Abstract
Concentration and temperature dependent studies of the circular dichroism of dianionic guanosine 5’-monophosphate (5’-GMP), where the cation was Na+, K+, or Rb+ ion, were done to obtain information regarding the nature of the self-assembled 5’-GMP species in aqueous solution, including G-quartets and other structures. Concentrations in the 0.05 M – 0.85 M range and temperatures in the 5–50 °C range were used. At the highest concentrations and 5 °C, Na2(5’-GMP) and K2(5’-GMP) formed a cholesteric phase, but Rb2(5’-GMP) did not. Evidence for antiparallel base stacking (stacked with opposite polarity; head to head) was observed for Rb2(5’-GMP), but not for the Na+ or K+ salts. This structure, believed to be G-quartets, had a melting temperature of 15 °C and dissociated into a second associated species as the temperature increased. The latter was present to the greatest extent at ~40 °C and it is characterized by a prominent negative CD band at 306 nm, which may be indicative of an X-DNA type of structure (an expanded G-quartet) or base-stacked monomers or dimers. The same negative band appeared at 310 nm in the CD spectra of K2(5’-GMP) and Na2(5’-GMP), but was much less intense in the latter case. K2(5’-GMP) also formed a non-cholesteric phase containing at least two different species, one more stable at low temperatures and the other more stable at higher temperatures, similar to Rb2(5’-GMP). 1H NMR spectroscopy was used to assist in the interpretation of the CD spectra.
Keywords: guanosine 5’-monophosphate, G-quartet, circular dichroism, self-assembly, alkali metal ions
1. Introduction
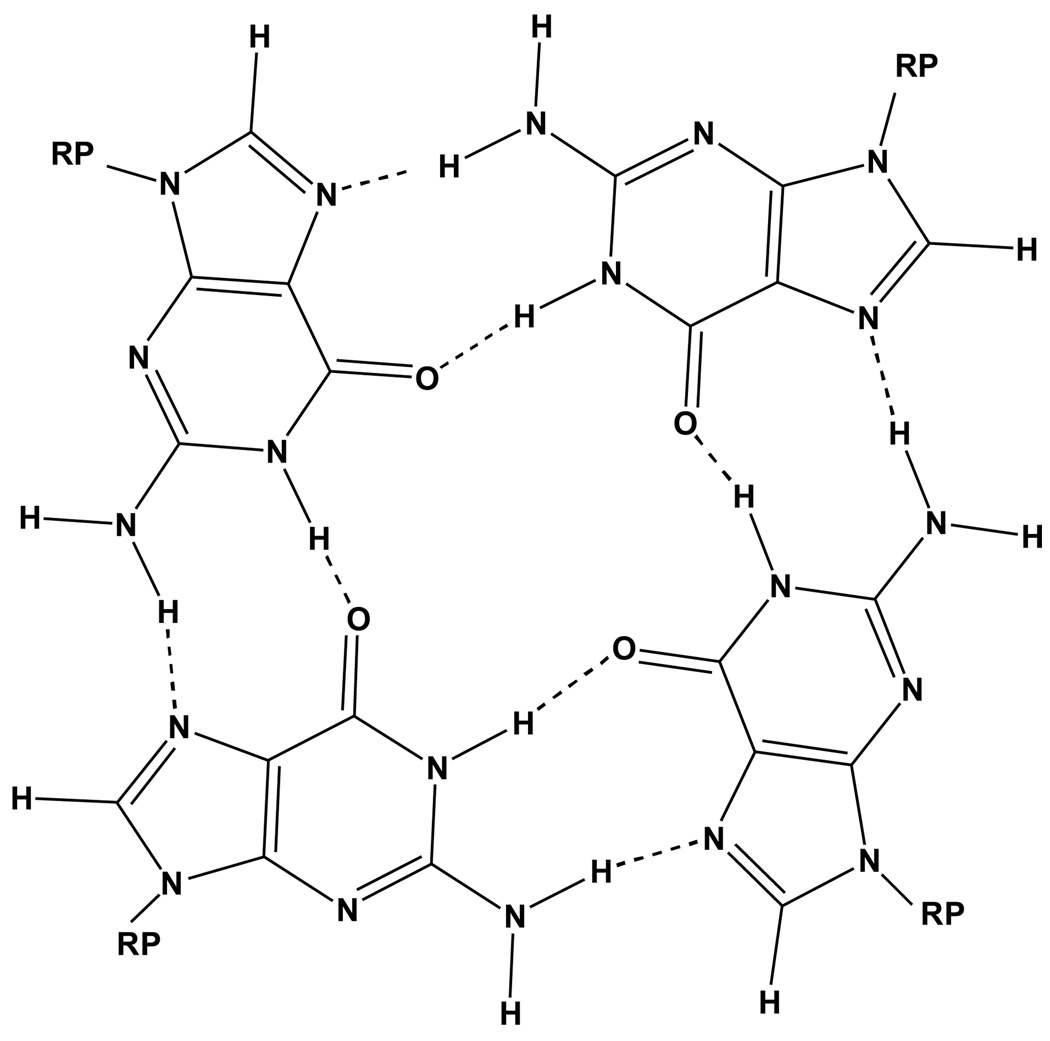
The self-assembly of the mononucleotide guanosine 5’-monophosphate (5’-GMP) has been under investigation for nearly 40 years1–5, but questions about the structure of the species formed in aqueous solution in the presence of Na+, K+, or Rb+ and about the solid state structure, remain unanswered. In the 1970’s, fiber diffraction6–8 and NMR spectroscopic9–13 investigations of the gels and solutions indicated the formation of stacked cyclic tetramers (G-quartets) held together by Hoogsteen hydrogen bonds between the guanine bases (Chart 1).
Chart 1.
Among the methods used to study this and related systems, NMR spectroscopy has been used the most often. The 1H NMR spectra of the self-assemblies of 5’-GMP in aqueous solution at low temperatures revealed that the spectra varied, depending on the particular cation present.11,12 This was ascribed to the difference in the sizes of the Na+, K+, and Rb+ ions12,13 and their possible location within the center of the stacks of G-quartets. Na+ is small enough to fit in the central cavity, but K+ and Rb+ are too large and must be located between the planes of the G-quartets. This has been confirmed by X-ray structure determinations of some guanosine-rich fragments of telomeric sequences which are found at the ends of chromosomes, and which form G-quadruplexes.14–19 Not only do each of these cations have a different 1H NMR spectrum, but each shows that multiple species are present in 5’-GMP solutions.20–23
Dynamic light scattering has been used to estimate the number of G-quartets in a stack in aqueous solution. This number varies depending on the cation, nucleotide concentration, temperature, and pH.24–26 For 5’-GMP, the estimates range from 9 26 to 87 24, and as high as 133 for 2-deoxy-5’-GMP.27 An SEM investigation of 5’-GMP deposited from aqueous solution on colloidal carbon disks revealed that when a large excess of Na+ was present, very long rods with an average diameter of 2000 nm could be grown, indicative of a large number of parallel stacks of G-quartets.23 In the case of large amounts of K+ ion, two different morphologies were observed, one of which appeared to be the G-quartet and the other was postulated to be that of a continuously hydrogen-bonded helix.1,8,28 With Rb+ as the cation, yet another morphology was apparent.
