Abstract
T cells deficient for CD28 have reduced ability to expand and survive, but still cause graft-versus-host disease (GVHD). Inducible costimulator (ICOS), the third member of the CD28 family, is expressed on antigen-activated T cells and has unique roles in T-cell activation and effector function. We hypothesized that ICOS contributes to the development of GVHD in the absence of B7:CD28/CTLA4 costimulation. In this study, we evaluated the roles of CD28, CTLA4 and ICOS in the pathogenesis of acute GVHD under myeloablative allogeneic bone marrow transplantation (BMT). Unexpectedly, we found that blocking CD28 and CTLA4 signals using the clinically relevant reagent, CTLA4-Ig, results in the enhancement of GVHD severity mediated by CD4+ T cells, and such treatment does not add any benefit to blockade of ICOS. In contrast, selectively blocking CD28 and ICOS but not CTLA4 prevents GVHD more effectively than blocking either CD28 or ICOS alone. Taken together, these results indicate that CD28 and ICOS are synergistic in promoting GVHD, whereas the CTLA4-signal is required for T-cell tolerance regardless of ICOS signaling. Thus, blocking CD28 and ICOS while sparing CTLA4 represents a promising approach for abrogating pathogenic T-cell responses after allogeneic BMT.
Introduction
Graft-versus-host disease (GVHD) remains the major complication of allogenic hematopoietic cell transplantation (HCT), resulting in high morbidity and mortality (1). GVHD is initiated by mature donor T cells that recognize disparate histocompatibility antigens of the recipient. An efficient T-cell response requires costimulatory signals delivered by antigen presenting cells (APCs) in addition to signals delivered through the TCR after recognition of specific antigen (2). CD28 has been well characterized and is the most effective co-stimulatory molecule expressed by naïve and activated T cells. Costimulation through CD28 regulates multiple aspects of T-cell function including cytokine secretion, proliferation and cell survival (3, 4). By using CD28-deficient mice, we (5, 6) and others have found that CD28 costimulation plays an important role in the development of GVHD, although T-cell activation and GVHD can still proceed in the absence of CD28. Furthermore, T-cell responses to high affinity or high abundance antigens, often present in transplant recipients, are far less dependent on CD28 costimulation than T-cell responses to low affinity or low abundance antigens (7-9). This makes it difficult to induce transplantation tolerance by blocking the CD28-signal alone.
CTLA4, the second member of the CD28 family, competes with CD28 binding to the same ligands (B7.1 and B7.2, B7 hereafter) and delivers an inhibitory signal to T-cell activation (10). Inducible costimulator (ICOS) was identified as the third member of the CD28 family (11). ICOS is expressed on T-cell surface after activation, and has unique roles in T-cell activation and differentiation (12, 13), germinal center formation and immunoglobulin class switching (14, 15). ICOS ligand, B7h, is constitutively expressed at low levels on APCs and is upregulated by TNFα or LPS (16, 17). Additional studies have suggested that CD28 and ICOS play distinct roles in T-cell differentiation, the CD28-signal being responsible for T-cell activation and the ICOS signal for certain effector functions (18-21).
In cardiac transplantation models, blockade of B7h/ICOS interaction produced a modest but significant prolongation of graft survival (20, 22). Efficiency was increased with delayed rather than early blockade, indicating an effect on primed T cells (23). Furthermore, the co-blockade of B7:CD28/CTLA4 and ICOS ligand:ICOS pathways was significantly more effective in prolonging graft survival than blocking either alone (22, 24). The role of ICOS in GVHD is complex, as ICOS blockade exacerbated acute GVHD but inhibited chronic GVHD in a non-irradiated parent-into-F1 model (25). However, recent studies indicated that blocking ICOS ameliorated GVHD in myleoablative BMT models mediated by CD4+ and CD8+ T cells (26, 27), with distinct effects in CD4+ versus CD8+ T cells in one model of single MHC antigen disparity (28). In this study, we tested the hypothesis that ICOS may play a significant role in the development of GVHD in the absence of B7:CD28/CTLA4 binding and found that selectively blocking B7:CD28 and ICOS ligand:ICOS while sparing B7:CTLA4 interactions most effectively prevent acute GVHD.
Materials and Methods
Mice
ICOS-deficient mice on C57BL/6 (B6) background were kindly provided by Dr. Chen Dong (MD Anderson Cancer Center, Houston, TX) (12, 29). CD28/ICOS-deficient mice on B6 background were kindly provided by Dr. Tak Mak (Ontario Cancer Institute, Toronto, Canada). B6, B6.C-H2bm12 (bm12), B6.C-H2bm1 (bm1), CD28-deficient, and B6.SJL-Ly5a Ptprca Pep3b (B6.Ly5.1) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). (B6.Ly5.1 × bm12)F1 mice were bred at H. Lee Moffitt Cancer Center & Research Institute (Tampa, FL). Experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee.
