2006;14:23–28. [DOI] [PubMed] [Google Scholar]