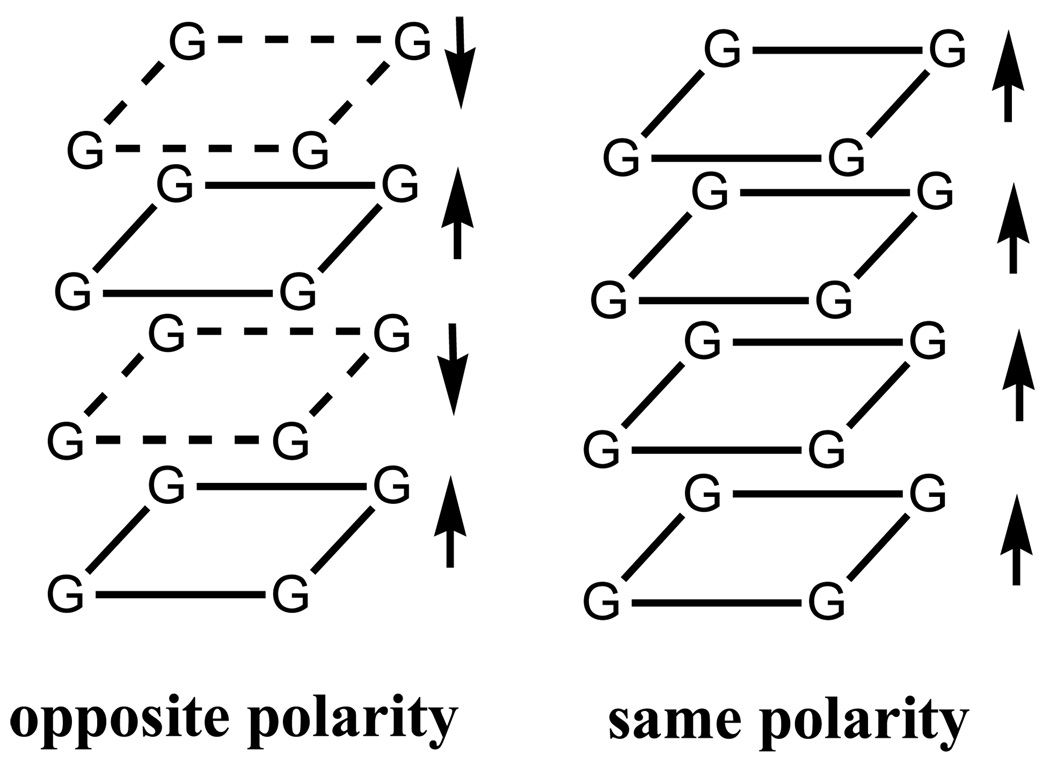
Circular dichroism is a technique that is quite sensitive to the conformation of anisotropic molecules and chiral super assemblies, and has been used extensively in the study of nucleic acids29,30 and G-quadruplex structures.30–36 Information about the way in which the bases, base pairs, or G-quartets are stacked, either with the same polarity (parallel) or opposite polarity (antiparallel), can be obtained (Chart 2).31–37 Generally, the CD spectrum of parallel stranded structures, such as d[TG4T]4, contain a negative band at about 240 nm and a positive band at 260–265 nm, while antiparallel quadruplex structures, such as d[G4T4G4]2 have a negative band at 260 nm and a positive band at 295 nm.29,31 Cation effects are important in determining the conformations of G-quadruplexes, and frequently high concentrations of Na+ lead to antiparallel structures and K+ to parallel structures. In some cases, it is possible to detect a mixture of structures or conformers existing simultaneously in a solution by comparison of the CD spectrum with those of known conformations.32,38–42 For example, Lee et al. showed that d[G4T4]n in the presence of 200 mM Na+ had an antiparallel conformation, but in the presence of 200 mM K+ it was a mixture of a parallel and antiparallel conformations.41
Chart 2.
G-quadruplexes
A large number of structural motifs and folding patterns are known for G-rich sequences that form G-quadruplexes and G-quartets4,5 and in some of these, a mixture of polarities occurred within a single structure.31,37,38 That is, some of the bases were stacked with the same polarity, also known as head-tail stacking, and others were stacked with opposite polarities (head-head stacking). Masiero et al. have shown that the observed CD spectra are dependent on the polarities of the base stacking rather than whether the associated strands are aligned parallel or antiparallel.31 For example, both d[G3T4G3]2 and [d(TGBrGGT)]2 have bases stacked with mixed polarities and very similar CD spectra, however, the former has a folded antiparallel strand structure and the latter contains parallel strands. CD spectra of this type of quadruplex bear some of the characteristics of both the parallel and antiparallel structures with some red shifting of the features. For example, d[G3T4G3]2 has a negative band at about 240, a positive band at 255, dip at 270, and a positive band at 290 nm.
CD spectroscopy has also been employed in the study of the self-assembly of monomeric guanosine and guanosine monophosphates.36,43,44 Depending on the concentration, temperature, and cation, the mononucleotides can exist in an unassembled, isotropic self-assembled or a cholesteric liquid crystalline state.35 The latter of these is most commonly found at high nucleotide concentrations and low temperature. The liquid crystalline phases formed by 3’-GMP, 5’-GMP, and 3’–5’cGMP are reported to be left-handed cholesterics as indicated by their negative CD spectra.43
In this paper we report an extensive investigation of the CD spectra of the Na+, K+, and Rb+ salts of 5’-GMP at various concentrations and temperatures. Consistent with NMR and other data on these systems, different CD spectra were observed for each of the different cations, although the Na+ and K+ salts both exhibited spectra characteristic of cholesteric phases at high concentration and low temperature.
2. Experimental
Materials and Methods
Na2(5’-GMP), H2(5’-GMP)•H2O, D2O, DCl, and NaOD were purchased from Sigma-Aldrich and used as received. K2(5’-GMP) and Rb2(5’-GMP) were prepared by titrating 1.0 g of H2(5’-GMP)•H2O in 15 mL of deionized H2O with 0.20 M KOH or RbOH to a pH of 7.8–8.0. The solutions were lyophilized to obtain the solids. Solutions for CD measurements (pH* 7.9–8.6) were prepared by dissolving the solid compounds in D2O and the concentrations were determined by UV spectroscopy at 252 nm (ε=1.37 × 104 M−1cm−1). D2O was used to prepare the solutions so that the state of self-association could be checked by 1H NMR spectroscopy. Solutions were stored at 5 °C for at least 24 hr prior to measurement and maintained at this temperature until use. CD measurements were obtained on a JASCO J-810 spectrometer with a JASCO PTC-348w1 temperature controller (±0.2 °C at low temperature; ±1.0 °C at high temperature) using 0.1 mm quartz cells. The scan rate was 50 nm/min, band width = 2 nm and N2 purge of 15 cfm/min. Samples were equilibrated in the spectrometer for 20 min at the measurement temperature. An additional 10 min equilibration revealed no changes in the spectra. The temperature dependent measurements on the most concentrated solutions were done twice with two different sample preparations. 1H NMR spectra were obtained on a 500 MHz Varian INOVA spectrometer with variable temperature capability. The same solutions that were used for the CD measurements were used for NMR measurements. The spectra were referenced to internal tetramethylammonium ion at 3.185 ppm. Suppression of residual H2O was not employed because it affected the relative intensities of resonances of 5’-GMP aggregates.