T-cell purification and transplantation
Our protocol for T-cell purification using a magnetic cell separation system has been described previously (6, 28), and the purity of T cells used for transplantation ranged from 91-97%. In non-myeloablative transplantation models, recipient mice (B6.bm1) were exposed to 600 cGy total body irradiation (TBI) at 120 cGy/min, a dose range that is immunosuppressive but not lethal for this strain of mice. Purified CD8+ T cells from different donors on B6 background were suspended in PBS and injected via the tail vein into 7 to 8-wk-old irradiated recipients within 24 h after irradiation. In myeloablative models, (B6 × bm12)F1 mice were exposed to 1100-1200 cGy and BALB/c mice to 800-900 cGy TBI. T-cell depleted (TCD) BM cells alone or in combination with purified Thy1.2+ cells from indicated donors were injected via the tail vein to recipients within 24 hrs after irradiation. Recipient mice were monitored every other day for clinical signs of GVHD, such as ruffled fur, hunched back, lethargy or diarrhea, and mortality.
Administration of antibodies (Abs)
Murine CTLA4-Ig and control L6-Ig, kindly provided by Robert Peach (Bristol-Myers Squibb, Princeton, NJ), were injected i.p. at 100 μg/mouse every other day for 14 days starting on the day 0 as described in our previous work (30). Anti-ICOS mAb (hybridoma 7E.17G9.G1, rIgG2b; produced at National Cell Culture, Minneapolis, MN) or irrelevant rat IgG was injected i.p. at 200 μg /mouse daily from day 0 to 5, then 3 times weekly until day 28 after BMT as described elsewhere (27).
Immuno-fluorescence analysis
Two-, 3- or 4-color flow cytometry was performed to measure the expression of surface molecules and intracellular cytokines according to standard techniques. FITC-labeled anti-CD4, biotin-labeled anti-FasL, PE-labeled anti-CD4, anti-IFNγ, anti-TNFα, anti-IgG isotype control, and Cy-chrome labeled anti-CD4 were purchased from BD-Pharmingen (San Diego, CA). PE-labeled anti-IgG2a was purchased from Caltag (Burlingame, CA). Biotin-labeled anti-Ly5.1 mAb was prepared in our laboratory. Biotinylated Abs were detected with streptavidin-Cy-chrome or streptavidin-APC. The level of FasL expression was presented as mean fluorescence index (MFI), which equals the mean fluorescence intensity of cells stained with a specific mAb divided by the mean fluorescence intensity of cells stained with isotype control.
Cytokine and histopathological analysis
Blood samples were obtained from BMT recipients at the time specified, and cytokine analysis was performed using a cytometric bead array kit as described previously (28). Histopathology on small intestine, liver, and skin was assessed by an expert pathologist (C. L.) using coded samples as previously described (31).
Statistical Analysis
For comparison of recipient survival among groups in GVHD experiments, the log-rank test was used to determine the statistical significance. To compare the engraftment and expansion of donor T cells, a student's t-test was used.
Results
Blocking the ligands of CD28 and CTLA4 exacerbated GVHD induced by CD4+ T cells after allogeneic BMT
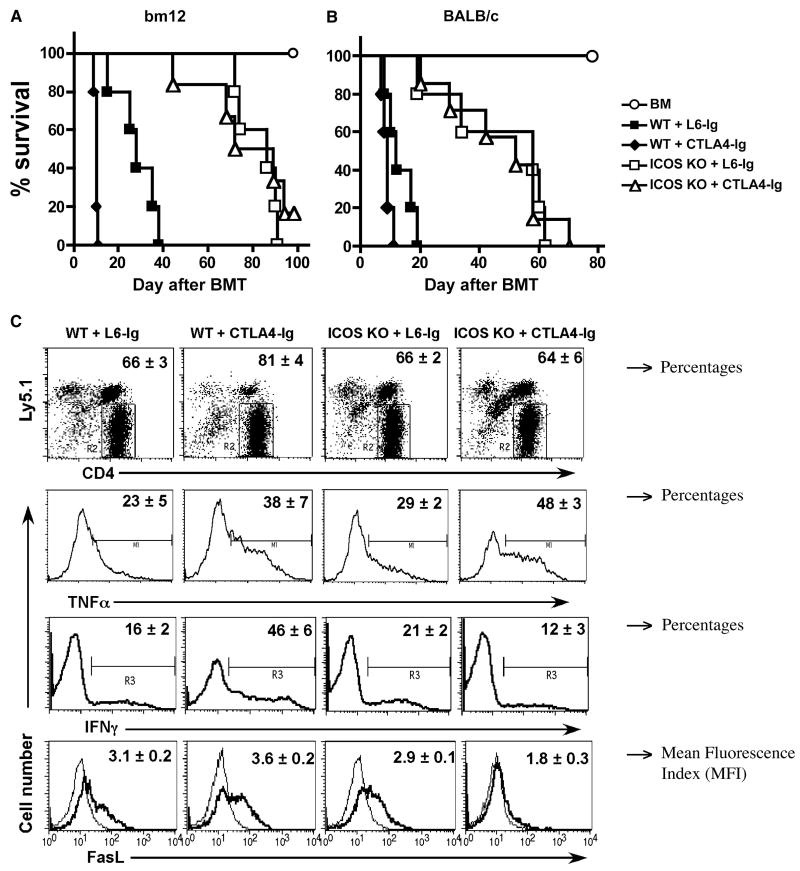
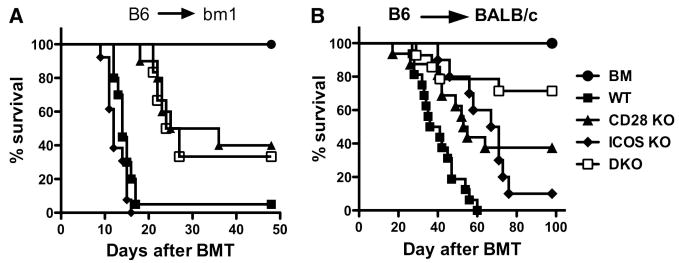
It is generally believed that CD28 and ICOS deliver positive costimulation while CTLA4 delivers negative costimulation to T-cell responses. This concept predicts that co-blockade of CD28 and ICOS while sparing CTLA4 should lead to a high degree of control of T cell alloresponses in transplantation. However, such a predication has not been previously proven in the context of allogeneic BMT. To address the role of CD28, ICOS and CTLA4 in the development of GVHD, we first examined the effect of blocking CD28 and CTLA4 in the presence or absence of ICOS costimulation after myeloablative BMT in mice. CTLA4-Ig was used to block B7 as an effective CD28 and CTLA4 antagonist (32). The B6 → bm12 BMT model was initially used where only MHC II was incompatible between donor and recipient. CD4+ cells were purified from WT or ICOS-/- B6 donors and injected into lethally irradiated bm12 mice. These recipients were divided into two groups and treated with L6-Ig or CTLA4-Ig. As shown in our previous work and others'(26-28), ICOS-/- CD4+ T cells induced significantly delayed GVHD (Fig. 1A). Surprisingly, under these conditions, treatment with CTLA4-Ig actually accelerated GVHD caused by WT CD4+ T cells in bm12 recipients compared to control treatment (p < 0.01, Fig. 1A). However, the treatment had no effect on GVHD induced by ICOS-/- T cells. To confirm the result, we used a fully MHC mismatched B6 → BALB/c BMT model, and found that treatment with CTLA4-Ig also accelerated GVHD induced by CD4+ donor T cells (p < 0.05) (Fig. 1B). These data differ from other studies showing that blocking B7:CD28/CTLA4 interactions results in reduction of GVHD rather than acceleration (33-37). The major difference between our current study and the previous studies is that GVHD was induced only by CD4+ T cells in our study whereas GVHD was induced by both CD4+ and CD8+ T cells in the other studies. In our study, negative regulation through CTLA4 dominates over the positive regulation through CD28 on CD4+ T cells, which is consistent with two recent reports that B7 plays an essential role in tolerance on alloreactive CD4+ T cells in MHC II-mismatched transplantation models (38, 39). The dominant negative role of CTLA4 over the positive role of CD28 on CD4+ T cells may be attributed to the down-regulation of immune responses through B7:CTLA4 ligation on effector T cells via T-T or T-Treg interactions (40, 41).
Fig. 1. Role of CD28, CTLA4 and ICOS in GVHD induced by CD4 T cells in myeloablative TBI model.
Lethally irradiated (B6.Ly5.1 × bm12)F1 (A) or BALB/c (B) mice were transplanted with TCD-BM alone or plus purified CD4+ cells at 1 × 106/mouse from WT or ICOS-/- B6 donors. L6-Ig or CTLA4-Ig was injected i.p. at 100 μg/mouse every other day for a total of eight doses. Data were obtained for one experiment in each model, and 5-6 mice were included in each group. (C) BMT was set up as in panel A, recipient spleen was collected 6 days after transplantation. Splenocytes were stained individually for surface expression of FasL, intercellular expression of IFNγ or TNFα, in combination with surface expression of CD4 and Ly5.1. The expression of surface FasL (MFI) and intracellular IFNγ or TNFα (% positive) are shown on gated CD4+/Ly5.1- donor cells. The thin lines represent cells stained with isotype control mAb, while thick lines with specific mAb for FasL. The results represent two replicate experiments. (D) BMT was set up as in panel A, peripheral blood was collected from each recipient on day 14. The levels of TNFα, IFNγ, IL-5, IL-2 and IL-4 in the recipient serum were measured as described in Materials and Methods. The levels of IL-2 and IL-4 were under detection level (not shown). The data were pooled from two replicate experiments, and each data point represents a cytokine concentration in one individual mouse.
To understand the underlying mechanisms, we measured T cell activation and expansion in myeloablative B6 → bm12 BMT model. In 6-day T cell transfer experiments, the absolute numbers of WT donor T cells (CD4+Ly5.1-) were 4.8 ± 2.1 and 6.7 ± 2.0 × 105/spleen in the recipients that were treated with L6-Ig or CTLA4-Ig, respectively. The absolute numbers of ICOS-/- donor T cells were 8.1 ± 0.4 and 3.5 ± 1.2 × 105/spleen in the recipients that were treated with L6-Ig or CTLA4-Ig, respectively. There was no significant difference (p > 0.05) comparing any two groups, indicating that the CD28, CTLA4 and/or ICOS signals did not have a significant effect on the early expansion of donor CD4 T cells. These data are in agreement with the previous studies by our group and others, which showed that blockade of ICOS had no effect on T-cell proliferation (26, 28). As blockade of CD28 reduces T-cell proliferation, we reasoned that additional CTLA4 blockade would reverse the effect of CD28 blockade and thus combinational blockade of CD28, CTLA4 and ICOS did not have a significant impact on CD4+ T-cell proliferation in vivo.