3. Results
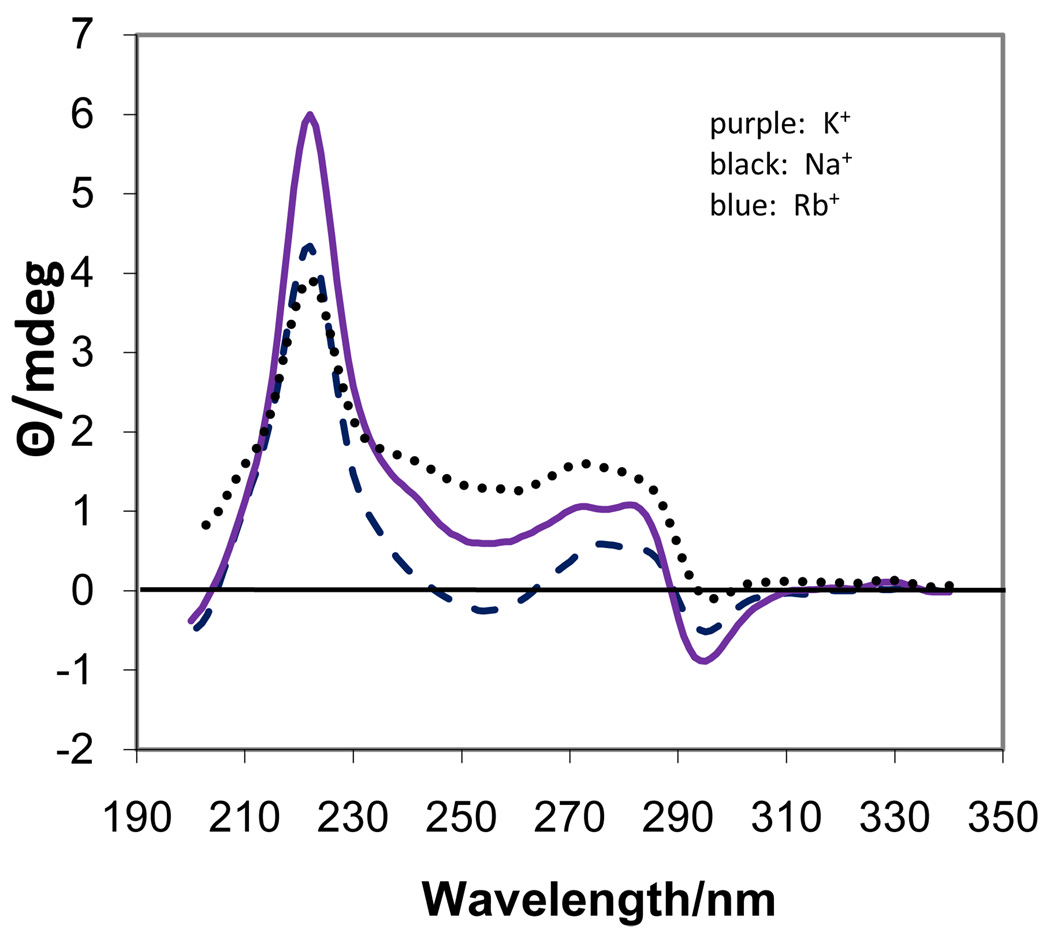
In order to investigate the self-assembly behavior of 5’-GMP it is necessary to perform experiments at various concentrations and temperatures. This is in contrast to guanosine oligomers and telomeric sequences which form associated structures in solution at low concentrations and room temperature. All three of the M2(5’-GMP) salts in solution at 0.05 M and either 5 or 20 °C had the same CD spectrum (Fig. 1; 5 °C data not shown) and were completely dissociated, which was confirmed by the 1H NMR in D2O where a single resonance at 8.22 ppm is observed for the H8 proton irrespective of the cation.
Figure 1.
CD spectra of 0.05 M M2(5’-GMP) at 20 °C.
Na2(5’-GMP)
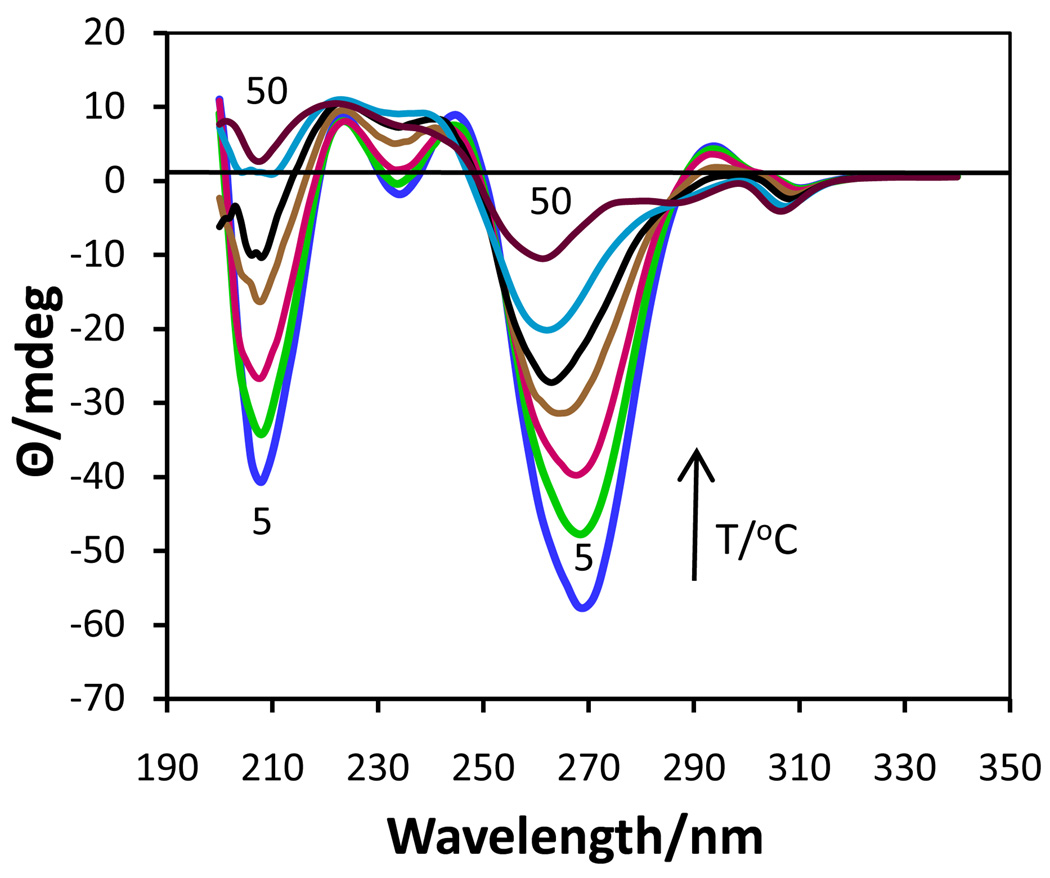
At 0.85 M, Na2(5’-GMP) exists as a cholesteric phase at all temperatures between 5 and 30 °C with very intense negative CD bands at 208 nm and 270 nm which gradually decrease in intensity as the temperature increases (Fig.2). Above 30 °C, the two negative bands begin to develop additional features as well as continuing to decrease in intensity. Another experiment with 0.50 M Na2(5’-GMP) at three temperatures (5, 20, 50 °C) showed that this solution was cholesteric at the two lower temperatures, but completely dissociated at 50 °C.
Figure 2.
CD spectra of 0.85 M Na2(5’-GMP) from 5 to 50 °C.