We further measured the expression of IFNγ, TNFα and FasL, because each plays an important role in the induction of GVHD by donor CD4+ cells. Six days after BMT, donor T cells in recipient spleen were evaluated for their intracellular expression of IFNγ, TNFα (% positive cells) and surface expression of FasL (MFI) (Fig. 1C). In separate experiments, we assessed how Th1/Th2 cytokines were affected by blockade of CD28, ICOS or both by measuring IL-2, IL-4, IL-5, IFNγ and TNFα in recipient sera 14 days after transplantation (Fig. 1D). On day 6, treatment with CTLA4-Ig increased expression of IFNγ, TNFα and FasL on WT T cells as compared to the treatment with L6-Ig (p < 0.05 for each effector molecule) (Fig. 1C). These data support that co-blockade of CD28 and CTLA4 accelerated GVHD induced by CD4+ T cells (Fig. 1 A and B). Absence of ICOS (ICOS-/- T cells) had little or no effect on the expression of these effector molecules at the single cell level on day 6 (Fig. 1C), but significantly suppressed TNFα and IFNγ but not IL-5 production in recipient sera on day 14 (Fig. 1D). These results confirmed the previous studies by us and others (26-28) that the decreased production of Th1 cytokines likely contributed to the reduced ability of ICOS-/- CD4+ T cells to cause GVHD. CTLA4-Igtreatment on ICOS-/- T cells significantly decreased IFNγ (p < 0.05) and FasL (p < 0.01), whereas TNFα was significantly increased (p < 0.01) as compared with control treatment on day 6. Furthermore, the cytokine profile was very similar on day 14 in the recipients of ICOS-/- T cells after treatment with CTLA4-Ig or L6-Ig (Fig. 1D). Taken together, these data may explain why treatment with CTLA4-Ig did not further reduce GVHD induced by ICOS-/- CD4+ T cells (Fig. 1 A and B).
Blocking ICOS (anti-ICOS) and CD28 (KO) while sparing CTLA4 prevents GVHD mediated by CD4+ T cells
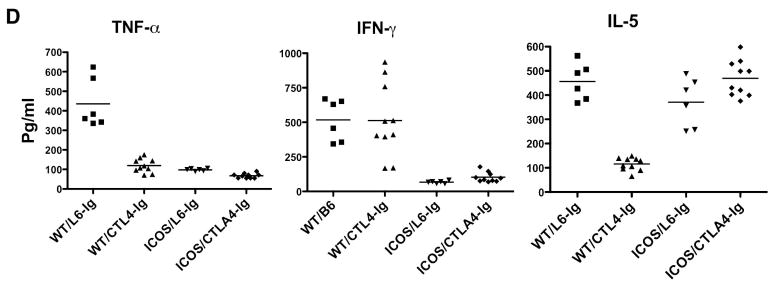
Our previous work showed that CTLA4-signal plays a protective role in GVHD development (5, 30). We therefore hypothesized that blocking ICOS and CD28 while sparing CTLA4 would ameliorate GVHD under the condition where the CD28 absence alone was ineffective in preventing lethality. To test this hypothesis, CD28-/- B6 mice were used as donors and BALB/c recipients were given BM supplemented with CD4+ T cells and then treated with antagonistic anti-ICOS mAb to block ICOS. Using this strategy, we found that blockade of ICOS (p = 0.002) but not the absence of CD28 alone (p = 0.1) significantly delayed GVHD lethality of the recipients (Fig. 2A). However, blockade of ICOS and the absence of CD28 were able to prevent GVHD lethality in more than 80% recipients as well as significantly reduce weight loss (Fig. 2 A and B) more effectively than either blockade of ICOS alone (p = 0.02) or the absence of CD28 alone (p = 0.009). Furthermore, blockade of ICOS and absence of CD28 significantly improved pathology scores in intestine, liver and lung tissues compared with intact costimulation, blockade of ICOS, or absence CD28 alone (Fig. 2C). We therefore concluded that CD28 and ICOS contribute synergistically in the development of GVHD induced by CD4 T cells.
Fig. 2. Role of CD28 and ICOS in the development of GVHD induced by CD4+ T cells in myeloablative TBI model.
Lethally irradiated BALB/c mice were transplanted with TCD-BM alone or TCD-BM plus 2 × 106 CD4+ T cells from WT or CD28 KO B6 donors. A group of recipients with WT or CD28 KO cells were treated with anti-ICOS mAb or irrelevant control as described in Materials and Methods. Recipient survival (A), weight loss (B), and pathology scores (C) are shown. The data are from one experiment with 5-6 recipients each group, and similar outcome was observed in another experiment where T cell dose and anti-ICOS treatment were somewhat different.