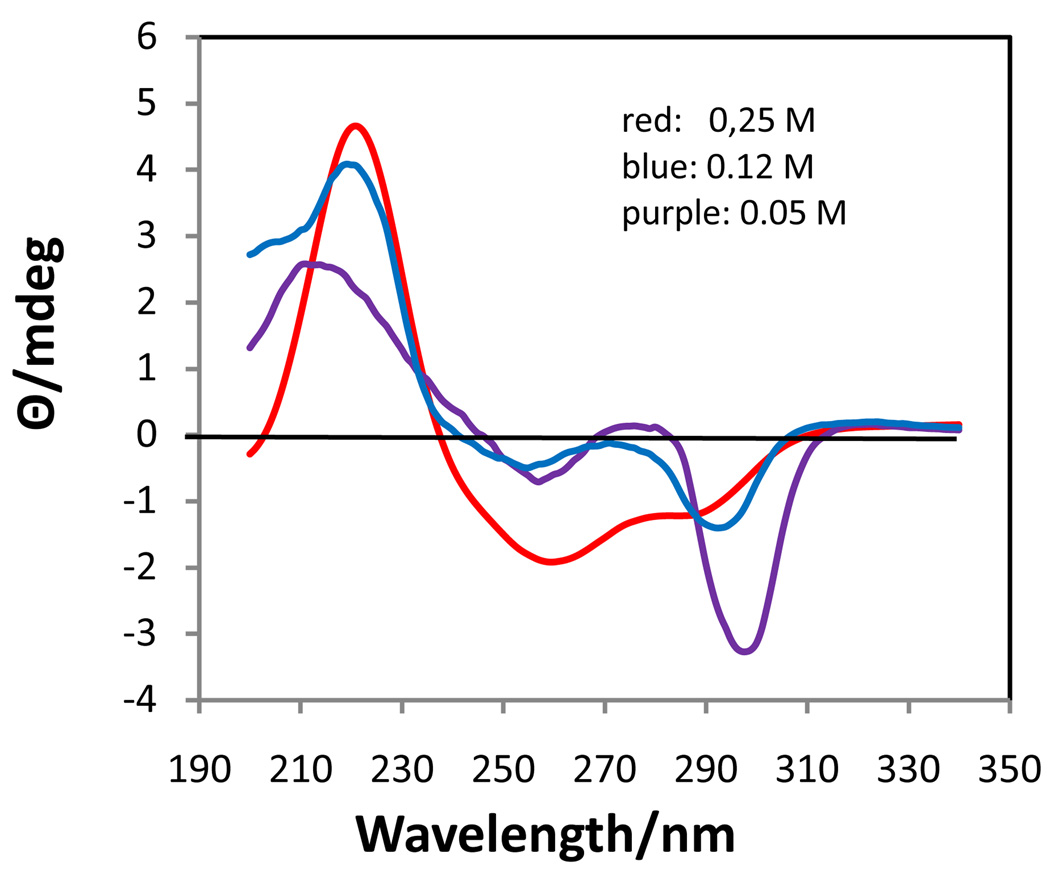
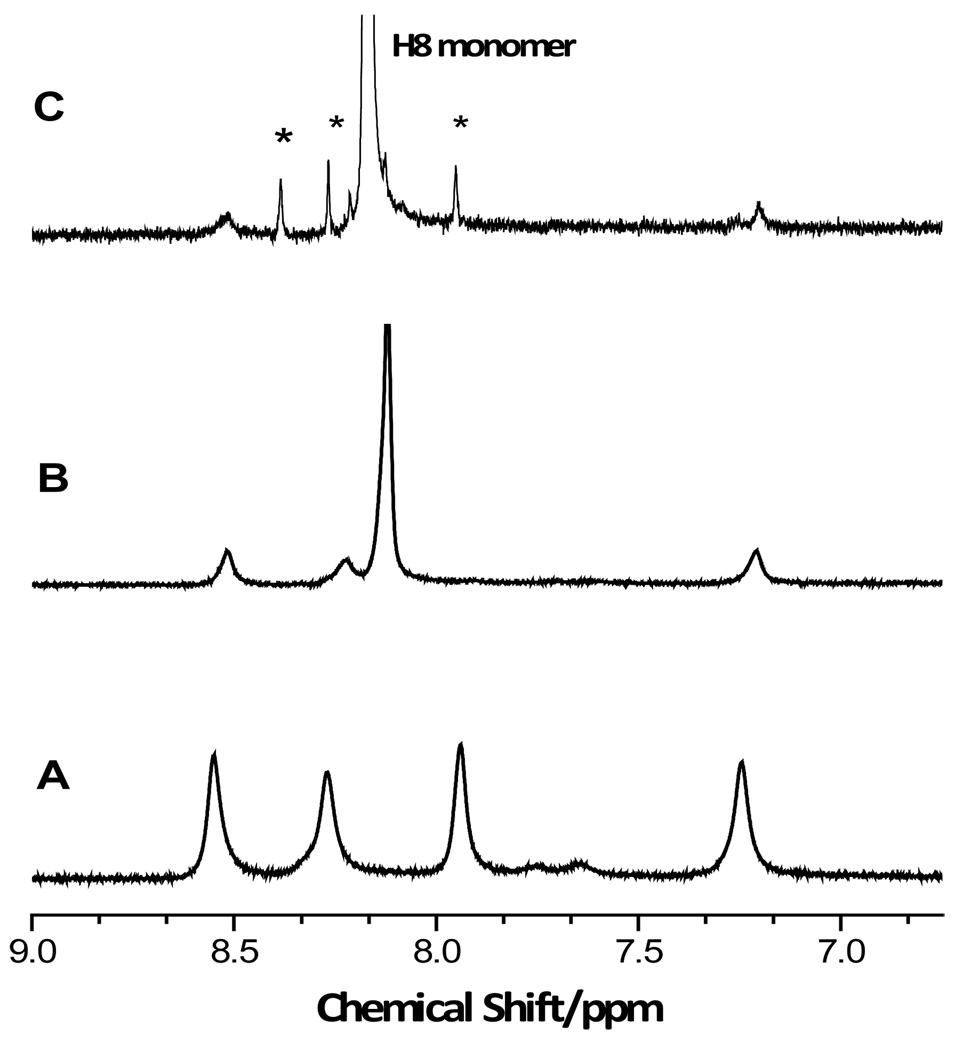
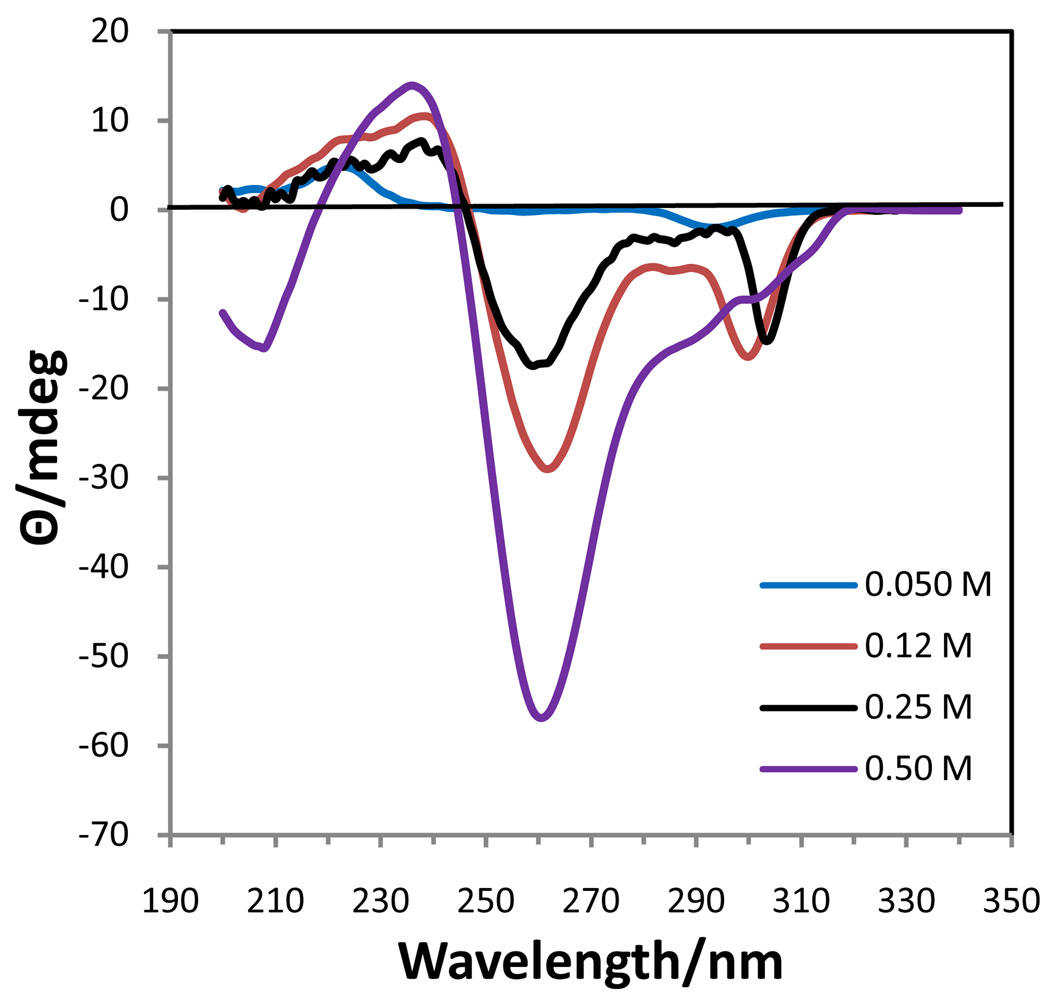
In order to gain more information about the associated (non-cholesteric) species, the CD spectra at 5 °C of three different concentrations (0.25, 0,12, 0.05 M) were obtained (Fig. 3). At 0.25 M the CD spectrum was that of an associated species of Na2(5’-GMP) with a positive band at 221 nm and a negative band at 260 nm and an additional dip at 288 nm. The H8 region of the 1H NMR spectrum at this concentration and temperature showed incipient formation of G-quartets by the presence of extremely weak and broad resonances at 8.52 and 7.20 ppm. The other two bands that appear in the spectrum of G-quartets at high concentration, 8.27 and 7.25 ppm, have been tentatively assigned as a dimer22 and monomers (stacked), respectively. Characteristic of the occurrence of base stacking, both the H8 and H1’ resonances of the monomer were shifted upfield by 0.05 ppm from their positions in 0.05 M solution (8.22 and 5.92 ppm, respectively). The 1H NMR spectra of 0.69, 0.50, and 0.25 M Na2(5’-GMP) at 5 °C are shown in Figure 4. The spectra of the associated species were the same at all concentrations, simply decreasing in intensity as the concentration decreased, although the monomer (or stacked monomer) resonance shifted with respect to concentration.
Figure 3.
CD spectra of Na2(5’-GMP) at various concentrations and 5 °C.
Figure 4.
1H NMR spectra of Na2(5’-GMP) in D2O at 5 °C: (A) 0.69 M; (B) 0.50 M; (C) 0.25 M, * indicates impurity/artifact.
At 0.12 M a negative band of moderate intensity was present at 299 nm with a reduction in the intensity of the 260 nm band. This spectrum was similar to that of the monomer at 0.05 M, but had a greater negative intensity in the 290–300 nm region. The 1H NMR spectrum at 5 °C did not show the presence of any G-quartets, but a slight upfield shift of 0.02 ppm of the H8 and H1’ resonances compared to 0.05 M solution supports the presence of a small amount of stacked monomer, although less than present in the 0.25 M solution.
K2(5’-GMP)
From temperature-dependent studies of two concentrated K2(5’-GMP) solutions (0.73 M and 0.77 M) it was observed that two different phases can be formed, an assembled structure with a complex CD spectrum (Fig. 5) and a cholesteric liquid crystalline phase with the same CD spectrum as the cholesteric phases of d(5’-GMP) in 1 M KCl 44 and our concentrated Na2(5’-GMP) solutions (See Fig 2). It is clear from the comparison of 0.77 M spectrum at 50 °C with that of the monomer (Fig. 1) that the former still contained assembled 5’-GMP species. By noting that the intensity changes for the (−)270 and (−)288 nm bands were opposite that of the (−)309 nm band in the non-cholesteric solution (Fig. 6), it was determined that there were at least two different types of assembled species present. The intensity changes were essentially linear with temperature, so a melting temperature could not be determined. A concentration dependent experiment revealed that at 0.50 M the cholesteric phase still was formed, but at ≤0.25 M isotropic associated species were present (Fig. 7). These data agree with the observations from NMR spectroscopy and scanning electron microscopy23 in which evidence for two different morphologies were identified. The 1H NMR spectrum of 0.73 M K2(5’-GMP) at 2 °C showed several broad, discrete resonances in the H8 region, similar to those observed in the stacked G-quartet structure of Na2(5’-GMP) but at different chemical shifts. In addition, very broad and overlapped resonances