The role of CD28, CTLA4 and ICOS in T-cell expansion and cytokine production
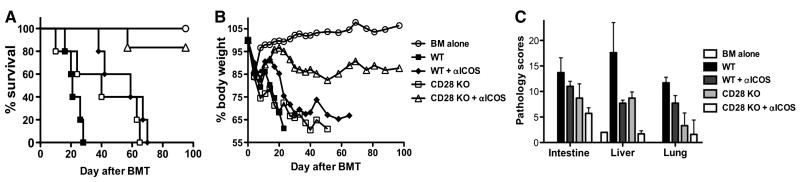
To elucidate the mechanisms by which simultaneous blockade of CD28 and ICOS prevent GVHD, we measured expansion of donor CD4+ T cells in recipient spleens. CD4+ T cells were purified from WT or CD28-deficient B6 mice and transferred together with TCD-BM from B6 Ly5.1+ donor into irradiated BALB/c recipients. Donor T cells were identified as CD4+H2b+Ly5.1- in recipient spleens 6 days after transplantation. The absolute number of donor CD4+ cells was an average of 8.2 ± 1.8 × 105 and 6.9 ± 1.6 × 105 per mouse for WT and CD28 KO cells respectively (p = 0.6), indicating both WT and CD28 KO CD4+ T cells had similar potential to expand in vivo. Treatment with anti-ICOS mAb actually increased expansion of WT donor CD4+ T cells (Fig. 3A, p = 0.05). However, treatment with anti-ICOS reduced expansion of CD28 KO cells, because the absolute number of CD28 KO cells was significantly fewer than those of WT cells and CD28 KO cells with control treatment (Fig. 3A, p < 0.05). These results indicate that CD4+ T-cell expansion depends on both CD28 and ICOS.
Fig. 3. Role of CD28, CTLA4 and ICOS in GVHD induced by CD4+ T cells.
Lethally irradiated BALB/c mice were transplanted with TCD-BM from normal B6 Ly5.1+ mice or plus purified CD4+ cells from WT or CD28-/- B6 donors. Half of the recipients were also treated with anti-ICOS or control mAb. (A) Six days after BMT, recipient spleen was collected and stained for expression of CD4, Ly5.1 and H2b. Data show absolute number of donor T cells (CD4+Ly5.1- H2b+) in individual mouse (n = 3 in each group), which represents one of 2 replicate experiments with similar setting. (B) In separate experiments as described in A, recipient peripheral blood samples were collected 3 weeks after BMT. The level of TNFα in recipient serum was shown in individual mouse (n = 5 or 6 per group), and the data were pooled from 2 replicate experiments.
Th1 cytokines (i.e. IFNγ and TNFα) play a critical role in GVHD induced by CD4+ T cells (42, 43). We asked how serum cytokines were affected by blockade of CD28, ICOS or both by measuring IL-2, IL-4, IL-17, IFNγ and TNFα in recipient serum at 18 days after transplantation (Fig. 3B). At this time point, the levels of IL-2, IL-4 and IL-17 were very low or undetectable, and IFNγ was detectable but not significantly different among the groups (data not shown). Absence of CD28 or blockade of ICOS alone reduced TNFα production, but the reduction was not significant (Fig. 3B, p > 0.05). However, absence of CD28 and blockade of ICOS significantly reduced TNFα production than either alone (Fig. 3B, p = 0.01 in both cases). Together, these data indicate that blocking CD28 and ICOS while sparing CTLA4 resulted in reduction of T cell expansion and TNFα production during the development of GVHD induced by donor CD4+ T cells.
The role of CD28, CTLA4 and ICOS in GVHD mediated by CD8+ or CD4+ plus CD8+ T cells
Since clinical HCT typically includes CD8+ T cells, studies were performed to determine whether the absence of CD28 and/or ICOS expression on donor CD8+ T cells would influence GVHD lethality when WT, CD28 KO, ICOS KO, or CD28/ICOS double knockout (DKO) mice were used as source of donor CD8+ T cells (Fig. 4A). Cohorts of MHC class I disparate bm1 mice were sublethally irradiated and given purified CD8+ T cells from one of the aforementioned donor strains. Whereas donor CD8+ T cells from WT and ICOS KO had comparable survival, recipients of CD28-deficient CD8+ T cells had a significant prolonged survival (P < 0.01). However, CD8+ T cells from DKO mice did not further prolong survival. In previous studies using the same model system, CD25-depleted CD8+ T cells from ICOS KO mice resulted in a significantly reduced GVHD lethality rate (27). Whether the difference between these 2 studies is related to using a CD25-depleted versus CD25-repleted T cell graft is unknown. Nonetheless, our studies indicated that the absence of ICOS did not have a major effect on GVHD lethality in this CD8+ T cell only mediated GVHD lethality model.
Fig. 4. The role of CD28 and ICOS on GVHD induced by CD8+ T cells alone or by CD4+ and CD8+ T cells.