from a less ordered structure were present in this region, but not observed for Na2(5’-GMP). It has been previously postulated that the latter arose from a continuously hydrogen-bonded structure formed by breaking two H-bonds in the G-quartet and connecting the fragments.28 Changes in the H8 region of the NMR spectra at 0.73, 0.50, 0.25, and 0.10 M at 5 °C showed that at least two different associated species were present in the system (Supp. Mat. Fig S1).
Figure 5.
CD spectra of 0.77 M K2(5’-GMP) from 5 to 50 °C.
Figure 6.
Change in ellipticity with temperature at selected wavelengths in 0.77 M K2(5’-GMP).
Figure 7.
CD spectra of K2(5’-GMP)at various concentrations and 5 °C.
Rb2(5’-GMP)
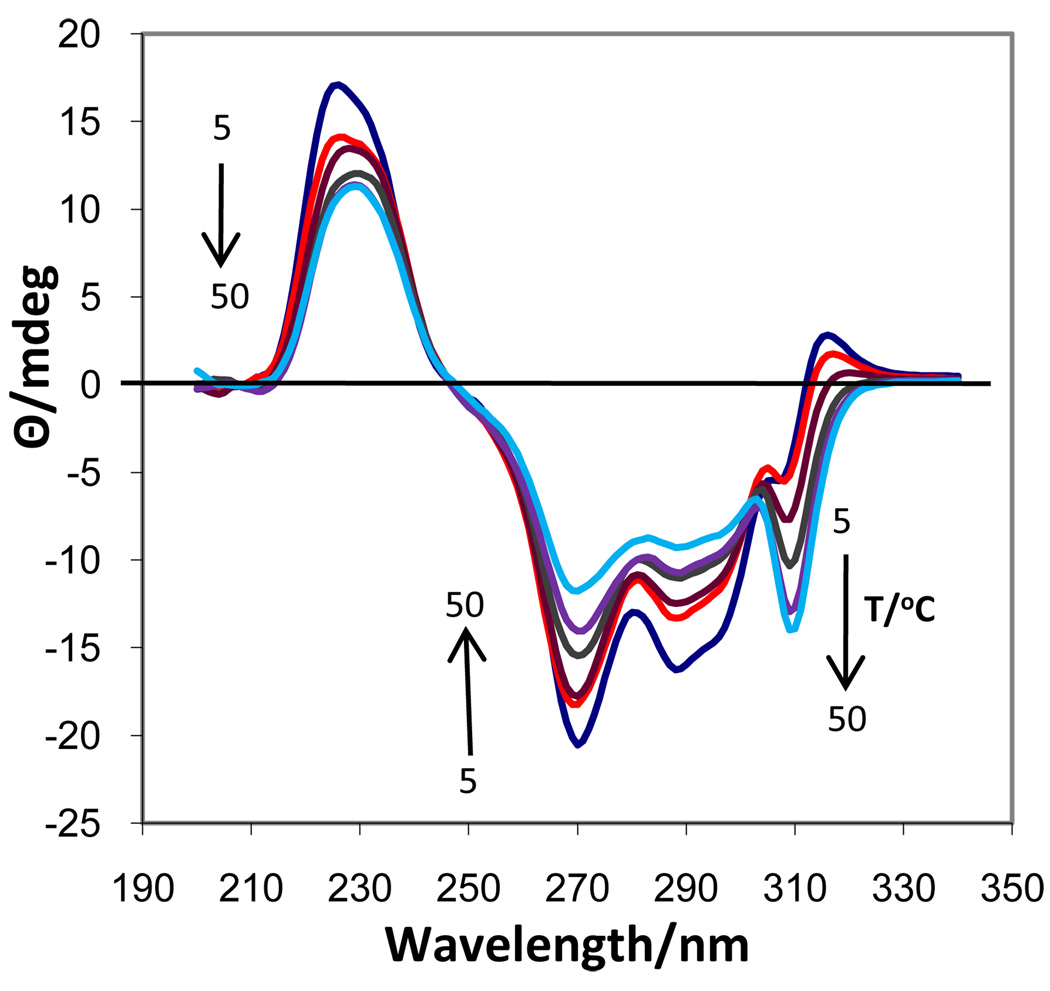
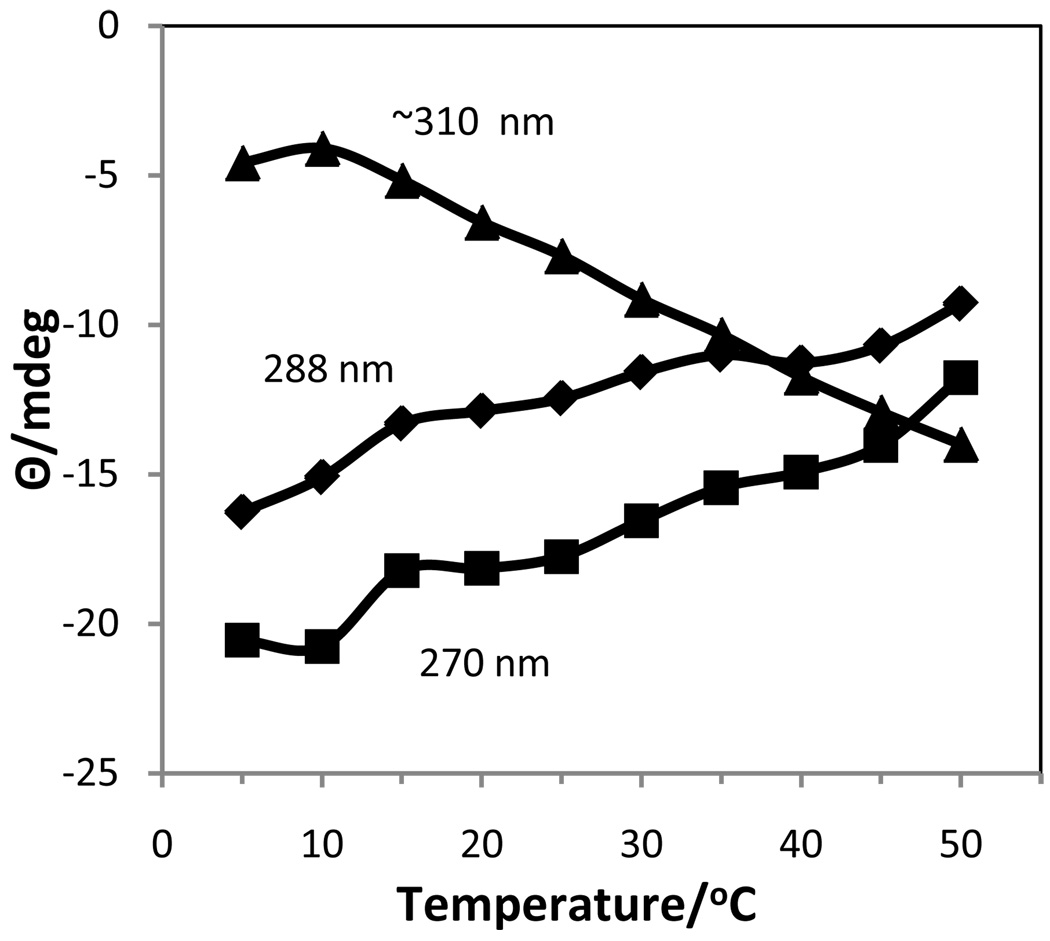
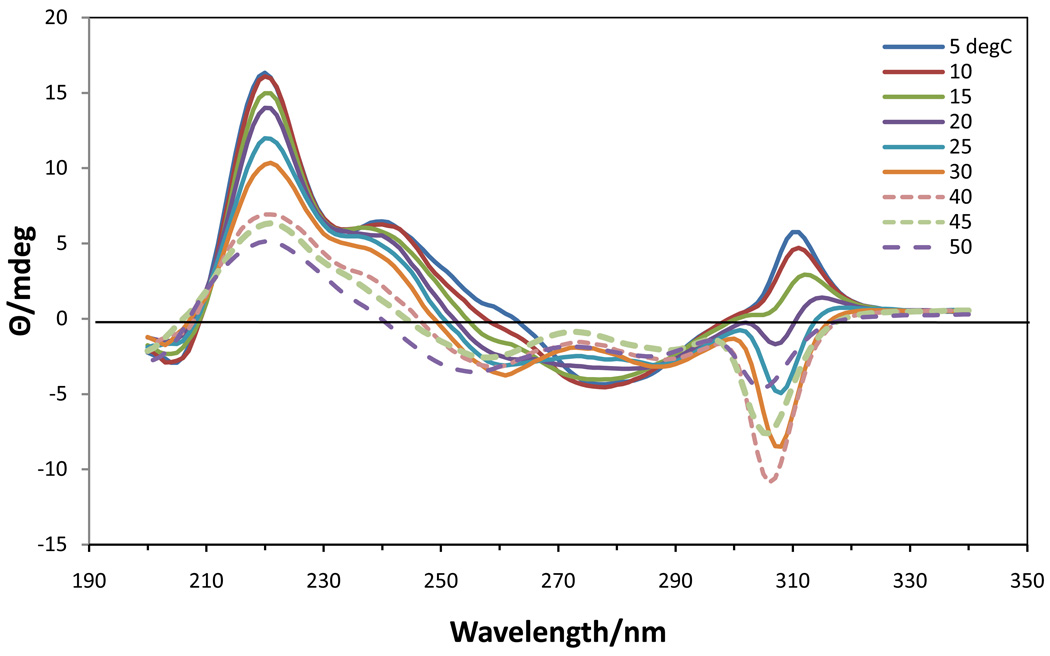
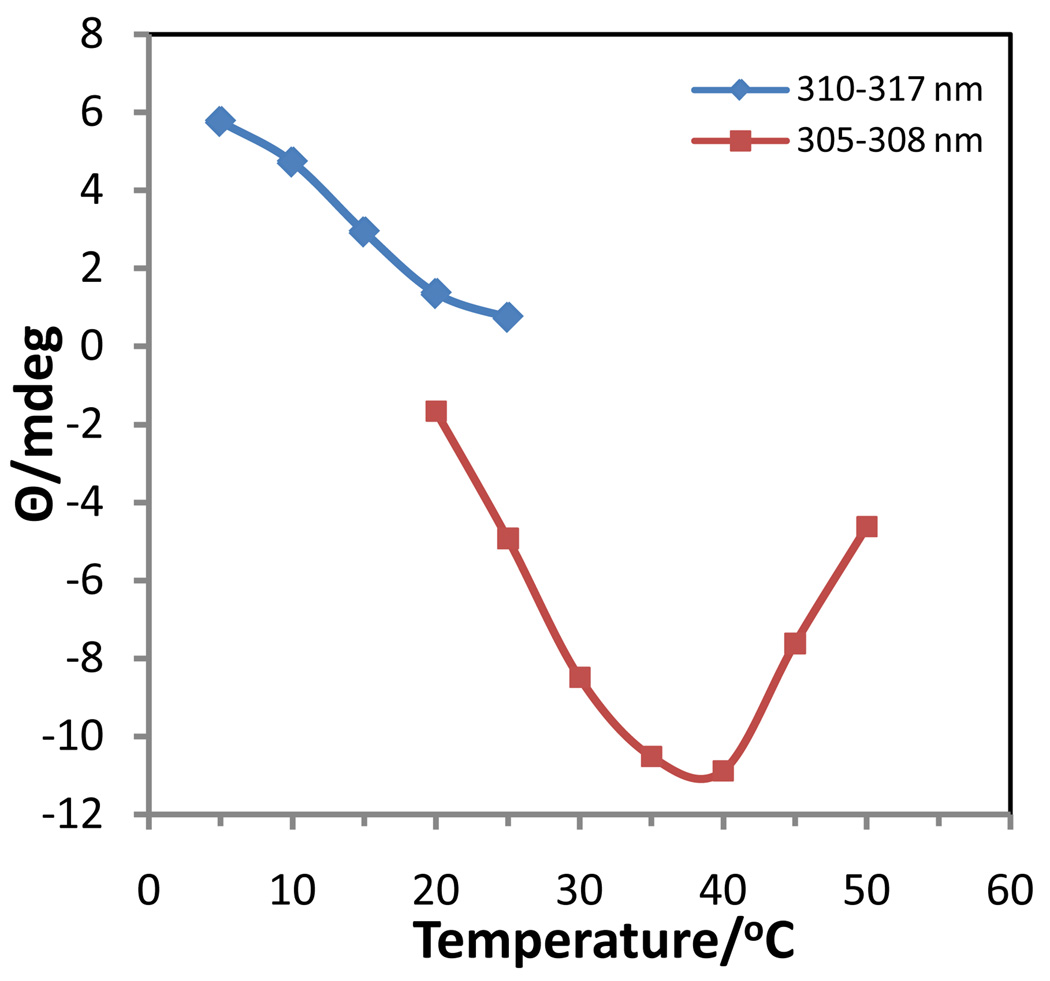
No CD investigation of the effect of Rb+ on the self-association of 5’-GMP has been previously reported. The temperature dependent CD spectra (Fig. 8) of 0.63 M Rb2(5’-GMP) clearly showed the presence of two different associated structures, but no cholesteric phase. Between 5 and ~20 °C, the spectra contained bands at (+)240, (−)277, (+)311 nm that were representative of a structure in which G-quartets were stacked in an antiparallel fashion, 30,34 similar to that found for Na[d(GpG)] with RbCl.35 From ~20–40 °C a second type of associated species was present in solution with the same negative band at 308 nm as observed in associated K2(5’-GMP) in this temperature range. This band reached a maximum intensity at 40 °C and then decreased in intensity as this second associated species gradually dissociated into monomers. By plotting the intensities vs. temperature for the positive and negative bands in the 305–315 nm region (Fig. 9), it was possible to determine an approximate melting temperature of 15 °C for the lower temperature species (antiparallel stacked G-quartets) and to observe the formation and subsequent dissociation of the higher temperature species. The CD spectra at the higher temperatures bear some resemblance to that of 0.12 M Na2(5’-GMP) at 5 °C, but the negative band was shifted in the 0.63 M Rb2(5’-GMP) and it was much more intense.