(A) B6 bm1 mice were sublethally irradiated and transplanted with 1 × 106 purified CD8+ cells per recipient from WT, CD28 KO, ICOS KO, or DKO B6 donors. Recipient survival is shown, and the data are from 2 replicate experiments with 6-15 recipients per group. CD28 KO vs. WT: p < 0.01. (B) Lethally irradiated BALB/c mice were transplanted with TCD-BM alone or plus 1-2 × 106 T cells (CD4 and CD8) from WT, CD28 KO, ICOS KO or dKO B6 donors. Recipient survival is shown, and the data are pooled from 3 replicate experiments with 11-16 mice per group. CD28 KO vs. WT: p < 0.001; ICOS KO vs. WT: p < 0.001; CD28 vs. ICOS: p = 0.7; DKO vs. CD28 KO: p = 0.06; DKO vs. ICOS KO: p = 0.01.
Since clinical HCT grafts contain both CD4+ and CD8+ T cells, we performed studies in which B6 WT, CD28 KO, ICOS KO or DKO mice were used as a source of donor CD4+ and CD8+ T cells into lethally irradiated BALB/c recipients that differ in both major and minor histocompatibility antigens. As shown in Fig. 4B, CD28-/- or ICOS-/- T cells induced significantly less GVHD compared to WT T cells (p < 0.001) (Fig. 1A). There was no difference in recipient survival between CD28-/- and ICOS-/- cells (p = 0.7). Moreover, DKO T cells induced less GVHD than CD28-/- (p = 0.06) or ICOS-/- (p = 0.01) T cells. Thus, in a CD4+ T cell-driven and CD8+ T cell-facilitated model system that more closely simulates clinical allogeneic HCT, the absence of both CD28 and ICOS provided the highest GVHD protective effects.
Discussion
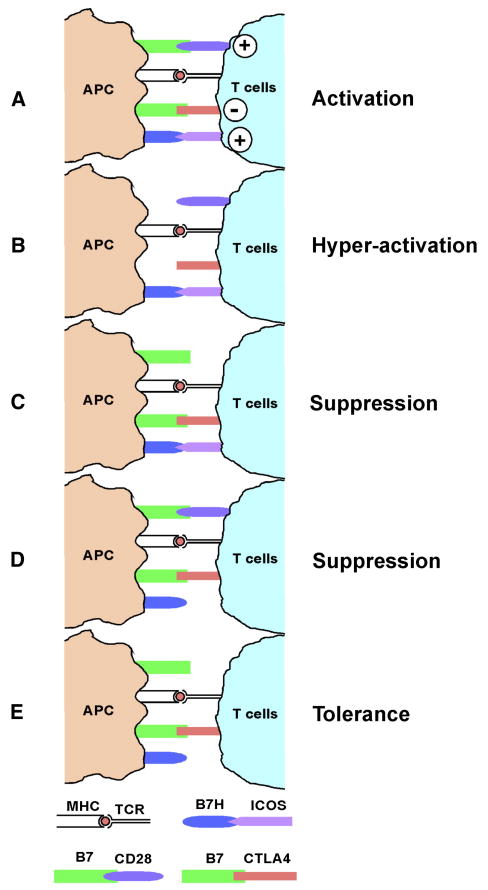
By using murine BMT models, which are representative of clinical allogeneic HCT settings, the current study demonstrated that CD28 and ICOS signaling positively regulated T-cell responses to alloantigens and supported GVHD development in an additive or synergistic manner, whereas CTLA4 was a negative regulator (Fig. 5A). Under the situation where GVHD is exclusively induced by CD4+ T cells or primarily driven by CD4+ and facilitated through CD8+ T cells, blockade of B7 (both CTLA4 and CD28) results in hyper-activation of allogeneic T cell response (Fig. 5B); Blockade of CD28 or ICOS results in suppression (Fig. 5C and 5D); and blockade of both CD28 and ICOS while sparing CTLA4 leads to T cell tolerance (Fig. 5E).
Fig. 5. The role of CD28 and ICOS on GVHD induced exclusively by CD4+ cells or primarily driven by CD4+ and facilitated by CD8+ T cells.
CD28 and ICOS signaling positively regulated T-cell responses to alloantigens and supported GVHD development in an additive or synergistic manner, whereas CTLA4 was a negative regulator (A). Blockade of B7 (both CTLA4 and CD28) results in hyper-activation of allogeneic T cell response (B); Blockade of CD28 or ICOS results in suppression (C and D); Blockade of both CD28 and ICOS while sparing CTLA4 leads to T cell tolerance (E).
An elegant in vitro study by Nurieva et al. showed that in the absence of positive costimulation mediated by CD28 and ICOS, negative costimulatory molecules including CTLA4 and PD-1 actively instruct T cells to develop into tolerant cells characterized by inactivation of intrinsic signaling and transcriptional programs (44). The current study extends those in vitro findings in clinically relevant models of GVHD and shows that T-cell immunity and tolerance are determined by the combination of costimulatory signals. Our study also provides direct evidence to support blocking CD28 and ICOS signals while sparing CTLA4-signal as an effective approach to prevent GVHD through manipulation of the CD28 family of costimulatory molecules in vivo. Currently, more selective CD28 blockade rather than B7 blockade, e.g. belatacept and non-activating CD28-specific Ab, have been produced (45, 46), and a fully humanized Ab against human ICOS was also generated (47). These reagents can be used in the translation of our research finding into clinical practice in allogeneic HCT.