Figure 8.
CD spectra of 0.63 M Rb2(5’-GMP) from 5 to 50 °C.
Figure 9.
Change in ellipticity with temperature at selected wavelengths in 0.0.63 M Rb2(5’GMP).
4. Discussion
The type of CD spectra that we have observed for the cholesteric phases in Na2(5’-GMP) and K2(5’-GMP) was interpreted by Gottarelli et al. to be typical of a left-handed helix of stacked G-quartets which are arranged into a left-handed superhelix.44 This was based on comparison of the spectra with that of the cholesteric phase of the of right-handed poly d(G)n, where n ≥3,45 and by the use of Manguin’s model extended to the absorption region.43 However, more recently Wu and Kwan22 have determined from a thorough 1H NMR study that self-assembled Na2(5’-GMP) forms a right-handed helical structure in which one G-quartet has a syn conformation about the glycosidic bond and the adjacent G-quartet has the anti conformation with stacking of G-quartets in a head to tail fashion (parallel, same polarity). Since their NMR measurements were made at the same temperature and slightly higher concentration than our CD experiments, this raises a question about the actual structure of the cholesteric phase in the Na+ and K+ salts. Both of these salts had the same CD spectrum in the cholesteric phase (Fig 2), which is consistent with the extensive observations of parallel stacking in K+-containing d(G) n oligonucleotides and telomeric sequences.29 As a result of the weaker self-assembly of 5’-GMP in the presence of Rb+, a cholesteric phase was not observed in the Rb2(5’-GMP) solutions.
Telomeric sequences and other G-rich species which are known to have antiparallel stacking (opposite polarity) of G-quartets, such as the hairpin d(G4T4G4), exhibit CD spectra with a negative band at 260 nm and a positive band at ~290 nm.36–38 Of the three 5’-GMP salts studied, only in concentrated Rb2(5’-GMP) in the 5–20 °C range did we observe the typical CD spectrum for antiparallel stacking (opposite polarity) of G-quartets, although it was somewhat red-shifted ((−) 277, (+)311 nm) (Fig. 8). This associated Rb2(5’-GMP) species had a Tm =15 °C and dissociated into a new type of associated structure. Although both the Na+ and K+ salts had negative bands in the 260 nm region as well, a positive band ~290 nm was not observed for either of them. Although antiparallel stacking might have been anticipated for Na2(5’-GMP) from the presence of some syn and anti 5’-GMP residues, both CD and NMR22 measurements agree with a parallel stacking motif.
A negative band at 300–310 nm was observed in all three salts of 5’-GMP in the more dilute solutions and in the concentrated solutions at the higher temperatures, but with much lesser intensity in the Na2(5’-GMP) system. For Na2(5’-GMP) and Rb2(5’-GMP) the intensity increased and subsequently decreased as the temperature was increased, while the other CD bands all decreased in absolute intensity. This was interpreted to indicate the presence of a second associated species (the monomer intensity was very weak in this region of the spectrum). Although only an increase in intensity was observed for concentrated K2(5’-GMP) (Figure 5), an examination of the concentration-dependent data in Figure 7 showed that the intensity at ~300 nm did follow the same pattern of increase followed by decrease. The intensities were: 0.50 M, −10.0; 0.25 M, −14.5; 0.125 M, −11.4; 0.05 M, −2.0.
The species responsible for 300–310 nm band was in equilibrium with that producing the negative 260–270 nm band in all of the salts. A negative CD band at this wavelength is an unusual feature for G-quadruplex structures, and could be attributed to several different types of associated structures. A negative band at or near this wavelength has also been observed in Z-DNA.30,46 However, CD spectra of Z-DNA species usually also have strong negative band around 200–210 nm, while in our spectra this band was positive in all non-cholesteric assemblies. Therefore it seems unlikely that a Z-DNA structure was present in the solutions. Another possibility is the presence of an X-DNA structure for one of the assembled species. The CD spectra of 0.12 and 0.25 M K2(5’-GMP) solutions are similar in appearance, but red-shifted compared to the CD spectrum of poly[d(AT)] with 1.3 mM CsCl in 82% ethanol,30 for which an X-DNA structure has been suggested. Yet another possibility is that of a symmetric dimer structure which has been postulated as the origin of the 8.27 ppm resonance in 1.0 M Na2(5’-GMP) solution at 5 °C.22 However, we cannot observe this resonance in our less concentrated solutions (Fig. 4) because it is overlapped by the intense monomer resonance under the same conditions where the negative 310 nm CD band was observed. Furthermore, an H-bonded G-dimer would be expected to be in rapid exchange with monomer, resulting an averaged NMR signal, unless the dimer was present in a highly ordered, cation-stablized stack, as suggested previously.47 At present our best hypothesis is that the negative CD band in the 310 nm region indicates the formation of stacked monomers or stacked dimers. This is consistent with the upfield shift of the H8 and H1’ “monomer” resonances, characteristic of base stacking.
The CD spectra of non-cholesteric, associated K2(5’-GMP) is the most complex of the three salts. One associated species, represented by CD bands (+ 228), (−)270 and (−)288 nm, predominated at the lower temperatures and a second species, with an additional band at (–)310 nm, formed at higher temperatures and existed in equilibrium with the low temperature species. The second species is postulated to be stacked monomers or stacked dimers (see supra). However, it is known from NMR experiments28 and SEM studies23 that K2(5’-GMP) can exist in at least two different structural motifs, a G-quartet and possibly a doubly H-bonded helix. The negative CD bands at 270 and 288 nm might be attributed to separate structures or conformations, although the temperature dependence of their intensities appeared to link them together (Fig. 6).
5. Conclusions
Although the known presence of syn and anti conformations about the glycosidic bond in the self-assembled Na2(5’-GMP) would suggest that the stacking of the G-quartets would be of opposite polarity, both the CD spectra and recent NMR22 studies show that they stacked with the same polarity. The only clear evidence for stacking with opposite polarity was observed in concentrated Rb2(5’-GMP) at below 15–20°C. Cholesteric phases were observed in both Na2(5’-GMP) and K2(5’-GMP) at high concentrations and low temperatures, but not in Rb2(5’-GMP). At least two different associated structures were present in the K2(5’-GMP) and in the Rb2(5’-GMP) systems as witnessed by opposite CD spectral intensity changes of two or more bands as the temperature was varied. This is consistent with the complexity of the 1H NMR spectra which could also mean that the associated conformations in these latter systems were less symmetrically arranged than in Na2(5’-GMP). The rather unusual negative CD band in the 310 nm region has been attributed to highly stacked monomers or dimers.
Supplementary Material
Acknowledgements
The authors thank the NIH SCORE program (S0-6-08194) for support of this work. JAW also thanks Dr. Trista Robichaud for technical assistance.