Acknowledgments
We thank Drs. Claudio Anasetti for his helpful discussion of this project, Yaming Liang for his technical assistance, and Drs. Chen Dong for ICOS KO and Tak Mak for CD28/ICOS DKO mice. We are grateful for the technical assistance provided by Flow Cytometry and Mouse Core Facility at the Moffitt Cancer Center.
This work was supported by National Institutes of Health Grants CA118116 and CA143812 to X.-Z.Y; 2R01 L56067, AI34495 and P01 AI 056299 to BR; Canadian Institutes of Health Research grant MOP 84544 to W.-K. S.
Footnotes
Authorship and Conflict of Interest Statements: The authors declare no competing financial conflict of interest.
References
- 1.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–389. doi: 10.1038/35077251. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science (New York, NY. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 3.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nauture Reviews. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 4.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annual review of immunology. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 5.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Sharpe AH, Vallera DA. Opposing roles of CD28:B7 and CTLA-4:B7 pathways in regulating in vivo alloresponses in murine recipients of MHC disparate T cells. Journal of Immunology. 1999;162:6368–6377. [PubMed] [Google Scholar]
- 6.Yu XZ, Martin PJ, Anasetti C. Role of CD28 in acute graft-versus-host disease. Blood. 1998;92:2963–2970. [PubMed] [Google Scholar]
- 7.Bachmann MF, McKall-Faienza K, Schmits R, et al. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity. 1997;7:549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 8.Kundig TM, Shahinian A, Kawai K, et al. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 9.Yu XZ, Martin PJ, Anasetti C. CD28 signal enhances apoptosis of CD8 T cells after strong TCR ligation. Journal of Immunology. 2003;170:3002–3006. doi: 10.4049/jimmunol.170.6.3002. [DOI] [PubMed] [Google Scholar]
- 10.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 11.Hutloff A, Dittrich AM, Beier KC, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 12.Dong C, Juedes AE, Temann UA, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. comment. [DOI] [PubMed] [Google Scholar]
- 13.McAdam AJ, Greenwald RJ, Levin MA, et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. comment. [DOI] [PubMed] [Google Scholar]
- 14.Dong C, Temann UA, Flavell RA. Cutting edge: critical role of inducible costimulator in germinal center reactions. Journal of Immunology. 2001;166:3659–3662. doi: 10.4049/jimmunol.166.6.3659. [DOI] [PubMed] [Google Scholar]
- 15.Tafuri A, Shahinian A, Bladt F, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. comment. [DOI] [PubMed] [Google Scholar]
- 16.Swallow MM, Wallin JJ, Sha WC. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- 17.Yoshinaga SK, Whoriskey JS, Khare SD, et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 18.Coyle AJ, Gutierrez-Ramos JC. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nature immunology. 2001;2:203–209. doi: 10.1038/85251. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalo JA, Tian J, Delaney T, et al. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nature immunology. 2001;2:597–604. doi: 10.1038/89739. comment. [DOI] [PubMed] [Google Scholar]
- 20.Ozkaynak E, Gao W, Shemmeri N, et al. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nature immunology. 2001;2:591–596. doi: 10.1038/89731. comment. [DOI] [PubMed] [Google Scholar]
- 21.Rottman JB, Smith T, Tonra JR, et al. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nature immunology. 2001;2:605–611. doi: 10.1038/89750. comment. [DOI] [PubMed] [Google Scholar]
- 22.Kosuge H, Suzuki J, Gotoh R, et al. Induction of immunologic tolerance to cardiac allograft by simultaneous blockade of inducible co-stimulator and cytotoxic T-lymphocyte antigen 4 pathway. Transplantation. 2003;75:1374–1379. doi: 10.1097/01.TP.0000061601.26325.82. [DOI] [PubMed] [Google Scholar]
- 23.Harada H, Salama AD, Sho M, et al. The role of the ICOS-B7h T cell costimulatory pathway in transplantation immunity. Journal of Clinical Investigation. 2003;112:234–243. doi: 10.1172/JCI17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo L, Fujino M, Kimura H, et al. Simultaneous blockade of co-stimulatory signals, CD28 and ICOS, induced a stable tolerance in rat heart transplantation. Transplant immunology. 2003;12:41–48. doi: 10.1016/S0966-3274(03)00016-9. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa S, Nagamatsu G, Watanabe M, et al. Opposing effects of anti-activation-inducible lymphocyte-immunomodulatory molecule/inducible costimulator antibody on the development of acute versus chronic graft-versus-host disease. Journal of Immunology. 2001;167:5741–5748. doi: 10.4049/jimmunol.167.10.5741. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard VM, Eng JM, Ramirez-Montagut T, et al. Absence of inducible costimulator on alloreactive T cells reduces graft versus host disease and induces Th2 deviation. Blood. 2005;106:3285–3292. doi: 10.1182/blood-2005-01-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, et al. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and faciliates engraftment of allogeneic bone marrow (BM) Blood. 2005;105:3372–3380. doi: 10.1182/blood-2004-10-3869. [DOI] [PubMed] [Google Scholar]