Footnotes
Supplemental Material Available
1H NMR spectra of 0.73, 0.50, 0.25, and 0.10 M K2(5’-GMP) and 0.56, 0.40, and 0.25 M Rb2(5’-GMP) in D2O at 5 °C. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Gellert M, Lipsett MN, Davies DR. Proc. Natl. Acad. Sci. USA. 1962;48:2013–2018. doi: 10.1073/pnas.48.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chantot JF, Sarocchi M-T, Guschlbauer W. Biochimie. 1972;53:347–354. doi: 10.1016/s0300-9084(71)80101-3. [DOI] [PubMed] [Google Scholar]
- 3.Guschlbauer W, Chantot J-F, Thiele D. J. Biomol. Struct. Dyn. 1990;8:491–511. doi: 10.1080/07391102.1990.10507825. [DOI] [PubMed] [Google Scholar]
- 4.Davis JT. Angew. Chem., Int. Ed. 2004;43:668–698. doi: 10.1002/anie.200300589. [DOI] [PubMed] [Google Scholar]
- 5.Huppert JL. Chem. Soc. Rev. 2008;37:1375–1384. doi: 10.1039/b702491f. [DOI] [PubMed] [Google Scholar]
- 6.Sasisekharan V, Zimmerman SB, Davies DR. J. Mol. Biol. 1975;92:171–179. doi: 10.1016/0022-2836(75)90221-1. [DOI] [PubMed] [Google Scholar]
- 7.Tougard P, Chantot J-F, Guschlbauer W. Biochim. Biophys. Acta. 1973;308:9–16. [PubMed] [Google Scholar]
- 8.Zimmerman SB. J. Mol. Biol. 1976;106:663–672. doi: 10.1016/0022-2836(76)90257-6. [DOI] [PubMed] [Google Scholar]
- 9.Howard FB, Frazier J, Miles HT. Biopolymers. 1975;16:791–809. doi: 10.1002/bip.1977.360160407. [DOI] [PubMed] [Google Scholar]
- 10.Pinnavaia TJ, Miles HT, Becker ED. J. Am. Chem. Soc. 1975;97:7198–7200. doi: 10.1021/ja00857a059. [DOI] [PubMed] [Google Scholar]
- 11.Pinnavaia TJ, Marshall CL, Mettler CM, Fisk CL, Miles HT, Becker ED. J. Am. Chem. Soc. 1978;100:3625–3627. [Google Scholar]
- 12.Borzo M, Detellier C, Laszlo P, Paris AJ. J. Am. Chem. Soc. 1980;102:1124–1134. [Google Scholar]
- 13.Detellier C, Laszlo P. J. Am. Chem. Soc. 1980;102:1135–1141. [Google Scholar]
- 14.Laughlan G, Murchie AIH, Norman DG, Moore MH, Moody PCE, Lilley DMJ, Luisi B. Science. 1994;265:520–524. doi: 10.1126/science.8036494. [DOI] [PubMed] [Google Scholar]
- 15.Phillips K, Dauter Z, Murchie AIH, Lilley DMJ, Luisi B. J. Mol. Biol. 1997;273:171–182. doi: 10.1006/jmbi.1997.1292. [DOI] [PubMed] [Google Scholar]
- 16.Carceres C, Wright G, Gouyette C, Parkinson G, Subirana JA. Nucl. Acids Res. 2004;32:1097–1102. doi: 10.1093/nar/gkh269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horvath MP, Schultz SC. J. Mol. Biol. 2001;310:367–377. doi: 10.1006/jmbi.2001.4766. [DOI] [PubMed] [Google Scholar]
- 18.Haider S, Parkinson GN, Neidle S. J. Mol. Biol. 2002;320:189–200. doi: 10.1016/S0022-2836(02)00428-X. [DOI] [PubMed] [Google Scholar]
- 19.Parkinson GN, Lee MPH, Neidle S. Nature. 2002;417:876–880. doi: 10.1038/nature755. [DOI] [PubMed] [Google Scholar]
- 20.Bouhoutsos-Brown E, Marshall CL, Pinnavaia TJ. J. Am. Chem. Soc. 1982;104:6576–6584. [Google Scholar]
- 21.Walmsley JA, Barr RG, Bouhoutsos-Brown E, Pinnavaia TJ. J. Phys. Chem. 1984;88:2599–2605. [Google Scholar]
- 22.Wu G, Kwan IC. J. Am. Chem. Soc. 2009;131:3180–3182. doi: 10.1021/ja809258y. [DOI] [PubMed] [Google Scholar]
- 23.Hightower JB, Olmos DR, Walmsley JA. J. Phys. Chem. B. 2009;113:12214–12219. doi: 10.1021/jp904383y. [DOI] [PubMed] [Google Scholar]
- 24.Wong A, Ida R, Spindler L, Wu G. J. Am. Chem. Soc. 2005;127:6990–6998. doi: 10.1021/ja042794d. [DOI] [PubMed] [Google Scholar]
- 25.Jurga-Nowak H, Banachowicz E, Dobek A, Patkowski A. J. Phys. Chem. B. 2004;108:2744–2750. [Google Scholar]
- 26.Eimer W, Dorfmüller Th. J. Phys. Chem. 1992;96:6790–6800. [Google Scholar]
- 27.Spindler L, Drevenšek Olenik I, Čopič M, Romih R, Cerar J, Škerjanc J, Mariani P. Eur. Phys. J. E. 2002;7:95–102. doi: 10.1140/epje/e2004-00037-0. [DOI] [PubMed] [Google Scholar]
- 28.Walmsley JA, Burnett JF. Biochemistry. 1999;38:14063–14068. doi: 10.1021/bi9900370. [DOI] [PubMed] [Google Scholar]
- 29.Berova N, Nakanishi K, Woody RW, editors. Circular Dichroism, Principles and Applications, 2nd ed. New York: Wiley-VCH; 2000. [Google Scholar]
- 30.Kypr J, Kejnovská I, Renčiuk D, Vorlíčková M. Nucl. Acids Res. 2009;37:1713–1725. doi: 10.1093/nar/gkp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masiero S, Trotta R, Pieraccini S, De Tito S, Perone R, Randazzo A, Spada GP. Org. Biomol. Chem. 2010;8:2683–2692. doi: 10.1039/c003428b. [DOI] [PubMed] [Google Scholar]
- 32.Paramasivan S, Rujan I, Bolton PH. Methods. 2007;43:324–331. doi: 10.1016/j.ymeth.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Davis JT, Spada GP. Chem. Soc. Revs. 2007;36:296–313. doi: 10.1039/b600282j. [DOI] [PubMed] [Google Scholar]
- 34.Holm AIS, Kohler B, Hoffman SV, Nielsen SB. Biopolymers. 2010;93:429–433. doi: 10.1002/bip.21354. [DOI] [PubMed] [Google Scholar]
- 35.Gottarelli G, Lena S, Masiero S, Pieraccini S, Spada GP. Chirality. 2008;20:471–485. doi: 10.1002/chir.20459. [DOI] [PubMed] [Google Scholar]
- 36.Gottarelli G, Masiero S, Spada GP. Enantiomer. 1998;3:429–438. [Google Scholar]
- 37.Gray DM, Wen J-D, Gray CW, Repges R, Repges C, Raabe G, Fleischhauer J. Chirality. 2008;20:431–440. doi: 10.1002/chir.20455. [DOI] [PubMed] [Google Scholar]
- 38.Abu-Ghazalah RM, Macgregor RB., Jr Biophys. Chem. 2009;141:180–185. doi: 10.1016/j.bpc.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Viglaský V, Bauer L, Tlučková K. Biochemistry. 2010;49:2110–2120. doi: 10.1021/bi902099u. [DOI] [PubMed] [Google Scholar]
- 40.Petraccone L, Erra E, Esposito V, Randazzo A, Mayol L, Nasti L, Barone G, Giancola C. Biochemistry. 2004;43:4877–4884. doi: 10.1021/bi0300985. [DOI] [PubMed] [Google Scholar]
- 41.Lee JY, Yoon J, Kihm HW, Kim DS. Biochemistry. 2008;47:3389–3396. doi: 10.1021/bi702013d. [DOI] [PubMed] [Google Scholar]
- 42.Ambrus A, Chen D, Dai J, Bialis T, Jones RA, Yang D. Nucl. Acids Res. 2006;34:2723–2735. doi: 10.1093/nar/gkl348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottarelli G, Proni G, Spada GP. Liquid Crystals. 1997;22:563–566. [Google Scholar]
- 44.Gottarelli G, Proni G, Spada GP. Enantiomer. 1996;1:201–209. [Google Scholar]
- 45.Gottarelli G, Spada GP. In: Circular Dichroism, 2nd ed. Berova N, Nakanishi K, Wood RW, editors. New York: Wiley-VCH; 2000. pp. 547–561. [Google Scholar]
- 46.Nadler A, Diederichsen U. Eur. J. Org. Chem. 2008:1544–1549. [Google Scholar]
- 47.Petersen SB, Led JJ, Johnston ER, Grant DM. J. Am. Chem. Soc. 1982;104:5007–5015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.