- 28.Yu XZ, Liang Y, Nurieva RI, Guo F, Anasetti C, Dong C. Opposing Effects of ICOS on Graft-versus-Host Disease Mediated by CD4 and CD8 T Cells. J Immunol. 2006;176:7394–7401. doi: 10.4049/jimmunol.176.12.7394. [DOI] [PubMed] [Google Scholar]
- 29.Nurieva RI, Duong J, Kishikawa H, et al. Transcriptional regulation of th2 differentiation by inducible costimulator. Immunity. 2003;18:801–811. doi: 10.1016/s1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 30.Yu XZ, Bidwell SJ, Martin PJ, Anasetti C. CD28-specific antibody prevents graft-versus-host disease in mice. Journal of Immunology. 2000;164:4564–4568. doi: 10.4049/jimmunol.164.9.4564. [DOI] [PubMed] [Google Scholar]
- 31.Liang Y, Liu C, Djeu JY, et al. Beta2 integrins separate graft-versus-host disease and graft-versus-leukemia effects. Blood. 2008;111:954–962. doi: 10.1182/blood-2007-05-089573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24:233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Blazar BR, Taylor PA, Boyer MW, Panoskaltsis-Mortari A, Allison JP, Vallera DA. CD28/B7 interactions are required for sustaining the graft-versus-leukemia effect of delayed post-bone marrow transplantation splenocyte infusion in murine recipients of myeloid or lymphoid leukemia cells. Journal of Immunology. 1997;159:3460–3473. [PubMed] [Google Scholar]
- 34.Blazar BR, Taylor PA, Linsley PS, Vallera DA. In vivo blockade of CD28/CTLA4: B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility comple×barrier in mice. Blood. 1994;83:3815–3825. [PubMed] [Google Scholar]
- 35.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Gray GS, Vallera DA. Coblockade of the LFA1:ICAM and CD28/CTLA4:B7 pathways is a highly effective means of preventing acute lethal graft-versus-host disease induced by fully major histocompatibility complex-disparate donor grafts. Blood. 1995;85:2607–2618. [PubMed] [Google Scholar]
- 36.Miller WP, Srinivasan S, Panoskaltsis-Mortari A, et al. GVHD after haploidentical transplantation: a novel, MHC-defined rhesus macaque model identifies CD28- CD8+ T cells as a reservoir of breakthrough T-cell proliferation during costimulation blockade and sirolimus-based immunosuppression. Blood. 2010;116:5403–5418. doi: 10.1182/blood-2010-06-289272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Speiser DE, Bachmann MF, Shahinian A, Mak TW, Ohashi PS. Acute graft-versus-host disease without costimulation via CD28. Transplantation. 1997;63:1042–1044. doi: 10.1097/00007890-199704150-00028. [DOI] [PubMed] [Google Scholar]
- 38.Kurtz J, Raval F, Vallot C, Der J, Sykes M. CTLA-4 on alloreactive CD4 T cells interacts with recipient CD80/86 to promote tolerance. Blood. 2009;113:3475–3484. doi: 10.1182/blood-2008-01-133736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, Riella LV, Boenisch O, et al. Paradoxical functions of B7: CD28 costimulation in a MHC class II-mismatched cardiac transplant model. Am J Transplant. 2009;9:2837–2844. doi: 10.1111/j.1600-6143.2009.02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor PA, Lees CJ, Fournier S, Allison JP, Sharpe AH, Blazar BR. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions [corrections] J Immunol. 2004;172:34–39. doi: 10.4049/jimmunol.172.1.34. [DOI] [PubMed] [Google Scholar]
- 41.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker MB, Riley RL, Podack ER, Levy RB. Graft-versus-host-disease-associated lymphoid hypoplasia and B cell dysfunction is dependent upon donor T cell-mediated Fas-ligand function, but not perforin function. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1366–1371. doi: 10.1073/pnas.94.4.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graubert TA, DiPersio JF, Russell JH, Ley TJ. Perforin/granzyme-dependent and independent mechanisms are both important for the development of graft-versus-host disease after murine bone marrow transplantation. Journal of Clinical Investigation. 1997;100:904–911. doi: 10.1172/JCI119606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nurieva R, Thomas S, Nguyen T, et al. T-cell tolerance or function is determined by combinatorial costimulatory signals. Embo J. 2006 doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poirier N, Blancho G, Vanhove B. A more selective costimulatory blockade of the CD28-B7 pathway. Transpl Int. 24:2–11. doi: 10.1111/j.1432-2277.2010.01176.x. [DOI] [PubMed] [Google Scholar]
- 46.Poirier N, Azimzadeh AM, Zhang T, et al. Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci Transl Med. 2:17ra10. doi: 10.1126/scitranslmed.3000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tajima N, Tezuka K, Tanimoto A, et al. JTA-009, a fully human antibody against human AILIM/ICOS, ameliorates graft-vs-host reaction in SCID mice grafted with human PBMCs. Experimental hematology. 2008;36:1514–1523. doi: 10.1016/j.exphem.2008.06.004. [DOI] [PubMed] [Google Scholar]